Dactylospongia elegans—A Promising Drug Source: Metabolites, Bioactivities, Biosynthesis, Synthesis, and Structural-Activity Relationship
Abstract
:1. Introduction
2. Secondary Metabolites of D. elegans
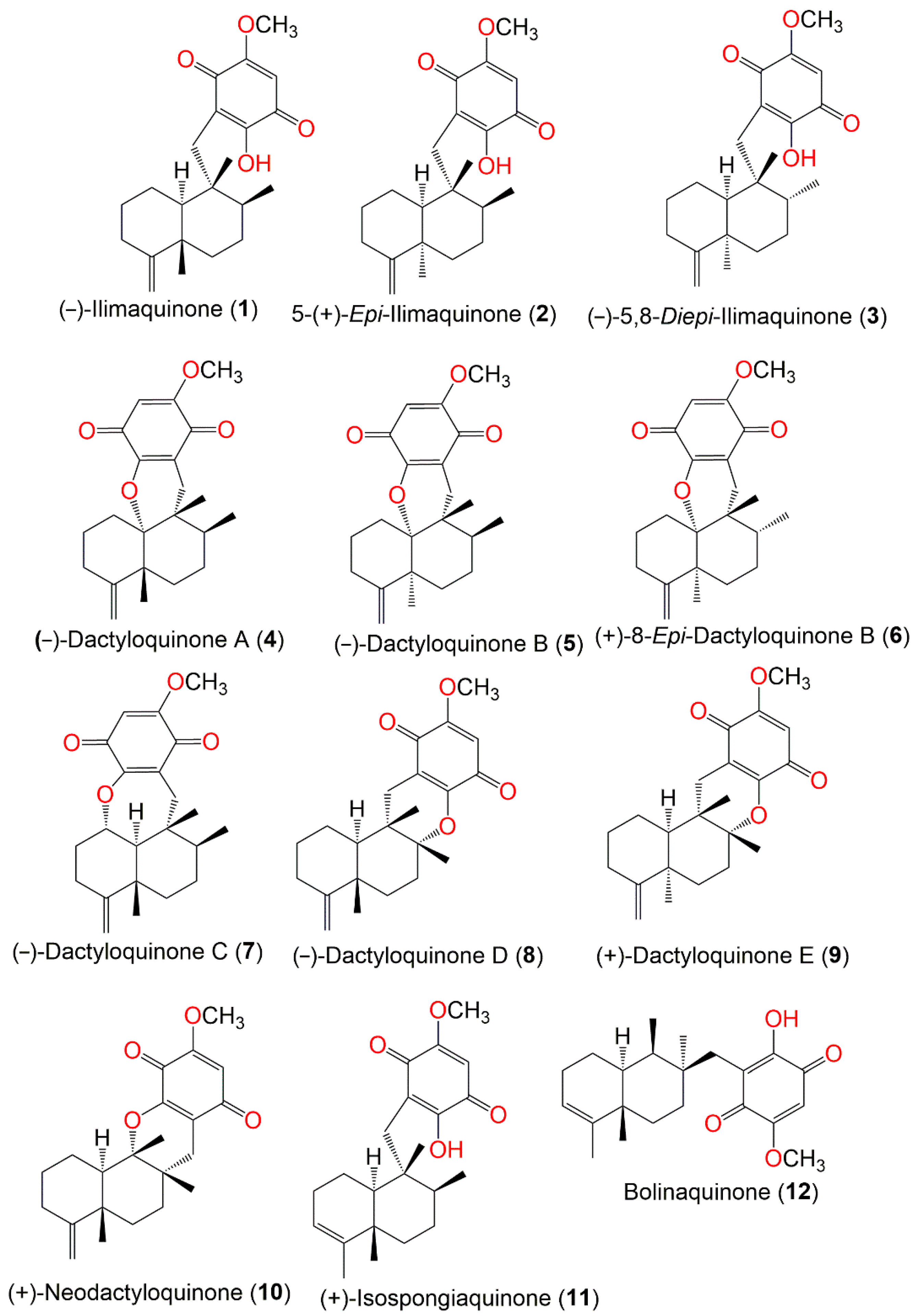
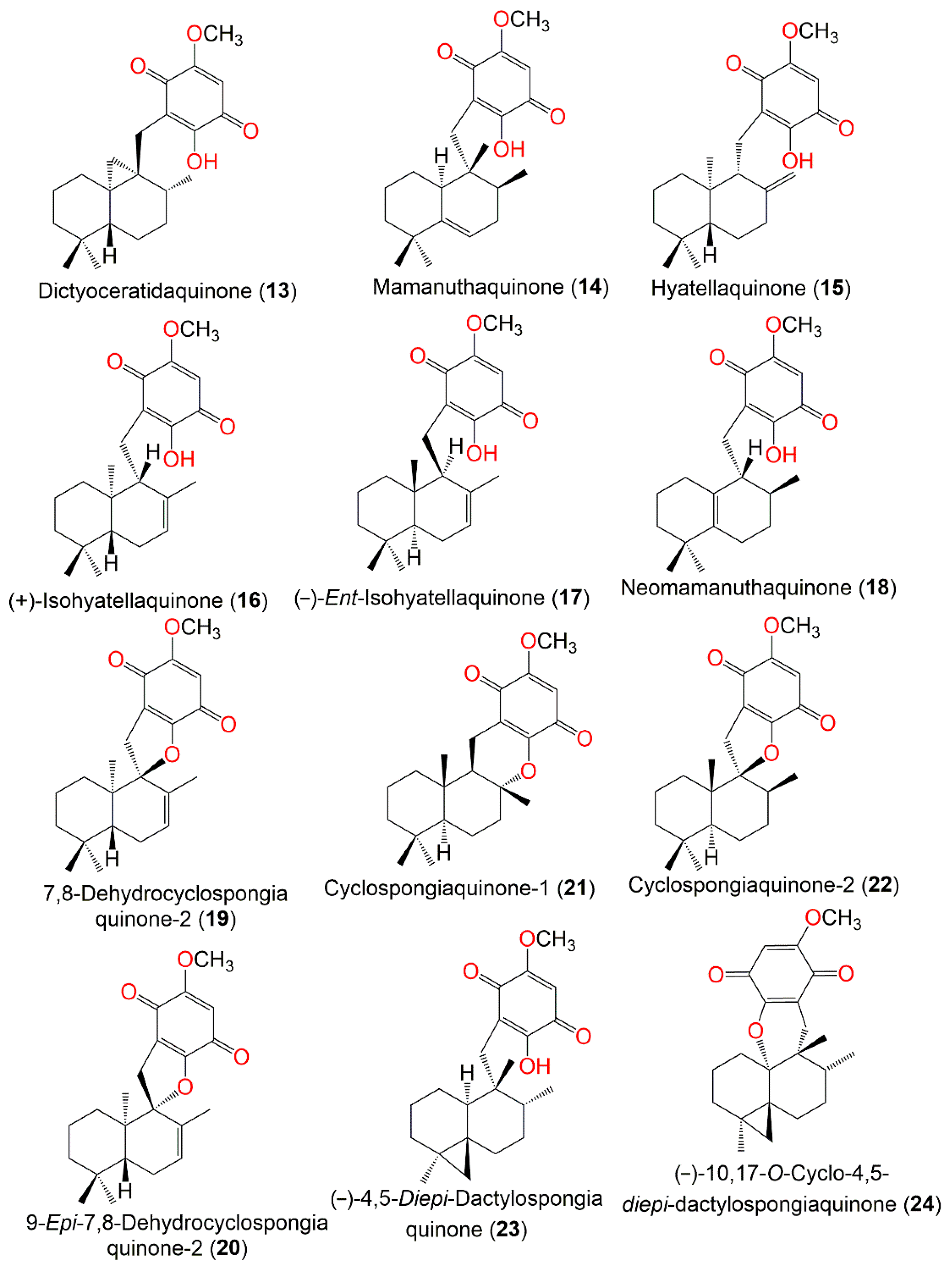
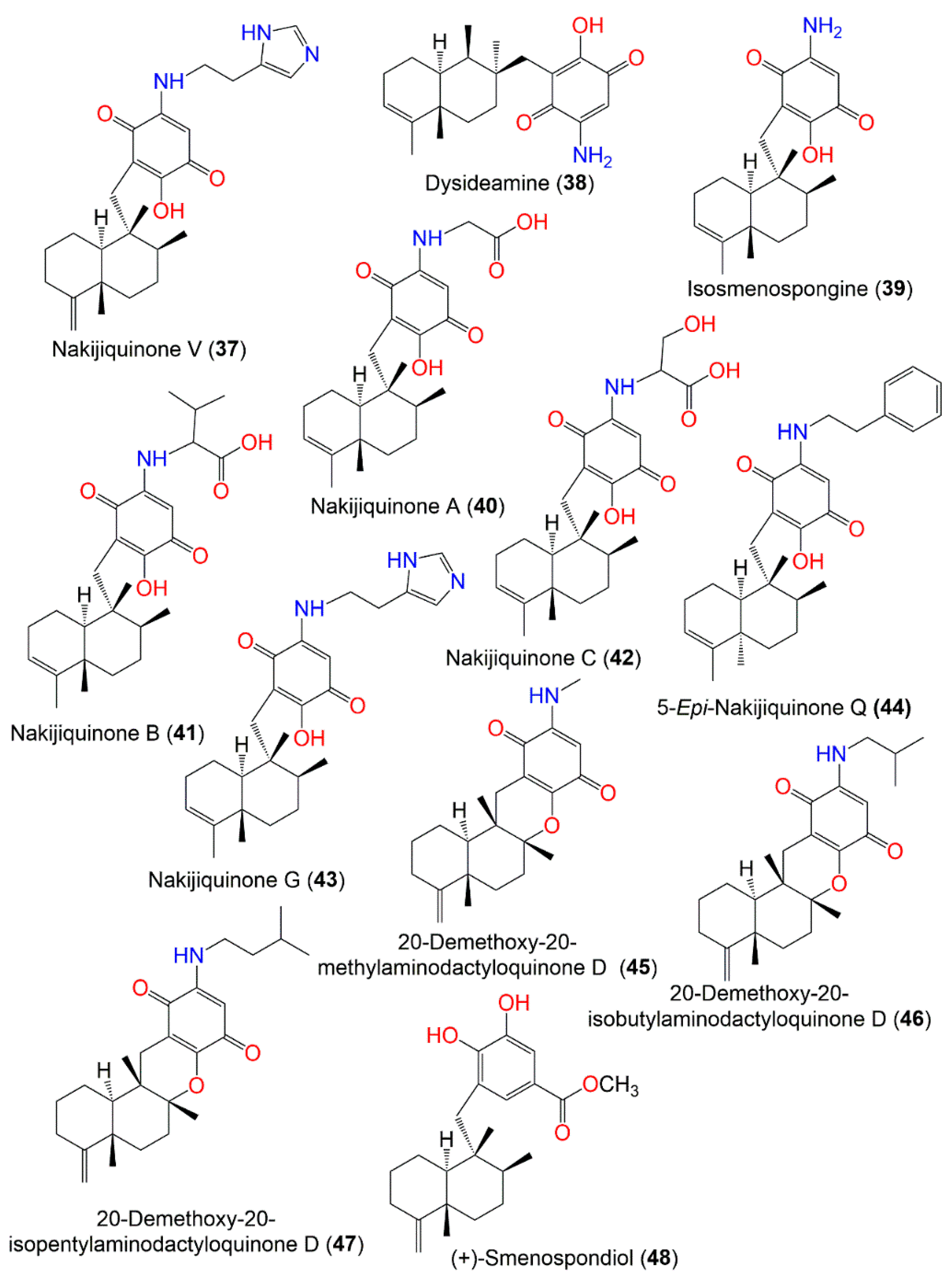
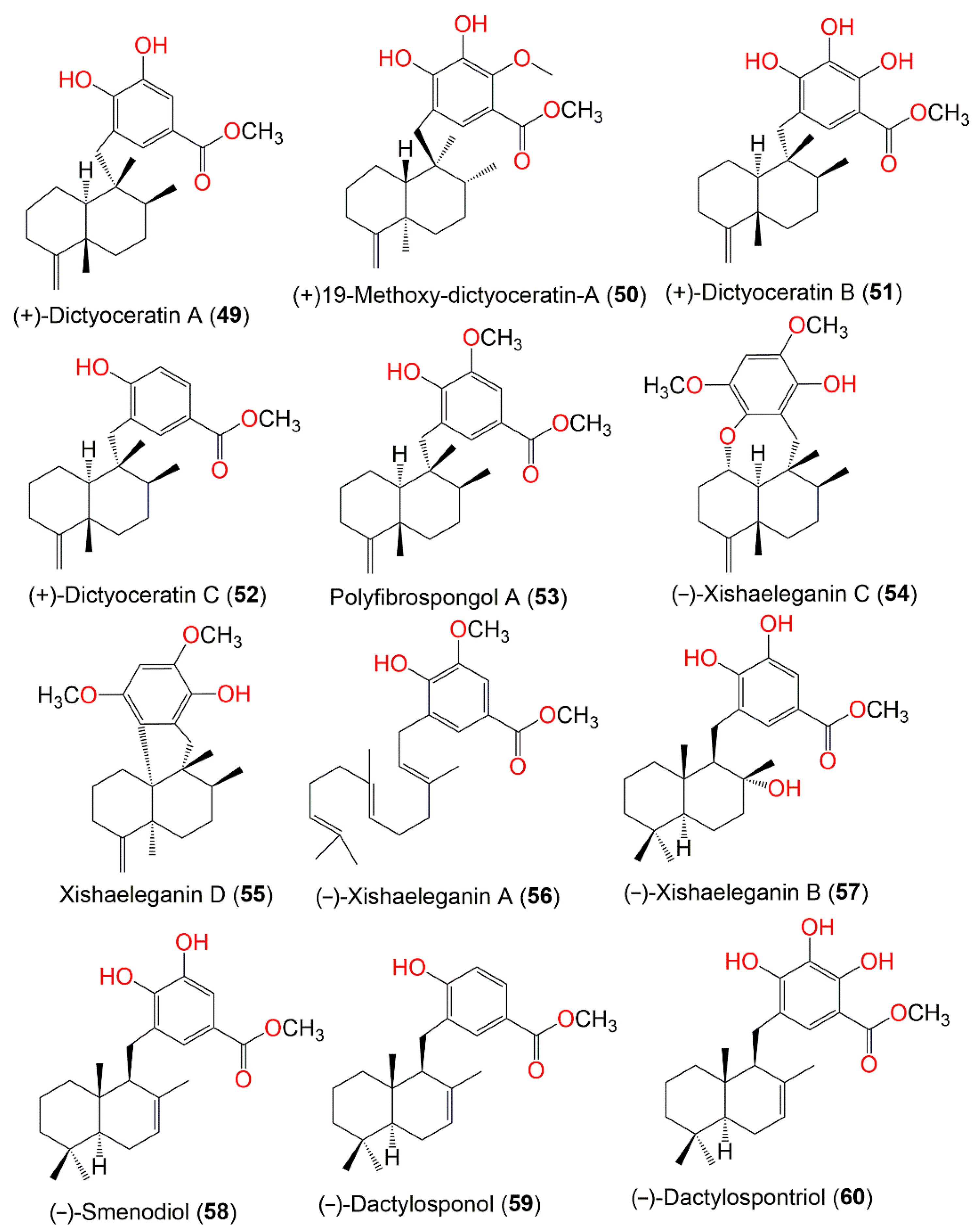
| The Chemical Structures of Compounds 1–12 (Figure 1), 13–24 (Figure 2), 25–36 (Figure 3), 37–48 (Figure 4), 49–60 (Figure 5), 61–70 (Figure 6), 71–80 (Figure 7), 81–91 (Figure 8), and 92–101 (Figure 9) are illustrated. Compound Name | Extract/Fraction | Mol. Wt. | Mol. Formula | City, Country | Ref. |
|---|---|---|---|---|---|
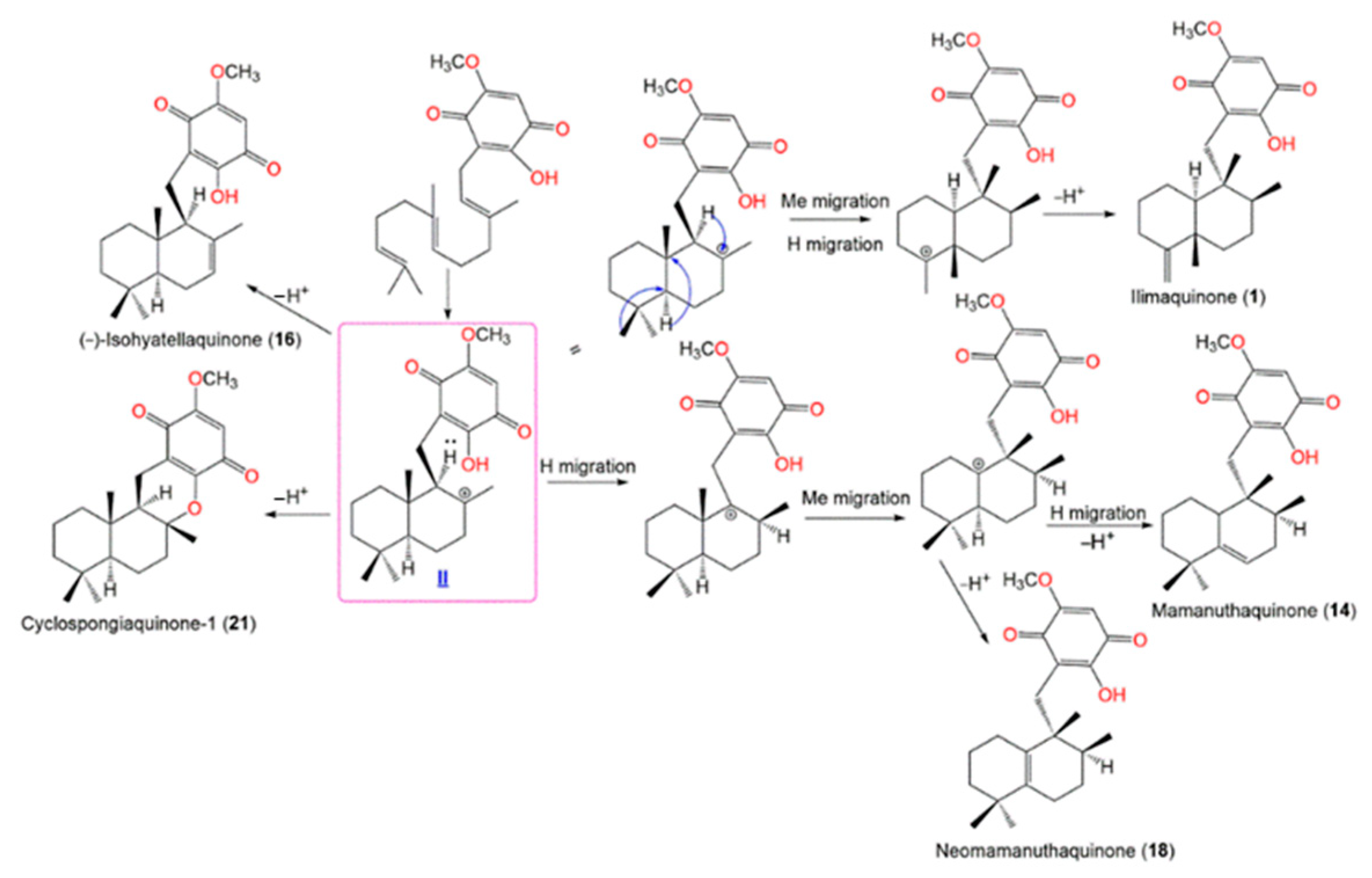
| (−)-Ilimaquinone (1) | CH2Cl2 fraction of MeOH extract | 358 | C22H30O4 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | Pulan Tiga, Sabah, Malaysia | [17] | |
| CH2Cl2 fraction of MeOH extract | - | - | Pelorus Island, the Great Barrier Reef, Queensland, Australia | [18] | |
| EtOAc fraction of MeOH extract | - | - | Coral reef of Ishigaki Island, Okinawa, Japan | [19] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [20] | |
| EtOAc fraction of CH2Cl2 of MeOH extract | - | - | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] | |
| 90% and 100% MeOH fraction of RP-18 CC of MeOH extract | - | - | Pugh Shoal, northeast of Truant Island, Australia | [22] | |
| CH2Cl2 fraction of H2O extract | - | - | * Coast of Malaysia * Coast of Palau | [23] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [24] | |
| RP-18 CC, 60% MeOH/H2O of MeOH extract | - | - | Towo’e Beach Tahuna Bay, Sangihe Islands North Sulawesi, Indonesia | [25] | |
| CH2Cl2 fraction of MeOH extract | - | - | Sheraton Caverns, Kauai, Hawaii | [26] | |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| 5-(+)-Epi-Ilimaquinone (2) | CH2Cl2 fraction of MeOH extract | 358 | C22H30O4 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| EtOAc fraction of MeOH extract | - | - | Coral reef of Ishigaki Island, Okinawa, Japan | [19] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [20] | |
| 90% and 100% MeOH fraction of RP-18 CC/MeOH extract | - | - | Pugh Shoal, northeast of Truant Island, Australia | [22] | |
| n-Hexane fraction of MeOH extract | - | - | Island of Ambon, Indonesia | [28] | |
| CH2Cl2 fraction of MeOH extract | - | - | Sheraton Caverns, Kauai, Hawaii | [26] | |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| (−)-5,8-Diepi-Ilimaquinone (3) | CH2Cl2 fraction of H2O extract | 358 | C22H30O4 | * Coast of Malaysia * Coast of Palau | [23] |
| (−)-Dactyloquinone A (4) | EtOAc fraction of MeOH extract | 356 | C22H28O4 | Coral reef of Ishigaki Island, Okinawa, Japan | [29] |
| EtOAc fraction of MeOH extract | - | - | Coral reef of Ishigaki Island, Okinawa, Japan | [19] | |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| (−)-Dactyloquinone B (5) | EtOAc fraction of MeOH extract | 356 | C22H28O4 | Coral reef of Ishigaki Island, Okinawa, Japan | [29] |
| EtOAc fraction of MeOH extract | - | - | Coral reef of Ishigaki Island, Okinawa, Japan | [19] | |
| 90% and 100% MeOH fraction of RP-18 CC of MeOH extract | - | - | Pugh Shoal, northeast of Truant Island, Australia | [22] | |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| (+)-8-Epi-Dactyloquinone B (6) | CH2Cl2 fraction of H2O extract | 356 | C22H28O4 | * Coast of Malaysia * Coast of Palau | [23] |
| (−)-Dactyloquinone C (7) | EtOAc fraction of MeOH extract | 356 | C22H28O4 | Coral reef of Ishigaki Island, Okinawa, Japan | [19] |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| (−)-Dactyloquinone D (8) | EtOAc fraction of MeOH extract | 356 | C22H28O4 | Coral reef of Ishigaki Island, Okinawa, Japan | [19] |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| (+)-Dactyloquinone E (9) | EtOAc fraction of MeOH extract | 356 | C22H28O4 | Coral reef of Ishigaki Island, Okinawa, Japan | [19] |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| (+)-Neodactyloquinone (10) | EtOAc fraction of MeOH extract | 356 | C22H28O4 | Coral reef of Ishigaki Island, Okinawa, Japan | [30] |
| (+)-Isospongiaquinone (11) | CH2Cl2 fraction of MeOH extract | 358 | C22H30O4 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| n-Hexane fraction of MeOH extract | - | - | Island of Ambon, Indonesia | [28] | |
| Bolinaquinone (12) | CH2Cl2 fraction of MeOH extract | 358 | C22H30O4 | West Flores, Indonesia | [24] |
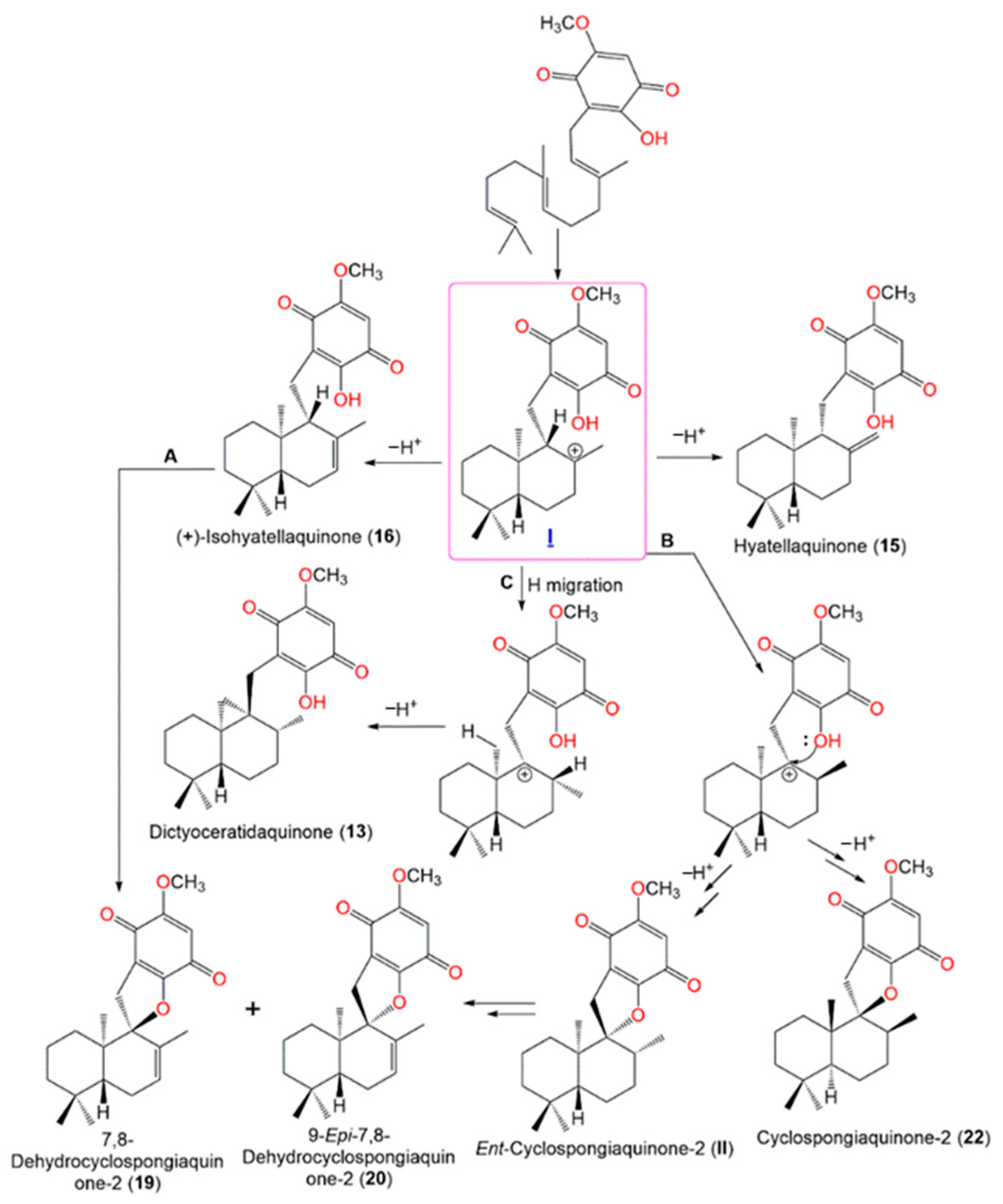
| Dictyoceratidaquinone (13) | EtOAc fraction of CH2Cl2 of MeOH extract | 358 | C22H30O4 | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] |
| Mamanuthaquinone (14) | EtOAc fraction of CH2Cl2 of MeOH extract | 358 | C22H30O4 | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba (Australia), | [21] |
| Hyatellaquinone (15) | EtOAc fraction of CH2Cl2 of MeOH extract | 358 | C22H30O4 | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] |
| (+)-Isohyatellaquinone (16) | EtOAc fraction of CH2Cl2 of MeOH extract | 358 | C22H30O4 | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] |
| (−)-Ent-Isohyatellaquinone (17) | EtOAc fraction of CH2Cl2 of MeOH extract | 358 | C22H30O4 | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] |
| Neomamanuthaquinone (18) | EtOAc fraction of CH2Cl2 of MeOH extract | 344 | C21H28O4 | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] |
| 7,8-Dehydrocyclospongiaquinone-2 (19) | EtOAc fraction of CH2Cl2 of MeOH extract | 356 | C22H28O4 | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] |
| 9-Epi-7,8-Dehydrocyclospongiaquinone-2 (20) | EtOAc fraction of CH2Cl2 of MeOH extract | 356 | C22H28O4 | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] |
| Cyclospongiaquinone-1 (21) | CH2Cl2 fraction of H2O extract | 358 | C22H30O4 | * Coast of Malaysia * Coast of Palau | [23] |
| Cyclospongiaquinone-2 (22) | CH2Cl2 fraction of H2O extract | 358 | C22H30O4 | * Coast of Malaysia * Coast of Palau | [23] |
| (−)-4,5-Diepi-Dactylospongiaquinone (23) | CH2Cl2 fraction of H2O extract | 358 | C22H30O4 | * Coast of Malaysia * Coast of Palau | [23] |
| (−)-10,17-O-Cyclo-4,5-diepi-dactylospongiaquinone (24) | CH2Cl2 fraction of H2O extract | 356 | C22H28O4 | * Coast of Malaysia * Coast of Palau | [23] |
| Smenospongine (25) | CH2Cl2 fraction of MeOH extract | 343 | C21H29NO3 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [20] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [31] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [24] | |
| RP-18 CC, 60% MeOH/H2O of MeOH extract | - | - | Towo’e Beach Tahuna Bay, Sangihe Islands North Sulawesi, Indonesia | [25] | |
| CH2Cl2 fraction of MeOH extract | - | - | Sheraton Caverns, Kauai, Hawaii | [26] | |
| 5-(+)-Epi-Smenospongine (26) | CH2Cl2 fraction of MeOH extract | 343 | C21H29NO3 | West Flores, Indonesia | [20] |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [24] | |
| n-Hexane fraction of MeOH extract | - | - | Island of Ambon, Indonesia | [28] | |
| Smenospongimine (27) | CH2Cl2/MeOH fractions of EtOH extract | 357 | C22H31NO3 | Yongxing Island, South China Sea | [32] |
| Smenospongine B (28) | 90% and 100% MeOH fraction of RP-18 CC of MeOH extract | 401 | C23H31NO5 | Pugh Shoal, northeast of Truant Island, Australia | [22] |
| Smenospongine C (29) | 90% and 100% MeOH fraction of RP-18 CC of MeOH extract | 415 | C24H33NO5 | Pugh Shoal, northeast of Truant Island, Australia | [22] |
| n-Hexane fraction of MeOH extract | - | - | Island of Ambon, Indonesia | [28] | |
| Smenospongorine (30) | CH2Cl2 fraction of MeOH extract | 399 | C25H37NO3 | West Flores, Indonesia | [20] |
| n-Hexane fraction of MeOH extract | - | - | Sheraton Caverns, Kauai, Hawaii | [26] | |
| CH2Cl2/MeOH fraction of EtOH extract | - | - | Yongxing Island, South China Sea | [32] | |
| 5-(+)-Epi-Smenospongorine (31) | CH2Cl2 fraction of MeOH extract | 399 | C25H37NO3 | West Flores, Indonesia | [20] |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [24] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [24] | |
| Smenospongiarine (32) | CH2Cl2 fraction of MeOH extract | 413 | C26H39NO3 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2/MeOH fraction of EtOH extract | - | - | Yongxing Island, South China Sea | [32] | |
| 5-(+)-Epi-Smenospongiarine (33) | CH2Cl2 fraction of MeOH extract | 413 | C26H39NO3 | * Similani island, Phuket, Thailand * Papua New Guinea | [15] |
| n-Hexane fraction of MeOH extract | - | - | Sheraton Caverns, Kauai, Hawaii | [26] | |
| Nakijiquinone D (34) | n-Hexane fraction of MeOH extract | 445 | C25H35NO6 | Island of Ambon, Indonesia | [28] |
| Smenospongidine (35) | CH2Cl2 fraction of MeOH extract | 447 | C29H37NO3 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [20] | |
| CH2Cl2 fraction of MeOH extract | - | - | Sheraton Caverns, Kauai, Hawaii | [26] | |
| 5-(+)-Epi-Smenospongidine (36) | CH2Cl2 fraction of MeOH extract | 447 | C29H37NO3 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [20] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [24] | |
| n-Hexane fraction of MeOH extract | - | - | Island of Ambon, Indonesia | [28] | |
| Nakijiquinone V (37) | RP-18 CC, 60% MeOH/H2O of MeOH extract | 437 | C26H35N3O3 | Towo’e Beach Tahuna Bay, Sangihe Islands, North Sulawesi, Indonesia | [25] |
| Dysideamine (38) | CH2Cl2 fraction of MeOH extract | 343 | C21H29NO3 | West Flores, Indonesia | [24] |
| Isosmenospongine (39) | n-Hexane fraction of MeOH extract | 343 | C21H29NO3 | Island of Ambon, Indonesia | [28] |
| Nakijiquinone A (40) | n-Hexane fraction of MeOH extract | 401 | C23H31NO5 | Island of Ambon, Indonesia | [28] |
| Nakijiquinone B (41) | n-Hexane fraction of MeOH extract | 443 | C26H37NO5 | Island of Ambon, Indonesia | [28] |
| Nakijiquinone C (42) | n-Hexane fraction of MeOH extract | 431 | C24H33NO6 | Island of Ambon, Indonesia | [28] |
| Nakijiquinone G (43) | n-Hexane fraction of MeOH extract | 437 | C26H35N3O3 | Island of Ambon, Indonesia | [28] |
| 5-Epi-Nakijiquinone Q (44) | n-Hexane fraction of MeOH extract | 447 | C29H37NO3 | Island of Ambon, Indonesia | [28] |
| 20-Demethoxy-20-methylaminodactyloquinone D (45) | CH2Cl2/MeOH fraction of EtOH extract | 355 | C22H29NO3 | Yongxing Island, South China Sea | [32] |
| 20-Demethoxy-20-isobutylaminodactyloquinone D (46) | CH2Cl2/MeOH fraction of EtOH extract | 397 | C25H35NO3 | Yongxing Island, South China Sea | [32] |
| 20-Demethoxy-20-isopentylaminodactyloquinone D (47) | CH2Cl2/MeOH fraction of EtOH extract | 411 | C26H37NO3 | Yongxing Island, South China Sea | [32] |
| (+)-Smenospondiol (48) | CH2Cl2 fraction of MeOH extract | 372 | C23H32O4 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [20] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [24] | |
| (+)-Dictyoceratin A (49) | CH2Cl2 fraction of MeOH extract | 372 | C23H32O4 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| n-Hexane fraction of MeOH extract | - | - | Sheraton Caverns, Kauai, Hawaii | [26] | |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| 19-Methoxy-dictyoceratin-A (50) | CH2Cl2/MeOH fractions of EtOH extract | 402 | C24H34O5 | Yongxing Island, South China Sea | [32] |
| (+)-Dictyoceratin B (51) | n-Hexane fraction of MeOH extract | 388 | C23H32O5 | Sheraton Caverns, Kauai, Hawaii | [26] |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| (+)-Dictyoceratin C (52) | CH2Cl2 fraction of MeOH extract | 356 | C23H32O3 | Pulan Tiga, Sabah, Malaysia | [17] |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [20] | |
| CH2Cl2 fraction of MeOH extract | - | - | West Flores, Indonesia | [24] | |
| EtOAc fraction of EtOH extract | - | - | Meishan coral reef, Sanya, China | [33] | |
| RP-18 CC, 60% MeOH/H2O of MeOH extract | - | - | Towo’e Beach Tahuna Bay, Sangihe Islands North Sulawesi, Indonesia | [25] | |
| n-Hexane fraction of MeOH extract | - | - | Sheraton Caverns, Kauai, Hawaii | [26] | |
| CH2Cl2/MeOH fraction of EtOH extract | - | - | Yongxing Island, South China Sea | [32] | |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| Polyfibrospongol A (53) | EtOAc fraction of EtOH extract | 386 | C24H34O4 | Meishan coral reef, Sanya, China | [33] |
| Et2O fraction of acetone extract | - | - | Xisha Island, Hainan, China | [27] | |
| (−)-Xishaeleganin C (54) | Et2O fraction of acetone extract | 372 | C23H32O4 | Xisha Island, Hainan, China | [27] |
| (+)-Xishaeleganin D (55) | Et2O fraction of acetone extract | 356 | C23H32O3 | Xisha Island, Hainan, China | [27] |
| (−)-Xishaeleganin A (56) | Et2O fraction of acetone extract | 386 | C24H34O4 | Xisha Island, Hainan, China | [27] |
| (−)-Xishaeleganin B (57) | Et2O fraction of acetone extract | 390 | C23H34O5 | Xisha Island, Hainan, China | [27] |
| (−)-Smenodiol (58) | CH2Cl2 fraction of MeOH extract | 372 | C23H32O4 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | Pelorus Island, the Great Barrier Reef, Queensland, Australia | [18] | |
| (−)-Dactylosponol (59) | CH2Cl2 fraction of MeOH extract | 356 | C23H32O3 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| (−)-Dactylospontriol (60) | CH2Cl2 fraction of MeOH extract | 388 | C23H32O5 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| (+)-Cyclospongiacatechol (61) | CH2Cl2 fraction of H2O extract | 388 | C23H32O5 | * Coast of Malaysia * Coast of Palau | [23] |
| Chromazonarol (62) | CH2Cl2 fraction of MeOH extract | 314 | C21H30O2 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| 8-Epi-Chromazonarol (63) | CH2Cl2 fraction of MeOH extract | 314 | C21H30O2 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of H2O extract | - | - | * Coast of Malaysia * Coast of Palau | [23] | |
| Pelorol (64) | CH2Cl2 fraction of MeOH extract | 372 | C23H32O4 | Pelorus Island, the Great Barrier Reef, Queensland, Australia | [18] |
| n-Hexane fraction of MeOH extract | - | - | Island of Ambon, Indonesia | [28] | |
| Nakijinol B (65) | 90% and 100% MeOH fraction of RP-18 CC of MeOH extract | 355 | C22H29NO3 | Pugh Shoal, northeast of Truant Island, Australia | [22] |
| Popolohuanone B (66) | CH2Cl2 fraction of CH2Cl2/MeOH extract | 623 | C42H57NO3 | Xisha Islands maritime space, South China Sea | [34] |
| Popolohuanone C (67) | CH2Cl2 fraction of CH2Cl2/MeOH extract | 623 | C42H57NO3 | Xisha Islands maritime space, South China Sea | [34] |
| Popolohuanone G (68) | CH2Cl2 fraction of CH2Cl2/MeOH extract | 642 | C42H56O4 | Xisha Islands maritime space, South China Sea | [34] |
| Popolohuanone H (69) | CH2Cl2 fraction of CH2Cl2/MeOH extract | 623 | C42H57NO3 | Xisha Islands maritime space, South China Sea | [34] |
| Popolohuanone I (70) | CH2Cl2 fraction of CH2Cl2/MeOH extract | 623 | C42H57NO3 | Xisha Islands maritime space, South China Sea | [34] |
| (−)-Dactyltronic acid A (71) | CH2Cl2 fraction of MeOH extract | 362 | C21H30O5 | Pulan Tiga, Sabah, Malaysia | [17] |
| EtOAc fraction of EtOH extract | - | - | Meishan coral reef, Sanya, China | [33] | |
| (−)-Dactyltronic acid B (72) | CH2Cl2 fraction of MeOH extract | 362 | C21H30O5 | Pulan Tiga, Sabah, Malaysia | [17] |
| EtOAc fraction of EtOH extract | - | - | Meishan coral reef, Sanya, China | [33] | |
| (+)-Dactylolactone A (73) | EtOAc fraction of MeOH extract | 404 | C23H32O6 | Coral reef of Ishigaki Island, Okinawa, Japan | [30] |
| (+)-Dactylolactone B (74) | EtOAc fraction of MeOH extract | 404 | C23H32O6 | Coral reef of Ishigaki Island, Okinawa, Japan | [30] |
| (−)-Dactylolactone C (75) | EtOAc fraction of MeOH extract | 404 | C23H32O6 | Coral reef of Ishigaki Island, Okinawa, Japan | [30] |
| (−)-Dactylolactone D (76) | EtOAc fraction of MeOH extract | 404 | C23H32O6 | Coral reef of Ishigaki Island, Okinawa, Japan | [30] |
| (+)-Dactylospene B (77) | CH2Cl2/MeOH fraction of EtOH extract | 400 | C26H40O3 | Yongxing Island, South China Sea | [35] |
| (+)-Dactylospene C (78) | CH2Cl2/MeOH fraction of EtOH extract | 400 | C26H40O3 | Yongxing Island, South China Sea | [35] |
| (+)-Dactylospene D (79) | CH2Cl2/MeOH fraction of EtOH extract | 432 | C27H44O4 | Yongxing Island, South China Sea | [35] |
| (+)-Dactylospene E (80) | CH2Cl2/MeOH fraction of EtOH extract | 432 | C27H44O4 | Yongxing Island, South China Sea | [35] |
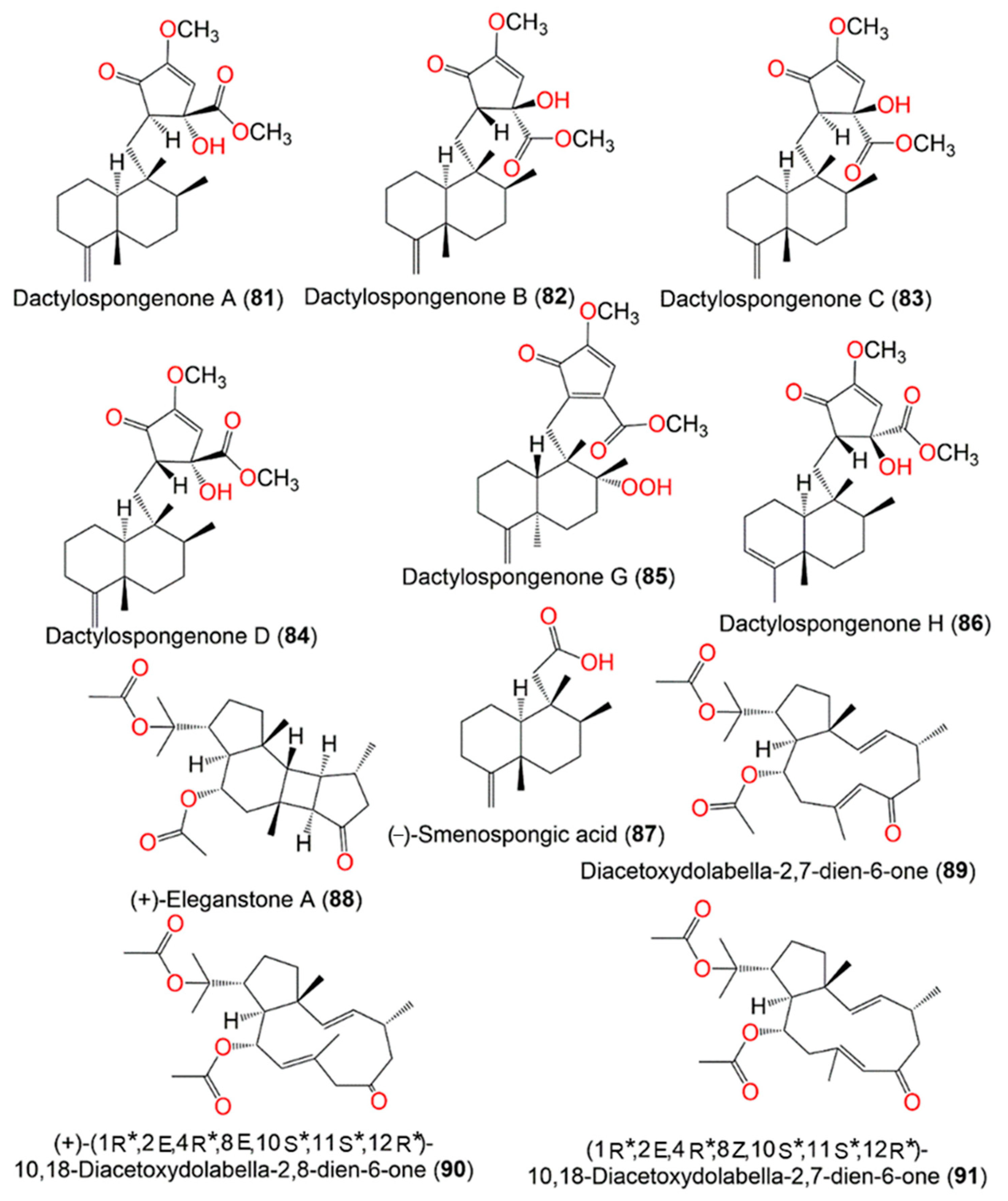
| Dactylospongenone A (81) | CH2Cl2 fraction of MeOH extract | 390 | C23H34O5 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | Pulan Tiga, Sabah, Malaysia | [17] | |
| Dactylospongenone B (82) | CH2Cl2 fraction of MeOH extract | 390 | C23H34O5 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | Pulan Tiga, Sabah, Malaysia | [17] | |
| Dactylospongenone C (83) | CH2Cl2 fraction of MeOH extract | 390 | C23H34O5 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | Pulan Tiga, Sabah, Malaysia | [17] | |
| Dactylospongenone D (84) | CH2Cl2 fraction of MeOH extract | 390 | C23H34O5 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | Pulan Tiga, Sabah, Malaysia | [17] | |
| Dactylospongenone G (85) | n-Hexane fraction of MeOH extract | 404 | C23H32O6 | Island of Ambon, Indonesia | [28] |
| Dactylospongenone H (86) | n-Hexane fraction of MeOH extract | 390 | C23H34O5 | Island of Ambon, Indonesia | [28] |
| (−)-Smenospongic acid (87) | CH2Cl2 fraction of MeOH extract | 250 | C16H26O2 | * Similani island, Phuket, Thailand * Papua New Guinea | [16] |
| CH2Cl2 fraction of MeOH extract | - | - | Pulan Tiga, Sabah, Malaysia | [17] | |
| (+)-Eleganstone A (88) | CH2Cl2 fraction of EtOH extract | 404 | C24H36O5 | Yongxing Island and Seven Connected Islets, South China Sea | [36] |
| Diacetoxydolabella-2,7-dien-6-one (89) | CH2Cl2 fraction of EtOH extract | 404 | C24H36O5 | Yongxing Island and Seven Connected Islets, South China Sea | [36] |
| (+)-(1R*,2E,4R*,8E,10S*,11S*,12R*)-10,18-Diacetoxydolabella-2,8-dien-6-one (90) | CH2Cl2 fraction of EtOH extract | 404 | C24H36O5 | Yongxing Island and Seven Connected Islets, South China Sea | [36] |
| (1R*,2E,4R*,7Z,10S*,11S*,12R*)-10,18-Diacetoxydolabella-2,7-dien- 6-one (91) | CH2Cl2 fraction of EtOH extract | 404 | C24H36O5 | Yongxing Island and Seven Connected Islets, South China Sea | [36] |
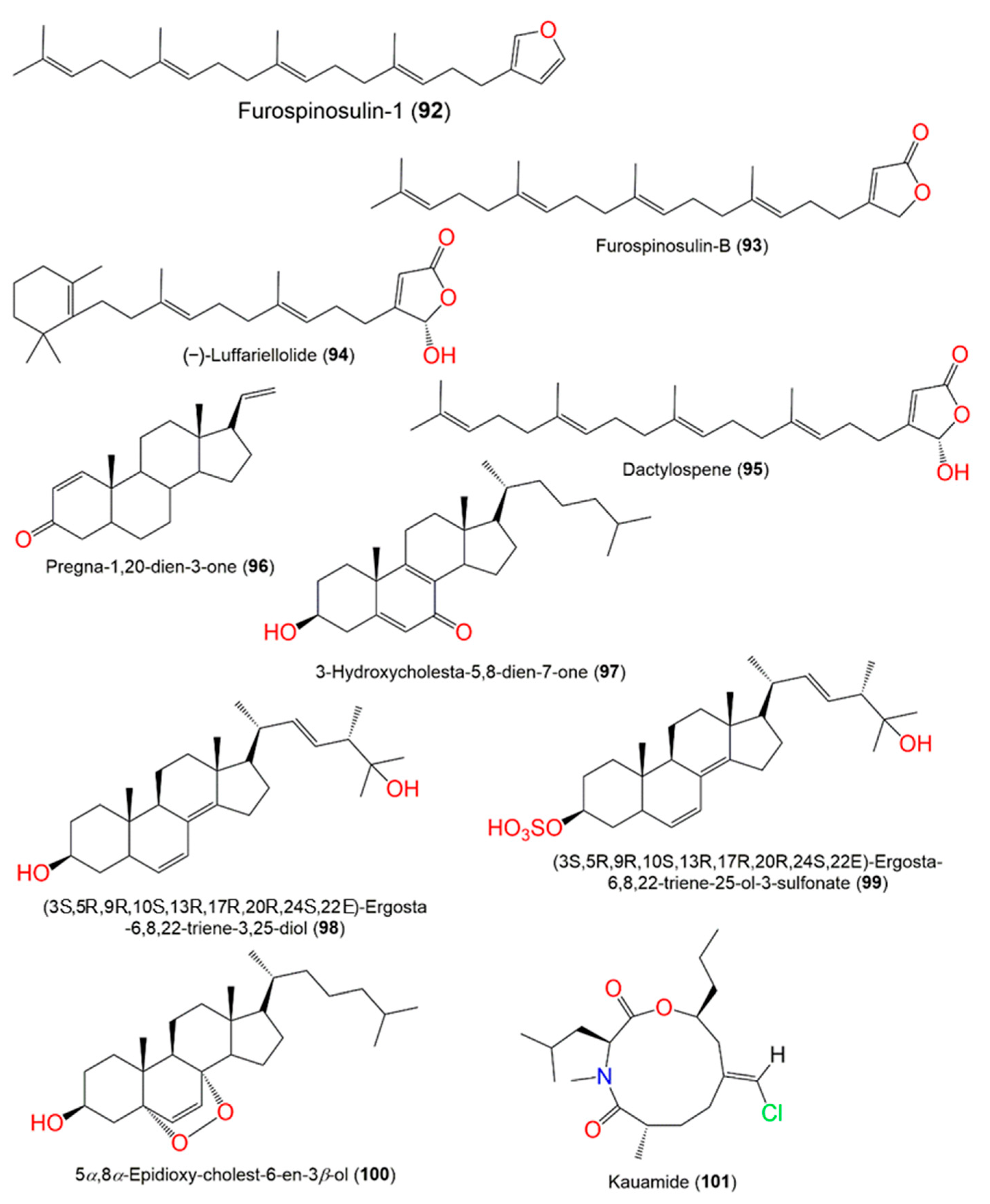
| Furospinosulin-1 (92) | EtOAc fraction of CH2Cl2 of MeOH extract | 354 | C25H38O | Coral Gardens dive site at the Inner Gneerings reef, a group of shoals near Mooloolaba, Australia | [21] |
| Furospinosulin B (93) | CH2Cl2/MeOH fractions of EtOH extract | 370 | C25H38O2 | Yongxing Island, South China Sea | [35] |
| (−)-Luffariellolide (94) | CH2Cl2/MeOH fractions of EtOH extract | 386 | C25H38O3 | Yongxing Island, South China Sea | [35] |
| (−)-Dactylospene A (95) | CH2Cl2/MeOH fractions of EtOH extract | 386 | C25H38O3 | Yongxing Island, South China Sea | [35] |
| Pregna-1,20-dien-3-one (96) | EtOAc fraction of EtOH extract | 298 | C21H30O | Meishan coral reef, Sanya, China | [33] |
| 3-Hydroxycholesta-5,8-dien-7-one (97) | EtOAc fraction of EtOH extract | 398 | C27H42O2 | Meishan coral reef, Sanya, China | [33] |
| (3S,5R,9R,10S,13R,17R,20R,24S,22E)-Ergosta-6,8,22-triene-3,25-diol (98) | CH2Cl2 fraction of CH2Cl2/MeOH extract | 412 | C28H44O2 | Xisha islands maritime space, South China Sea | [37] |
| (3S,5R,9R,10S,13R,17R,20R,24S,22E)-Ergosta-6,8,22-triene-25-ol-3-sulfonate (99) | CH2Cl2 fraction of CH2Cl2/MeOH extract | 492 | C28H44O5S | Xisha islands maritime space, South China Sea | [37] |
| 5α,8α-Epidioxy-cholest-6-en-3β-ol (100) | CH2Cl2 fraction of CH2Cl2/MeOH extract | 416 | C27H44O3 | Xisha islands maritime space, South China Sea | [37] |
| Kauamide (101) | n-Hexane fraction of MeOH extract | 357 | C19H33ClNO3 | Sheraton Caverns, Kauai, Hawaii | [25] |
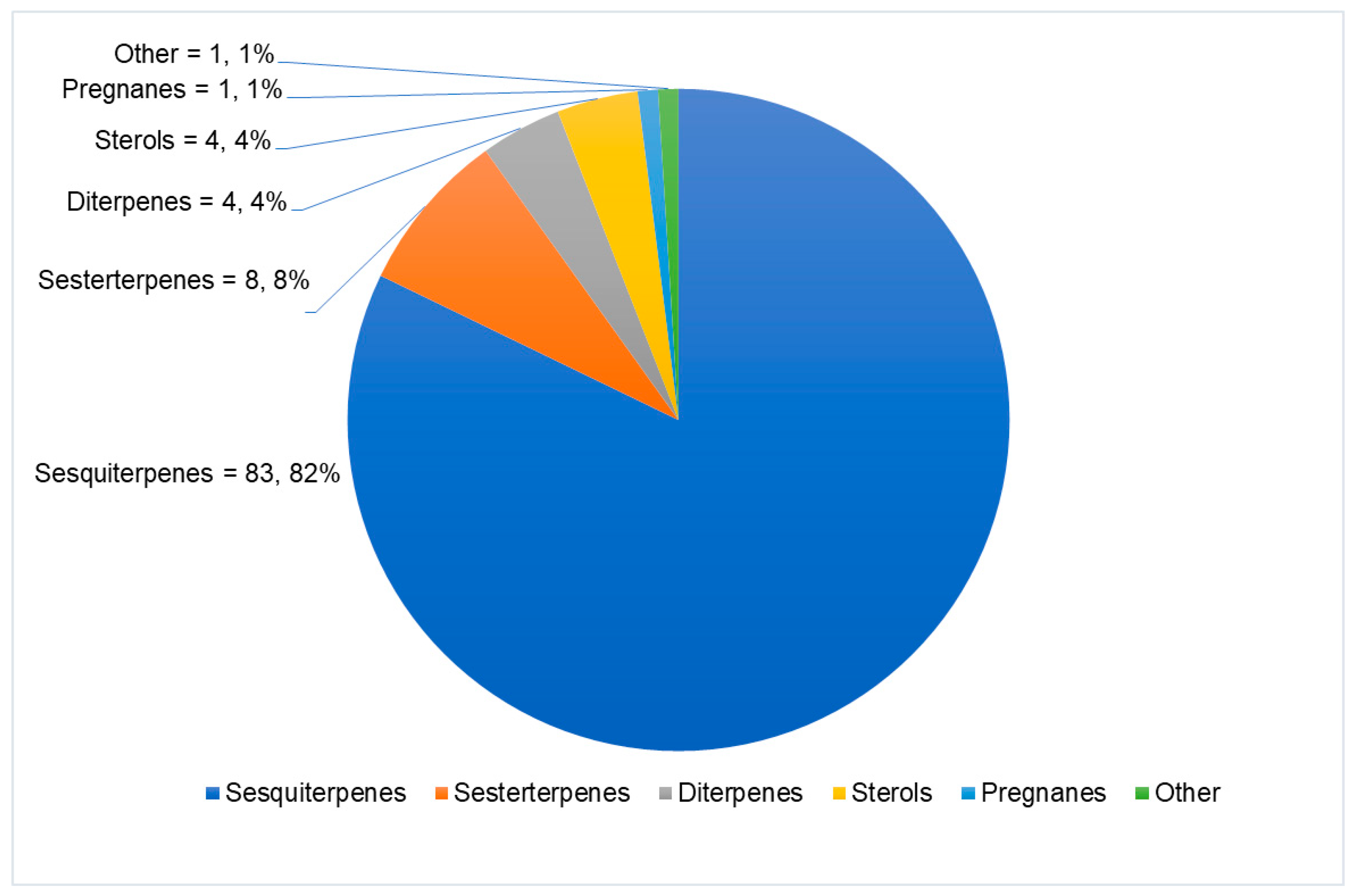
2.1. Sesquiterpenes
2.1.1. Sesquiterpenic Quinones/Hydroquinones
2.1.2. Sesquiterpenic Quinone/Hydroquinone Dimers
2.1.3. Sesquiterpene Tetronic Acids
2.2. Sesterterpenes
2.3. Diterpenes
2.4. Sterols and Pregnanes
2.5. Other Metabolites
3. Biosynthetic Pathways of D. elegans Metabolites
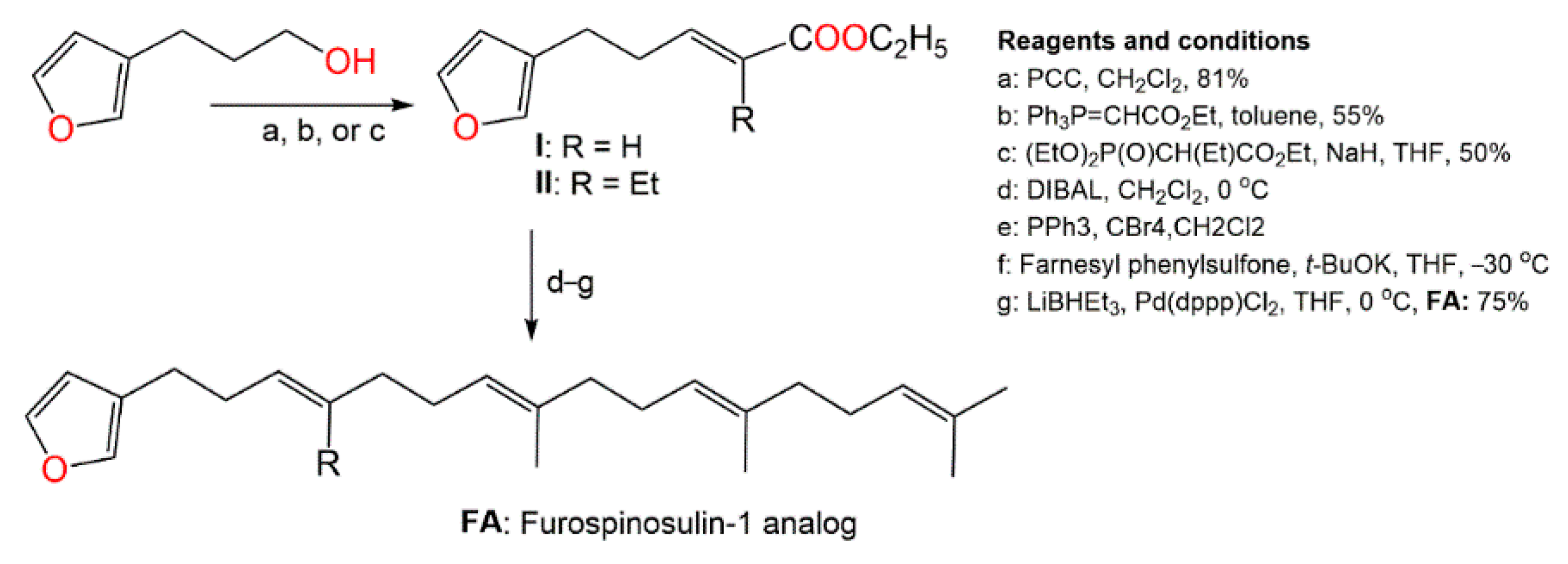
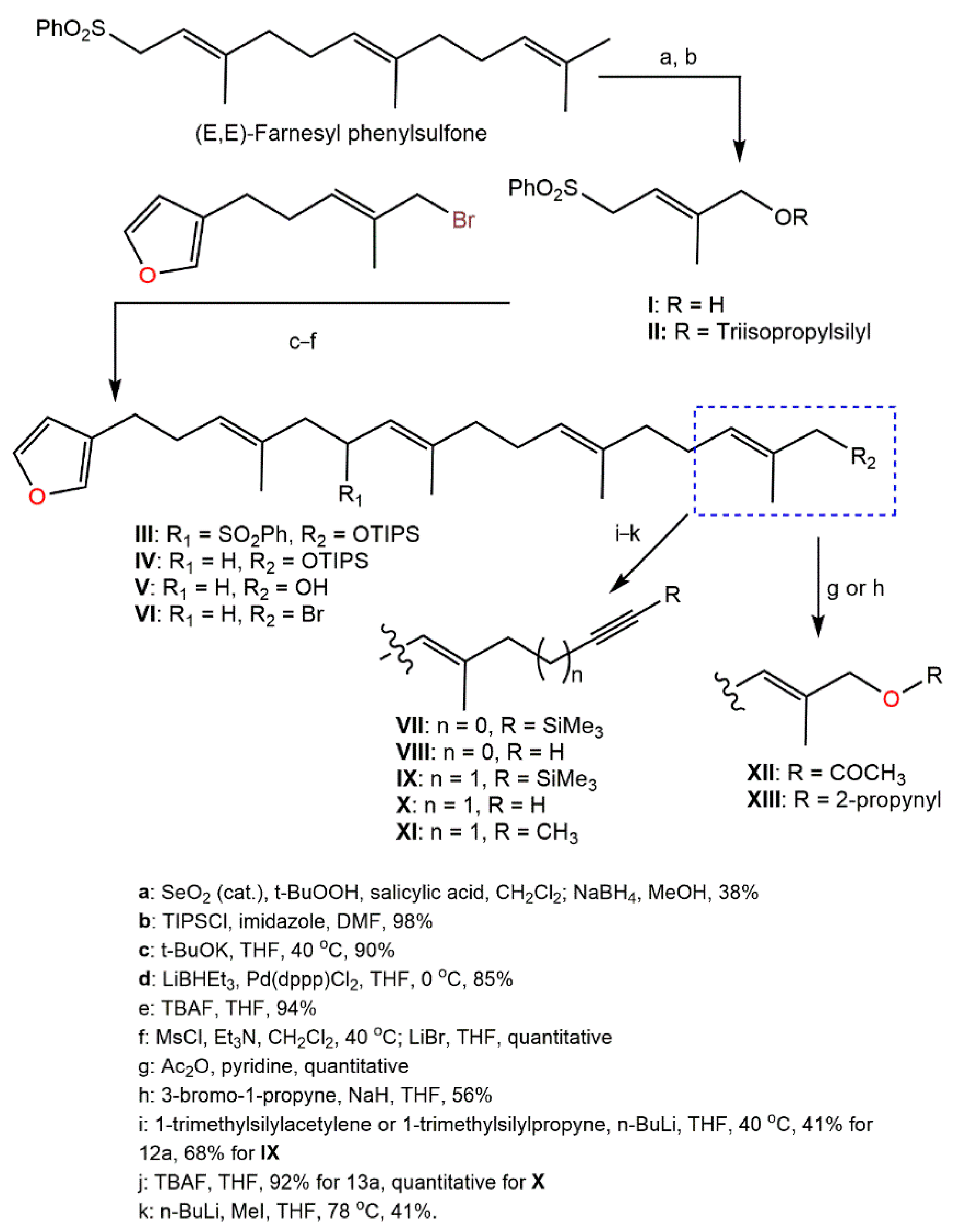
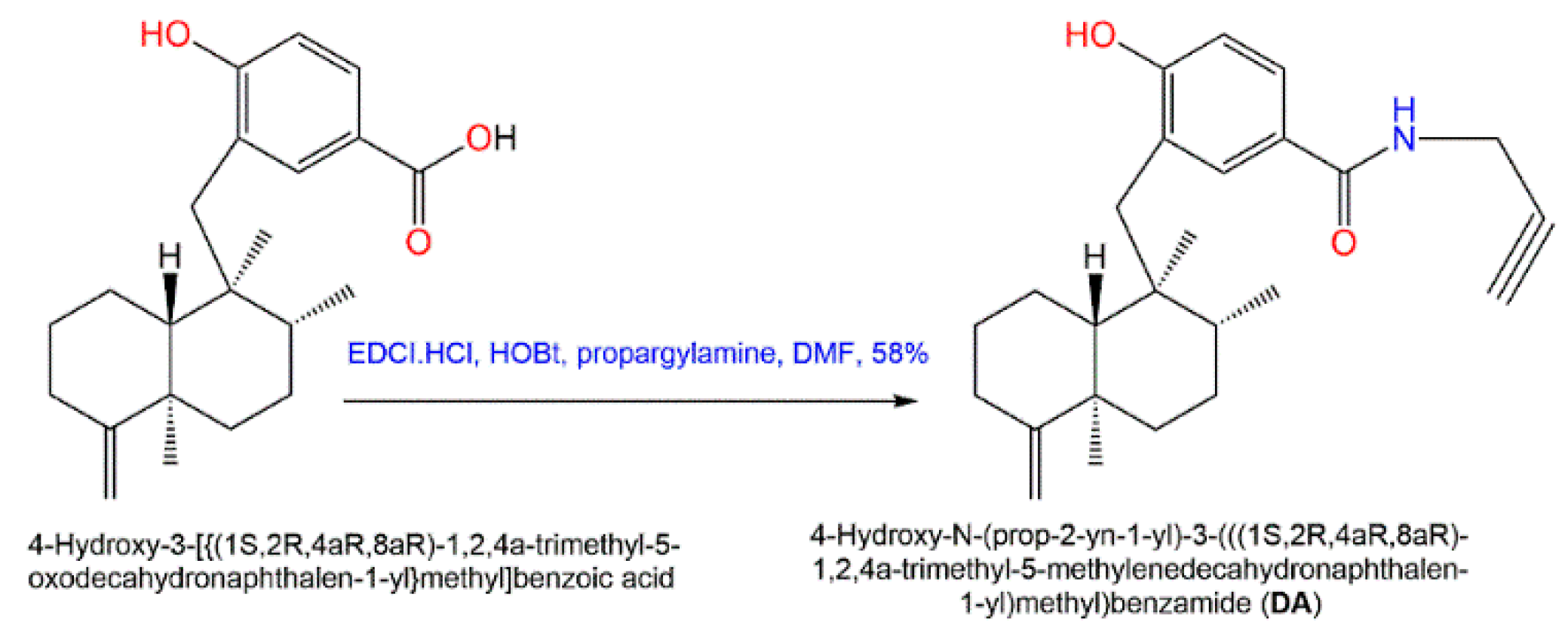
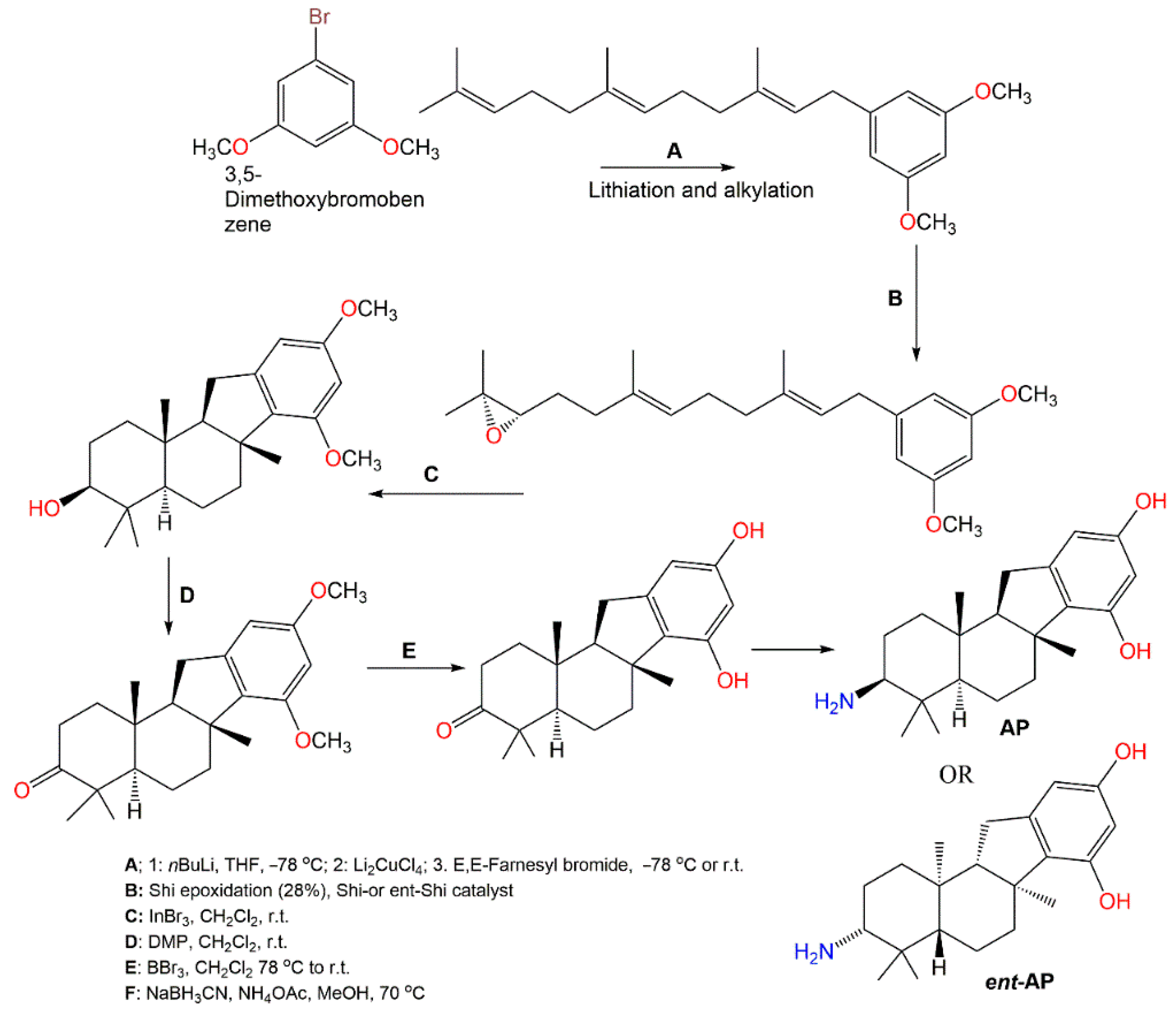
4. Synthesis of D. elegans Metabolites
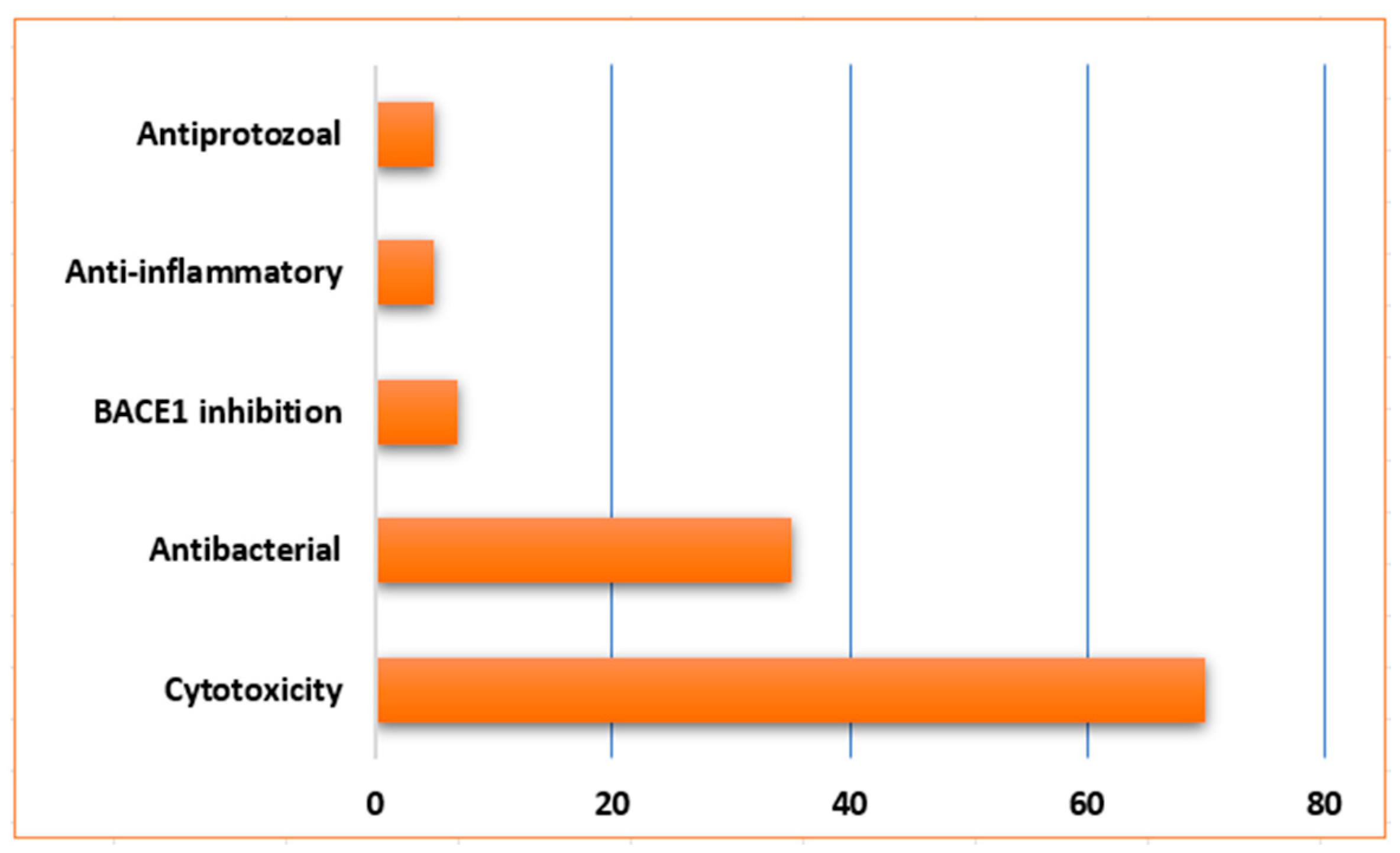
5. Activities of D. elegans Extracts and Fractions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kobayashi, J. Search for new bioactive marine natural products and application to drug development. Chem. Pharm. Bull. 2016, 64, 1079–1083. [Google Scholar] [CrossRef] [Green Version]
- Omar, A.M.; Mohamed, G.A.; Ibrahim, S. Chaetomugilins and chaetoviridins-promising natural metabolites: Structures, separation, characterization, biosynthesis, bioactivities, molecular docking, and molecular dynamics. J. Fungi 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Radjasa, O.K.; Vaske, Y.M.; Navarro, G.; Vervoort, H.C.; Tenney, K.; Linington, R.G.; Crews, P. Highlights of marine invertebrate-derived biosynthetic products: Their biomedical potential and possible production by microbial associants. Bioorg. Med. Chem. 2011, 19, 6658–6674. [Google Scholar] [CrossRef] [Green Version]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, G.A.; Ibrahim, S.R.M. Untapped potential of marine-associated Cladosporium species: An overview on secondary metabolites, biotechnological relevance, and biological activities. Mar. Drugs 2021, 19, 645. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.M.; Zhou, G.W.; Huang, H.; Wang, Y. The Cyanobacteria-dominated sponge Dactylospongia elegans in the South China Sea: Prokaryotic community and metagenomic insights. Front. Microbiol. 2017, 8, 1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentschel, U.; Hopke, J.; Horn, M.; Friedrich, A.B.; Wagner, M.; Hacker, J.; Moore, B.S. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl. Environ. Microbiol. 2002, 68, 4431–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, R.; Ruocco, N.; Viel, T.; Federico, S.; Zupo, V.; Costantini, M. Sponges and their symbionts as a source of valuable compounds in cosmeceutical field. Mar. Drugs 2021, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.J. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, J.H.; Lee, H.K. Microbial symbiosis in marine sponges. J. Microbiol. 2001, 39, 254–264. [Google Scholar]
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association-a review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galitz, A.; Nakao, Y.; Schupp, P.J.; Wörheide, G.; Erpenbeck, D. A soft spot for chemistry–current taxonomic and evolutionary implications of sponge secondary metabolite distribution. Mar. Drugs 2021, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Sladić, D.; Gasić, M.J. Reactivity and biological activity of the marine sesquiterpene hydroquinone avarol and related compounds from sponges of the order Dictyoceratida. Molecules 2006, 11, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, Y.; Fujioka, H.; Kita, Y. Synthesis of the marine pyrroloiminoquinone alkaloids, discorhabdins. Mar. Drugs 2010, 8, 1394–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, J.; Quiñoá, E.; Riguera, R.; Peters, B.M.; Abrell, L.M.; Crews, P. The structures and stereochemistry of cytotoxic sesquiterpene quinones from Dactylospongia elegans. Tetrahedron 1992, 48, 6667–6680. [Google Scholar] [CrossRef]
- López, M.D.; Quiñoá, E.; Riguera, R. Dactyltronic acids from the sponge Dactylospongia elegans. J. Nat. Prod. 1994, 57, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Goclik, E.; König, G.M.; Wright, A.D.; Kaminsky, R. Pelorol from the tropical marine sponge Dactylospongia elegans. J. Nat. Prod. 2000, 63, 1150–1152. [Google Scholar] [CrossRef]
- Mitome, H.; Nagasawa, T.; Miyaoka, H.; Yamada, Y.; van Soest, R.W. Dactyloquinones C, D and E novel sesquiterpenoid quinones, from the Okinawan marine sponge, Dactylospongia elegans. Tetrahedron 2002, 58, 1693–1696. [Google Scholar] [CrossRef]
- Aoki, S.; Kong, D.; Matsui, K.; Rachmat, R.; Kobayashi, M. Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukemia, K562 cells. Chem. Pharm. Bull. 2004, 52, 935–937. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.W.L.; Jankam, A.; Hooper, J.N.A.; Suksamrarn, A.; Garson, M.J. Stereochemical evaluation of sesquiterpene quinones from two sponges of the genus Dactylospongia and the implication for enantioselective processes in marine terpene biosynthesis. Tetrahedron 2008, 64, 6341–6348. [Google Scholar] [CrossRef]
- Ovenden, S.P.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. Sesquiterpene benzoxazoles and sesquiterpene quinones from the marine sponge Dactylospongia elegans. J. Nat. Prod. 2011, 74, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhou, Y.D.; Nagle, D.G. Inducers of hypoxic response: Marine sesquiterpene quinones activate HIF-1. J. Nat. Prod. 2013, 76, 1175–1181. [Google Scholar] [CrossRef] [Green Version]
- Arai, M.; Kawachi, T.; Sato, H.; Setiawan, A.; Kobayashi, M. Marine spongian sesquiterpene phenols, dictyoceratin-C and smenospondiol, display hypoxia-selective growth inhibition against cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 3155–3157. [Google Scholar] [CrossRef] [PubMed]
- Balansa, W.; Mettal, U.; Wuisan, Z.G.; Plubrukarn, A.; Ijong, F.G.; Liu, Y.; Schäberle, T.F. A New sesquiterpenoid aminoquinone from an Indonesian marine sponge. Mar. Drugs 2019, 17, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neupane, R.P.; Parrish, S.M.; Neupane, J.B.; Yoshida, W.Y.; Yip, M.; Turkson, J.; Harper, M.K.; Head, J.D.; Williams, P.G. Cytotoxic sesquiterpenoid quinones and quinols, and an 11-membered heterocycle, kauamide, from the Hawaiian marine sponge Dactylospongia elegans. Mar. Drugs 2019, 17, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Zhao, Q.; Gu, Y.-C.; Lan, L.; Wang, C.-Y.; Guo, Y.-W. Xishaeleganins A–D, sesquiterpenoid hydroquinones from Xisha marine sponge Dactylospongia elegans. Mar. Drugs 2022, 20, 118. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; de Voogd, N.; Kalscheuer, R.; Müller, W.E.G.; Chaidir; Proksch, P. Cytotoxic drimane meroterpenoids from the Indonesian marine sponge Dactylospongia elegans. Phytochem. Lett. 2017, 22, 154–158. [Google Scholar] [CrossRef]
- Mitome, H.; Nagasawa, T.; Miyaoka, H.; Yamada, Y.; van Soest, R.W. Dactyloquinones A and B, new sesquiterpenoid quinones from the Okinawan marine sponge Dactylospongia elegans. J. Nat. Prod. 2001, 64, 506–1508. [Google Scholar] [CrossRef] [PubMed]
- Mitome, H.; Nagasawa, T.; Miyaoka, H.; Yamada, Y.; van Soest, R.W. A new sesquiterpenoid quinone and other related compounds from the Okinawan marine sponge Dactylospongia elegans. J. Nat. Prod. 2003, 66, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Kong, D.; Matsui, K.; Kobayashi, M. Smenospongine, a spongean sesquiterpene aminoquinone, induces erythroid differentiation in K562 cells. Anti-Cancer Drugs 2004, 15, 363–369. [Google Scholar] [CrossRef]
- Yu, H.B.; Yin, Z.F.; Gu, B.B.; Zhang, J.P.; Wang, S.P.; Yang, F.; Lin, H.W. Cytotoxic meroterpenoids from the marine sponge Dactylospongia elegans. Nat. Prod. Res. 2021, 35, 1620–1626. [Google Scholar] [CrossRef]
- Zhong, R.; Shao, C.-L.; de Voogd, N.J.; Wang, C.-Y. Sesquiterpene derivatives and steroids from the sponge Dactylospongia elegans collected from the south China sea. Chem. Nat. Compd. 2014, 50, 759–761. [Google Scholar] [CrossRef]
- Li, J.; Wu, W.; Yang, F.; Liu, L.; Wang, S.P.; Jiao, W.H.; Xu, S.H.; Lin, H.W. Popolohuanones G–I, dimeric sesquiterpene quinones with IL-6 inhibitory activity from the marine sponge Dactylospongia elegans. Chem. Biodivers. 2018, 15, e1800078. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Gu, B.B.; Iwasaki, A.; Jiang, W.L.; Ecker, A.; Wang, S.P.; Yang, F.; Lin, H.W. Dactylospenes A–E, sesterterpenes from the marine sponge Dactylospongia elegans. Mar. Drugs 2020, 18, 491. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-B.; Gu, B.-B.; Wang, S.-P.; Cheng, C.-W.; Yang, F.; Li, H.-W. New diterpenoids from the marine sponge Dactylospongia elegans. Tetrahedron 2017, 73, 6657–6661. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yang, F.; Jiao, W.H.; Lin, H.W.; Xu, S.H. Two new steroids with cytotoxicity from the marine sponge Dactylospongia elegans collected from the South China Sea. Nat. Prod. Res. 2019, 33, 1340–1344. [Google Scholar] [CrossRef]
- Saide, A.; Damiano, S.; Ciarcia, R.; Lauritano, C. Promising activities of marine natural products against Hematopoietic Malignancies. Biomedicines 2021, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [Green Version]
- Boufridi, A.; Lachkar, D.; Erpenbeck, D.; Beniddir, M.A.; Evanno, L.; Petek, S.; Debitus, C.; Poupon, E. Ilimaquinone and 5-epi-ilimaquinone: Beyond a simple diastereomeric ratio, biosynthetic considerations from NMR-based analysis. Aust. J. Chem. 2017, 70, 743–750. [Google Scholar] [CrossRef]
- Yan, M.; Liu, Q. Differentiation therapy: A promising strategy for cancer treatment. Chin. J. Cancer 2016, 35, 3. [Google Scholar] [CrossRef] [Green Version]
- Amarante-Mendes, G.P.; Rana, A.; Datoguia, T.S.; Hamerschlak, N.; Brumatti, G. BCR-ABL1 Tyrosine kinase complex signaling transduction: Challenges to overcome resistance in chronic myeloid leukemia. Pharmaceutics 2022, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Aoki, S.; Sowa, Y.; Sakai, T.; Kobayashi, M. Smenospongine, a sesquiterpene aminoquinone from a marine sponge, induces G1 arrest or apoptosis in different leukemia cells. Mar. Drugs 2008, 6, 480–488. [Google Scholar] [PubMed]
- Kondracki, M.; Guyot, M. Smenospongine: A cytotoxic and antimicrobial aminoquinone isolated from Smenospongia sp. Tetrahedron Lett. 1987, 28, 5815–5818. [Google Scholar] [CrossRef]
- Kong, D.; Yamori, T.; Kobayashi, M.; Duan, H. Antiproliferative and antiangiogenic activities of smenospongine, a marine sponge sesquiterpene aminoquinone. Mar. Drugs 2011, 9, 154–161. [Google Scholar] [CrossRef] [Green Version]
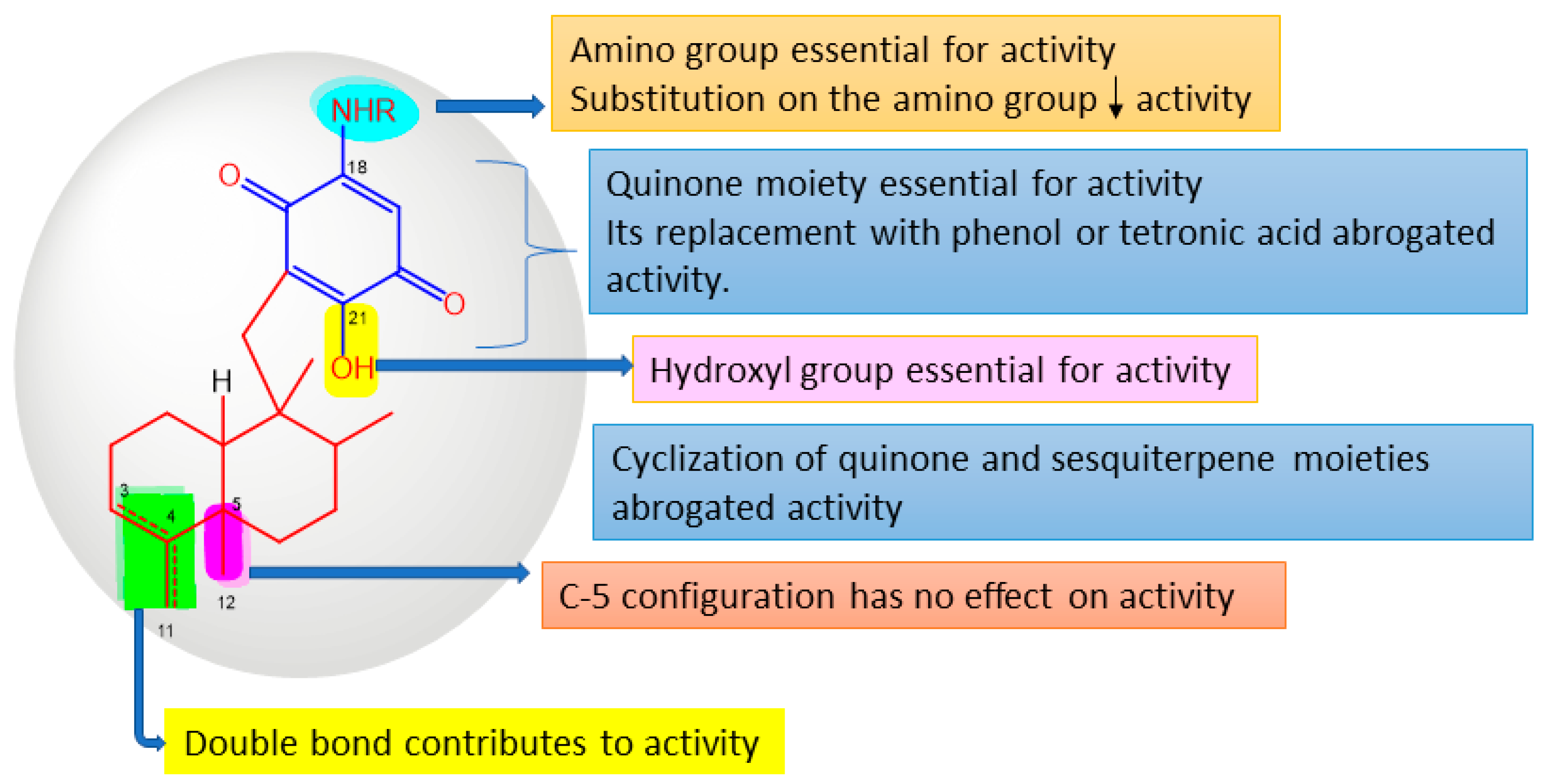
- Sumii, Y.; Kotoku, N.; Fukuda, A.; Kawachi, T.; Arai, M.; Kobayashi, M. Structure-activity relationship and in vivo anti-tumor evaluations of dictyoceratin-A and -C, hypoxia-selective growth inhibitors from marine sponge. Mar. Drugs 2015, 13, 7419–7432. [Google Scholar] [CrossRef] [PubMed]
- Sumii, Y.; Kotoku, N.; Fukuda, A.; Kawachi, T.; Sumii, Y.; Arai, M.; Kobayashi, M. Enantioselective synthesis of dictyoceratin-A (smenospondiol) and -C, hypoxia-selective growth inhibitors from marine sponge. Bioorg. Med. Chem. 2015, 23, 966–975. [Google Scholar] [CrossRef]
- Wang, B.; Bai, Z.Q.; Lin, X.P.; Yang, B.; Zhou, X.F.; Liu, Y.H. Chemical constituents of an endophytic fungus Aspergillus flavipes AIL8 obtained from mangrove Acanthus ilicifolius. Nat. Prod. Res. Dev. 2016, 28, 860–863. [Google Scholar]
- Pérez-García, E.; Zubía, E.; Ortega, M.J.; Carballo, J.L. Merosesquiterpenes from two sponges of the genus Dysidea. J. Nat. Prod. 2005, 68, 653–658. [Google Scholar] [CrossRef]
- Arai, M.; Kawachi, T.; Setiawan, A.; Kobayashi, M. Hypoxia-selective growth inhibition of cancer cells by furospinosulin-1, a furanosesterterpene isolated from an Indonesian marine sponge. ChemMedChem 2010, 5, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, N.; Arai, M.; Kawachi, T.; Fujioka, S.; Nakata, C.; Yamada, M.; Kobayashi, M. Studies on analogue synthesis and action-mechanism of furospinosulin-1, hypoxia-selective growth inhibitor from marine sponge. Symp. Chem. Nat. Prod. 2010, 52, 355–360. [Google Scholar]
- Tasdemir, D.; Bugni, T.S.; Mangalindan, G.C.; Concepcion, G.P.; Harper, M.K.; Ireland, C.M. Cytotoxic bromoindole derivatives and terpenes from the philippine marine sponge Smenospongia sp. Z. Naturforsch. C 2002, 57, 914–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, S.; Capon, R.J. Cometins (A-C), new furanosesterterpenes from an Australian marine sponge, Spongia sp. Aust. J. Chem. 1992, 45, 1255–1263. [Google Scholar] [CrossRef]
- Butler, M.S.; Capon, R.J. Beyond polygodial: New drimane sesquiterpene from a Southern marine sponge, Dysidea sp. Aust. J. Chem. 1993, 46, 1255–1267. [Google Scholar] [CrossRef]
- Poigny, S.; Huor, T.; Guyot, M.; Samadi, M. Synthesis of (−)-hyatellaquinone and revision of absolute configuration of naturally occurring (+)-hyatellaquinone. J. Org. Chem. 1999, 64, 9318–9320. [Google Scholar] [CrossRef]
- Liang, L.-F.; Kurtán, T.; Mándi, A.; Yao, L.-G.; Li, J.; Zhang, W.; Guo, Y.-W. Unprecedented diterpenoids as a PTP1B inhibitor from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller. Org. Lett. 2012, 15, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, N.; Fujioka, S.; Nakata, C.; Yamada, M.; Sumii, Y.; Kawachi, T.; Arai, M.; Kobayashi, M. Concise synthesis and structure activity relationship of furospinosulin-1, a hypoxia-selective growth inhibitor from marine sponge. Tetrahedron 2011, 67, 6673–6678. [Google Scholar] [CrossRef]
- Kotoku, N.; Nakata, C.; Kawachi, T.; Sato, T.; Guo, X.H.; Ito, A.; Sumii, Y.; Arai, M.; Kobayashi, M. Synthesis and evaluation of effective photoaffinity probe molecule of furospinosulin-1, a hypoxia-selective growth inhibitor. Bioorg. Med. Chem. 2014, 22, 2102–2112. [Google Scholar] [CrossRef]
- Yang, L.; Williams, D.E.; Mui, A.; Ong, C.; Krystal, G.; van Soest, R.; Andersen, R.J. Synthesis of pelorol and analogues: Activators of the inositol 5-phosphatase SHIP. Org. Lett. 2005, 7, 1073–1076. [Google Scholar] [CrossRef]
- Meimetis, L.G.; Nodwell, M.; Yang, L.; Wang, X.; Wu, J.; Harwig, C.; Stenton, G.R.; Mackenzie, L.F.; MacRury, T.; Patrick, B.O.; et al. Synthesis of SHIP1-activating analogs of the sponge meroterpenoid pelorol. Eur. J. Org. Chem. 2012, 2012, 5195–5207. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Rivera, A.P.; Uy, M.M. In vitro antioxidant and cytotoxic activities of some marine sponges collected off misamis oriental coast, Philippines. E-J. Chem. 2012, 9, 354–358. [Google Scholar] [CrossRef]






| Sponge Class | Compounds Classes |
|---|---|
| Calcarea | C27 to C29 ∆5,7,9(11),22 and C27 to C29 ∆5,7,22 sterols Amino alcohols |
| Hexactinellida | 5α(H)-Cholestan-3β-ol/cholest-5-en-3β-ol Ceramide glycosides |
| Homoscleromorpha | Steroidal alkaloids Peroxy-polyketides |
| Demospongiae | Pyrroloquinoline, azetidine, pyrrole-2-aminoimidazole, and pentacyclic guanidine alkaloids Norditerpene and norsesterterpene peroxides Tetramic acids Steroidal saponins and glycosides Isomalabaricane triterpenoids Bengamide and bengazoles Hydroxyimino- and 3β-hydroxymethyl-A-nor-sterols 3-Alkylpyridines/3-alkylpiperidines Renieramycins and polyacetylenes Pentacyclic hydroquinones/polyprenylated benzoquinones Adenine- and cyanthiwigin diterpenes Hypotaurocyamine (Sesquiterpene derivatives) Diterpene thio/iso/cyanides and formamides Sesquiterpene thio/iso/cyanides and formamides Aaptamines and bromotyrosines Suberitane-derived sesterterpenes Diterpene, sesquiterpene, and sesterterpenefurans/lactones Scalarane sesterterpenes/sesterterpene hydroquinones Thiazole polyketides Polybrominated diphenyl ethers |
| Compound Name | Biological Activity | Assay, Organism, or Cell Line | Biological Results | Ref. | |
|---|---|---|---|---|---|
| Compound | Positive Control | ||||
| (−)-Ilimaquinone (1) | Antitrypanosomal | Semiautomated microdilution/Trypanosoma brucei | 7.7 µg/mL (IC50) | Melarsoprol 0.0026 µg/mL (IC50) | [18] |
| Antimalarial | Semiautomated microdilution/P. falciparum clone K1 | 1743.0 µg/mL (IC50) | Chloroquine 91.0 µg/mL (IC50) | [18] | |
| Semiautomated microdilution/P. falciparum clone NF54 | 949.0 µg/mL (IC50) | Chloroquine 4.6 µg/mL (IC50) | [18] | ||
| Cytotoxicity | MTT/BC | 1.50 µg/mL (IC50) | Doxorubicin 0.29 µg/mL (IC50) | [21] | |
| MTT/NCI-H187 | 3.37 µg/mL (IC50) | Doxorubicin 0.06 µg/mL (IC50) | [21] | ||
| SRB/SF-268 | 2.7 µM (GI50) | Vehicle-DMSO | [22] | ||
| SRB/MCF-7 | 3.9 µM (GI50) | Vehicle -DMSO | [22] | ||
| SRB/H460 | 1.8 µM (GI50) | Vehicle -DMSO | [22] | ||
| SRB/HT-29 | 5.4 µM (GI50) | Vehicle-DMSO | [22] | ||
| SRB/CHO-K1 | 2.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| β-Secretase 1 inhibition | BACE1 | 65.0 µM (IC50) | - | [26] | |
| Cytotoxicity | MTT/U251 | 19.3 µM (CC50) | Vehicle-DMSO | [26] | |
| MTT/Panc-1 | 20.4 µM (CC50) | Vehicle-DMSO | [26] | ||
| Antibacterial | Broth microdilution/S. aureus USA300 LAC | 5.6 µg/mL (MIC) | Vancomycin 1.0 µg/mL (MIC) | [27] | |
| Broth microdilution/S. pyogenes ATCC 12344 | 2.8 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] | ||
| Broth microdilution/E. faecium Efm-HS0649 | 11.2 µg/mL (MIC) | Vancomycin ˃ 64.0 µg/mL (MIC) | [27] | ||
| 5-(+)-Epi-Ilimaquinone (2) | Cytotoxicity | A549 | 0.9 µg/mL (IC50) | - | [16] |
| HT-29 | 3.4 µg/mL (IC50) | - | [16] | ||
| B16F10 | 1.1 µg/mL (IC50) | - | [16] | ||
| P388 | 2.2 µg/mL (IC50) | - | [16] | ||
| Cytotoxicity | MTT/L5178Y | 2.23 µM (IC50) | Kahalalide F 4.30 µM (IC50) | [28] | |
| Antibacterial | Broth microdilution/S. aureus ATCC 25923 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | |
| Broth microdilution/S. aureus ATCC 700699 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | ||
| Cytotoxicity | MTT/U251 | 19.4 µM (CC50) | Vehicle-DMSO | [26] | |
| MTT/Panc-1 | 16.2 µM (CC50) | Vehicle-DMSO | [26] | ||
| Antibacterial | Broth microdilution/S. aureus USA300 LAC | 5.6 µg/mL (MIC) | Vancomycin 1.0 µg/mL (MIC) | [27] | |
| Broth microdilution/S. pyogenes ATCC 12344 | 2.8 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] | ||
| Broth microdilution/E. faecium Efm-HS0649 | 11.2 µg/mL (MIC) | Vancomycin ˃ 64.0 µg/mL (MIC) | [27] | ||
| (−)-Dactyloquinone A (4) | Antibacterial | Broth microdilution/S. pyogenes ATCC 12344 | 44.5 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] |
| Broth microdilution/E. faecium Efm-HS0649 | 22.2 µg/mL (MIC) | Vancomycin ˃ 64.0 µg/mL (MIC) | [27] | ||
| (−)-Dactyloquinone B (5) | Cytotoxicity | SRB/SF-268 | 32.0 µM (GI50) | Vehicle -DMSO | [22] |
| SRB/MCF-7 | 41.0 µM (GI50) | Vehicle -DMSO | [22] | ||
| SRB/H460 | 30.0 µM (GI50) | Vehicle -DMSO | [22] | ||
| SRB/HT-29 | 46.0 µM (GI50) | Vehicle -DMSO | [22] | ||
| SRB/CHO-K1 | 43.0 µM (GI50) | Vehicle -DMSO | [22] | ||
| Antibacterial | Broth microdilution/S. aureus USA300 LAC | 178.0 µg/mL (MIC) | Vancomycin 1.0 µg/mL (MIC) | [27] | |
| Broth microdilution/S. pyogenes ATCC 12344 | 22.2 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] | ||
| Broth microdilution/E. faecium Efm-HS0649 | 22.2 µg/mL (MIC) | Vancomycin ˃ 64.0 µg/mL (MIC) | [27] | ||
| (−)-Dactyloquinone C (7) | Antibacterial | Broth microdilution/S. aureus USA300 LAC | 11.1 µg/mL (MIC) | Vancomycin 1.0 µg/mL (MIC) | [27] |
| Broth microdilution/S. pyogenes ATCC 12344 | 5.6 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] | ||
| Broth microdilution/E. faecium Efm-HS0649 | 5.6 µg/mL (MIC) | Vancomycin ˃ 64.0 µg/mL (MIC) | [27] | ||
| (−)-Dactyloquinone D (8) | Antibacterial | Broth microdilution/S. pyogenes ATCC 12344 | 89.0 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] |
| Broth microdilution/E. faecium Efm-HS0649 | 178.0 µg/mL (MIC) | Vancomycin ˃ 64.0 µg/mL (MIC) | [27] | ||
| (+)-Dactyloquinone E (9) | Antibacterial | Broth microdilution/S. pyogenes ATCC 12344 | 22.2 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] |
| Broth microdilution/E. faecium Efm-HS0649 | 178.0 µg/mL (MIC) | Vancomycin ˃ 64.0 µg/mL (MIC) | [27] | ||
| (+)-Isospongiaquinone (11) | Cytotoxicity | MTT/L5178Y | 1.34 µM (IC50) | Kahalalide F 4.30 µM (IC50) | [28] |
| Antibacterial | Broth microdilution/S. aureus ATCC 25923 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | |
| Broth microdilution/S. aureus ATCC 700699 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 51299 | 50.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 35677 | 50.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 700221 | 50.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Mamanuthaquinone (14) | Cytotoxicity | MTT/BC | 2.61 µg/mL (IC50) | Doxorubicin 0.29 µg/mL (IC50) | [21] |
| MTT/NCI-H187 | 8.78 µg/mL (IC50) | Doxorubicin 0.06 µg/mL (IC50) | [21] | ||
| Hyatellaquinone (15) | Cytotoxicity | MTT/BC | 4.45 µg/mL (IC50) | Doxorubicin 0.29 µg/mL (IC50) | [21] |
| MTT/NCI-H187 | 10.90 µg/mL (IC50) | Doxorubicin 0.06 µg/mL (IC50) | [21] | ||
| MTT/BC | 1.50 µg/mL (IC50) | Doxorubicin 0.29 µg/mL (IC50) | [21] | ||
| (+)-Isohyatellaquinone (16) | Cytotoxicity | MTT/BC | 6.69 µg/mL (IC50) | Doxorubicin 0.29 µg/mL (IC50) | [21] |
| MTT/NCI-H187 | 11.52 µg/mL (IC50) | Doxorubicin 0.06 µg/mL (IC50) | [21] | ||
| Neomamanuthaquinone (18) | Cytotoxicity | MTT/BC | 8.42 µg/mL (IC50) | Doxorubicin 0.29 µg/mL (IC50) | [21] |
| 9-Epi-7,8-Dehydrocyclospongiaquinone-2 (20) | Cytotoxicity | MTT/BC | 7.38 µg/mL (IC50) | Doxorubicin 0.29 µg/mL (IC50) | [21] |
| MTT/NCI-H187 | 12.40 µg/mL (IC50) | Doxorubicin 0.06 µg/mL (IC50) | [21] | ||
| Smenospongine (25) | Cytotoxicity | A549 | 5.7 µg/mL (IC50) | - | [16] |
| HT-29 | 4.0 µg/mL (IC50) | - | [16] | ||
| B16F10 | 4.1 µg/mL (IC50) | - | [16] | ||
| P388 | 2.6 µg/mL (IC50) | - | [16] | ||
| MTT/U251 | 2.4 µM (CC50) | Vehicle-DMSO | [26] | ||
| β-Secretase 1 inhibition | BACE1 | 65.0 µM (IC50) | - | [26] | |
| Smenospongimine (27) | Cytotoxicity | CCK-8/DU145 | 3.5 µM (IC50) | Cisplatin 2.9 µM (IC50) | [32] |
| CCK-8/SW1990 | 4.2 µM (IC50) | Cisplatin 1.2 µM (IC50) | [32] | ||
| CCK-8/Huh7 | 2.3 µM (IC50) | Cisplatin 2.2 µM (IC50) | [32] | ||
| CCK-8/Panc-1 | 5.8 µM (IC50) | Cisplatin 4.6 µM (IC50) | [32] | ||
| Smenospongine B (28) | Cytotoxicity | SRB/SF-268 | 9.7 µM (GI50) | Vehicle-DMSO | [22] |
| SRB/MCF-7 | 10.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| SRB/H460 | 6.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| SRB/HT-29 | 6.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| SRB/CHO-K1 | 3.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| Smenospongine C (29) | Cytotoxicity | SRB/SF-268 | 20.0 µM (GI50) | Vehicle-DMSO | [22] |
| SRB/MCF-7 | 31.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| SRB/H460 | 14.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| SRB/HT-29 | 28.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| SRB/CHO-K1 | 18.0 µM (GI50) | Vehicle-DMSO | [22] | ||
| Antibacterial | Broth microdilution/S. aureus ATCC 25923 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | |
| Broth microdilution/S. aureus ATCC 700699 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | ||
| Smenospongorine (30) | Cytotoxicity | MTT/U251 | 19.4 µM (CC50) | Vehicle-DMSO | [26] |
| MTT/Panc-1 | 22.6 µM (CC50) | Vehicle-DMSO | [26] | ||
| CCK-8/DU145 | 4.2 µM (IC50) | Cisplatin 2.9 µM (IC50) | [32] | ||
| CCK-8/SW1990 | 4.4 µM (IC50) | Cisplatin 1.2 µM (IC50) | [32] | ||
| CCK-8/Huh7 | 3.0 µM (IC50) | Cisplatin 2.2 µM (IC50) | [32] | ||
| CCK-8/Panc-1 | 7.7 µM (IC50) | Cisplatin 4.6 µM (IC50) | [32] | ||
| Smenospongiarine (32) | Cytotoxicity | MTT/U251 | 4.5 µM (CC50) | Vehicle-DMSO | [26] |
| MTT/Panc-1 | 15.1 µM (CC50) | Vehicle-DMSO | [26] | ||
| CCK-8/DU145 | 6.1 µM (IC50) | Cisplatin 2.9 µM (IC50) | [32] | ||
| CCK-8/SW1990 | 5.9 µM (IC50) | Cisplatin 1.2 µM (IC50) | [32] | ||
| CCK-8/Huh7 | 3.7 µM (IC50) | Cisplatin 2.2 µM (IC50) | [32] | ||
| CCK-8/Panc-1 | 8.7 µM (IC50) | Cisplatin 4.6 µM (IC50) | [32] | ||
| 5-(+)-Epi-Smenospongiarine (33) | Cytotoxicity | A549 | 0.8 µg/mL (IC50) | - | [16] |
| HT-29 | 0.9 µg/mL (IC50) | - | [16] | ||
| B16F10 | 0.6 µg/mL (IC50) | - | [16] | ||
| P388 | 0.7 µg/mL (IC50) | - | [16] | ||
| Smenospongidine (35) | Cytotoxicity | MTT/U251 | 4.0 µM (CC50) | Vehicle-DMSO | [26] |
| MTT/Panc-1 | 12.6 µM (CC50) | Vehicle-DMSO | [26] | ||
| 5-(+)-Epi-Smenospongidine (36) | Cytotoxicity | A549 | 3.9 µg/mL (IC50) | - | [16] |
| HT-29 | 2.4 µg/mL (IC50) | - | [16] | ||
| B16F10 | 1.9 µg/mL (IC50) | - | [16] | ||
| P388 | 1.9 µg/mL (IC50) | - | [16] | ||
| MTT/L5178Y | 1.34 µM (IC50) | Kahalalide F 4.30 µM (IC50) | [28] | ||
| Antibacterial | Broth microdilution/S. aureus ATCC 25923 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | |
| Broth microdilution/E. faecalis ATCC 35677 | 50.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 700221 | 25.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Isosmenospongine (39) | Cytotoxicity | MTT/L5178Y | 1.69 µM (IC50) | Kahalalide F 4.30 µM (IC50) | [28] |
| Antibacterial | Broth microdilution/S. aureus ATCC 25923 | 25.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | |
| Broth microdilution/S. aureus ATCC 700699 | 12.5 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 29212 | 25.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 51299 | 25.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 35677 | 25.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 700221 | 25.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Nakijiquinone A (40) | Cytotoxicity | MTT/L5178Y | 6.48 µM (IC50) | Kahalalide F 4.30 µM (IC50) | [28] |
| Antibacterial | Broth microdilution/S. aureus ATCC 700699 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | |
| Broth microdilution/E. faecalis ATCC 51299 | 50.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Nakijiquinone B (41) | Antibacterial | Broth microdilution/S. aureus ATCC 25923 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] |
| Nakijiquinone G (43) | Cytotoxicity | MTT/L5178Y | 2.74 µM (IC50) | Kahalalide F 4.30 µM (IC50) | [28] |
| 5-Epi-Nakijiquinone Q (44) | Antibacterial | Broth microdilution/S. aureus ATCC 25923 | 25.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] |
| Broth microdilution/S. aureus ATCC 700699 | 50.0 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 35667 | 50.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| (+)-Dictyoceratin A (49) | Cytotoxicity | MTT/U251 | 2.8 µM (CC50) | Vehicle-DMSO | [26] |
| MTT/Panc-1 | 21.7 µM (CC50) | Vehicle-DMSO | [26] | ||
| Antibacterial | Broth microdilution/S. aureus USA300 LAC | 2.9 µg/mL (MIC) | Vancomycin 1.0 µg/mL (MIC) | [27] | |
| Broth microdilution/S. pyogenes ATCC 12344 | 2.9 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] | ||
| Broth microdilution/E. faecium Efm-HS0649 | 1.4 µg/mL (MIC) | Vancomycin ˃64.0 µg/mL (MIC) | [27] | ||
| (+)-19-Methoxy-dictyoceratin-A (50) | Cytotoxicity | CCK-8/DU145 | 24.4 µM (IC50) | Cisplatin 2.9 µM (IC50) | [32] |
| CCK-8/SW1990 | 21.4 µM (IC50) | Cisplatin 1.2 µM (IC50) | [32] | ||
| CCK-8/Huh7 | 17.4 µM (IC50) | Cisplatin 2.2 µM (IC50) | [32] | ||
| CCK-8/Panc-1 | 37.8 µM (IC50) | Cisplatin 4.6 µM (IC50) | [32] | ||
| (+)-Dictyoceratin B (51) | Cytotoxicity | MTT/U251 | 8.4 µM (CC50) | Vehicle-DMSO | [26] |
| MTT/Panc-1 | 54.6 µM (CC50) | Vehicle-DMSO | [26] | ||
| Antibacterial | Broth microdilution/S. aureus USA300 LAC | 12.1 µg/mL (MIC) | Vancomycin 1.0 µg/mL (MIC) | [27] | |
| Broth microdilution/S. pyogenes ATCC 12344 | 1.5 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] | ||
| Broth microdilution/E. faecium Efm-HS0649 | 3.0 µg/mL (MIC) | Vancomycin ˃ 64.0 µg/mL (MIC) | [27] | ||
| (+)-Dictyoceratin C (52) | Cytotoxicity | MTT/U251 | 4.1 µM (CC50) | Vehicle-DMSO | [26] |
| MTT/Panc-1 | 88.9 µM (CC50) | Vehicle-DMSO | [26] | ||
| CCK-8/DU145 | 8.3 µM (IC50) | Cisplatin 2.9 µM (IC50) | [32] | ||
| CCK-8/SW1990 | 7.9 µM (IC50) | Cisplatin 1.2 µM (IC50) | [32] | ||
| CCK-8/Huh7 | 6.9 µM (IC50) | Cisplatin 2.2 µM (IC50) | [32] | ||
| CCK-8/Panc-1 | 9.2 µM (IC50) | Cisplatin 4.6 µM (IC50) | [32] | ||
| (−)-Xishaeleganin C (54) | Antibacterial | Broth microdilution/S. aureus USA300 LAC | 11.1 µg/mL (MIC) | Vancomycin 1.0 µg/mL (MIC) | [27] |
| Broth microdilution/S. pyogenes ATCC 12344 | 2.8 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] | ||
| Broth microdilution/E. faecium Efm-HS0649 | 5.6 µg/mL (MIC) | Vancomycin ˃64.0 µg/mL (MIC) | [27] | ||
| (+)-Xishaeleganin D (55) | Antibacterial | Broth microdilution/S. pyogenes ATCC 12344 | 11.6 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] |
| (−)-Xishaeleganin B (57) | Antibacterial | Broth microdilution/S. aureus USA300 LAC | 1.5 µg/mL (MIC) | Vancomycin 1.0 µg/mL (MIC) | [27] |
| Broth microdilution/S. pyogenes ATCC 12344 | 1.5 µg/mL (MIC) | Vancomycin 0.25 µg/mL (MIC) | [27] | ||
| Broth microdilution/E. faecium Efm-HS0649 | 3.0 µg/mL (MIC) | Vancomycin ˃64.0 µg/mL (MIC) | [27] | ||
| Pelorol (64) | Antitrypanosomal | Semiautomated microdilution/Trypanosoma brucei | 17.4 µg/mL (IC50) | Melarsoprol 0.0026 µg/mL (IC50) | [18] |
| Antimalarial | Semiautomated microdilution/P. falciparum clone K1 | 786.0 µg/mL (IC50) | Chloroquine 91.0 µg/mL (IC50) | [18] | |
| Semiautomated microdilution/P. falciparum clone NF54 | 1911.0 µg/mL (IC50) | Chloroquine 4.6µg/mL (IC50) | [18] | ||
| Antibacterial | Broth microdilution/S. aureus ATCC 25923 | 6.25 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | |
| Broth microdilution/S. aureus ATCC 700699 | 3.125 µM (MIC) | Moxifloxacin 3.89 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 29212 | 12.5 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 51299 | 12.5 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 35677 | 25.0 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Broth microdilution/E. faecalis ATCC 700221 | 12.5 µM (MIC) | Ciprofloxacin 0.02 µM (MIC) | [28] | ||
| Nakijinol B (65) | Cytotoxicity | SRB/SF-268 | 24.0 µM (GI50) | Vehicle -DMSO | [22] |
| SRB/MCF-7 | 35.0 µM (GI50) | Vehicle -DMSO | [22] | ||
| SRB/H460 | 24.0 µM (GI50) | Vehicle -DMSO | [22] | ||
| SRB/HT-29 | 21.0 µM (GI50) | Vehicle -DMSO | [22] | ||
| SRB/CHO-K1 | 11.0 µM (GI50) | Vehicle -DMSO | [22] | ||
| (−)-Dactyltronic acid A (71) | Antibacterial | Broth microdilution/Vibrio parahemolyticus | 3.45 µM (MIC) | Ciprofloxacin 1.25 µM (MIC) | [33] |
| (−)-Dactyltronic acid B (72) | Antibacterial | Broth microdilution/Vibrio parahemolyticus | 3.45 µM (MIC) | Ciprofloxacin 1.25 µM (MIC) | [33] |
| (+)-Dactylospene B (77) | Anti-inflammatory | Griess reagent/LPS | 77.5% NO inhibition | - | [35] |
| (+)-Dactylospene C (78) | Cytotoxicity | CCK-8/DU145 | 13.35 µM (IC50) | Cisplatin 2.90 µM (IC50) | [35] |
| CCK-8/SW1990 | 7.40 µM (IC50) | Cisplatin 5.09 µM (IC50) | [35] | ||
| CCK-8/Huh7 | 2.37 µM (IC50) | Cisplatin 1.11 µM (IC50) | [35] | ||
| Anti-inflammatory | Griess reagent/LPS | 77.5% NO inhibition | - | [35] | |
| Dactylospongenone A (81) | Cytotoxicity | B16F10 | 2.1 µg/mL (IC50) | - | [16] |
| P388 | 0.6 µg/mL (IC50) | - | [16] | ||
| (−)-Luffariellolide (94) | Cytotoxicity | CCK-8/DU145 | 3.21 µM (IC50) | Cisplatin 2.90 µM (IC50) | [35] |
| CCK-8/SW1990 | 3.55 µM (IC50) | Cisplatin 5.09 µM (IC50) | [35] | ||
| CCK-8/Huh7 | 3.61 µM (IC50) | Cisplatin 1.11 µM (IC50) | [35] | ||
| CCK-8/Panc-1 | 5.21 µM (IC50) | Cisplatin 4.59 µM (IC50) | [35] | ||
| (−)-Dactylospene A (95) | Cytotoxicity | CCK-8/DU145 | 2.87 µM (IC50) | Cisplatin 2.90 µM (IC50) | [35] |
| CCK-8/SW1990 | 2.11 µM (IC50) | Cisplatin 5.09 µM (IC50) | [35] | ||
| CCK-8/Huh7 | 2.87 µM (IC50) | Cisplatin 1.11 µM (IC50) | [35] | ||
| CCK-8/Panc-1 | 7.59 µM (IC50) | Cisplatin 4.59 µM (IC50) | [35] | ||
| Pregna-1,20-dien-3-one (96) | Antibacterial | Broth microdilution/B. cereus | 4.19 µM (MIC) | Ciprofloxacin 1.25 µM (MIC) | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.R.M.; Fadil, S.A.; Fadil, H.A.; Hareeri, R.H.; Alolayan, S.O.; Abdallah, H.M.; Mohamed, G.A. Dactylospongia elegans—A Promising Drug Source: Metabolites, Bioactivities, Biosynthesis, Synthesis, and Structural-Activity Relationship. Mar. Drugs 2022, 20, 221. https://doi.org/10.3390/md20040221
Ibrahim SRM, Fadil SA, Fadil HA, Hareeri RH, Alolayan SO, Abdallah HM, Mohamed GA. Dactylospongia elegans—A Promising Drug Source: Metabolites, Bioactivities, Biosynthesis, Synthesis, and Structural-Activity Relationship. Marine Drugs. 2022; 20(4):221. https://doi.org/10.3390/md20040221
Chicago/Turabian StyleIbrahim, Sabrin R. M., Sana A. Fadil, Haifa A. Fadil, Rawan H. Hareeri, Sultan O. Alolayan, Hossam M. Abdallah, and Gamal A. Mohamed. 2022. "Dactylospongia elegans—A Promising Drug Source: Metabolites, Bioactivities, Biosynthesis, Synthesis, and Structural-Activity Relationship" Marine Drugs 20, no. 4: 221. https://doi.org/10.3390/md20040221
APA StyleIbrahim, S. R. M., Fadil, S. A., Fadil, H. A., Hareeri, R. H., Alolayan, S. O., Abdallah, H. M., & Mohamed, G. A. (2022). Dactylospongia elegans—A Promising Drug Source: Metabolites, Bioactivities, Biosynthesis, Synthesis, and Structural-Activity Relationship. Marine Drugs, 20(4), 221. https://doi.org/10.3390/md20040221









