Abstract
We successfully prepared a series of l-arginine Schiff bases acylated chitosan derivatives, aiming to improve the antioxidant activity and antimicrobial activity of chitosan by introducing a furan ring, pyridine ring, and l-arginine structure. The accuracy of the structures of ten compounds was characterized by FT-IR and 1H NMR. In terms of DPPH radical scavenging activity, except for compound CR3PCA, the scavenging rate of other compounds was higher than chitosan, especially CRCF and CRBF had strong scavenging abilities. At the same time, in the superoxide-radical scavenging activity assay, CRCF, CRBF, CR3PCA, CR2C3PCA, and CR2B3PCA were comparable to positive control at 1.60 mg/mL. Simultaneously, CRFF, CRCF, and CRBF had a certain inhibitory effect on Botrytis cinerea. Furthermore, the inhibitory effect of CRFF, CRCF, and CR3PCA on Staphylococcus aureus was very well, close to the positive control at 1.00 mg/mL. CRCF and CR2B3PCA showed better inhibitory effects on Escherichia coli than other compounds. The MTT assay was used to determine the cytotoxicity of the chitosan derivatives, which proved their safety to fibroblast cells. In summary, the study indicated that some of these compounds have the potential for further development and utilization in the preparation of antioxidants and antimicrobial agents.
1. Introduction
There is growing emphasis on the improvement of chitosan in recent years with a large number of synthetic chitosan derivatives demonstrating good biological activity [1,2,3,4]. Chitosan is a product of the deacetylation of chitin, which is widely found in nature and is the second largest natural polysaccharide on earth after cellulose [5]. Most of all, chitosan is the only cationic natural polysaccharide in nature, and it has certain antimicrobial and antioxidant activity in previous studies [2,6,7,8]. Furthermore, chitosan is non-toxic and harmless, which means that it has good biocompatibility and can be degraded by related enzymes after entering the human body [9]. Numerous researchers have demonstrated that chitosan derivatives have some antibacterial activity and antioxidant activity [10,11,12,13], including Amira A et al. who synthesized chitosan Schiff base derivatives containing different heterocyclic groups and determined their antimicrobial activity [14,15]. Cross-linked chitosan Schiff bases mixed with carboxymethyl chitosan (CMC) and graphene quantum dots (GQDs), might have the potential to be utilized for the treatment of Helicobacter pylori [16]. Methyl acrylate chitosan bearing p-nitrobenzaldehyde showed good capability at inhibiting the multidrug resistant (MDR) bacterial pathogens of skin burn infection [17]. Tan et al. synthesized α-lipoic acid grafted chitosan derivatives that could significantly improve the antioxidant activities, including superoxide anion, hydroxyl, and DPPH radicals scavenging activities, and reducing power [18]. Therefore, it is crucial to develop chitosan derivatives with high antimicrobial activity, good antioxidant activity, and low cytotoxicity.
l-arginine, as a precursor of many bioactive substances, can participate in regulating biological processes, and the metabolic pathways of l-arginine in vitro are as follows: l-arginine is decomposed into ornithine and urea under the action of arginase and then decomposed into citrulline and NO under the action of nitric oxide synthase [19]. At the same time, compounds containing guanidine have been widely studied and applied in pharmaceutical chemistry. The guanidine in l-arginine is protonated with a positive charge and interacts electrically with negatively charged bacterial cell surfaces to produce bacteriostatic effects [20]. In addition, studies have shown that l-arginine could induce disease resistance [21], attenuate oxidative stress, and inhibit the formation of atherosclerosis in hypercholesterolemia [22], etc.
There have been a series of studies on l-arginine-modified chitosan derivatives: Chitosan-N-arginine with different degrees of substitution was prepared and proved to have obvious antibacterial activity [23]. Chitosan-N-arginine/Hydroxypropyl methylcellose (HPMC) composite film was prepared by using HPMC as gelling agent and glycerin as a plasticizer, which had significant antibacterial activity and chitosan-N-arginine had no cytotoxicity to L929 cells [24]. 6-deoxy-6-arginine-modified chitosan not only increases the positive charge of the derivative with the guanidine of arginine but also retains the amino group after deprotection, showing a larger antibacterial circle than chitosan in antibacterial experiments [25]. Most studies focus on the modification of chitosan amino groups, such as forming amide bonds with the carboxyl groups of amino acids, respectively, or generating Schiff bases with the aldehyde groups of aldehydes [26,27,28]. However, few studies have connected amino acid Schiff bases to the chitosan framework.
Schiff bases are the product of the condensation of aldehydes/ketones and primary amines, and according to the different substituents, they can be divided into amines, ketones, semicarbazides, quinolines, azocyclics, guanidines, hydrazones, amino acids and so on [29,30]. The existence of the C=N double bond makes Schiff bases have a wide range of biological activities [31], and Schiff bases can form complexes with transition metals, lanthanides, actinides, and some main metallic elements, which have antimicrobial, antioxidant, anti-inflammatory, anticancer, and antitubercular activities [32,33]. So far, imine bonds were found in many drugs already approved for use, including furantoin, cimetidine, metformin, etc.
