Mechanism of Action and Therapeutic Potential of the β-Hairpin Antimicrobial Peptide Capitellacin from the Marine Polychaeta Capitella teleta
Abstract
:1. Introduction
2. Results and Discussion
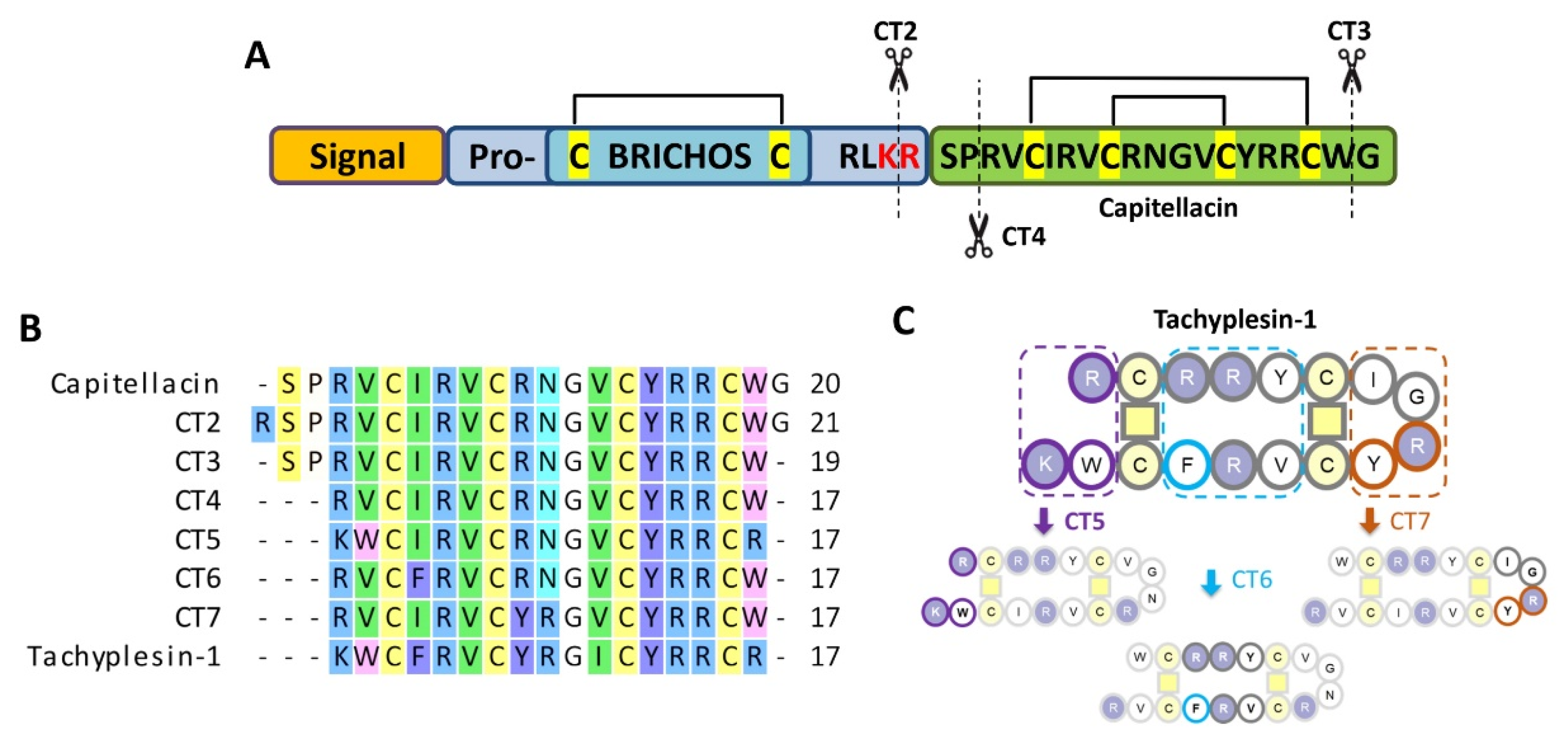
2.1. Design of Capitellacin Variants and Tachyplesin-Inspired Chimeric Analogs
2.2. Antimicrobial Activity of Capitellacin Analogs
2.3. The Structure of β-Turn Region Defines Low Membranotropic Activity of Capitellacin
2.4. Capitellacin Does Not Induce Bacterial Resistance
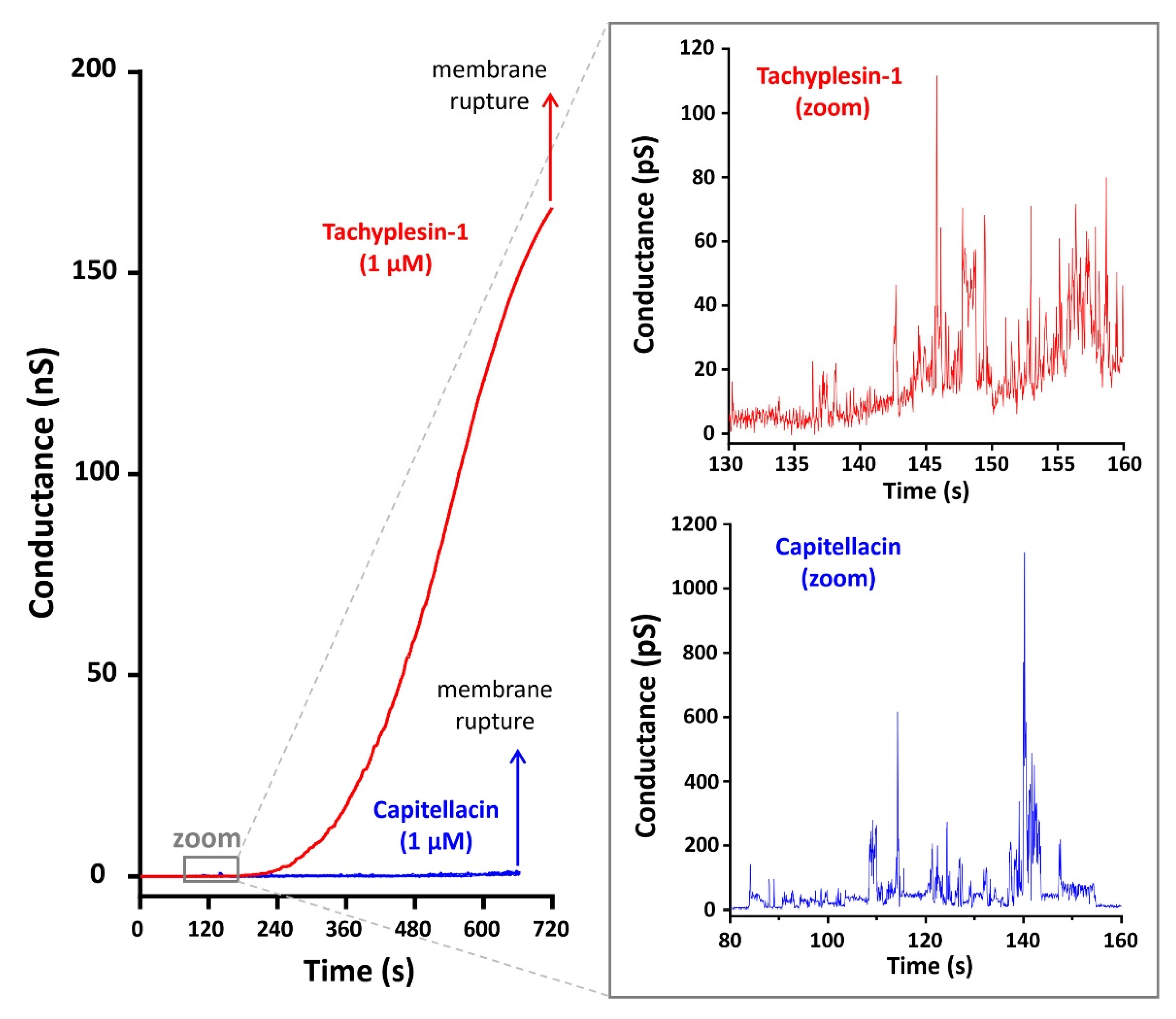
2.5. A Study of Capitellacin-Induced Conductance of Planar Lipid Membranes
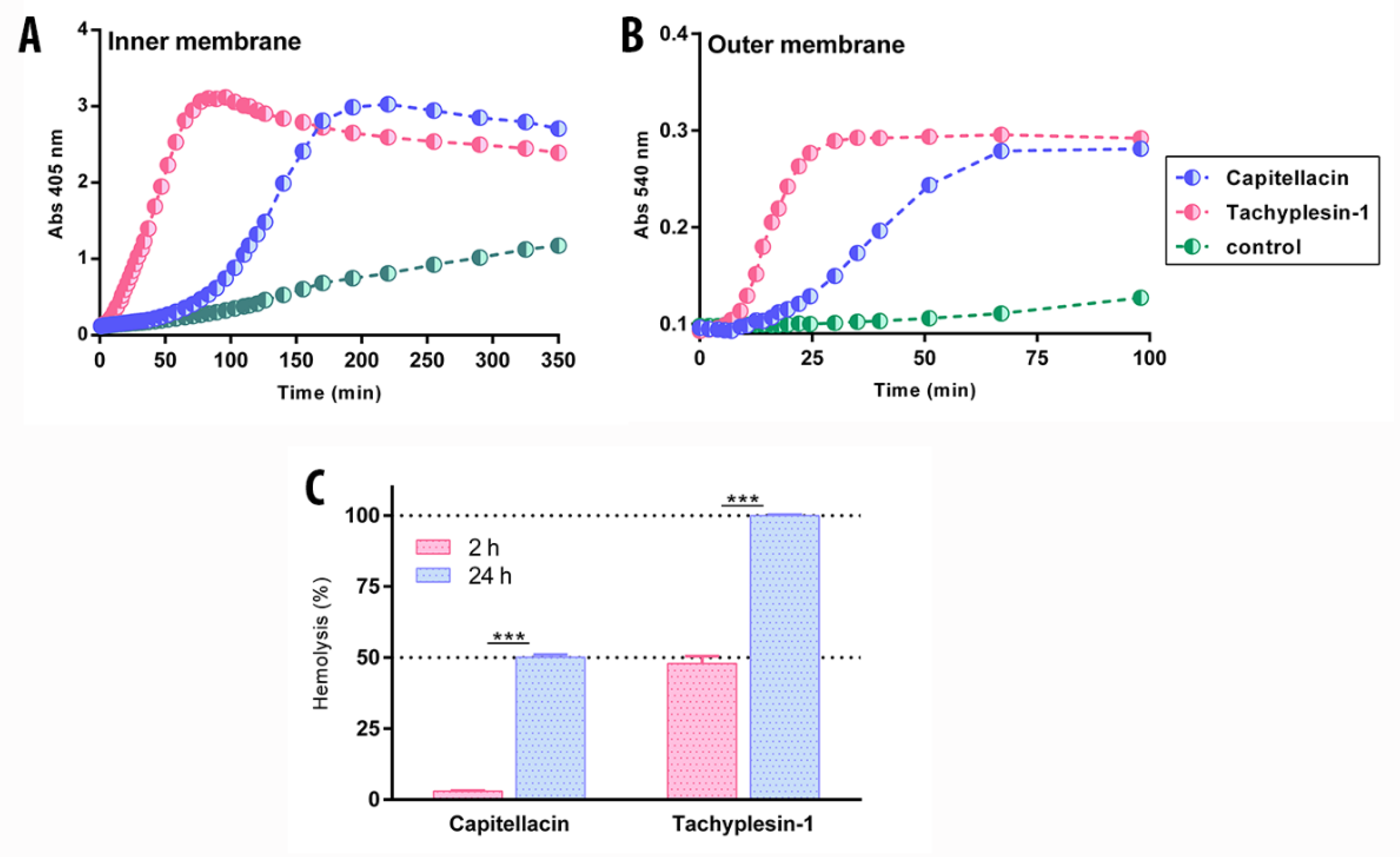
2.6. Capitellacin Demonstrates a Delayed Cell Membrane Permeability Effect as Compared to Tachyplesin-1
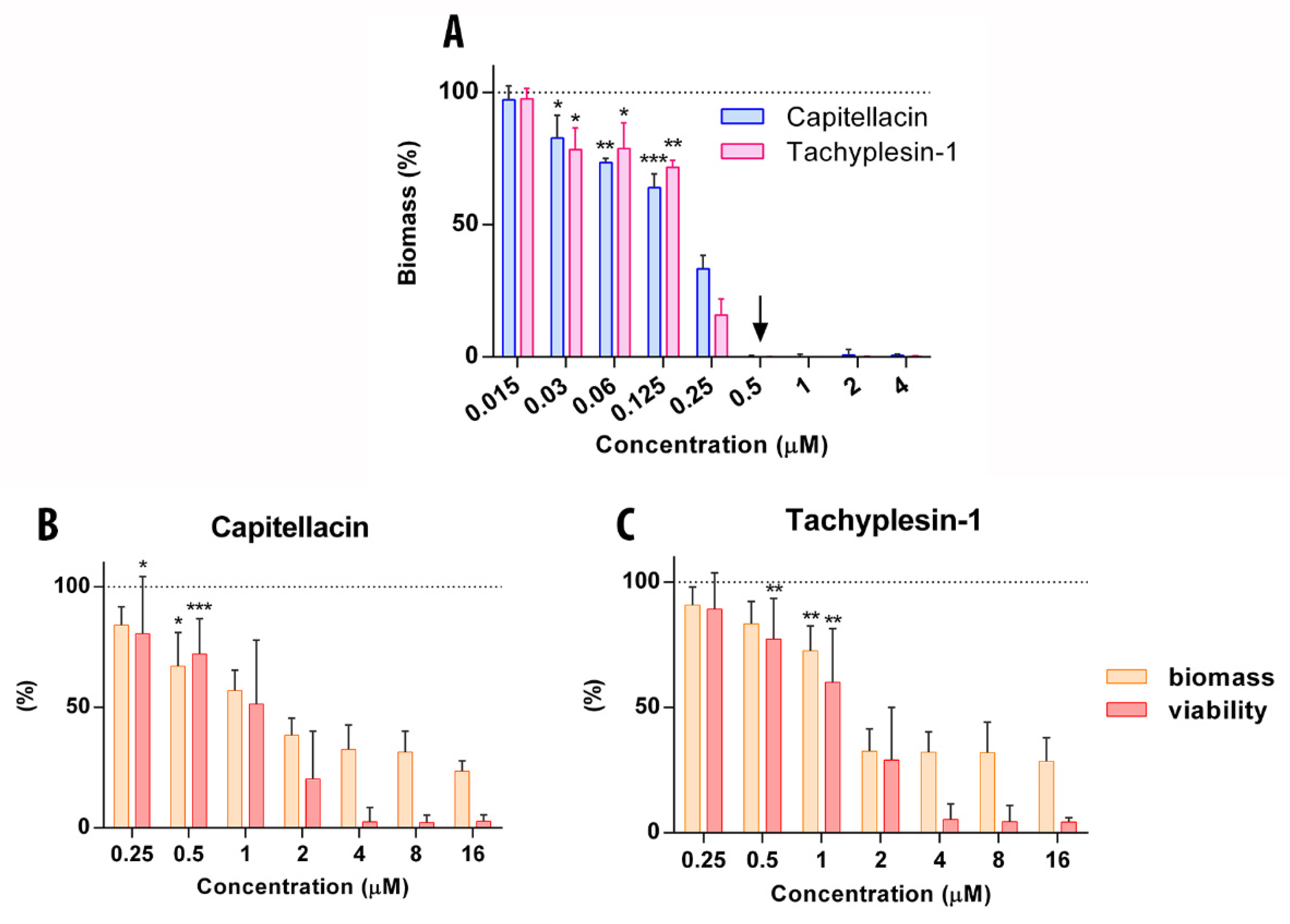
2.7. Capitellacin Exhibits Anti-Biofilm Activity against E. coli
3. Materials and Methods
3.1. Expression and Purification of the Antimicrobial Peptides
3.2. Antimicrobial Assays
3.3. Hemolytic Assay
3.4. The Effect of Peptides on the Permeability of the Outer and Cytoplasmatic Membrane of E. coli ML-35p
3.5. Measurement of Biofilm Activity
3.6. The Effect of Peptides on Established Biofilms
3.7. Resistance Induction of Peptides against Bacteria
3.8. Preparation of Planar Bilayers and Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional Cationic Host Defence Peptides and Their Clinical Applications. Cell. Mol. Life Sci. 2011, 68, 2161. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R. Road to Clinical Efficacy: Challenges and Novel Strategies for Antimicrobial Peptide Development. Future Microbiol. 