Purification, Chemical Characterization and Immunomodulatory Activity of a Sulfated Polysaccharide from Marine Brown Algae Durvillaea antarctica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characteristics of the Sulfated Polysaccharide DAP4
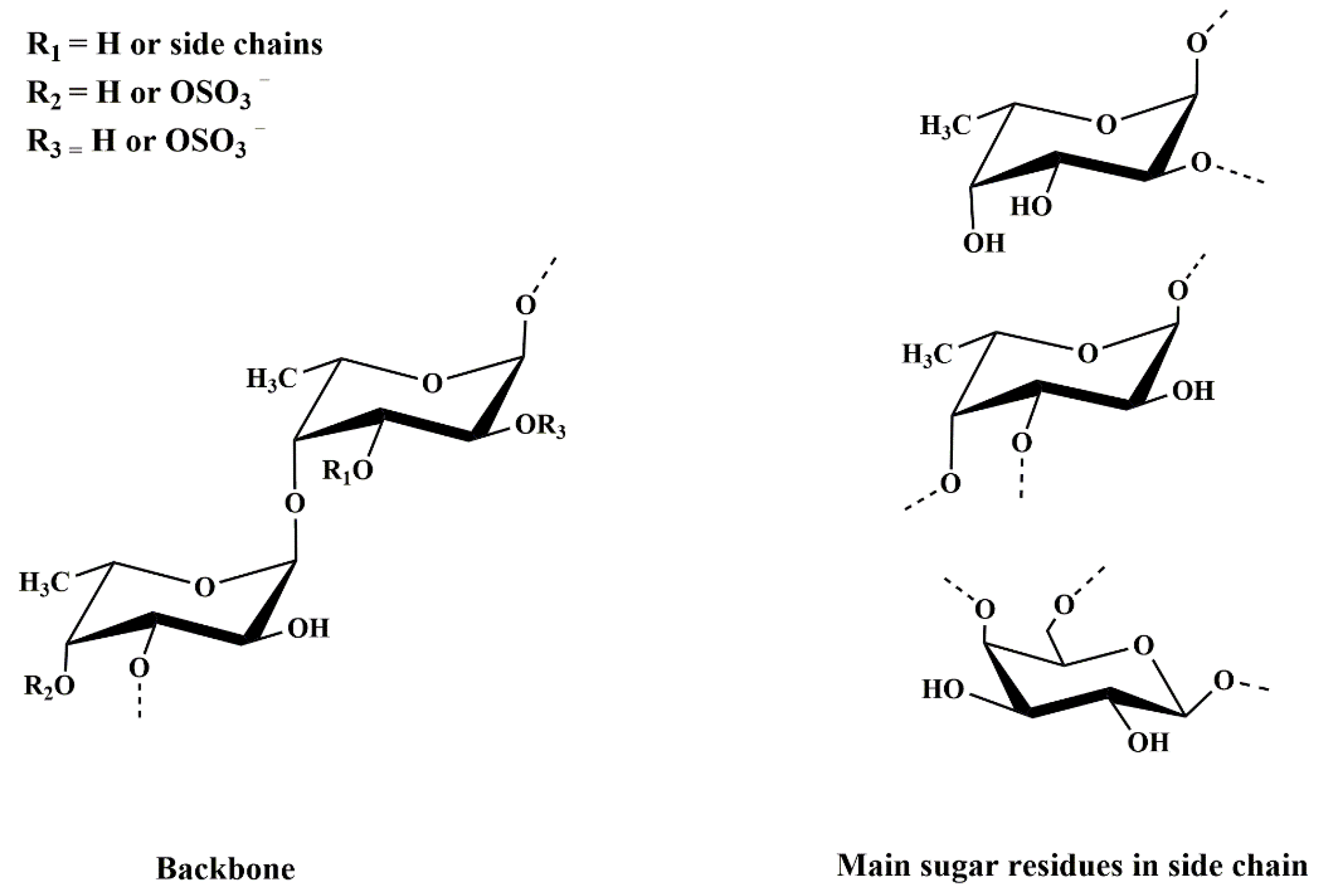
2.2. Structural Characterization of DAP4
2.2.1. Methylation Analysis
2.2.2. NMR Analysis
2.3. Immunomodulatory Activity
2.3.1. Effects of DAP4 on RAW264.7 Cell Viability
2.3.2. Effect of DAP4 on the Neutral Red Phagocytic Activity
2.3.3. Effect of DAP4 on Lymphocyte Proliferation
2.3.4. Effect of DAP4 on the Production NO
2.3.5. Effect of DAP4 on NK Cells Activity
3. Materials and Methods
3.1. Materials
3.2. Isolation and Purification of the Sulfated Polysaccharide
3.3. Composition Analysis
3.4. Purity and Molecular Weight
3.5. Desulfation and Methylation Analysis
3.6. Spectroscopy Analysis
3.7. Immunomodulatory Activity
3.7.1. Cell Cultures
3.7.2. Cytotoxicity Assay
3.7.3. Neutral Red Phagocytosis Assay
3.7.4. NO Production Assay
3.7.5. Lymphocyte Proliferation Assay
3.7.6. NK Cells Activity Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Manlusoc, J.K.; Hsieh, C.-L.; Hsieh, C.-Y.; Salac, E.S.; Lee, Y.-T.; Tsai, P.-W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers 2019, 11, 7. [Google Scholar]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 12. [Google Scholar]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar]
- Gong, Y.; Ma, Y.; Cheung, P.C.-K.; You, L.; Liao, L.; Pedisić, S.; Kulikouskaya, V. Structural characteristics and anti-inflammatory activity of UV/H2O2-treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food Chem. Toxicol. 2021, 152, 112157. [Google Scholar] [PubMed]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2021, 13, 100623. [Google Scholar]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar]
- Zvyagintseva, T.N.; Usoltseva, R.V.; Shevchenko, N.M.; Surits, V.V.; Imbs, T.I.; Malyarenko, O.S.; Besednova, N.N.; Ivanushko, L.A.; Ermakova, S.P. Structural diversity of fucoidans and their radioprotective effect. Carbohydr. Polym. 2021, 273, 118551. [Google Scholar]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Thuy, T.T.T.; Van, T.T.T.; Ly, B.M.; Nifantiev, N.E.; Usov, A.I. Preliminary investigation of a highly sulfated galactofucan fraction isolated from the brown alga Sargassum polycystum. Carbohydr. Res. 2013, 377, 48–57. [Google Scholar]
