Abstract
Antibiotic resistance and residues in aquaculture are a growing concern worldwide and consequently identifying favorable antibacterial compounds against aquatic pathogenic bacteria are gained more attention. Active compounds derived from marine microorganisms have shown great promise in this area. This review is aimed to make a comprehensive survey of anti-aquatic pathogenic bacterial compounds that were produced by marine microorganisms. A total of 79 compounds have been reported, covering literature from 1997 to 2021. The compounds are included in different structural classes such as polyketides, terpenoids, nitrogen compounds and others, and some of them present the potential to be developed into agents for the treatment of aquatic pathogenic bacteria.
1. Introduction
With the rapid and intensive development of aquaculture, many problems related to aquatic animal diseases and environmental pollution of the water body have gradually been exposed, which has seriously restricted the stable development of aquaculture [1]. The main pathogens of aquatic animals include bacteria, viruses and parasites etc. [2]. It is estimated that China’s annual losses caused by aquatic animal diseases are more than 10 billion yuan. Among them, the economic loss of aquatic animal caused by bacterial diseases accounts for 58%, which is the most serious factor leading to the economic loss of aquaculture [3].
Major bacterial pathogens are Vibrio, Aeromonas, Edwardsiella, Flavobacterium, Pseudomonas, and Micrococcus. Vibrio is the most important class of pathogens for bacterial diseases in marine aquaculture. Vibriosis has the characteristics of widespread area and high incidence. The common pathogenic Vibrio spp. in aquaculture includes Vibrio anguillarum, Vibrio harveyi, Vibrio parahaemolyticus, and Vibrio alginolyticus. V. anguillarum is the earliest studied pathogen with high pathogenicity to fish, and the symptom of infection is mainly sepsis. V. harveyi is a light-emitting bacterium and a main pathogen of aquatic animals, especially in the nursery and growing stages of shrimp [1]. The main symptoms of V. harveyi infection in fish are subcutaneous hemorrhage, redness of the anus, and sides of the skull. V. parahaemolyticus can cause inflammation and congestion on the surface of shrimp and marine fish, such as acute hepatopancreatic necrosis disease [4].
Aeromonas hydrophila is the main pathogen in the genus Aeromonas. Fish infected with A. hydrophila are prone to fulminant bleeding disorders, such as erythematosus of carp and loach, and “printing disease” (rotten skin) of catfish [5]. Edwardsiella tarda and Edwardsiella ictaluri are the most common aquatic pathogens in the genus Edwardsiella. E. tarda and E. ictaluri have been found in a variety of farmed freshwater and marine fish, such as eel, flounder, rainbow trout, and catfish. The disease caused by E. tarda or E. ictaluri is known as enteric septicemia of catfish (ESC) [6]. In addition, yellow mullet, black bass, goldfish, mullet can also be infected.
Flavobacterium columnare is a common pathogenic bacterium in the family Flavobacteriaceae, and usually attacks the skins, fins and gills of fish. The disease caused by F. columnare is often called “columnaris disease” [7]. The symptoms of disease are basically the same, including severe necrosis of gill tissue and skin ulceration from systemic infection. The pathogens of the genus Pseudomonas causing fish diseases are mainly Pseudomonas aeruginosa and Pseudomonas fluorescens etc. Pseudomonas can cause diseases in a variety of aquatic animals, such as hemorrhagic, red skin, rot skin, and ulcer diseases [8]. Micrococcus luteus is the main cause of hemorrhagic disease in Monopterus albus. After infection, the symptoms are diffuse bleeding on the surface, anal swelling and eversion of the anus, and cause high mortality [9].
The antibacterial compounds used in aquaculture are the same used in medicine and veterinary fields. Even though antibiotics are convenient and effective as drugs for the prophylaxis and treatment of bacterial diseases in aquaculture animals, the long-term application or abuse of antibiotics has made antibiotics less and less effective, and mutant pathogens often cause more severe disease. In addition, antibiotic residues in aquatic products directly threaten human health [10]. Therefore, it is urgent to develop new antibacterial agents for aquatic products.
Bio-derived drugs have the advantages of relatively safe, low toxicity, and easy degradation. They are ideal for finding safe and harmless antibacterial raw materials for the aquatic application. At present, marine microorganisms are an important resource for the development of antibacterial agents used in aquaculture. This review article outlines various anti-aquatic pathogenic bacterial molecules produced from marine microorganisms. The availability of these compounds will help develop various applications in the aquaculture field of antibiotics against aquatic bacterial pathogens.
2. Marine Bacterial Compounds against Aquatic Pathogenic Bacteria
Marine microbes, especially bacteria and fungi, are excellent producers of natural products with diverse structures and pharmacological activities, and marine microbes serve as valuable resources in the ongoing search for antibacterial compounds against aquatic pathogens [11,12].
A cyclic lipopeptide N3 produced by B. amyloliquefaciens M1 was identified as surfactin (1, Figure 1). The minimal inhibitory concentration (MIC) of the purified lipopeptide N3 against V. anguillarum was 1.5 μg/mL (Table 1) [13]. 3-(octahydro-9-isopropyl-2H-benzo[h]chromen-4-yl)-2-methylpropyl benzoate (2) and methyl 8-(2-(benzoyloxy)-ethyl)-hexahydro-4-((E)-pent-2-enyl)-2H-chromene-6-carboxylate (3) are two polyketides with activity against Vibrio vulnificus and were isolated from the ethyl acetate extract of B. amyloliquefaciens associated with edible red seaweed, Laurenciae papillosa. The compounds 2 and 3 demonstrated significant antibacterial activity against V. vulnificus (inhibitory zone diameter of 18.00 ± 1.00 mm and 16.67 ± 0.58 mm, 25 mcg on disk) [14]. Three polyketides from Bacillus amyloliquefaciens associated with seaweed Padina gymnospora were characterized as 11-(15-butyl-13-ethyl-tetrahydro-12-oxo-2H-pyran-13-yl) propyl-2-methylbenzoate (4), 9-(tetrahydro-12-isopropyl-11-oxofuran-10-yl)-ethyl-4-ethoxy-2-hydroxybenzoate (5), and 12-(aminomethyl)-11-hydroxyhexanyl-10-phenylpropanoate (6). Compounds 4–6 displayed significant antibacterial activities against V. vulnificus MTCC 1145, A. hydrophila MTCC 646, and V. vulnificus MTCC 1145 with inhibitory zone diameters of 16.33 ± 0.58 mm, 14.67 ± 1.15 mm, and 17.33 ± 1.00 mm (10 mcg on disk), respectively [15].