2011, 6, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial Peptides for Therapeutic Use: Obstacles and Realistic Outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Panteleev, P.V.; Tsarev, A.V.; Safronova, V.N.; Reznikova, O.V.; Bolosov, I.A.; Sychev, S.V.; Shenkarev, Z.O.; Ovchinnikova, T.V. Structure Elucidation and Functional Studies of a Novel β-Hairpin Antimicrobial Peptide from the Marine Polychaeta Capitella teleta. Mar. Drugs 2020, 18, 620. [Google Scholar] [CrossRef]
- Saravanan, R.; Mohanram, H.; Joshi, M.; Domadia, P.N.; Torres, J.; Ruedl, C.; Bhattacharjya, S. Structure, Activity and Interactions of the Cysteine Deleted Analog of Tachyplesin-1 with Lipopolysaccharide Micelle: Mechanistic Insights into Outer-Membrane Permeabilization and Endotoxin Neutralization. Biochim. Biophys. Acta (BBA)—Biomembr. 2012, 1818, 1613–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Guan, W.; Jin, G.; Zhao, H.; Jiang, X.; Dai, J. Mechanism of Tachyplesin I Injury to Bacterial Membranes and Intracellular Enzymes, Determined by Laser Confocal Scanning Microscopy and Flow Cytometry. Microbiol. Res. 2015, 170, 69–77. [Google Scholar] [CrossRef]
- Kushibiki, T.; Kamiya, M.; Aizawa, T.; Kumaki, Y.; Kikukawa, T.; Mizuguchi, M.; Demura, M.; Kawabata, S.; Kawano, K. Interaction between Tachyplesin I, an Antimicrobial Peptide Derived from Horseshoe Crab, and Lipopolysaccharide. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2014, 1844, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K.; Yoneyama, S.; Fujii, N.; Miyajima, K.; Yamada, K.; Kirino, Y.; Anzai, K. Membrane Permeabilization Mechanisms of a Cyclic Antimicrobial Peptide, Tachyplesin I, and Its Linear Analog. Biochemistry 1997, 36, 9799–9806. [Google Scholar] [CrossRef]
- Katsu, T.; Nakao, S.; Iwanaga, S. Mode of Action of an Antimicrobial Peptide, Tachyplesin I, on Biomembranes. Biol. Pharm. Bull. 1993, 16, 178–181. [Google Scholar] [CrossRef] [Green Version]
- Imura, Y.; Nishida, M.; Ogawa, Y.; Takakura, Y.; Matsuzaki, K. Action Mechanism of Tachyplesin I and Effects of PEGylation. Biochim. Biophys. Acta (BBA)—Biomembr. 2007, 1768, 1160–1169. [Google Scholar] [CrossRef] [Green Version]
- Yonezawa, A.; Kuwahara, J.; Fujii, N.; Sugiura, Y. Binding of Tachyplesin I to DNA Revealed by Footprinting Analysis: Significant Contribution of Secondary Structure to DNA Binding and Implication for Biological Action. Biochemistry 1992, 31, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.V.; Aleshina, G.M.; Balandin, S.V.; Krasnosdembskaya, A.D.; Markelov, M.L.; Frolova, E.I.; Leonova, Y.F.; Tagaev, A.A.; Krasnodembsky, E.G.; Kokryakov, V.N. Purification and Primary Structure of Two Isoforms of Arenicin, a Novel Antimicrobial Peptide from Marine Polychaeta Arenicola marina. FEBS Lett. 2004, 577, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoni, G.; De Poli, A.; Mardirossian, M.; Gambato, S.; Florian, F.; Venier, P.; Wilson, D.N.; Tossi, A.; Pallavicini, A.; Gerdol, M. Myticalins: A Novel Multigenic Family of Linear, Cationic Antimicrobial Peptides from Marine Mussels (Mytilus spp.). Mar. Drugs 2017, 15, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, P.; Vanhoye, D.; Amiche, M. Molecular Strategies in Biological Evolution of Antimicrobial Peptides. Peptides 2003, 24, 1669–1680. [Google Scholar] [CrossRef]
- Tasiemski, A.; Jung, S.; Boidin-Wichlacz, C.; Jollivet, D.; Cuvillier-Hot, V.; Pradillon, F.; Vetriani, C.; Hecht, O.; Sönnichsen, F.D.; Gelhaus, C.; et al. Characterization and Function of the First Antibiotic Isolated from a Vent Organism: The Extremophile Metazoan Alvinella pompejana. PLoS ONE 2014, 9, e95737. [Google Scholar] [CrossRef]