- Bilan, M.I.; Shashkov, A.S.; Usov, A.I. Structure of a sulfated xylofucan from the brown alga Punctaria plantaginea. Carbohydr. Res. 2014, 393, 1–8. [Google Scholar]
- Zhou, S.; Li, Q.; Wu, H.; Lu, Q. The pathogenic role of innate lymphoid cells in autoimmune-related and inflammatory skin diseases. Cell Mol. Immunol 2020, 17, 335–346. [Google Scholar]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol Rev. 2009, 22, 240–273. [Google Scholar] [PubMed] [Green Version]
- Taha, Z.; van Rensburg, J.H.J.; Yang, X. The Hippo Pathway: Immunity and cancer. Cancers 2018, 10, 94. [Google Scholar]
- Yu, J.; Dong, X.-D.; Jiao, J.-S.; Ji, H.-Y.; Liu, A.-J. Antitumor and immunoregulatory activities of a novel polysaccharide from Astragalus membranaceus on S180 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 189, 930–938. [Google Scholar] [PubMed]
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar]
- Smith, J.M.B.; Bayliss-Smith, T.P. Kelp-plucking: Coastal erosion facilitated by bull-kelp Durvillaea antarctica at subantarctic Macquarie Island. Antarct. Sci. 1998, 10, 431–438. [Google Scholar]
- Parvizi, E.; Craw, D.; Waters, J.M. Kelp DNA records late Holocene paleoseismic uplift of coastline, southeastern New Zealand. Earth Planet. Sci. Lett. 2019, 520, 18–25. [Google Scholar]
- Quiñones, J.; Díaz, R.; Dantagnan, P.; Hernández, A.; Valdes, M.; Lorenzo, J.M.; Cancino, D.; Sepúlveda, N.; Farías, J.G. Dietary inclusion of Durvillaea antarctica meal and rapeseed (Brassica napus) oil on growth, feed utilization and fillet quality of rainbow trout (Oncorhynchus mykiss ). Aquaculture 2021, 530, 735882. [Google Scholar]
- Castillo, E.; Duarte, L.F.; Corrales, N.; Álvarez, D.M.; Farías, M.A.; Henríquez, A.; Smith, P.C.; Agurto-Muñoz, C.; González, P.A. Anti-herpetic activity of macrocystis pyrifera and Durvillaea antarctica algae extracts against HSV-1 and HSV-2. Front. Microbiol 2020, 11, 2006. [Google Scholar]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar]
- Su, F.; Song, Q.; Zhang, C.; Xu, X.; Li, M.; Yao, D.; Wu, L.; Qu, X.; Guan, H.; Yu, G.; et al. A β-1,3/1,6-glucan from Durvillaea Antarctica inhibits tumor progression in vivo as an immune stimulator. Carbohydr. Polym. 2019, 222, 114993. [Google Scholar]
- Yang, Y.; Zhao, X.; Li, J.; Jiang, H.; Shan, X.; Wang, Y.; Ma, W.; Hao, J.; Yu, G. A beta-glucan from Durvillaea Antarctica has immunomodulatory effects on RAW264.7 macrophages via toll-like receptor 4. Carbohydr. Polym. 2018, 191, 255–265. [Google Scholar] [PubMed]