As usual, KOH is commonly added in the synthesis of amino acids Schiff bases, since most amino acids are insoluble in ethanol and methanol, adding strong bases can promote the dissolution of amino acids and the deprotonation of ligands [34,35]. However, the problem is that the synthetic Schiff bases are carboxylate, which is not conducive to the next step of the reaction to form amide bonds. The formation of Schiff bases between l-arginine and aldehydes has been studied extensively, for example: salicylaldehyde–arginine Schiff base, disalicylaldehyde–arginine Schiff base and metal–disalicylaldehyde–arginine Schiff base had high catalytic activity and remarkable selectivity under mild conditions [36]. Herein, a series of l-arginine Schiff bases acylated chitosan derivatives were reported: chitosan-l-arginine-furfural (CRFF), chitosan-l-arginine-5-methylfurfural (CRMF), chitosan-l-arginine-5-hydroxymethylfurfural (CRHMF), chitosan-l-arginine-5-chloro-furfural (CRCF), chitosan-l-arginine-5-bromo-furfural (CRBF), chitosan-l-arginine-2-pyridinecarboxaldehyde (CR2PCA), chitosan-l-arginine-3-pyridinecarboxaldehyde (CR3PCA), chitosan-l-arginine-4-pyridinecarboxaldehyde (CR4PCA), chitosan-l-arginine-2-chloro-3-pyridinecarboxaldehyde (CR2C3PCA), chitosan-l-arginine-2-bromo-3-pyridinecarboxaldehyde (CR2B3PCA), and their chemical structures were characterized by FT-IR and 1H NMR. The cytotoxicity was evaluated by MTT assay and the scavenging ability of superoxide radical and DPPH-radical were measured in this paper. Concurrently, two phytopathogenic fungi and two bacteria: Fusarium graminearum (F. graminearum), Botrytis cinerea (B. cinerea), Staphylococcus aureus (S. aureus), and Escherichia coli (E. coli) were selected to evaluate the antifungal property and antibacterial activity of the derivatives in vitro.
2. Results and Discussion
2.1. Chemical Synthesis and Characterization
2.1.1. FT-IR Spectra
Acylated chitosan derivatives with l-arginine Schiff bases were synthesized by a multi-step reaction (Scheme 1). The structures of chitosan derivatives were characterized by FT-IR and 1H NMR.
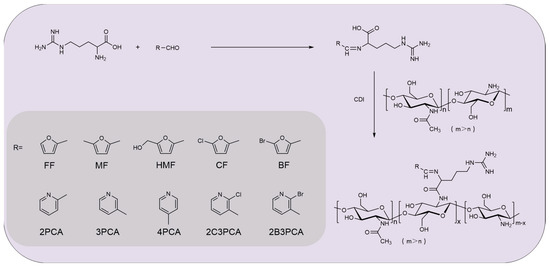
Scheme 1.
Synthesis pathway of chitosan derivatives.
First of all, as shown in Figure 1, we can see from the spectrum of raw material chitosan that the wide association peak near 3300 cm−1 includes O–H and N–H stretching absorption spectrum bands, the sway bending vibration of CH2 at 1411 cm−1, and the C–O stretching vibration of primary alcohol hydroxyl at 1074 cm−1. After the synthesis of ten compounds, it can be seen that the stretching vibration peak of NH2 disappears around 1526 cm−1, and a new wide peak appears around 1640 cm−1, which can be inferred to be the stretching vibration peak of C=N and C=O (amide bond). The stretching vibration peak at 1440 cm−1 is presumed to be CH2 bending vibration in l-arginine [37], the peak around 790 cm−1 is assumed to be the absorption peak of furan ring [38], and 750 cm−1 is attributed to the out-of-plane oscillations of C–H in the pyridine ring. In summary, it can be preliminarily proved that l-arginine Schiff bases combined successfully with chitosan.
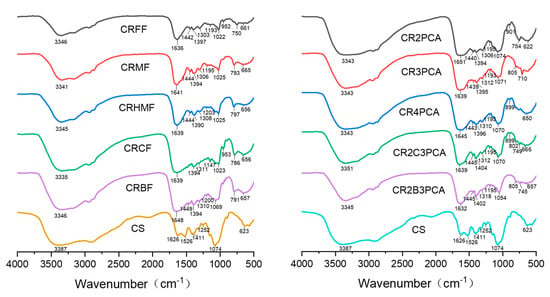
Figure 1.
Fourier Transform Infrared (FT-IR) spectra of chitosan and chitosan derivatives.
2.1.2. NMR Spectra
In order to compare the peak position of hydrogen atoms more directly, DMSO-d6 was selected as the solvent of chitosan and its derivatives. Usually, D2O is used as the solvent of chitosan in experiments, but the chitosan with molecular weight 8 kDa used in this experiment can be dissolved in DMSO-d6 under the condition of heating [39,40]. In Figure 2, The chemical shift of the hydrogen atom at the second position of chitosan is between 2.80 ppm and 3.00 ppm, and the characteristic peak of the hydrogen atom at the third to sixth positions of chitosan is between 3.20 ppm and 4.80 ppm [41]. After modification with l-arginine Schiff bases, the peak appears at 7.65 ppm, which is presumed to be the peak of hydrogen atoms on the C=N of Schiff bases. Additionally, the inactive hydrogen atom on l-arginine peaks at about 2.52 ppm and 1.50 ppm, respectively, which has also been mentioned in previous studies [42]. In addition, the active hydrogen peaks between 6.00–8.00 ppm on the furan ring and 7.00–9.00 ppm on the pyridine ring. These results can further prove the successful synthesis of chitosan derivatives modified with l-arginine Schiff bases.
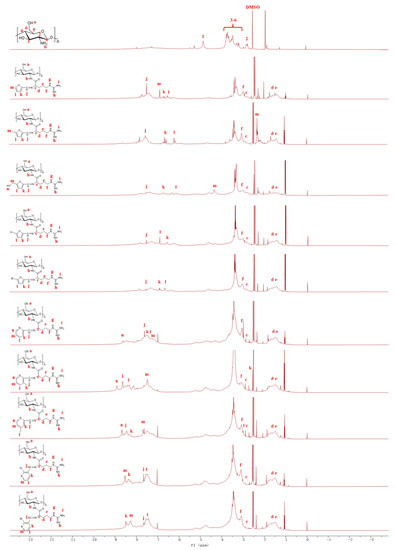
Figure 2.
1H NMR spectra of chitosan and chitosan derivatives.
2.1.3. Yields and Degree of Substitution (DS) Analysis
The yields and DS of CRFF, CRMF, CRHMF, CRCF, CRBF, CR2PCA, CR3PCA, CR4PCA, CR2C3PCA and CR2B3PCA are shown in Table 1. Thus indirectly proved the successful synthesis of chitosan derivatives.

Table 1.