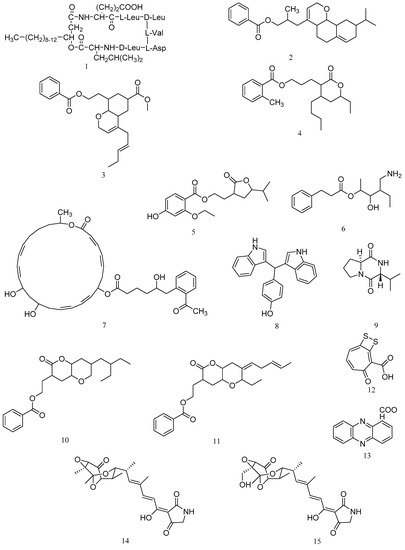
Figure 1.
Structures of marine bacterial compounds against aquatic pathogenic bacteria, surfactin (1), 3-(octahydro-9-isopropyl-2H-benzo[h]chromen-4-yl)-2-methylpropyl benzoate (2), methyl 8-(2-(benzoyloxy)-ethyl)-hexahydro-4-((E)-pent-2-enyl)-2H-chromene-6-carboxylate (3), 11-(15-butyl-13-ethyl-tetrahydro-12-oxo-2H-pyran-13-yl) propyl-2-methylbenzoate (4), 9-(tetrahydro-12-isopropyl-11-oxofuran-10-yl)-ethyl-4-ethoxy-2-hydroxybenzoate (5), 12-(aminomethyl)-11-hydroxyhexanyl-10-phenylpropanoate (6), 7-O-6′-(2”-acetylphenyl)-5′-hydroxyhexanoate-macrolactin (7), 7,7-bis(3-indolyl)-p-cresol (8), cyclo-(S-Pro-R-Val) (9), 2-(7-(2-Ethylbutyl)-2,3,4,4a,6,7-hexahydro-2-oxopyrano-[3,2b]-pyran-3-yl)-ethyl benzoate (10), 2-((4Z)-2-ethyl-octahydro-6-oxo-3-((E)-pent-3-enylidene)-pyrano-[3,2b]-pyran-7-yl)-ethyl benzoate (11), tropodithietic acid (12), phenazine-1-carboxylic acid (13), tirandamycin A (14), and tirandamycin B (15).

Table 1.
Marine bacterial compounds against aquatic pathogenic bacteria.
Antibacterial aryl-crowned polyketide, 7-O-6′-(2”-acetylphenyl)-5′-hydroxyhexanoate-macrolactin (7) was isolated from Bacillus subtilis MTCC 10,403 associated with brown seaweed Anthophycus longifolius. The MIC assay showed that compound 7 displayed potential antibacterial activities against significant Gram-negative pathogens with MIC of 3.12 μg/mL against V. vulnificus, 6.25 μg/mL against A. hydrophilla, 12.5 μg/mL against V. parahaemolyticus and P. aeruginosa [16]. Two compounds including 7,7-bis(3-indolyl)-p-cresol (8) and cyclo-(S-Pro-R-Val) (9) were isolated from the strain of Bacillus megaterium LC derived from the marine sponge Haliclona oculata. Compound 8 displayed antibacterial activity at MIC values of 0.05 μg/mL and 0.005 μg/mL against V. vulnificus and M. luteus. Compound 9 showed antimicrobial activity at MIC value of 0.05 μg/mL against V. parahaemolyticus [17].
O-heterocyclic derivatives with antibacterial properties were isolated from B. subtilis MTCC 10,407 associated with brown seaweed Sargassum myriocystum, and identified as 2-(7-(2-Ethylbutyl)-2,3,4,4a,6,7-hexahydro-2-oxopyrano-[3,2b]-pyran-3-yl)-ethyl benzoate (10) and 2-((4Z)-2-ethyl-octahydro-6-oxo-3-((E)-pent-3-enylidene)-pyrano-[3,2b]-pyran-7-yl)-ethyl benzoate (11). Compounds 10 and 11 showed significant antibacterial activity (inhibitory zone diameters of 17.66 ± 0.58 mm and 15.3 ± 1.0 mm, 10 μg on disk) against A. hydrophilla [18].
An antimicrobial compound produced by Pseudovibrio sp. P12, a common and abundant coral-associated bacterium, was identified as tropodithietic acid (12), with the MIC value of 0.5 μg/mL against Vibrio coralliilyticus and Vibrio owensii [19]. A phenazine derivative against V. anguillarum was isolated from Pseudomonas aeruginosa strain PA31x and demonstrated to be phenazine-1-carboxylic acid (13) with the MIC value of 50 μg/mL for V. anguillarum [20]. Tirandamycins A (14) and B (15) were isolated from the crude extract of Streptomyces tirandamycinicus sp. nov., a novel marine sponge-derived actinobacterium. Compounds 14 and 15 showed potent antibacterial activity against Streptococcus agalactiae with MIC values of 2.52 and 2.55 μg/mL, respectively [21].
3. Marine Fungal Compounds against Aquatic Pathogenic Bacteria
3.1. Marine Aspergillus
Marine fungi have become the main source of natural products of marine microorganisms due to their complex genetic background, structural diversity and high yields of metabolites. New natural products derived from marine fungi account for about 60% of total marine microbial new natural products and the most studied genera are Aspergillus and Penicillium [22].
A bisabolane-type sesquiterpenoid, (−)-sydonic acid (16), was isolated from marine-derived fungus Aspergillus sp. associated with the sponge Xestospongia testudinaria (Figure 2). Compound 16 exhibited significant inhibiting activity against V. Parahaemolyticus and V. anguillarum with MIC values of 10.0 and 5.00 μM (Table 2) [23]. A new polyketide, asperochrin A (17), was isolated from Aspergillus ochraceus MA-15, which was isolated from the rhizospheric soil of marine mangrove plant Bruguiera gymnorrhiza. Compound 17 displayed significant antibacterial activity against A. hydrophilia, V. anguillarum and V. harveyi, with MIC values of 8 μg/mL, 16 μg/mL and 8 μg/mL, respectively [24]. A new prenylated phenol derivative, terreprenphenol A (18), was isolated from Aspergillus terreus EN-539, which was obtained from the marine red alga Laurencia okamurai. Compound 18 displayed potent activity against A. hydrophila, P. aeruginosa, and V. harveyi with MIC values of 2, 2, and 4 μg/mL, respectively [25].
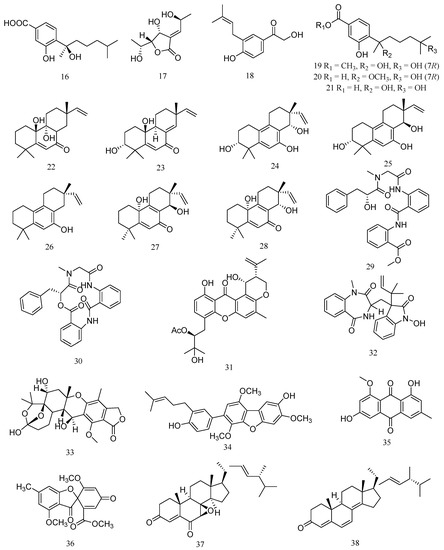
Figure 2.
Structures of marine Aspergillus-derived compounds against aquatic pathogenic bacteria, (−)-sydonic acid (16), asperochrin A (17), terreprenphenol A (18), ent-aspergoterpenin C (19), 7-O-methylhydroxysydonic acid (20), hydroxysydonic acid (21), aspewentin D (22), aspewentin F (23), aspewentin G (24), aspewentin H (25), aspewentin A (26), aspewentin I (27), aspewentin J (28), seco-clavatustide B (29), clavatustide B (30), aspergixanthone I (31), 3-((1-hydroxy-3-(2-methylbut-3-en-2-yl)-2-oxoindolin-3-yl)methyl)-1-methyl-3,4-dihydrobenzo[e][1,4]diazepine-2,5-dione (32), austalide R (33), 4-methyl-3”-prenylcandidusin A (34), questin (35), trypacidin (36), 7β,8β-epoxy-(22E,24R)-24-methylcholesta-4,22-diene-3,6-dione (37), and ergosta-4, 6, 8(14), 22-tetraene-3-one (38).

Table 2.
Marine Aspergillus-derived compounds against aquatic pathogenic bacteria.
Two new bisabolane-type sesquiterpenoid derivatives, ent-aspergoterpenin C (19) and 7-O-methylhydroxysydonic acid (20), and a known bisabolane sesquiterpenoid, hydroxysydonic acid (21), were isolated from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Compound 19 exhibited antibacterial activities against E. tarda, P. aeruginosa, V. harveyi, and V. parahaemolyticus with MIC value of 8.0 μg/mL. Compound 20 exhibited antibacterial activities against E. tarda, V. anguillarum, A. hydrophilia, V. harveyi, and V. parahaemolyticus with MIC value of 8.0 μg/mL. Compound 21 exhibited more potent activities against A. hydrophilia, E. tarda, V. anguillarum and V. harveyi with MIC value of 4.0 μg/mL [26].
Four new 20-nor-isopimarane diterpenoids, aspewentins D, F, G and H (22–25), and a known congener, aspewentin A (26), were isolated from the deep-sea sediment-derived Aspergillus wentii SD-310. Compounds 22–26 showed inhibitory activity against the aquatic pathogens M. luteus, E. tarda, V. harveyi, P. aeruginosa, and V. parahemolyticus with MIC value of 4.0 μg/mL [27]. Meanwhile, two uncommon 20-nor-isopimarane diterpenoid epimers, aspewentin I (27) and aspewentin J (28) were also isolated from A. wentii SD-310. Compounds 27 and 28 showed antibacterial activities against E. tarda, V. harveyi, and V. parahaemolyticus with MIC value of 8.0 μg/mL [28].
Two aminobenzoic peptide, seco-clavatustide B (29) and clavatustide B (30), were characterized from the Ascidian-derived endophytic fungus Aspergillus clavatus AS-107. Compounds 29 exhibited potent activity against A. hydrophilia, with a MIC value of 8.2 μM, while compound 30 showed antibacterial activity against P. aeruginosa, with a MIC value of 8.8 μM [29]. A new prenylxanthone derivative, aspergixanthone I (31), was isolated from the marine-derived fungus Aspergillus sp. ZA-01. Compound 31 showed the strongest antibacterial activity against V. parahemolyticus (MIC = 1.56 μM), V. anguillarum (MIC = 1.56 μM) and V. alginolyticus (MIC = 3.12 μM) [30].
A new tryptophan derived alkaloid, 3-((1-hydroxy-3-(2-methylbut-3-en-2-yl)-2-oxoindolin-3-yl)methyl)-1-methyl-3,4-dihydrobenzo[e][1,4]diazepine-2,5-dione (32), and a new meroterpenoid, austalide R (33), were isolated from the fungus Aspergillus sp., isolated from the Mediterranean sponge Tethya aurantium. Compound 32 showed significant antibacterial activities against V. harveyi and V. natriegens, with MIC value of 1 μg/mL. Compound 33 displayed the better potential activity against V. harveyi with a MIC value of 0.1 μg/mL [31]. A prenylcandidusin derivative, 4-methyl-3”-prenylcandidusin A (34), was isolated from the coral-derived fungus Aspergillus tritici SP2-8-1. Compound 34 displayed stronger antibacterial activities against strains of V. vulnificus, V. rotiferianus, and V. campbellii, with MIC values ranging from 7 to 15 μg/mL [32].
Bioassay-guided fractionation resulted in the isolation of an antibacterial compound against V. harveyi, questin (35), from the marine-derived Aspergillus flavipes strain HN4-13. Compound 35 exhibited the same anti-V. harveyi activity as streptomycin sulfate (MIC 31.25 μg/mL) [1]. Trypacidin (36) was isolated from Aspergillus fumigatus HX-1 associated with Clams. Compound 36 showed the same anti-V. harveyi activity as streptomycin sulfate, with a MIC value of 31.25 µg/mL [33]. 7β,8β-epoxy-(22E,24R)-24-methylcholesta-4,22-diene-3,6-dione (37) and ergosta-4, 6, 8(14), 22-tetraene-3-one (38) were steroids isolated from the deep sea-derived fungus Aspergillus penicillioides SD-311. Compound 37 showed antibacterial activity against V. anguillarum with MIC value of 32.0 µg/mL. Compound 38 exhibited inhibitory activity against E. tarda and M. luteus, with MIC value of 16 μg/mL [34].
3.2. Marine Penicillium
Two new phenolic bisabolane sesquiterpenes, peniciaculins A (39) and B (40), a new nor-bisabolane derivative, 1-hydroxyboivinianin A (41), and a known bisabolene, (7S,11S)-(+)-12-hydroxysydonic acid (42), were isolated from the deep-sea sediment-derived Penicillium aculeatum SD-321 (Figure 3). Compound 39 exhibited antibacterial activity against V. alginolyticus with MIC value of 2.0 μg/mL, while compounds 40 and 41 showed inhibitory activity against E. tarda and V. harveyi, with MIC values of 8.0 and 4.0 μg/mL, respectively. Compound 42 exhibited significant antibacterial activity against V. parahemolyticus, with MIC value of 0.5 μg/mL (Table 3) [35].
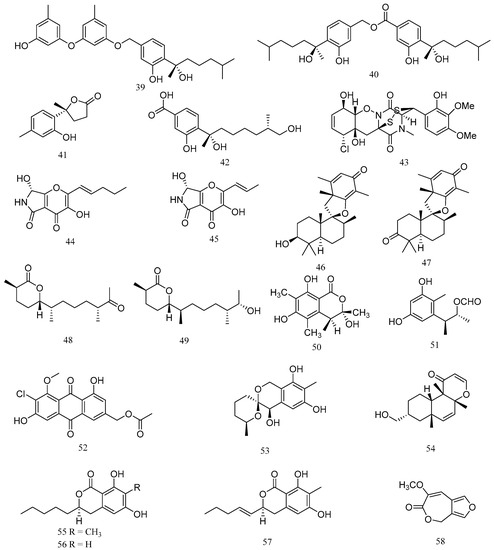
Figure 3.
Structures of marine Penicillium-derived compounds against aquatic pathogenic bacteria, peniciaculin A (39), peniciaculin B (40), 1-hydroxyboivinianin A (41), (7S,11S)-(+)-12-hydroxysydonic acid (42), adametizine A (43), pyranonigrin F (44), pyranonigrin A (45), chermesin A (46), chermesin B (47), chermesiterpenoid B (48), chermesiterpenoid C (49), (3S,4S)-sclerotinin A (50), citrinin H2 (51), 20-acetoxy-7-chlorocitreorosein (52), 9-dehydroxysargassopenilline A (53), 1,2-didehydropeaurantiogriseol E (54), penicisimpin A (55), penicisimpin B (56), penicisimpin C (57), and penicillilactone A (58).

Table 3.
Marine Penicillium-derived compounds against aquatic pathogenic bacteria.
A new bisthiodiketopiperazine derivative, adametizine A (43), was isolated from marine-sponge derived fungus Penicillium adametzioides AS-53. Compound 43 showed antibacterial activities against A. hydrophilia, V. harveyi and V. parahaemolyticus, with MIC values of 8, 32, and 8 μg/mL, respectively [36]. A new polyAS-53-dione derivative, pyranonigrin F (44), and a related known compound, pyranonigrin A (45), were isolated from an endophytic fungus Penicillium brocae MA-231, which was obtained from the fresh tissue of the marine mangrove plant Avicennia marina. Compounds 44 and 45 displayed significant activity against V. harveyi and V. parahaemolyticus with MIC values of 0.5 μg/mL [37].
Two new spiromeroterpenoids, chermesins A (46) and B (47), were isolated from an endophytic fungus Penicillium chermesinum EN-480, which was isolated from the inner tissue of the marine red alga Pterocladiella tenuis. Compounds 46 and 47 displayed significant activity against M. luteus, with MIC value of 8 μg/mL [38]. Meanwhile, two new sesquiterpenoids, chermesiterpenoids B (48) and C (49), were isolated from P. chermesinum EN-480. Compound 48 and 49 exhibited antibacterial activities against V. anguillarum, V. parahaemolyticus and M. luteus, with MIC values of 0.5, 16, and 64 μg/mL, and 1, 32, and 64 μg/mL, respectively [39].
(3S,4S)-sclerotinin A (50) and citrinin H2 (51) were isolated from the deep sea-derived fungus Penicillium citrinum NLG-S01-P1. Compounds 50 and 51 displayed relatively stronger activities against V. vulnificus and V. campbellii, with MIC values ranging from 15 to 17 μg/mL, respectively [40]. A new chlorinated metabolite, 20-acetoxy-7-chlorocitreorosein (52), was isolated from Penicillium citrinum HL-5126, an endophytic fungus that was isolated from the mangrove Bruguiera sexangula var. rhynchopetala collected in the South China Sea. Compound 52 exhibited antibacterial activity against V. parahaemolyticus, with a MIC value of 10 μM [41]. Two new polyketide derivatives, 9-dehydroxysargassopenilline A (53) and 1,2-didehydropeaurantiogriseol E (54), were isolated from the deep sea-derived fungus Penicillium cyclopium SD-413. Compounds 53 and 54 exhibited inhibitory activities against E. tarda, M. luteus, V. anguillarum, and V. harveyi, with MIC values ranging from 4 to 32 μg/mL [42].
Three new dihydroisocoumarin derivatives, penicisimpins A–C (55–57), were isolated from Penicillium simplicissimum MA-332, a fungus that was isolated from the rhizosphere of the marine mangrove plant Bruguiera sexangula var. rhynchopetala. Compounds 55–57 exhibited broad-spectrum inhibitory activities with various MIC values ranging from 4 to >64 mg/mL, with compound 55 showing highest activities against P. aeruginosa, V. parahaemolyticus, and V. harveyi, with MIC value of 4 μg/mL, while compounds 56 and 57 exhibited moderate activities against the tested strains [43]. One novel 7-membered lactone derivative, penicillilactone A (58), was isolated from the sponge-derived fungus Penicillium sp. LS54. Compound 58 showed antibacterial activity against V. harveyi, with a MIC value of 8 μg/mL [44].
3.3. Marine Fungi Belonging to Genera Other Than Aspergillus and Penicillium
Four new cladosporol derivatives, cladosporols F−I (59−62), the known cladosporol C (63), and its new epimer, cladosporol J (64) (Figure 4), were isolated from the marine algal-derived endophytic fungus Cladosporium cladosporioides EN-399. Compounds 59−64 showed inhibitory activities against M. luteus and V. harveyi, with MIC values of 4−128 μg/mL (Table 4) [45]. Pandangolide 1 (65) was isolated from Cladosporium cladosporioides MA-299, an endophytic fungus that was isolated from the leaves of the mangrove plant Bruguiera gymnorrhiza. Compound 65 exhibited inhibitory activity against E. ictalurid, with MIC value of 4.0 μg/mL [46]. Meanwhile, two new 12-membered macrolides, thiocladospolides A (66) and D (67), were isolated from the same strain, C. cladosporioides MA-299. Compounds 66 and 67 exhibited significant activity against E. tarda and E. ictalurid, with MIC value of 1 μg/mL, respectively [47]. Furthermore, Cladocladosin A (68), along with two new sulfur-containing macrolides, thiocladospolides F and G (69 and 70), were obtained from C. cladosporioides MA-299. Compounds 68-70 showed antibacterial activities against E. tarda and V. anguillarum, with MIC values ranging from 1.0 to 4.0 μg/mL [48].
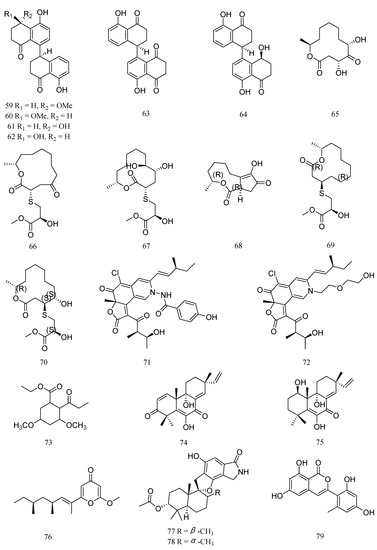
Figure 4.
Structures of anti-aquatic pathogenic bacterial compounds isolated from marine fungi belonging to genera other than Aspergillus and Penicillium, cladosporol F (59), cladosporol G (60), cladosporol H (61), cladosporol I (62), cladosporol C (63), cladosporol J (64), pandangolide 1 (65), thiocladospolide A (66), thiocladospolide D (67), cladocladosin A (68), thiocladospolide F (69), thiocladospolide G (70), chaetoviridide A (71), chaetoviridide B (72), ethyl 3,5-dimethoxy-2-propionylphenylacetate (73), libertellenone M (74), libertellenone A (75), fusolanone B (76), stachybomycin E (77), stachybotrylactam acetate (78), and trichophenol A (79).

Table 4.
Anti-aquatic pathogenic bacterial compounds isolated from marine fungi belonging to genera other than Aspergillus and Penicillium.
Two novel compounds, chaetoviridides A (71) and B (72), were isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Compounds 71 and 72 exhibited relatively stronger activities against V. rotiferianus and V. vulnificus, with MIC values ranging from 7 to 8 μg/mL, respectively [49]. Ethyl 3,5-dimethoxy-2-propionylphenylacetate (73) was isolated from the fermentation of Engyodontium album derived from deep sea sediment. Compound 73 showed inhibitory activities against V. vulnificus with MIC value of 15.6 μg/mL [50]. Two pimarane diterpenes, libertellenones M (74) and A (75) were isolated from the culture extract of Eutypella sp. D-1 derived from high-latitude soil of the Arctic. Compound 74 and 75 displayed inhibitory activity against V. vulnificus, with a MIC value of 16 μg/mL [51].
Fusolanone B (76) was isolated from a mangrove endophytic fungus Fusarium solani HDN15-410. Compound 76 showed inhibitory activity against V. parahaemolyticus, with a MIC value of 6.25 μg/mL [52]. A new phenylspirodrimane, stachybomycin E (77), and a known compound, stachybotrylactam acetate (78), were isolated from the marine-derived fungus Stachybotrys sp. SCSIO 40434. Compounds 77 and 78 showed moderate antibacterial activities against M. luteus SCSIO ML01, with a MIC value of 8 μg/mL [53]. One new isocoumarin derivative, trichophenol A (79), was isolated from Trichoderma citrinoviride A-WH-20-3, an endophyte from the marine red alga Laurencia okamurai. Compound 79 displayed inhibitory activity against Pseudoalteromonas citrea, with a MIC of 16 μg/mL [54].
4. Concluding Remarks and Future Prospects
Since Cephalosporins were isolated from the secondary metabolites of marine-derived fungus Acremonium chrysogenum in 1945, especially since the 1990s, more than 20,000 inspirational natural products with diverse structures and potential bioactivities have been discovered in marine microbes [55]. From our literature review, although marine microbial secondary metabolites have been shown to have diverse biological activities [11,56], there are relatively few reports evaluating their antibacterial activity against aquatic pathogens [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Among the 79 active molecules against aquatic bacterial pathogens, there are only 15 compounds derived from marine bacteria, accounting for 19%. In contrast, antibacterial compounds derived from marine fungi accounted for more than 80%, and were isolated mainly from two genera Aspergillus (23, 29%) and Penicillium (20, 25%) (Figure 5). When it comes to structural classes, polyketides, terpenoids and nitrogen-containing compounds are the three major structural types of marine microbial-derived anti-aquatic pathogenic bacterial active molecules, accounting for 57%, 25%, and 15%, respectively (Figure 6).
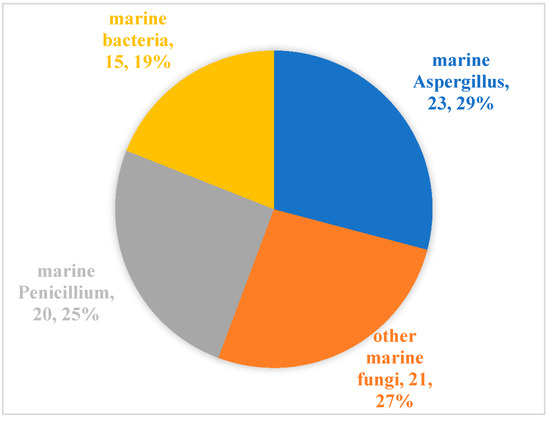
Figure 5.
Distribution of marine microorganisms capable of producing active molecules against aquatic pathogenic bacteria.
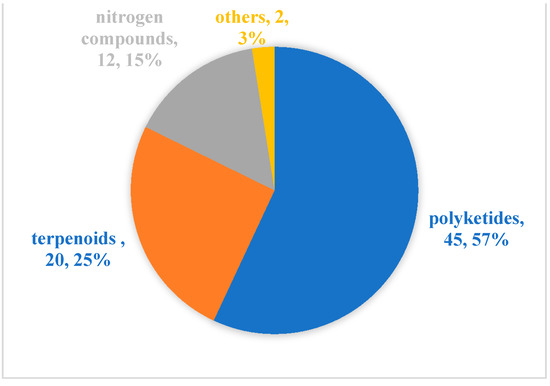
Figure 6.
Structural distribution of the anti-aquatic pathogenic bacterial molecules.
As reported in this review, some marine microbial-derived natural products have good potential against aquatic bacterial pathogens, but there are very few in-depth reports of the in vivo antibacterial efficacy and safety of active molecules [20]. Therefore, further research should focus on the in vivo bacteriostatic effect and safety of marine microbial-derived active compounds against aquatic pathogens in this field.
At present, a limited number of reports on the mechanism of action of anti-aquatic pathogenic active compounds have focused on the changes in bacterial morphology, the inhibition of growth, and the damage in cell membrane and cell wall [13,16,57]. More research is needed to study the mechanism of action of the compounds at the molecular or genetic level.
In conclusion, based on the huge demand for environmentally friendly antibiotic alternatives in the aquaculture industry, further research should deeply evaluate the antibacterial efficacy and safety of marine microbial active molecules in vivo, and investigate their mechanism of action.
Author Contributions
Writing—original draft preparation, S.G. and Z.Z.; writing—review and editing, L.G.; funding acquisition, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Postgraduate Research and Practice Innovation Program of Jiangsu Province (SJCX21_1483).
Institutional Review Board Statement
Not applicable.
Acknowledgments
We thank Fei Zhang for her technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, L.; Wang, C. Optimized production and isolation of antibacterial agent from marine Aspergillus flavipes against Vibrio harveyi. 3 Biotech 2017, 7, 383. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Zhu, W.; Xu, F. Bioassay-guided fractionation and identification of active substances from the fungus Aspergillus tubingensis against Vibrio anguillarum. Biotechnol. Biotechnol. Equip. 2016, 30, 602–606. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Lu, Y.; Wang, L.; Li, X.; Li, S.; Che, J.; Xu, Y. Research progress of antibiotic alternatives therapy against bacteriosis in aquaculture. Feed Livest. 2015, 4, 18–22. (In Chinese) [Google Scholar]
- Ge, H.; Ni, Q.; Chen, Z.; Li, J.; Zhao, F. Effects of short period feeding polysaccharides from marine macroalga, Ulva prolifera on growth and resistance of Litopenaeus vannamei against Vibrio parahaemolyticus infection. J. Appl. Phycol. 2019, 31, 2085–2092. [Google Scholar] [CrossRef]
- Xu, F.; Peng, L.; Cai, T.; Hu, L.; Zhou, Z.; Zhu, S. Research progress of the antimicrobial effects of Chinese herbal medicines to aquatic animal pathogens. J. Aquac. 2016, 37, 48–52. (In Chinese) [Google Scholar]
- Wagner, B.A. The epidemiology of bacterial diseases in food-size channel catfish. J. Aquat. Anim. Health 2002, 14, 263–272. [Google Scholar] [CrossRef]
- Schrader, K.K.; Ibrahim, M.A.; Abd-Alla, H.I.; Cantrell, C.L.; Pasco, D.S. Antibacterial activities of metabolites from Vitis rotundifolia (Muscadine) roots against fish pathogenic bacteria. Molecules 2018, 23, 2761. [Google Scholar] [CrossRef]
- Teng, T.; Liang, L.G.; Xie, J. Research progress on pathogenic bacteria of conventional freshwater fish. Jiangsu Agric. Sci. 2015, 43, 8–12. (In Chinese) [Google Scholar]
- Peng, B.; Yang, G.Y.; Chen, X.L.; Zhan, A.S.; He, M.L. Isolation and identification of Micrococcus luteus in rice field eel (Monopterus albus) in Sichuan province. J. Shanghai Ocean Univ. 2011, 20, 405–411. (In Chinese) [Google Scholar]
- Guo, L.; Guo, J.; Xu, F. Optimized extraction process and identification of antibacterial substances from Rhubarb against aquatic pathogenic Vibrio harveyi. 3 Biotech 2017, 7, 377. [Google Scholar] [CrossRef]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current status and future prospects of marine natural products (MNPs) as antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, L.; Li, X.; Xu, X.; Guo, J.; Wang, X.; Yang, W.; Xu, F.; Li, F. Enhanced production of questin by marine-derived Aspergillus flavipes HN4-13. 3 Biotech 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.M.; Rong, Y.J.; Zhao, M.X.; Song, B.; Chi, Z.M. Antibacterial activity of the lipopetides produced by Bacillus amyloliquefaciens M1 against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Appl. Microbiol. Biotechnol. 2014, 98, 127–136. [Google Scholar] [CrossRef]
- Chakraborty, K.; Thilakan, B.; Raola, V.K.; Joy, M. Antibacterial polyketides from Bacillus amyloliquefaciens associated with edible red seaweed Laurenciae papillosa. Food Chem. 2017, 218, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Previously undescribed antibacterial polyketides from heterotrophic Bacillus amyloliquefaciens associated with seaweed Padina gymnospora. Appl. Biochem. Biotechnol. 2018, 184, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Thilakan, B.; Kizhakkekalam, V.K. Antibacterial aryl-crowned polyketide from Bacillus subtilis associated with seaweed Anthophycus longifolius. J. Appl. Microbiol. 2018, 124, 108–125. [Google Scholar] [CrossRef]
- Cuong, P.V.; Cuc, N.T.K.; Quyen, V.T.; Binh, P.T.; Kiem, P.V.; Nam, N.H.; Dat, N.T. Antimicrobial constituents from the Bacillus megaterium IC isolated from marine sponge Haliclona oculata. Nat. Prod. Sci. 2014, 20, 202–205. [Google Scholar]
- Chakraborty, K.; Thilakan, B.; Chakraborty, R.D.; Raola, V.K.; Joy, M. O-heterocyclic derivatives with antibacterial properties from marine bacterium Bacillus subtilis associated with seaweed, Sargassum myriocystum. Appl. Microbiol. Biotechnol. 2017, 101, 569–583. [Google Scholar] [CrossRef]
- Raina, J.B.; Tapiolas, D.; Motti, C.A.; Foret, S.; Seemann, T.; Tebben, J.; Willis, B.L.; Bourne, D.G. Isolation of an antimicrobial compound produced by bacteria associated with reef-building corals. PeerJ 2016, 4, e2275. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, X.; Kuang, S.; Liu, G.; Zhang, C.; Sun, C. Antagonistic activity and mode of action of phenazine-1-carboxylic acid, produced by marine bacterium Pseudomonas aeruginosa PA31x, against Vibrio anguillarum in vitro and in a Zebrafish in vivo model. Front. Microbiol. 2017, 8, 289. [Google Scholar] [CrossRef]
- Huang, X.; Kong, F.; Zhou, S.; Huang, D.; Zheng, J.; Zhu, W. Streptomyces tirandamycinicus sp. nov. a novel marine sponge-derived actinobacterium with antibacterial potential against Streptococcus agalactiae. Front. Microbiol. 2019, 10, 482. [Google Scholar] [CrossRef]
- Guo, L.; Cao, X.; Yang, S.; Wang, X.; Wen, Y.; Zhang, F.; Chen, H.; Wang, L. Characterization, solubility and antibacterial activity of inclusion complex of questin with hydroxypropyl-β-cyclodextrin. 3 Biotech 2019, 9, 123. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Shao, C.L.; Yang, R.Y.; Zheng, C.J.; Chen, Y.Y.; Fu, X.M.; Qian, P.Y.; She, Z.G.; de Voogd, N.J.; et al. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.M.; Meng, L.H.; Wang, B.G. Polyketides from the marine mangrove-derived fungus Aspergillus ochraceus MA-15 and their activity against aquatic pathogenic bacteria. Phytochem. Lett. 2015, 12, 232–236. [Google Scholar]
- Li, H.L.; Li, X.M.; Yang, S.Q.; Meng, L.H.; Li, X.; Wang, B.G. Prenylated phenol and benzofuran derivatives from Aspergillus terreus EN-539, an endophytic fungus derived from marine red alga Laurencia okamurai. Mar. Drugs 2019, 17, 605. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Li, X.M.; Yin, X.L.; Li, X.; Wang, B.G. Antimicrobial sesquiterpenoid derivatives and monoterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Mar. Drugs 2019, 17, 563. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Li, X.M.; Li, X.; Xu, G.M.; Liu, Y.; Wang, B.G. Aspewentins D-H, 20-nor-isopimarane derivatives from the deep sea sediment-derived fungus Aspergillus wentii SD-310. J. Nat. Prod. 2016, 79, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Li, X.; Li, X.M.; Xu, G.M.; Liu, Y.; Wang, B.G. 20-nor-isopimarane epimers produced by Aspergillus wentii SD-310, a fungal strain obtained from deep sea sediment. Mar. Drugs 2018, 16, 440. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, X.M.; Hu, X.Y.; Li, X.; Chi, L.P.; Li, H.L.; Wang, B.G. Antibacterial metabolites from Ascidian-derived fungus Aspergillus clavatus AS-107. Phytochem. Lett. 2019, 34, 30–34. [Google Scholar] [CrossRef]
- Zhu, A.; Zhang, X.W.; Zhang, M.; Li, W.; Ma, Z.Y.; Zhu, H.J.; Cao, F. Aspergixanthones I-K, new anti-Vibrio prenylxanthones from the marine-derived fungus Aspergillus sp. ZA-01. Mar. Drugs 2018, 16, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Debbab, A.; Wray, V.; Lin, W.H.; Schulz, B.; Trepos, R.; Pile, C.; Hellio, C.; Proksch, P.; Aly, A.H. Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014, 55, 2789–2792. [Google Scholar] [CrossRef]
- Wang, W.; Liao, Y.; Tang, C.; Huang, X.; Luo, Z.; Chen, J.; Cai, P. Cytotoxic and antibacterial compounds from the coral-derived fungus Aspergillus tritici SP2-8-1. Mar. Drugs 2017, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, S.; Chen, H.; Zhang, Z.; Li, X.; Wang, W.; Guo, L. Bioassay-guided isolation and characterization of antibacterial compound from Aspergillus fumigatus HX-1 associated with Clam. 3 Biotech 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Yang, S.Q.; Li, X.M.; Li, X.D.; Wang, B.G.; Li, X. A new steroid with 7β,8β-epoxidation from the deep sea-derived fungus Aspergillus penicillioides SD-311. J. Asian Nat. Prod. Res. 2021, 23, 884–891. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.M.; Xu, G.M.; Zhang, P.; Wang, B.G. Antimicrobial phenolic bisabolanes and related derivatives from Penicillium aculeatum SD-321, a deep sea sediment-derived fungus. J. Nat. Prod. 2015, 78, 844–849. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.M.; Meng, L.H.; Jiang, W.L.; Xu, G.M.; Huang, C.G.; Wang, B.G. Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J. Nat. Prod. 2015, 78, 1294–1299. [Google Scholar] [CrossRef]
- Meng, L.H.; Li, X.M.; Liu, Y.; Wang, B.G. Polyoxygenated dihydropyrano [2,3-c]pyrrole-4,5-dione derivatives from the marine mangrove-derived endophytic fungus Penicillium brocae MA-231 and their antimicrobial activity. Chin. Chem. Lett. 2015, 26, 610–612. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.M.; Liu, Y.; Zhang, P.; Wang, J.N.; Wang, B.G. Chermesins A-D: Meroterpenoids with a drimane-type spirosesquiterpene skeleton from the marine algal-derived endophytic fungus Penicillium chermesinum EN-480. J. Nat. Prod. 2016, 79, 806–811. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, X.M.; Yang, S.Q.; Liu, H.; Meng, L.H.; Wang, B.G. Three new sesquiterpenoids from the algal-derived fungus Penicillium chermesinum EN-480. Mar. Drugs 2020, 18, 194. [Google Scholar] [CrossRef]
- Wang, W.; Liao, Y.; Zhang, B.; Gao, M.; Ke, W.; Li, F.; Shao, Z. Citrinin monomer and dimer derivatives with antibacterial and cytotoxic activities isolated from the deep sea-derived fungus Penicillium citrinum NLG-S01-P1. Mar. Drugs 2019, 17, 46. [Google Scholar] [CrossRef]
- He, K.Y.; Zhang, C.; Duan, Y.; Huang, G.L.; Yang, C.Y.; Lu, X.R.; Zheng, C.J.; Chen, G.Y. New chlorinated xanthone and anthraquinone produced by a mangrove-derived fungus Penicillium citrinum HL-5126. J. Antibiot. 2017, 70, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Li, X.M.; Li, X.; Yang, S.Q.; Shi, X.S.; Li, H.L.; Wang, B.G. Antibacterial alkaloids and polyketide derivatives from the deep sea-derived fungus Penicillium cyclopium SD-413. Mar. Drugs 2020, 18, 553. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, X.M.; Wang, B.G. Penicisimpins A–C, three new dihydroisocoumarins from Penicillium. Phytochem. Lett. 2016, 17, 114–118. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, L.; Fang, F.; He, S. Penicillilactone A, a novel antibacterial 7-membered lactone derivative from the sponge-associated fungus Penicillium sp. LS54. Nat. Prod. Res. 2019, 33, 2466–2470. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Mándi, A.; Antus, S.; Li, X.; Zhang, P.; Liu, Y.; Kurtán, T.; Wang, B.G. Characterization of cladosporols from the marine algal-derived endophytic fungus Cladosporium cladosporioides EN-399 and configurational revision of the previously reported Cladosporol derivatives. J. Org. Chem. 2017, 82, 9946–9954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Z.; Li, X.M.; Li, X.; Yang, S.Q.; Meng, L.H.; Wang, B.G. Polyketides from the mangrove-derived endophytic fungus Cladosporium cladosporioides. Mar. Drugs 2019, 17, 296. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Li, X.M.; Yang, S.Q.; Meng, L.H.; Wang, B.G. Thiocladospolides A-D, 12-membered macrolides from the mangrove-derived endophytic fungus Cladosporium cladosporioides MA-299 and structure revision of pandangolide 3. J. Nat. Prod. 2019, 82, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Z.; Li, X.M.; Meng, L.H.; Wang, B.G. Cladocladosin A, an unusual macrolide with bicyclo 5/9 ring system, and two thiomacrolides from the marine mangrove-derived endophytic fungus, Cladosporium cladosporioides MA-299. Bioorg. Chem. 2020, 101, 103950. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liao, Y.; Chen, R.; Hou, Y.; Ke, W.; Zhang, B.; Gao, M.; Shao, Z.; Chen, J.; Li, F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar. Drugs 2018, 16, 61. [Google Scholar] [CrossRef]
- Wang, W.; Chen, R.; Luo, Z.; Wang, W.; Chen, J. Two new benzoate derivatives and one new phenylacetate derivative from a marine-derived fungus Engyodontium album. Nat. Prod. Res. 2017, 31, 758–764. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Wang, B. Bioactive pimarane diterpenes from the Arctic fungus Eutypella sp. D-1. Chem. Biodivers. 2018, 15, 1700501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qiao, L.; Zhang, X.; Sun, C.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Fusaricates H-K and fusolanones A-B from a mangrove endophytic fungus Fusarium solani HDN15-410. Phytochemistry 2019, 158, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Mou, P.; Zhang, Q.; Peng, J.; Jiang, X.; Zhang, L.; Zhou, Z.; Zhang, C.; Zhu, Y. Antibacterial phenylspirodrimanes from the marine-derived fungus Stachybotrys sp. SCSIO 40434. Fitoterapia 2021, 152, 104937. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Hou, X.L.; Song, Y.P.; Wang, B.G.; Ji, N.Y. Cyclonerane sesquiterpenes and an isocoumarin derivative from the marine-alga-endophytic fungus Trichoderma citrinoviride A-WH-20-3. Fitoterapia 2020, 141, 104469. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Fenner, A.M. Drug discovery from marine microbes. Microb. Ecol. 2013, 65, 800–806. [Google Scholar] [CrossRef]
- Barzkar, N.; Tamadoni Jahromi, S.; Poorsaheli, H.B.; Vianello, F. Metabolites from marine microorganisms, micro, and macroalgae: Immense scope for pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, F.; Wang, X.; Chen, H.; Wang, Q.; Guo, J.; Cao, X.; Wang, L. Antibacterial activity and action mechanism of questin from marine Aspergillus flavipes HN4-13 against aquatic pathogen Vibrio harveyi. 3 Biotech 2019, 9, 14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).