- Yang, Y.; Hu, T.; Li, J.; Xin, M.; Zhao, X. Structural characterization and effect on leukopenia of fucoidan from Durvillaea antarctica. Carbohydr. Polym. 2021, 256, 117529. [Google Scholar] [PubMed]
- Ponce, N.M.A.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Cordoba, O.; Damonte, E.B.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 2019, 478, 18–24. [Google Scholar] [PubMed]
- Wang, P.; Zhao, X.; Lv, Y.; Liu, Y.; Lang, Y.; Wu, J.; Liu, X.; Li, M.; Yu, G. Analysis of structural heterogeneity of fucoidan from Hizikia fusiforme by ES-CID-MS/MS. Carbohydr. Polym. 2012, 90, 602–607. [Google Scholar] [PubMed]
- Mathlouthi, M.; Koenig, J.L. Vibrational Spectra of Carbohydrates. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Horton, D., Eds.; Academic Press: Cambridge, MA, USA, 1987; Volume 44, pp. 7–89. [Google Scholar]
- Liu, X.; Hao, J.; He, X.; Wang, S.; Cao, S.; Qin, L.; Mao, W. A rhamnan-type sulfated polysaccharide with novel structure from Monostroma angicava Kjellm (Chlorophyta) and its bioactivity. Carbohydr. Polym. 2017, 173, 732–748. [Google Scholar]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar]
- Mitić, Ž.; Cakić, M.; Nikolić, G.M.; Nikolić, R.; Nikolić, G.S.; Pavlović, R.; Santaniello, E. Synthesis, physicochemical and spectroscopic characterization of copper(II)-polysaccharide pullulan complexes by UV–vis, ATR-FTIR, and EPR. Carbohydr. Res. 2011, 346, 434–441. [Google Scholar]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Kalinovsky, A.I.; Miansong, Z.; Changheng, L.; Malyarenko, O.; Zueva, A.O.; Zvyagintseva, T.N.; Ermakova, S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017, 175, 654–660. [Google Scholar]
- He, W.; Sun, H.; Su, L.; Zhou, D.; Zhang, X.; Shanggui, D.; Chen, Y. Structure and anticoagulant activity of a sulfated fucan from the sea cucumber Acaudina leucoprocta. Int. J. Biol. Macromol. 2020, 164, 87–94. [Google Scholar]
- Li, N.; Mao, W.; Yan, M.; Liu, X.; Xia, Z.; Wang, S.; Xiao, B.; Chen, C.; Zhang, L.; Cao, S. Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga Codium divaricatum. Carbohydr. Polym. 2015, 121, 175–182. [Google Scholar]
- Bilan, M.I.; Ustyuzhanina, N.E.; Shashkov, A.S.; Thanh, T.T.T.; Bui, M.L.; Tran, T.T.V.; Bui, V.N.; Nifantiev, N.E.; Usov, A.I. A sulfated galactofucan from the brown alga Hormophysa cuneiformis (Fucales, Sargassaceae). Carbohydr. Res. 2018, 469, 48–54. [Google Scholar] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [PubMed]
- Matsuhiro, B.; Zu, I.E.; Jashes, M. Sulfated polysaccharides from Durvillaea antarctica. Hydrobiologia 1996, 321, 77–81. [Google Scholar]
- Lim, S.J.; Wan, A.W.M.; Schiehser, S. Structural elucidation of fucoidan from Cladosiphon okamuranus (Okinawa mozuku). Food Chem. 2019, 272, 222–226. [Google Scholar] [PubMed]
- Mak, W.; Hamid, N.; Liu, T. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [PubMed]
- Liu, X.; Xi, X.; Jia, A.; Zhang, M.; Cui, T.; Bai, X.; Shi, Y.; Liu, C. A fucoidan from Sargassum fusiforme with novel structure and its regulatory effects on intestinal microbiota in high-fat diet-fed mice. Food Chem. 2021, 358, 129908. [Google Scholar] [PubMed]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [PubMed]
- Wen, Y.; Gao, L.; Zhou, H.; Ai, C.; Huang, X.; Wang, M.; Zhang, Y.; Zhao, C. Opportunities and challenges of algal fucoidan for diabetes management. Trends Food Sci. Technol. 2021, 111, 628–641. [Google Scholar]
- Lorbeer, A.J.; Charoensiddhi, S.; Lahnstein, J.; Lars, C.; Franco, C.M.M.; Bulone, V.; Zhang, W. Sequential extraction and characterization of fucoidans and alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J. Appl. Phycol. 2017, 29, 1515–1526. [Google Scholar]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [PubMed]
- Flaminio, M.J.B.F.; Antczak, D.F. Inhibition of lymphocyte proliferation and activation: A mechanism used by equine invasive trophoblast to escape the maternal immune response. Placenta 2005, 26, 148–159. [Google Scholar] [PubMed]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [PubMed]
- Vazquez-Torres, A.; Stevanin, T.; Jones-Carson, J.; Castor, M.; Read, R.C.; Fang, F.C. Chapter 26-Analysis of nitric oxide-dependent antimicrobial actions in macrophages and mice. In Methods in Enzymology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2008; Volume 437, pp. 521–538. [Google Scholar]
- Cerwenka, A.; Lanier, L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunololy 2001, 1, 41–49. [Google Scholar]
- Han, J.G.; Syed, A.Q.; Kwon, M. Antioxident, immunomodulatory and anticancer activity of fucoidan isolated from Fucus vesiculosus. J. Biotechnol. 2008, 136, S571. [Google Scholar]
- Peng, Y.; Song, Y.; Wang, Q. In vitro and in vivo immunomodulatory effects of fucoidan compound agents. Int. J. Biol. Macromol. 2019, 127, 48–56. [Google Scholar]