Yields and the DS of chitosan derivatives.
2.1.4. Thermal Gravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG)
Water-absorption and degradation behavior of chitosan and chitosan derivatives can be evaluated by TGA. In Figure 3, chitosan has two stages of weight loss, the first stage is around 30–120 °C with a loss of about 11% by weight for the evaporation of water molecules in chitosan; the second stage starts at 200 °C and reaches around 700 °C with a weight loss of about 74%, which is caused by the breakage and burning of the chitosan molecular chain [43,44]. In this case, chitosan reaches its maximum weight loss at 210 °C with a weight loss of 30.85%, which may be caused by the decomposition of the sugar rings at this point [45]. After modification, the chitosan derivatives have about four stages of weight loss, with a slower decomposition rate and higher thermal stability than chitosan. In the second stage, the maximum decomposition rates of the chitosan derivatives (CRFF, CRMF, CRHMF, CRCF, CRBF, CR2PCA, CR3PCA, CR4PCA, CR2C3PCA, CR2B3PCA) occurred at 198 °C, 207 °C, 199 °C, 210 °C, 201 °C, 207 °C, 206 °C, 208 °C, 203 °C, 201 °C, at which time the mass fractions were: 83.95%, 83.84%, 81.68%, 84.57%, 89.68%, 86.41%, 87.51%, 83.52%, 82.36%, 88.39%, and the weight losses were: 16.05%, 16.16%, 18.32%, 15.43%, 10.32%, 13.59% 12.49%, 16.48%, 17.64%, 11.61%.
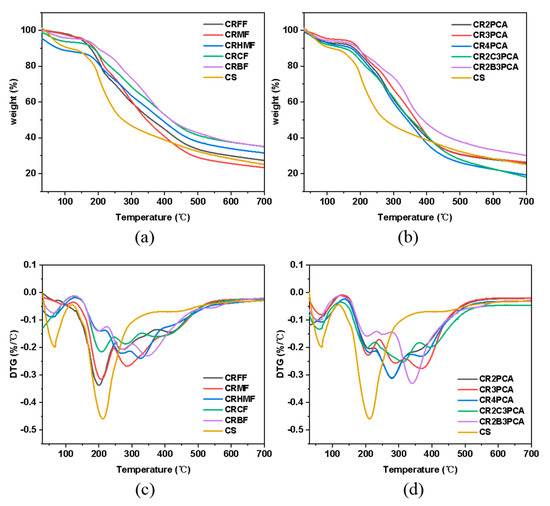
Figure 3.
TGA (a,b) and DTG (c,d) curves of chitosan and chitosan derivatives.
2.2. Antioxidant Activity
Superoxide anion radical (·), as active intermediates in organism metabolism, is the product of single electron reduction of oxygen molecules. Abnormal levels of superoxide anion radicals in organisms are associated with a variety of diseases, such as diabetes, atherosclerosis, tumors, etc. The scavenging ability of ten newly synthesized compounds on superoxide anion free radical is shown in Figure 4, and the scavenging rate is higher than that of chitosan. According to the reaction process of the superoxide anion radical scavenging experiment, NAD+ and PMS-H2 are generated by the oxidation of NADH by PMS. PMS-H2 will generate superoxide anion radicals with oxygen in the air while NBT turns into NBT-H2, a blue compound when it encounters superoxide anion radicals. If the system contains antioxidants, the superoxide anion first associates with the antioxidants, and the production of NBT-H2 is reduced, leading to a decrease in the absorbance of the solution. We can infer that the ability of halogenated compounds to bind superoxide anion radicals is higher than that of other compounds, especially CR2B3PCA, CRCF, CRBF, and CR2C3PCA, which are comparable to Vitamin C (VC) at 1.60 mg/mL, the scavenging rates of superoxide anion radical were 100.00%, 99.37%, 95.95%, 95.45%, and 95.13%, respectively.
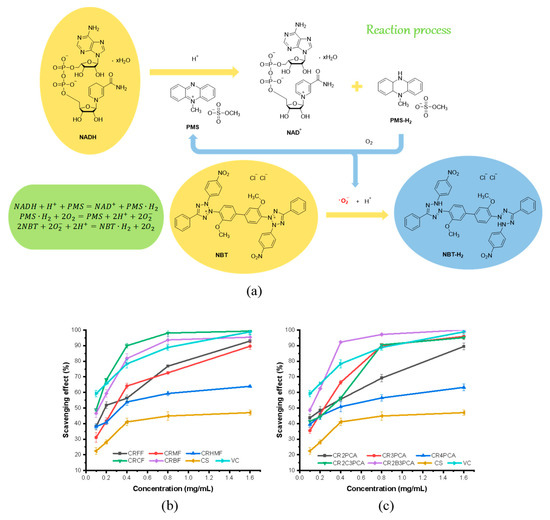
Figure 4.
Reaction process of superoxide-radical scavenging activity assay (a), superoxide radical-scavenging capacity of chitosan and chitosan derivatives (b,c).
1,1-Diphenyl-2-picrylhydrazyl (DPPH) is a commonly used reagent to study the scavenging ability of free radicals, its structure is very stable due to electron delocalization. When DPPH is dissolved in ethanol, the solution appears purple and has a maximum absorption wavelength of 517 nm. The lone pair electron on the N atom connected with the trinitrobenzene ring can form a stable DPPH-H molecule when it meets the antioxidant, and the purple of the original solution will fade into yellow. Therefore, if the compound is a hydrogen donor, the absorbance of the solution will decrease, and the absorbance is linear with the concentration, thus reflecting its antioxidant activity. The scavenging ability of DPPH free radical was shown in Figure 5, the scavenging rate of other compounds was higher than that of chitosan except for compound CR3PCA. On the whole, CRCF, CRBF, and CR2PCA had strong scavenging abilities, and the scavenging ability of DPPH free radicals reached 89.01%, 82.04%, and 80.92% at the concentration of 1.60 mg/mL. In addition, CRCF, CRBF, CR4PCA, CR3PCA, and CR2C3PCA still showed a strong upward trend at the highest test concentrations, with obvious concentration dependence.
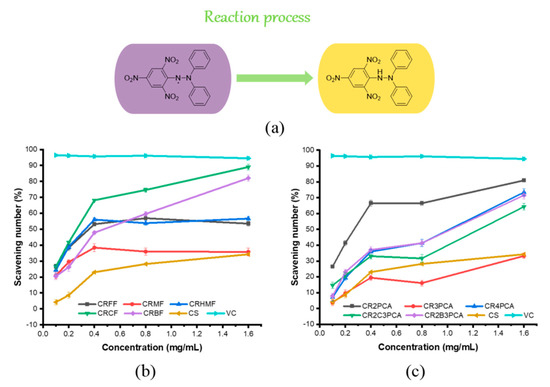
Figure 5.
Reaction process of DPPH-radical scavenging ability assay (a), DPPH radical-scavenging capacity of chitosan and chitosan derivatives (b,c).
2.3. Antifungal Activity
F. graminearum is an epidemic plant fungus, which mainly occurs in the flowering stage and filling stage of the wheat growth period. If there is sufficient rain and high temperature, it is suitable for the growth of F. graminearum [46]. By the time wheat reaches maturity, it will have suffered a large loss of production if it is infected by F. graminearum. At the same time, it produces a variety of mycotoxins that can be toxic to humans and animals after use. B. cinerea has been widely studied as a model fungus, it can infect grapes, tomatoes, cucumbers, strawberries, and other crops, resulting in reduced production, so the design of more specific and effective fungicides is extremely important. There are two possible explanations for the inhibition mechanism of chitosan on fungi: one of them is that chitosan affects the activity of related enzymes in the cell wall, destroys the cell wall, and thus inhibits the growth of fungi, the other is that chitosan interacts with the cell membrane, increasing the permeability of the cell membrane and leaking the intracellular substances, leading to the death of fungi [2,47]. Studies have shown that introducing guanidine groups can enhance the ability of chitosan to inhibit fungi [48].
As shown in Figure 6a,b, all the synthesized compounds exhibit a concentration-dependent manner on B. cinerea, and the inhibitory effect of compounds containing furan rings was higher than that of compounds containing pyridine rings. Among them, the inhibition rates of CRFF, CRCF, and CRBF at 1.00 mg/mL were 70.22%, 71.07%, and 67.83%, respectively. Compared with chitosan, the antifungal activity of CRFF, CRCF, and CRBF was achieved at 32.66%, 33.51%, and 30.27% at the highest concentration tested, respectively. The five compounds containing pyridine ring showed little difference in their inhibitory activity against B. cinerea, and the inhibitory activity increased with the increase of concentration.
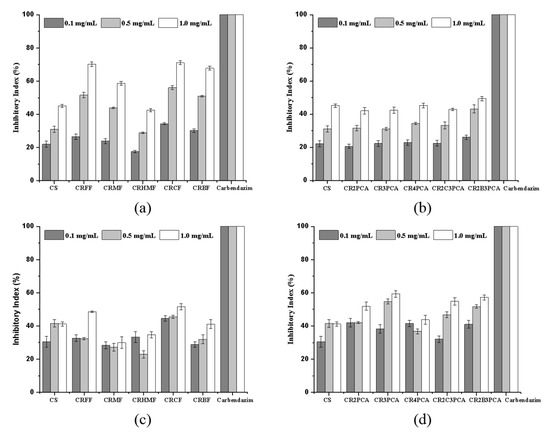
Figure 6.
Antifungal activity of chitosan and chitosan derivatives against B. cinerea (a,b) and F. graminearum (c,d).
In Figure 6c,d, at the concentration of 1.00 mg/mL, the inhibition rate of CR3PCA was the highest (59.29%), followed by 57.15% of CR2B3PCA, 54.87% of CR2C3PCA and 51.99% of CR2PCA. In the series of compounds containing furan rings, at this concentration, the inhibition rate of CRCF was 51.63%, followed by that of CRFF was 48.58%. Even in the case of low concentration, at the concentration of 0.10 mg/mL, the inhibition rate was 44.50% for CRCF, 42% for CR2PCA, 41.50% for CR4PCA, and 41.03% for CR2B3PCA. At the same time, it can be seen that there is little difference between l-arginine Schiff bases containing furan rings and pyridine rings in the results of inhibition of F. graminearum. Similarly, halogens, hydroxyl groups, and methyl groups contained in heterocyclic Schiff bases have no significant effect on the inhibition effect.
2.4. Antibacterial Activity
As shown in Figure 7, at the concentration of 1.00 mg/mL, the inhibition rates of CRFF, CRMF, CRHMF, CRCF, CRBF, CR3PCA, and CR2B3PCA against S. aureus were 99.17%, 99.35%, 97.56%, 99.47%, 97.30%, 99.31%, 96.98%, respectively. Gram-negative bacteria and Gram-positive bacteria have different cell wall compositions. Gram-positive bacteria (S. aureus) are composed of multilayer reticular peptidoglycan linked to teichoic acid and have a thicker cell wall. Peptidoglycan is a disaccharide unit containing a tetraceptide side chain, in which the amino acids in the tetraceptide vary by bacterial species. The teichoteic acid main chain is alternately linked by alcohol and phosphoric acid molecules, and the side chain is a single glucose or d-Ala. For Gram-negative bacteria (E. coli), the cell wall is thin and contains a single layer of peptidoglycan covered by an outer lipid membrane. From the experimental results, the sample solution at 0.10 mg/mL of gold glucose-inhibition ability is low, but at a high concentration (1.00 mg/mL) inhibition ability is very strong. Previous studies have speculated that chitosan derivatives form polymer membranes on cell surfaces that block the entry of nutrients [49]. For Gram-negative bacteria (E. coli), there is little difference between 0.50 mg/mL and 1.00 mg/mL in the inhibition ability of bacteria. It is speculated that the free positive charge and guanidine carried by chitosan can combine with the negatively charged components of the cell wall, resulting in the leakage of substances inside the cell holes and disturbing the normal physiological activities of bacteria [50].
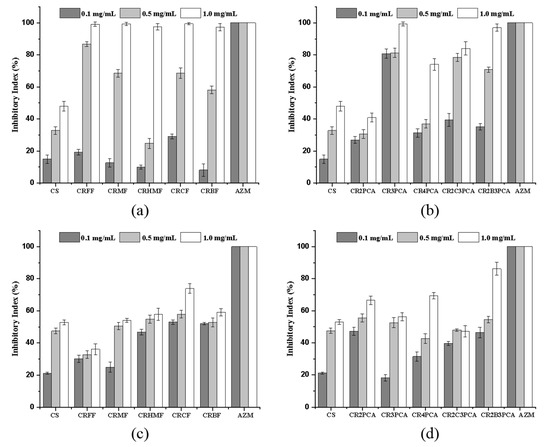
Figure 7.
Antibacterial activity of chitosan and chitosan derivatives against S. aureus (a,b) and E. coli (c,d).
2.5. Cytotoxicity Analysis
The cytotoxicity profile of chitosan and ten chitosan derivatives was evaluated in vitro by the MTT method, using L929 mice fibroblast cells line (L929, obtained from China Center for Type Culture Collection, Shandong Luye Pharmaceutical Co., Ltd., Yantai, China), which have a high differentiation capacity and ease of culture in vitro. The mechanism of MTT assay is that succinate dehydrogenase in the mitochondria of living cells restore exogenous MTT (3-(4,5-dimethyl-2-thizolyl)-2,5-diphenyltertazolium bromide) into formazan (a purple crystal insoluble in water). DMSO was added to dissolve formazan, and the number of cells was measured by measuring the absorbance of the solution. The cytotoxicity of the samples as shown in Figure 8a,b, it can be seen that CRFF, CRMF, CRHMF, CR2PCA, and CR3PCA were hardly toxic to L929 cells in the concentration range of 1–1000 µg/mL, while CRCF, CRBF, CR4PCA, CR2C3PCA, and CR2B3PCA had some cytotoxicity at 1000 µg/mL. For all samples, within the concentration range of the test sample, the cell viability exceeded 80%, which intuitively indicated low cytotoxicity. The morphology of the cells can be seen in Figure 8c. The normal cell morphology is spindle-shaped and adheres well to the cell wall, while the sample is toxic, the cells have a rounded morphology and no longer adhere to the cell wall. In conclusion, the non-toxicity of the chitosan derivatives was initially confirmed by the L929 cytotoxicity test and further experimental studies will be conducted if practical applications are considered.
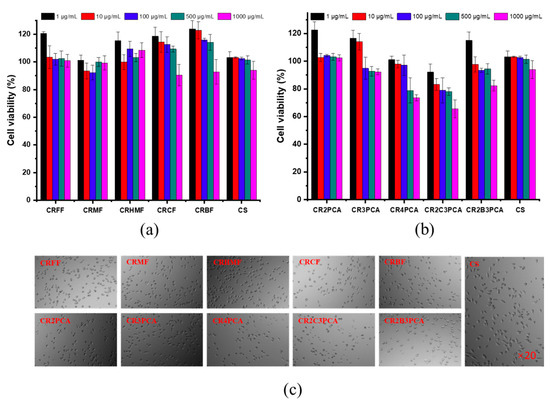
Figure 8.
Cytotoxicity of chitosan and chitosan derivatives (a,b) and the sample concentration in the cell growth pictures (c) was 1000 μg/mL.
3. Materials and Methods
3.1. Materials
Chitosan with a molecular weight of 8 kDa and 93% N-deacetylation was purchased from Golden-Shell Pharmaceutical Co., Ltd. (Zhejiang, China). l-Arginine and methanol were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Furfural, 5-methylfurfural, 5-hydroxymethylfurfural, 5-chloro-furfural, 5-bromo-furfural, 2-pyridinecarboxaldehyde, 3-pyridinecarboxaldehyde, 4-pyridinecarboxaldehyde, 2-chloro-3-pyridinecarboxaldehyde, 2-bromo-3-pyridinecarboxaldehyde, 1,1′-carbonyldiimidazole (CDI) and dimethyl sulfoxide (DMSO) were purchased from Merck Life Science (Shanghai) Co., Ltd., Shanghai, China. Ethanol absolute was obtained from Fuyu Fine Chemicals Co., Ltd., Tianjin, China. Chloramphenicol and azithromycin were purchased from Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Purified water was supplied by Wahaha Group Co., Ltd., Shandong, China.
3.2. Synthesis of Chitosan Derivatives
3.2.1. Synthesis of l-Arginine Schiff Bases
l-arginine (1.74 g, 0.01 mol) was weighed and dispersed in 30 mL methanol at 60 °C for 2 h. The methanol solution with excess aldehydes was dropped into the above solution, heated to 70 °C, and reacted for 2 h after everything had dissolved. The solvent was removed by vacuum rotary evaporation, and excess aldehydes were removed by repeated washing and filtration with ethanol. The methods were slightly modified from those reported in the previous literature [36].
3.2.2. Synthesis of Chitosan Derivatives
A total of 0.01 mol of l-arginine Schiff base was dissolved in 5 mL DMSO, and equal mole of CDI (1.62 g) was added to it for 12 h, then chitosan (0.005 mol, 0.8 g) was added, the reaction lasted for 24 h. After the reaction, ethanol was used for precipitation, washing, and lyophilization.
3.3. Analytical Methods
3.3.1. Fourier Transform Infrared (FT-IR) Spectroscopy
The changes of functional groups of all chitosan derivatives were characterized by Nicolet IS 50 Fourier Transform Infrared Spectrometer (Thermo, Waltham, MA, USA), the test sample was scanned 16 times, and the infrared spectrum with a spectral range of 4000–400 cm−1 was obtained at a resolution of 4 cm−1. The dried samples were ground and pressed with KBr for testing at 20 °C.
3.3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
The 1H NMR spectra of chitosan derivatives were recorded on Bruker AVIII 500 spectrometer (500MHz, Switzerland, provided by Bruker Tech. and Serv. Co., Ltd., Beijing, China), using DMSO as solvent. And the degrees of substitution (DS) of chitosan derivatives were calculated from the 1H NMR spectra.
3.3.3. Degrees of Substitution (DS)
The calculation formula of DS was as follows: DS = Ha/Hb (where Ha represents the integral area of a hydrogen atom in the C=N of the compound, Hb represents the integral area of the 1-position carbon bonded hydrogen proton on the main chain of chitosan.
3.3.4. Thermal Stability
Thermal stability characterization of chitosan and its derivatives were characterized by Mettler 5 MP thermogravimetric analyzer (Mettler Toledo, Greifensee, Switzerland). The powdered samples were placed into a crucible and heated at 10 min−1 under a dynamic nitrogen atmosphere at a flow rate of 25 mL min−1 from 30 °C to 700 °C.
3.4. Antioxidant Assays
3.4.1. Superoxide-Radical Scavenging Activity Assay
This experiment was measured according to the previous method [51]. The samples were freeze-dried to a constant weight and then added with deionized water to form a solution of 10 mg/mL, and then diluted with water into the solution to be tested with different concentrations: 0.20, 0.40, 0.80, 1.60, 3.20 mg/mL, respectively. Tris (Hydroxymethyl)aminoethane was configured with concentrated hydrochloric acid to form a Tris-HCl buffer (pH = 8.0). Concurrently, nicotinamide adenine dinucleotide reduced (NADH, 36.60 mg), nitro blue tetrazolium (NBT, 24.50 mg), and phenazine methosulfate (PMS, 1.80 mg) were weighed and dissolved in Tris-HCl buffer to a volume of 100 mL, respectively. For the experimental group, the solution was diluted to different concentrations and then 0.50 mL of NADH, 0.50 mL of NBT, and 0.50 mL of PMS were added. The blank group was replaced with deionized water, and the control group was replaced with Tris-HCl buffer instead of NADH solution, and then shaken well and reacted at room temperature for 5 min. After that, the absorbance of sample solutions with different concentrations at 560 nm was measured with a microplate reader. The formula of the superoxide-radical scavenging ability of chitosan derivatives is as follows:
where Aexperimental is the absorbance of the experimental group at 560 nm, Acontrol is the absorbance of the control group at 560 nm, and Ablank is the absorbance of the control group at 560 nm.
3.4.2. DPPH-Radical Scavenging Ability Assay
Firstly, DPPH-ethanol solution was prepared: 35.50 mg DPPH was weighed and dissolved in anhydrous ethanol and transferred to a 500 mL volumetric bottle with anhydrous ethanol for constant volume. Then the sample solution was then prepared: the chitosan derivatives were freeze-dried to a constant weight and weigh 80 mg to prepare a sample mother solution of 10 mg/mL. The sample mother solution was diluted into the same volume (1.00 mL) but with different concentrations of 0.01 mg/mL, 0.02 mg/mL, 0.04 mg/mL, 0.08 mg/mL, and 0.16 mg/mL solutions. Then 2.00 mL DPPH-ethanol solution was added to the solution and reacted at room temperature for 20 min. After that, the absorbance of sample solutions with different concentrations at 517 nm was measured with a microplate reader. The blank group replaced the sample solution with the same volume of deionized water, and in the control group, DPPH ethanol was replaced with the same volume of anhydrous ethanol, excluding the interference of the sample solution’s own color. The formula of the DPPH radical scavenging ability for chitosan derivatives was as follows:
where Aexperimental is the absorbance of the experimental group at 517 nm, Acontrol is the absorbance of the control group at 517 nm, Ablank is the absorbance of the blank group at 517 nm. All experiments were repeated three times.
3.5. Antifungal Assays
The inhibition of chitosan derivatives on fungal pathogens was measured by mycelial growth rate method [52]. We selected two plant-pathogenic fungi: F. graminearum and B. cinerea, which have been selected as “The Top 10 fungal pathogens in molecular plant pathology” [53].
In this experiment, the mycelial growth rate method was used to test the inhibitory ability of chitosan derivatives on plant fungi, and the experimental methods were as follows: First, all compounds were dried to a constant weight, and then dissolved in sterile deionized water into a sample solution of 10 mg/mL. Solid media of different volumes were added to prepare the final concentration of 0.10 mg/mL, 0.50 mg/mL and 1.00 mg/mL. The positive control was carbendazim, and the blank control was sterile deionized water. After the frozen fungi were rejuvenated, the fungi were evenly distributed on the surface of solid medium by plate coating method, and cultured under the condition of temperature of 28 °C and humidity of 60% for 48 h. The 5 mm cake was taken with fungus cake puncher, inoculated in the center of solid medium containing different concentrations of compounds, and then cultured at the same humidity and temperature. Finally, the growth diameter of mycelia was measured by cross crossing method. The formula of fungal inhibition rates of chitosan derivatives was as follows:
where Dsample is the diameter of sample medium and Dblank is the diameter of blank medium. All experiments were repeated three times.
3.6. Antibacterial Assay
Two kinds of model organisms were selected to test the antibacterial activity: S. aureus and E. coli. The plate colony counting method was used to measure the antibacterial activity of chitosan derivatives. The method is described in the previous experiment [54,55], the solid medium is mixed with the compound solution and finally prepared into 0.10 mg/mL, 0.50 mg/mL, and 1.00 mg/mL culture medium, in order to prepare the solid medium containing different concentrations of compounds. The pure colonies were selected and inoculated in a liquid medium after rejuvenating the frozen bacteria, and the bacterial solution was diluted after 4 h of growth. The concentration was measured as 105 CFU/mL by absorbance, and 0.10 mL of the diluted liquid was absorbed and applied evenly on the surface of the solid medium containing the compound with a coating stick. The colony number was measured after growing at 37 °C for 12 h. The formula for bacteria inhibition rates of chitosan derivatives was as follows:
where Asample is the colony number of the sample to be tested. Ablank is the colony number of the blank sample. All experiments were repeated three times.
3.7. Cytotoxicity Assay
The cytotoxicity of chitosan and chitosan derivatives on L929 cells was determined by MTT assay at different concentrations of 1, 10, 100, 500, and 1000 μg/mL in vitro. L929 cells were incubated at 37 °C and 5% CO2. By cell recovery and passaging, L929 cells in the logarithmic growth phase (1 × 104 cells/mL) were inoculated in 96-well plates. Cells were incubated to monolayer growth, then the supernatant was aspirated and discarded, followed by repeated washes with phosphate buffer solution (PBS). A series of concentrations of chitosan derivatives solution diluted in a culture medium was added into 96-well plates and incubated for 48 h. 20 µL of MTT solution and 80 µL of medium were added to each well, after 4 h of incubation, supernatants were removed, and 100 μL DMSO was added. The absorbance of each well at 490 nm was measured by a microplate reader (SuPerMax 3100, Flash, CN) and the cell viability was calculated according to the following formula:
where Asample is the absorbance of the sample group at 490 nm, Ablank is the absorbance of the blank group at 490 nm, Anegative is the absorbance of the negative group at 490 nm. All experiments were repeated three times.
3.8. Statistical Analysis
The mean and standard deviation (SD) were calculated (n = 3) and one-way analysis of variance (ANOVA) and multiple comparison test (TUKEY) were used to analyze and compare the data. The results showed significant p < 0.05, indicating that the comparison between groups was statistically significant.
4. Conclusions
In this work, ten chitosan derivatives containing l-arginine Schiff base were prepared and their structural accuracy was verified by FT-IR and 1H NMR. Compared with chitosan, the antioxidant activities of the ten compounds were greatly improved, and they also had certain antifungal activities. Furthermore, these compounds also showed significant improvement in their ability to inhibit S. aureus. Schiff bases containing guanidine were grafted to chitosan by forming an amide group, which enriches the types of chitosan derivatives and expands the application range of chitosan derivatives as fungicides and antioxidants. It is hoped that this research will be further developed and utilized in agriculture or biomedical fields.
Author Contributions
Conceptualization, J.C. and Z.G.; methodology, J.C and L.W.; software, Q.M.; formal analysis.; writing—original draft preparation, J.C.; writing—review and editing, J.C., Y.S.; visualization, J.C.; supervision, Y.S.; project administration, Z.G.; funding acquisition, Z.G., W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Deployment Project of Center for Ocean Mega-Science of CAS (COMS2020J04) and Youth Innovation Promotion Association CAS (2020219).
Data Availability Statement
All data contained in the manuscript are available from the authors.
Acknowledgments
We would like to thank Institutional center for shared technologies and facilities of Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences for their assistance with conventional FT-IR, 1H NMR and Thermal stability. We would also like to thank the editors and reviewers for their helpful comments to improve this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a potential natural com-pound to manage plant diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Masson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mu, M.; Fan, R.; Zou, B.; Guo, G. Functionalized chitosan as a promising platform for cancer immunotherapy: A review. Carbohydr. Polym. 2022, 290, 119452. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Qin, C.Q.; Li, H.R.; Xiao, Q.; Liu, Y.; Zhu, J.C.; Du, Y.M. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Progress Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.L.; Ge, M.Y.; Wang, L.; Fang, Y.; Wang, Y.L.; Xie, G.L.; Sun, G.C. Antibacterial Mechanism of Chitosan and its Applications in Protection of Plant from Bacterial Disease. Asian J. Chem. 2013, 25, 10033–10036. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, Q.; Dong, F.; Feng, Y.; Guo, Z. Phenolic antioxidants-functionalized quaternized chitosan: Synthesis and antioxidant properties. Int. J. Biol. Macromol. 2013, 53, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.A.; Saad, G.R.; Abdelhamid, I.A.; Abdel-Aziz, M.M.; Taha, H.A.; Abou El Dahab, M.M.; Elsabee, M.Z. Chitosan Schiff bases/AgNPs: Synthesis, characterization, antibiofilm and preliminary anti-schistosomal activity studies. Polym. Bull. 2022, 1–26. [Google Scholar] [CrossRef]
- Hamed, A.A.; Abdelhamid, I.A.; Saad, G.R.; Elkady, N.A.; Elsabee, M.Z. Synthesis, characterization and antimicrobial activity of a novel chitosan Schiff bases based on heterocyclic moieties. Int. J. Biol. Macromol. 2020, 153, 492–501. [Google Scholar] [CrossRef]
- Hamed, A.A.; Saad, G.R.; Abdelhamid, I.A.; Elwahy, A.H.M.; Abdel-Aziz, M.M.; Elsabee, M.Z. Chitosan Schiff bases-based polyelectrolyte complexes with graphene quantum dots and their prospective biomedical applications. Int. J. Biol. Macromol. 2022, 208, 1029–1045. [Google Scholar] [CrossRef]
- Ali, S.S.; Kenawy, E.-R.; Sonbol, F.I.; Sun, J.; Al-Etewy, M.; Ali, A.; Huizi, L.; El-Zawawy, N.A. Pharmaceutical Potential of a Novel Chitosan Derivative Schiff Base with Special Reference to Antibacterial, Anti-Biofilm, Antioxidant, Anti-Inflammatory, Hemocompatibility and Cytotoxic Activities. Pharm. Res. 2018, 36, 5. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Li, Q.; Guo, Z. Synthesis and characterization of α-lipoic acid grafted chitosan derivatives with antioxidant activity. React. Funct. Polym. 2022, 172, 105205. [Google Scholar] [CrossRef]
- Blachier, F.; Darcyvrillon, B.; Sener, A.; Duee, P.H.; Malaisse, W.J. Arginine Metabolism in Rat Enterocytes. Biochim. Biophys. Acta 1991, 1092, 304–310. [Google Scholar] [CrossRef]
- Kim, S.-H.; Semenya, D.; Castagnolo, D. Antimicrobial drugs bearing guanidine moieties: A review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef]
- Zheng, Y.; Sheng, J.; Zhao, R.; Zhang, J.; Lv, S.; Liu, L.; Shen, L. Preharvest l-arginine Treatment Induced Postharvest Disease Resistance to Botrysis cinerea in Tomato Fruits. J. Agric. Food Chem. 2011, 59, 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Kauter, K.; Withers, K.; Sernia, C.; Brown, L. Chronic l-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats. Food Funct. 2013, 4, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wan, Y.; Zhao, M.; Liu, Y.; Zhang, S. Preparation and characterization of antimicrobial chitosan-N-arginine with different degrees of substitution. Carbohydr. Polym. 2011, 83, 144–150. [Google Scholar] [CrossRef]
- Song, J.; Feng, H.; Wu, M.; Chen, L.; Xia, W.; Zhang, W. Preparation and characterization of arginine-modified chitosan/hydroxypropyl methylcellose antibacterial film. Int. J. Biol. Macromol. 2020, 145, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Han, Q.; Zhang, F.; Meng, X.; Liu, B. Preparation, characterization and antibacterial properties of 6-deoxy-6-arginine modified chitosan. Carbohydr. Polym. 2020, 230, 115635. [Google Scholar] [CrossRef] [PubMed]
- Anush, S.M.; Vishalakshi, B.; Kalluraya, B.; Manju, N. Synthesis of pyrazole-based Schiff bases of Chitosan: Evaluation of antimicrobial activity. Int. J. Biol. Macromol. 2018, 119, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.B.; Aotegen, B.; Zhong, Z.M. Synthesis, characterization and biological activity of C-6-Schiff bases derivatives of chitosan. Int. J. Biol. Macromol. 2017, 105, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Gavalyan, V.B. Synthesis and characterization of new chitosan-based Schiff base compounds. Carbohydr. Polym. 2016, 145, 37–47. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, H.J.; Li, S.J.; Li, Z.; Jiang, M.Y. Synthesis, antimicrobial activity of Schiff base compounds of cinnamaldehyde and amino acids. Bioorg. Med. Chem. Lett. 2016, 26, 809–813. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, J.Y. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Hameed, A.; al-Rashida, M.; Uroos, M.; Abid Ali, S.; Khan, K.M. Schiff bases in medicinal chemistry: A patent review (2010–2015). Expert Opin. Ther. Pat. 2017, 27, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Malekshah, R.E.; Shakeri, F.; Khaleghian, A.; Salehi, M. Developing a biopolymeric chitosan supported Schiff-base and Cu(II), Ni (II) and Zn(II) complexes and biological evaluation as pro-drug. Int. J. Biol. Macromol. 2020, 152, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.F.G.; Attjioui, M.; Ferreira, A.P.G.; Moerschbacher, B.M.; Cavalheiro, E.T.G. New series of metal complexes by amphiphilic biopolymeric Schiff bases from modified chitosans: Preparation, characterization and effect of molecular weight on its biological applications. Int. J. Biol. Macromol. 2020, 145, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Z.S.; Yen, Z.H.; Le, Z.F.; Zhu, X.D.; Huang, Q.H. Synthesis, characterization and antifungal activity of copper(ii), zinc(ii), cobalt(ii) and nickel(ii) complexes derived from 2-chlorobenzaldehyde and glycine. Synth. React. Inorg. Met. 1994, 24, 1453–1460. [Google Scholar]
- Koh, L.L.; Ranford, J.O.; Robinson, W.T.; Svensson, J.O.; Tan, A.L.C.; Wu, D.Q. Model for the reduced Schiff base intermediate between amino acids and pyridoxal: Copper(II) complexes of N-(2-hydroxbenzyl)amino acids with nonpolar side chains and the crystal structures of Cu(N-(2-hydroxbenzyl)-D,L-alanine)(phen) center dot H2O and Cu(N-(2-hydroxybenzyl)-D,L-alanine)(imidazole). Inorg. Chem. 1996, 35, 6466–6472. [Google Scholar]
- Mao, J.; Li, N.; Li, H.; Hu, X. Novel Schiff base complexes as catalysts in aerobic selective oxidation of β-isophorone. J. Mol. Catal. A Chem. 2006, 258, 178–184. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Amiri, A.; Shanbedi, M.; Maghrebi, M.; Baniadam, M. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method. Colloids Surf. B Biointerfaces 2012, 92, 196–202. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G.Y. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. A Polym. Chem. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Bi, R.; Yue, L.; Niazi, S.; Khan, I.M.; Sun, D.; Wang, B.; Wang, Z.P.; Jiang, Q.X.; Xia, W.S. Facile synthesis and antibacterial activity of geraniol conjugated chitosan oligosaccharide derivatives. Carbohydr. Polym. 2021, 251, 117099. [Google Scholar] [CrossRef]
- Mao, S.F.; Wang, B.; Yue, L.; Xia, W.S. Effects of citronellol grafted chitosan oligosaccharide derivatives on regulating anti-inflammatory activity. Carbohydr. Polym. 2021, 262, 117972. [Google Scholar] [CrossRef]
- Mi, Y.; Li, Q.; Miao, Q.; Tan, W.; Zhang, J.; Guo, Z. Enhanced antifungal and antioxidant activities of new chitosan derivatives modified with Schiff base bearing benzenoid/heterocyclic moieties. Int. J. Biol. Macromol. 2022, 208, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, S.; Qin, Y.; Xing, R.; Li, K.; Yu, H.; Yue, Y.; Li, Y.; Li, P. Synthesis and antibacterial activities of basic amino acidmodified chitosan derivatives. Haiyang Kexue 2017, 41, 24–29. [Google Scholar]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Raju, L.; Stesho Crystalin Lazuli, A.R.; Udaya Prakash, N.K.; