Fillet Fish Fortified with Algal Extracts of Codium tomentosum and Actinotrichia fragilis, as a Potential Antibacterial and Antioxidant Food Supplement
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity and MIC Determination
2.2. Antioxidant Activity
2.3. Cytotoxicity Assay
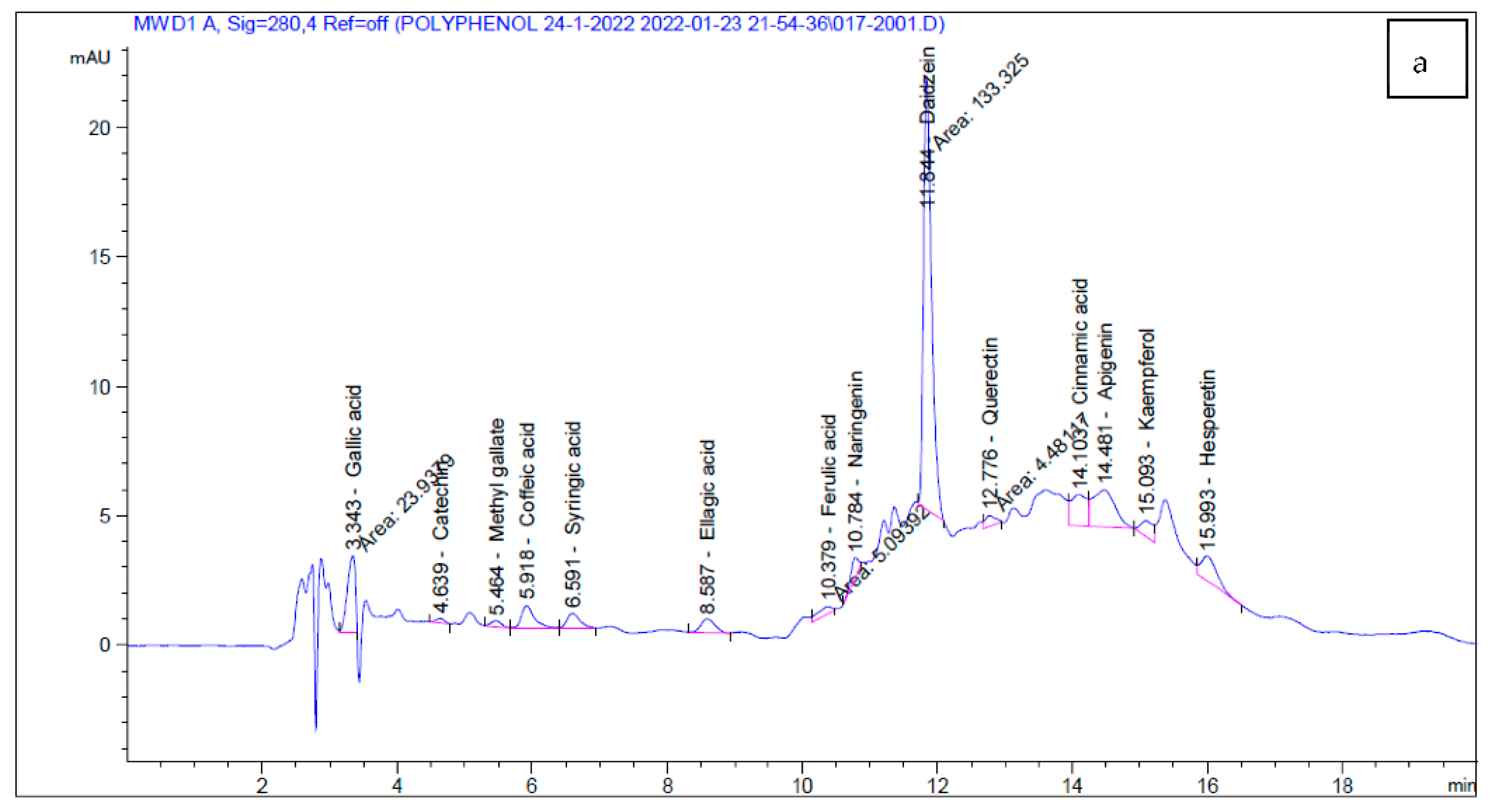
2.4. HPLC Analysis
2.5. Sensory Evaluation of Fillet Fish Fortified with Algal Extracts
3. Materials and Methods
3.1. Algae Collection
3.2. Preparation of Algal Extracts
3.3. Antibacterial Activity and MIC Determination
3.4. Determination of Total Phenolic Contents
3.5. Determination of Total Flavonoid Contents
3.6. Antioxidant Potentials and DPPH Radical Scavenging Activity
3.7. HPLC Analysis
3.8. Cytotoxicity Assay
3.9. Sensory Evaluation of Fillet Fish Fortified with Algal Extracts
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Feky, S.E.; Abd El Hafez, M.S.M.; Abd El Moneim, N.A.; Ibrahim, H.A.H.; Okbah, M.A.; Ata, A.; El Sedfy, A.S.; Hussein, A. Cytotoxic and Antimicrobial Activities of Two New Sesquiterpenoids from Red Sea Brittle Star Ophiocoma dentata. Sci. Rep. 2022, 12, 8209. [Google Scholar] [CrossRef]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine Natural Products as Source of New Drugs: A Patent Review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Abd El Hafez, M.S.M.; El Aziz Okbah, M.A.; Ibrahim, H.A.H.; Hussein, A.A.E.R.; El Moneim, N.A.A.; Ata, A. First Report of Steroid Derivatives Isolated from Starfish Acanthaster planci with Anti-Bacterial, Anti-Cancer and Anti-Diabetic Activities. Nat. Prod. Res. 2022, 36, 5545–5552. [Google Scholar] [CrossRef]
- Abd El Hafez Et Al, M.S. Biological Activities of Secondary Metabolites from Turbinaria triquetra (Phaeophyceae), Hypnea cornuta (Florideophyceae), and Ulva prolifera (Ulvophyceae) Methanolic Extracts. Egypt. J. Aquat. Biol. Fish. 2022, 26, 1227–1246. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study. Mar. Drugs 2021, 19, 178. [Google Scholar] [CrossRef]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Environmental Impact on Seaweed Phenolic Production and Activity: An Important Step for Compound Exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef]
- Abd El Hafez, M.S.M.; ElKomy, R.G.; Saleh, H.; Aboul-Ela, H.M. Extracts of the Green Algae Ulva Prolifera Possess Antioxidant and Antibacterial Activities In Vitro. Egypt. J. Aquat. Biol. Fish. 2020, 24, 267–280. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Hassan, S.S.U.; Bungau, S.; Si, Y.; Xu, H.; Rahman, M.H.; Behl, T.; Gitea, D.; Pavel, F.-M.; Corb Aron, R.A.; et al. Chemically Diverse and Biologically Active Secondary Metabolites from Marine Phylum Chlorophyta. Mar. Drugs 2020, 18, 493. [Google Scholar] [CrossRef]
- Pasdaran, A.; Hamedi, A.; Mamedov, N.A. Antibacterial and Insecticidal Activity of Volatile Compounds of Three Algae Species of Oman Sea. Int. J. Second. Metab. 2016, 3, 66–73. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive Properties and Potentials Cosmeceutical Applications of Phlorotannins Isolated from Brown Seaweeds: A Review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.; Pinto, D.C.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Abd El Hafez, M.S.M.; Abd El-Wahab, M.G.; Seadawy, M.G.; El-Hosseny, M.F.; Beskales, O.; Saber Ali Abdel-Hamid, A.; El Demellawy, M.A.; Ghareeb, D.A. Characterization, In-Silico, and In-Vitro Study of a New Steroid Derivative from Ophiocoma dentata as a Potential Treatment for COVID-19. Sci. Rep. 2022, 12, 5846. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.-S.; Jena, M. Beneficial Effects of Seaweeds and Seaweed-Derived Bioactive Compounds: Current Evidence and Future Prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Alghazeer, R.; Howell, N.K.; El-Naili, M.B.; Awayn, N. Anticancer and Antioxidant Activities of Some Algae from Western Libyan Coast. Nat. Sci. 2018, 10, 1–24. [Google Scholar] [CrossRef][Green Version]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and Antioxidant Activity of Phlorotannins Extracted from the Brown Seaweed Cystoseira compressa in Streptozotocin-Induced Diabetic Rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef]
- Swanson, B.G. Tannins and Polyphenols. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5729–5733. [Google Scholar]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780367395094. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant Activities of Enzymatic Extracts from Brown Seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Corona, G.; Coman, M.M.; Guo, Y.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Effect of Simulated Gastrointestinal Digestion and Fermentation on Polyphenolic Content and Bioactivity of Brown Seaweed Phlorotannin-Rich Extracts. Mol. Nutr. Food Res. 2017, 61, 1700223. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E. Phenolic Compounds in the Brown Seaweed Ascophyllum Nodosum: Distribution and Radical-Scavenging Activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Li, Z.; Mou, Q. The Anti-Allergic Activity of Polyphenol Extracted from Five Marine Algae. J. Ocean Univ. China 2015, 14, 681–684. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.; Ryan, L.; Bonham, M. The Impact of a Single Dose of a Polyphenol-Rich Seaweed Extract on Postprandial Glycaemic Control in Healthy Adults: A Randomised Cross-Over Trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Neuroprotective Effects of Marine Algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential Pharmacological Applications of Polyphenolic Derivatives from Marine Brown Algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Tanna, B.; Brahmbhatt, H.R.; Mishra, A. Phenolic, Flavonoid, and Amino Acid Compositions Reveal That Selected Tropical Seaweeds Have the Potential to Be Functional Food Ingredients. J. Food Process. Preserv. 2019, 43, e14266. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-Proliferative and Potential Anti-Diabetic Effects of Phenolic-Rich Extracts from Edible Marine Algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Urquiaga, I.; Leighton, F. Plant Polyphenol Antioxidants and Oxidative Stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef]
- Pereira, L. Therapeutic and Nutritional Uses of Algae; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781498755382. [Google Scholar]
- Ravikumar, S.; Jacob Inbaneson, S.; Suganthi, P. Seaweeds as a Source of Lead Compounds for the Development of New Antiplasmodial Drugs from South East Coast of India. Parasitol. Res. 2011, 109, 47–52. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging Role of Phenolic Compounds as Natural Food Additives in Fish and Fish Products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural Phenol Polymers: Recent Advances in Food and Health Applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef]
- Arguelles, E.D.L.R. Evaluation of Nutritional Composition and in Vitro Antioxidant and Antibacterial Activities of Codium intricatum Okamura from Ilocos Norte (Philippines). Jordan J. Biol. Sci. 2020, 13, 375–382. [Google Scholar]
- Koz, F.F.Y.; Yavasoglu, N.U.K.; Demirel, Z.; Sukatar, A.; Ozdemir, G. Antioxidant and Antimicrobial Activities of Codium fragile (Suringar) Hariot (Chlorophyta) Essential Oil and Extracts. Asian J. Chem. 2009, 21, 1197–1209. [Google Scholar]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Elkhateeb, M.I.; El-Bitar, A.M.H.; Saleh, S.R.; Abdelreheem, A.M.A. Evaluation of Bioactive Phytochemical Characterization, Antioxidant, Antimicrobial, and Antihemolytic Properties of Some Seaweeds Collected from Red Sea Coast, Egypt. Egypt. J. Aquat. Biol. Fish. 2021, 25, 417–436. [Google Scholar] [CrossRef]
- Salem, W.M.; Galal, H.; Nasr El-deen, F. Screening for Antibacterial Activities in Some Marine Algae from the Red Sea (Hurghada, Egypt). Afr. J. Microbiol. Res. 2011, 5, 2160–2167. [Google Scholar] [CrossRef]
- Alghazeer, R.; Whida, F.; Abduelrhman, E.; Gammoudi, F.; Naili, M. In Vitro Antibacterial Activity of Alkaloid Extracts from Green, Red and Brown Macroalgae from Western Coast of Libya. Afr. J. Biotechnol. 2013, 12, 7086–7091. [Google Scholar]
- Eldrin De Los Reyes, A.; Arsenia Basaran, S. Bioactive Properties of Sargassum siliquosum J. Agardh (Fucales, Ochrophyta) and Its Potential as Source of Skin-Lightening Active Ingredient for Cosmetic Application. J. Appl. Pharm. Sci. 2020, 10, 51–58. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Olugbami, J.O.; Gbadegesin, M.A.; Odunola, O.A. In Vitro Evaluation of the Antioxidant Potential, Phenolic and Flavonoid Contents of the Stem Bark Ethanol Extract of Anogeissus leiocarpus. Afr. J. Med. Med. Sci. 2014, 43, 101–109. [Google Scholar]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and Free Radical Scavenging Activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Baskaran, K.; Rathi, M.A.; Nirmaladevi, N. Free Radical Scavenging Activity of Methanolic Extract of Marine Red Algae Actinotrichia fragilis. Asian J. Pharm. Pharmacol. 2019, 5, 876–883. [Google Scholar] [CrossRef]
- Jeyaprakash, R.R.K. HPLC Analysis of Flavonoids in Acanthophora specifera (Red Seaweed) Collected from Gulf of Mannar, Tamilnadu, India. Int. J. Sci. Res. 2017, 6, 69–72. [Google Scholar]
- Bilanglod, A.; Petthongkhao, S.; Churngchow, N. Phenolic and Flavonoid Contents Isolated from the Red Seaweed, Acanthophora spicifera. In Proceedings of the 31st Annual Meeting of the Thai Society for Biotechnology and International Conference (TSB2019), Phuket, Thailand, 10–12 November 2019; pp. 189–196. [Google Scholar]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MS n: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate Composition, Phenolic Content and In Vitro Antioxidant Activity of Aqueous Extracts of the Seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. Effect of Addition of the Extracts on the Oxidative Stability of Canola Oil Unde. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive Phenolic Compounds from Algae. Bioact. Compd. Mar. Foods Plant Anim. Sources 2013, 6, 113–129. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent Developments in the Application of Seaweeds or Seaweed Extracts as a Means for Enhancing the Safety and Quality Attributes of Foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibition of Listeria monocytogenes by Oregano, Cranberry and Sodium Lactate Combination in Broth and Cooked Ground Beef Systems and Likely Mode of Action through Proline Metabolism. Int. J. Food Microbiol. 2008, 128, 317–324. [Google Scholar] [CrossRef]
- Lin, Y.T.; Labbe, R.G.; Shetty, K. Inhibition of Listeria monocytogenes in Fish and Meat Systems by Use of Oregano and Cranberry Phytochemical Synergies. Appl. Environ. Microbiol. 2004, 70, 5672–5678. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Thorkelsson, G.; Jacobsen, C.; Hamaguchi, P.Y.; Ólafsdóttir, G. Inhibition of Haemoglobin-Mediated Lipid Oxidation in Washed Cod Muscle and Cod Protein Isolates by Fucus vesiculosus Extract and Fractions. Food Chem. 2010, 123, 321–330. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant Activities and Phenolic Contents of Three Red Seaweeds (Division: Rhodophyta) Harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial Effect of Phlorotannins from Marine Brown Algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Helidoniotis, F.; Shaw, K.J.; Svoronos, D. Distribution of Bromophenols in Species of Marine Algae from Eastern Australia. J. Agric. Food Chem. 1999, 47, 2367–2373. [Google Scholar] [CrossRef]
- Aleem, A.A. Contribution to the Study of the Marine Algae of the Red Sea. III—Marine Algae from Obhor, in the Vicinity of Jeddah. Bull. Fac. Sci. K.A.U. Jeddah 1978, 2, 99–118. [Google Scholar]
- Kadaikunnan, S.; Rejiniemon, T.S.; Khaled, J.M.; Alharbi, N.S.; Mothana, R. In-Vitro Antibacterial, Antifungal, Antioxidant and Functional Properties of Bacillus amyloliquefaciens. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 9. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; ACADEMIC PRESS, INC.: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and Antioxidant Properties of Extracts of Japanese Persimmon Leaf Tea (Kakinoha-Cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Saraiva, S.C.; Sobral, A.J.F.N.; Cardoso, S.M. Characterization of Phenolic Constituents and Evaluation of Antioxidant Properties of Leaves and Stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef]
- Panda, S.; Ravindran, B. Isolation of Human PBMCs. Bio-Protocol 2013, 3, e323. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]


| Strains | Inhibition Zone Diameter (mm) 1 | |
|---|---|---|
| Codium tomentosum (Green Algae) | Actinotrichia fragilis (Red Algae) | |
| Gram-positive bacteria | ||
| Staphylococcus aureus (ATCC25923) | 22 ± 0.04 a | 18 ± 0.05 b |
| Streptococcus pyogenes (EMCC1772) | 20 ± 0.01 a | 16 ± 0.01 b |
| Gram-negative bacteria | ||
| Escherichia coli (ATCC25922) | 14 ± 0.08 a | 12 ± 0.05 b |
| Klebsiella pneumonia (ATCC700603) | 18 ± 0.02 a | 14 ± 0.07 b |
| Strains | MIC (mg/mL) 1 | |
|---|---|---|
| Codium tomentosum | Actinotrichia fragilis | |
| Gram-positive bacteria | ||
| Staphylococcus aureus (ATCC25923) | 0.4 ± 0.05 b | 0.8 ± 0.03 a |
| Streptococcus pyogenes (EMCC1772) | 0.4 ± 0.04 b | 0.8 ± 0.02 a |
| Gram-negative bacteria | ||
| Escherichia coli (ATCC25922) | 0.8 ± 0.01 b | 1.2 ± 0.03 a |
| Klebsiella pneumonia (ATCC700603) | 0.6 ± 0.04 b | 1.2 ± 0.01 a |
| Test 1 | Codium tomentosum | Actinotrichia fragilis |
|---|---|---|
| Total phenolic content (mg/g of extract) | 32.28 ± 1.63 a | 19.96 ± 1.28 b |
| Total flavonoid content (mg/g of extract) | 4.54 ± 1.48 c | 3.86 ± 1.02 d |
| Extracts | DPPH (IC50) μg/mL |
|---|---|
| Ascorbic acid | 22.71 ± 0.03 c |
| Codium tomentosum | 75.32 ± 0.07 b |
| Actinotrichia fragilis | 94.43 ± 0.02 a |
| Concentration (µg/mL) | Inhibition % | Viability % |
|---|---|---|
| 250 | 69 | 31 |
| 125 | 69 | 31 |
| 62.5 | 64 | 36 |
| 31.2 | 30 | 70 |
| 15.6 | 29 | 71 |
| 7.8 | 27 | 73 |
| IC50 = 33.7 ± 1.02 µg/mL | ||
| Concentration (µg/mL) | Inhibition % | Viability % |
|---|---|---|
| 250 | 73 | 27 |
| 125 | 60 | 40 |
| 62.5 | 47 | 53 |
| 31.2 | 39 | 61 |
| 15.6 | 37 | 63 |
| 7.8 | 11 | 89 |
| IC50 = 51.0 ± 1.14 µg/mL | ||
| Phenolic Compounds | Codium tomentosum | Actinotrichia fragilis |
|---|---|---|
| Conc. (µg/g) | ||
| Gallic acid | 174.32 | 303.68 |
| Chlorogenic acid | ND | 29.50 |
| Catechin | 29.92 | 87.10 |
| Methyl gallate | 12.91 | 0.00 |
| Caffeic acid | 70.21 | 12.79 |
| Syringic acid | 50.03 | 0.00 |
| Pyro catechol | ND | 0.00 |
| Rutin | ND | 22.73 |
| Ellagic acid | 232.69 | 190.62 |
| Coumaric acid | ND | 0.00 |
| Vanillin | ND | 3.36 |
| Ferulic acid | 26.13 | 15.99 |
| Naringenin | 61.09 | 23.77 |
| Daidzein | 791.39 | 10.47 |
| Quercetin | 48.03 | 65.78 |
| Cinnamic acid | 35.51 | 6.10 |
| Apigenin | 224.88 | 30.28 |
| Kaempferol | 77.28 | 0.00 |
| Hesperetin | 75.90 | 0.00 |
| Appearance | Consistency | Tenderness | Flavor (Odor and Taste) | Overall Acceptance | |
|---|---|---|---|---|---|
| T1 (Control) | 4.60 ± 1.06 a | 5.07 ± 1.03 a | 3.53 ± 1.69 b | 4.13 ± 1.64 a | 4.53 ± 1.18 b |
| T2 (1% of C. tomentosum extract) | 5.80 ± 1.15 a | 5.67 ± 1.18 a | 5.13 ± 1.41 a | 5.60 ± 1.63 a | 5.93 ± 1.10 a |
| T3 (1% A. fragilis extract) | 4.73 ± 1.16 a | 4.87 ± 1.19 a | 4.80 ± 1.61 ab | 4.67 ± 1.11 a | 4.80 ± 0.77 b |
| T4 (0.5% of C. tomentosum and 0.5% of A. fragilis extracts) | 5.13 ± 1.41 a | 5.67 ± 1.05 a | 5.40 ± 1.59 a | 5.33 ± 1.39 a | 5.47 ± 1.45 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafez, M.S.M.A.E.; Rashedy, S.H.; Abdelmotilib, N.M.; El-Hassayeb, H.E.A.; Cotas, J.; Pereira, L. Fillet Fish Fortified with Algal Extracts of Codium tomentosum and Actinotrichia fragilis, as a Potential Antibacterial and Antioxidant Food Supplement. Mar. Drugs 2022, 20, 785. https://doi.org/10.3390/md20120785
Hafez MSMAE, Rashedy SH, Abdelmotilib NM, El-Hassayeb HEA, Cotas J, Pereira L. Fillet Fish Fortified with Algal Extracts of Codium tomentosum and Actinotrichia fragilis, as a Potential Antibacterial and Antioxidant Food Supplement. Marine Drugs. 2022; 20(12):785. https://doi.org/10.3390/md20120785
Chicago/Turabian StyleHafez, Mohamed S. M. Abd El, Sarah H. Rashedy, Neveen M. Abdelmotilib, Hala E. Abou El-Hassayeb, João Cotas, and Leonel Pereira. 2022. "Fillet Fish Fortified with Algal Extracts of Codium tomentosum and Actinotrichia fragilis, as a Potential Antibacterial and Antioxidant Food Supplement" Marine Drugs 20, no. 12: 785. https://doi.org/10.3390/md20120785
APA StyleHafez, M. S. M. A. E., Rashedy, S. H., Abdelmotilib, N. M., El-Hassayeb, H. E. A., Cotas, J., & Pereira, L. (2022). Fillet Fish Fortified with Algal Extracts of Codium tomentosum and Actinotrichia fragilis, as a Potential Antibacterial and Antioxidant Food Supplement. Marine Drugs, 20(12), 785. https://doi.org/10.3390/md20120785