- Li, B.; Wei, X.-J.; Sun, J.-L.; Xu, S.-Y. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed, Hizikia fusiforme. Carbohydr. Res. 2006, 341, 1135–1146. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Terho, T.T.; Hartiala, K. Method for determination of the sulfate content of glycosaminoglycans. Anal. Biochem. 1971, 41, 471–476. [Google Scholar]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar]
- Qin, L.; He, M.; Yang, Y. Anticoagulant-active sulfated arabinogalactan from Chaetomorpha linum: Structural characterization and action on coagulation factors. Carbohydr. Polym. 2020, 242, 116394. [Google Scholar]
- Harris, P.J.; Henry, R.J.; Blakeney, A.B.; Stone, B.A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr. Res. 1984, 127, 59–73. [Google Scholar]
- Zhao, X.; Jiao, G.; Yang, Y. Structure and immunomodulatory activity of a sulfated agarose with pyruvate and xylose substitutes from Polysiphonia senticulosa Harvey. Carbohydr. Polym. 2017, 176, 29–37. [Google Scholar]
- Lysle, D.T.; Coussons, M.E.; Watts, V.J.; Bennett, E.H.; Dykstra, L.A. Morphine-induced alterations of immune status: Dose dependency, compartment specificity and antagonism by naltrexone. J. Pharmacol. Exp. Ther. 1993, 265, 1071. [Google Scholar]
- Bi, H.; Han, H.; Li, Z.; Ni, W.; Chen, Y.; Zhu, J.; Gao, T.; Hao, M.; Zhou, Y. A water-soluble polysaccharide from the fruit bodies of Bulgaria inquinans (Fries) and its anti-malarial activity. Evid. Based Complement. Altern. Med. 2011, 2011, 973460. [Google Scholar]
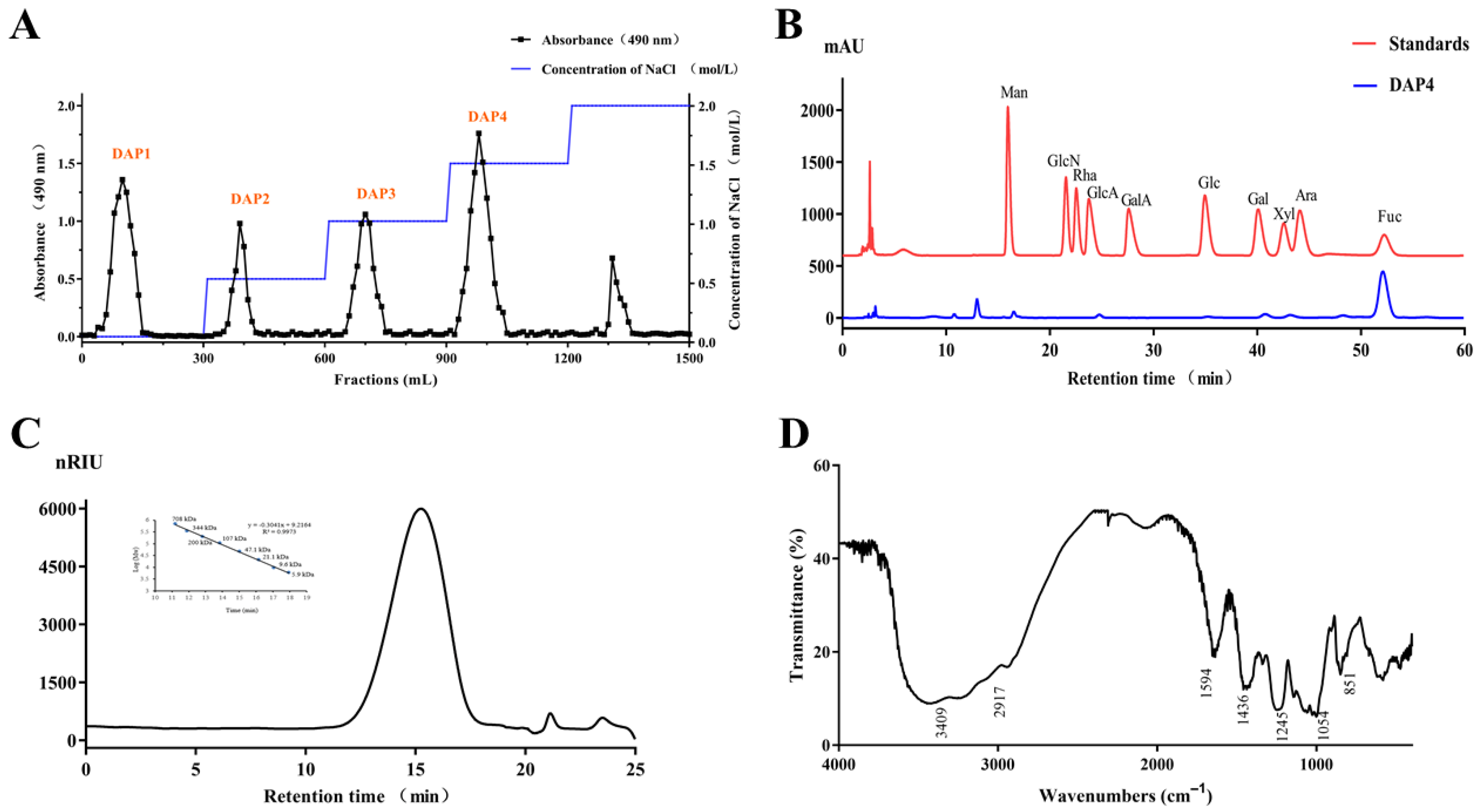






| Sample | Total Sugar (%) | Protein (%) | Sulfate (%) | Mw (kDa) | Monosaccharide Composition (Molar Ratio %) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | GlcA | Glc | Gal | Xyl | Fuc | |||||
| DAP | 69.8 | 3.4 | 20.7 | nd | 9.7 | 11.8 | 19.7 | 18.1 | 5.9 | 34.8 |
| DAP1 | 78.2 | 2.1 | 4.5 | 754 | 9.2 | nd a | 82.1 | 3.4 | nd a | 5.3 |
| DAP2 | 76.7 | 1.0 | 15.6 | 532 | 23.5 | 0.9 | 23.7 | 9.5 | 6.8 | 35.6 |
| DAP3 | 82.3 | nd | 17.7 | 368 | 17.8 | 2.3 | 4.3 | 32.1 | 4.2 | 39.3 |
| DAP4 | 75.9 | 0.2 | 22.3 | 92 | 2.9 | 1.2 | nd a | 3.5 | 4.5 | 87.9 |
| Methylate Alditol Acetate | Molar Percent Ratio | Linkage Pattern | |
|---|---|---|---|
| DAP4 | DAP4-Ds | ||
| 1,5-Di-O-acrtyl-2,3,4-tri-O-methyl-d-xylitol | 3.22 | 3.41 | Xylp-(1→ |
| 1,5-Di-O-acetyl-2,3,4-tri-O-methyl-l-fucitol | 7.68 | 6.42 | Fucp-(1→ |
| 1,5-Di-O-acetyl-2,3,4,6-tetra-O-methyl-d-galacitol | 1.47 | 1.13 | Galp-(1→ |
| 1,3,5-Tri-O-acetyl-2,4-di-O-methyl-l-fucitol | 24.73 | 37.92 | →3)-Fucp-(1→ |
| 1,4,5-Tri-O-acetyl-2,3-di-O-methyl-l-fucitol | 21.42 | 28.23 | →4)-Fucp-(1→ |
| 1,2,5-Tetra-O-acetyl-3,4-di-O-methyl-fucitol | 5.24 | 7.54 | →2)-Fucp-(1→ |
| 1,3,4,5-Tetra-O-acetyl-2-O-methyl-fucitol | 24.43 | 12.01 | →3,4)-Fucp-(1→ |
| 1,2,4,5-Tri-O-acetyl-3-O-methyl-l-fucitol | 8.36 | nd a | →2,4)-Fucp-(1→ |
| 1,4,5-Tri-O-acetyl-2,3,6-tri-O-methyl-d-galactitol | 2.21 | 3.34 | →4)-Galp-(1→ |
| 1,4,5,6-Tetra-O-acetyl-2,3-di-O-methyl-d-galactitol | 1.24 | nd a | →4,6)-Galp-(1→ |
| Residue b | Chemical Shifts (ppm) a | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| A | 5.57/100.08 | 4.64/82.29 | 3.96/73.59 | 4.29/80.21 | 3.90/68.31 | 1.43/17.22 |
| B | 5.50/95.23 | 4.21/76.15 | 4.60/75.76 | 4.71/82.12 | 3.90/68.31 | 1.43/17.22 |
| C | 5.44/97.79 | 4.29/82.29 | 4.11/73.69 | 4.04/70.32 | 3.68/68.31 | 1.43/17.22 |
| D | 5.41/110.16 | 3.85/73.42 | --/-- | --/-- | --/-- | --/-- |
| E | 5.34/99.20 | 4.29/73.59 | 4.11/77.94 | 3.96/74.71 | 3.90/68.31 | 1.30/17.22 |
| F | 5.34/97.79 | 4.29/73.59 | 4.11/77.94 | 4.23/78.32 | 4.01/69.7 | 1.30/17.22 |
| G | 5.16/99.20 | 4.29/73.40 | 4.03/73.59 | 4.34/79.95 | 4.01/69.7 | 1.30/17.22 |
| H | 4.52/104.70 | --/-- | --/-- | --/-- | --/-- | 3.80/62.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Xu, H.; He, Y.; Liang, C.; Wang, K.; Cao, J.; Qu, C.; Miao, J. Purification, Chemical Characterization and Immunomodulatory Activity of a Sulfated Polysaccharide from Marine Brown Algae Durvillaea antarctica. Mar. Drugs 2022, 20, 223. https://doi.org/10.3390/md20040223
Qin L, Xu H, He Y, Liang C, Wang K, Cao J, Qu C, Miao J. Purification, Chemical Characterization and Immunomodulatory Activity of a Sulfated Polysaccharide from Marine Brown Algae Durvillaea antarctica. Marine Drugs. 2022; 20(4):223. https://doi.org/10.3390/md20040223
Chicago/Turabian StyleQin, Ling, Hui Xu, Yingying He, Chen Liang, Kai Wang, Junhan Cao, Changfeng Qu, and Jinlai Miao. 2022. "Purification, Chemical Characterization and Immunomodulatory Activity of a Sulfated Polysaccharide from Marine Brown Algae Durvillaea antarctica" Marine Drugs 20, no. 4: 223. https://doi.org/10.3390/md20040223
APA StyleQin, L., Xu, H., He, Y., Liang, C., Wang, K., Cao, J., Qu, C., & Miao, J. (2022). Purification, Chemical Characterization and Immunomodulatory Activity of a Sulfated Polysaccharide from Marine Brown Algae Durvillaea antarctica. Marine Drugs, 20(4), 223. https://doi.org/10.3390/md20040223