- Tasiemski, A.; Schikorski, D.; Le Marrec-Croq, F.; Pontoire-Van Camp, C.; Boidin-Wichlacz, C.; Sautière, P.-E. Hedistin: A Novel Antimicrobial Peptide Containing Bromotryptophan Constitutively Expressed in the NK Cells-like of the Marine Annelid, Nereis diversicolor. Dev. Comp. Immunol. 2007, 31, 749–762. [Google Scholar] [CrossRef]
- Shinnar, A.E.; Butler, K.L.; Park, H.J. Cathelicidin Family of Antimicrobial Peptides: Proteolytic Processing and Protease Resistance. Bioorg. Chem. 2003, 31, 425–436. [Google Scholar] [CrossRef]
- Dong, N.; Ma, Q.; Shan, A.; Lv, Y.; Hu, W.; Gu, Y.; Li, Y. Strand Length-Dependent Antimicrobial Activity and Membrane-Active Mechanism of Arginine- and Valine-Rich β-Hairpin-Like Antimicrobial Peptides. Antimicrob. Agents Chemother. 2012, 56, 2994–3003. [Google Scholar] [CrossRef] [Green Version]
- Panteleev, P.V.; Bolosov, I.A.; Ovchinnikova, T.V. Bioengineering and Functional Characterization of Arenicin Shortened Analogs with Enhanced Antibacterial Activity and Cell Selectivity. J. Pept. Sci. 2016, 22, 82–91. [Google Scholar] [CrossRef]
- Gennaro, R.; Zanetti, M.; Benincasa, M.; Podda, E.; Miani, M. Pro-Rich Antimicrobial Peptides from Animals: Structure, Biological Functions and Mechanism of Action. Curr. Pharm. Des. 2002, 8, 763–778. [Google Scholar] [CrossRef]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Blaskovich, M.A.T.; Cooper, M.A. Structure–Activity and –Toxicity Relationships of the Antimicrobial Peptide Tachyplesin-1. ACS Infect. Dis. 2017, 3, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Panteleev, P.V.; Ovchinnikova, T.V. Improved Strategy for Recombinant Production and Purification of Antimicrobial Peptide Tachyplesin I and Its Analogs with High Cell Selectivity. Biotechnol. Appl. Biochem. 2017, 64, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and Consequences of Bacterial Resistance to Antimicrobial Peptides. Drug Resist. Updates 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Elliott, A.G.; Huang, J.X.; Neve, S.; Zuegg, J.; Edwards, I.A.; Cain, A.K.; Boinett, C.J.; Barquist, L.; Lundberg, C.V.; Steen, J.; et al. An Amphipathic Peptide with Antibiotic Activity against Multidrug-Resistant Gram-Negative Bacteria. Nat. Commun. 2020, 11, 3184. [Google Scholar] [CrossRef]
- Spohn, R.; Daruka, L.; Lázár, V.; Martins, A.; Vidovics, F.; Grézal, G.; Méhi, O.; Kintses, B.; Számel, M.; Jangir, P.K.; et al. Integrated Evolutionary Analysis Reveals Antimicrobial Peptides with Limited Resistance. Nat. Commun. 2019, 10, 4538. [Google Scholar] [CrossRef] [PubMed]
- Shenkarev, Z.O.; Balandin, S.V.; Trunov, K.I.; Paramonov, A.S.; Sukhanov, S.V.; Barsukov, L.I.; Arseniev, A.S.; Ovchinnikova, T.V. Molecular Mechanism of Action of β-Hairpin Antimicrobial Peptide Arenicin: Oligomeric Structure in Dodecylphosphocholine Micelles and Pore Formation in Planar Lipid Bilayers. Biochemistry 2011, 50, 6255–6265. [Google Scholar] [CrossRef] [PubMed]
- Shamova, O.V.; Orlov, D.S.; Balandin, S.V.; Shramova, E.I.; Tsvetkova, E.V.; Panteleev, P.V.; Leonova, Y.F.; Tagaev, A.A.; Kokryakov, V.N.; Ovchinnikova, T.V. Acipensins—Novel Antimicrobial Peptides from Leukocytes of the Russian Sturgeon Acipenser Gueldenstaedtii. Acta Nat. 2014, 6, 99–109. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.M.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of Bacterial Biofilm Formation and Swarming Motility by a Small Synthetic Cationic Peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, V.; Malini, A. Antimicrobial Resistance Pattern in Escherichia coli Causing Urinary Tract Infection among Inpatients. Indian J. Med. Res. 2014, 139, 945–948. [Google Scholar]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli Biofilm: Development and Therapeutic Strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human Host Defense Peptide LL-37 Prevents Bacterial Biofilm Formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-C.; Lee, M.-Y.; Kim, J.-Y.; Kim, H.; Jung, M.; Shin, M.-K.; Lee, W.-K.; Cheong, G.-W.; Lee, J.R.; Jang, M.-K. Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm. Molecules 2019, 24, 4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radzig, M.A.; Koksharova, O.A.; Khmel’, I.A. Antibacterial Effects of Silver Ions on Growth of Gram-Negative Bacteria and Biofilm Formation. Mol. Genet. Microbiol. Virol. 2009, 24, 194–199. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside Antibiotics Induce Bacterial Biofilm Formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.F.; Gustafsson, I.; Baquero, F.; Martinez, J.L. Antibiotics as Intermicrobial Signaling Agents Instead of Weapons. Proc. Natl. Acad. Sci. USA 2006, 103, 19484–19489. [Google Scholar] [CrossRef] [Green Version]
- Panteleev, P.V.; Bolosov, I.A.; Kalashnikov, A.À.; Kokryakov, V.N.; Shamova, O.V.; Emelianova, A.A.; Balandin, S.V.; Ovchinnikova, T.V. Combined Antibacterial Effects of Goat Cathelicidins with Different Mechanisms of Action. Front. Microbiol. 2018, 9, 2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panteleev, P.V.; Balandin, S.V.; Ovchinnikova, T.V. Effect of Arenicins and Other β-Hairpin Antimicrobial Peptides on Pseudomonas aeruginosa PAO1 Biofilms. Pharm. Chem. J. 2017, 50, 715–720. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Reconstitution of Cell Membrane Structure in Vitro and Its Transformation into an Excitable System. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef]


| Peptide | Recombinant Peptide Final Yield, mg/L | RP-HPLC Retention Time, min 1 | Hydrophobicity Index 2 | Calculated [M+H]+ Monoisotopic Mass, Da 3 | Measured Monoisotopic m/z Value 4 |
|---|---|---|---|---|---|
| Cap * | 6.1 | 38.5 | −0.215 | 2379.16 | 2379.27 |
| CT2 | 5.5 | 38.0 | −0.419 | 2535.26 | 2535.28 |
| CT3 | 5.1 | 38.3 | −0.205 | 2322.13 | 2322.31 |
| CT4 | 5.2 | 37.8 | −0.388 | 2138.05 | 2138.54 |
| CT5 | 5.1 | 33.5 | −0.565 | 2167.08 | 2167.42 |
| CT6 | 6.4 | 37.7 | −0.188 | 2172.03 | 2172.32 |
| CT7 | 4.6 | 40.8 | 0.041 | 2187.07 | 2187.43 |
| Tach-1 | 7.2 | 37.5 | −0.518 | 2264.10 | 2263.73 |
| Bacteria | Minimum Inhibitory Concentration (µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cap * | CT2 | CT3 | CT4 | CT5 | CT6 | CT7 | Tach-1 | |
| Gram-positive | ||||||||
| Micrococcus luteus B-1314 | 4 | 1 | 4 | 4 | 8 | 4 | 1 | 2 |
| Staphylococcus aureus 209P | 8 | 4 | 8 | 4 | 8 | 4 | 2 | 1 |
| Bacillus subtilis B-886 | 16 | 8 | 32 | 16 | 32 | 16 | 4 | 2 |
| Geometric mean (µM) ** | 8 | 3.17 | 10.08 | 6.35 | 12.7 | 6.35 | 2 | 1.59 |
| Gram-negative | ||||||||
| E. coli MDR CI 1057 | 0.5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.125 |
| E. coli BW25113 | 0.5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.25 |
| E. coli ML-35p | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 0.125 |
| E. coli ATTC 25922 | 0.5 | 0.25 | 0.5 | 0.5 | 0.5 | 0.25 | 0.25 | 0.25 |
| E. coli CI 3600 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.25 | 0.06 |
| E. coli CI 214 | 1 | 0.25 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 0.06 |
| E. cloacae CI 4172 | 4 | 2 | 8 | 4 | 4 | 2 | 2 | 0.25 |
| A. baumanii CI 2675 | 0.25 | 0.125 | 0.25 | 0.125 | 0.5 | 0.25 | 0.125 | 0.016 |
| P. aeruginosa PAO1 | 2 | 1 | 2 | 1 | 4 | 2 | 1 | 0.5 |
| K. pneumonia ATCC 700603 | 4 | 2 | 4 | 2 | 4 | 2 | 4 | 1 |
| Geometric mean (µM) ** | 1 | 0.46 | 1.09 | 0.59 | 1.09 | 0.55 | 0.55 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safronova, V.N.; Panteleev, P.V.; Sukhanov, S.V.; Toropygin, I.Y.; Bolosov, I.A.; Ovchinnikova, T.V. Mechanism of Action and Therapeutic Potential of the β-Hairpin Antimicrobial Peptide Capitellacin from the Marine Polychaeta Capitella teleta. Mar. Drugs 2022, 20, 167. https://doi.org/10.3390/md20030167
Safronova VN, Panteleev PV, Sukhanov SV, Toropygin IY, Bolosov IA, Ovchinnikova TV. Mechanism of Action and Therapeutic Potential of the β-Hairpin Antimicrobial Peptide Capitellacin from the Marine Polychaeta Capitella teleta. Marine Drugs. 2022; 20(3):167. https://doi.org/10.3390/md20030167
Chicago/Turabian StyleSafronova, Victoria N., Pavel V. Panteleev, Stanislav V. Sukhanov, Ilia Y. Toropygin, Ilia A. Bolosov, and Tatiana V. Ovchinnikova. 2022. "Mechanism of Action and Therapeutic Potential of the β-Hairpin Antimicrobial Peptide Capitellacin from the Marine Polychaeta Capitella teleta" Marine Drugs 20, no. 3: 167. https://doi.org/10.3390/md20030167
APA StyleSafronova, V. N., Panteleev, P. V., Sukhanov, S. V., Toropygin, I. Y., Bolosov, I. A., & Ovchinnikova, T. V. (2022). Mechanism of Action and Therapeutic Potential of the β-Hairpin Antimicrobial Peptide Capitellacin from the Marine Polychaeta Capitella teleta. Marine Drugs, 20(3), 167. https://doi.org/10.3390/md20030167






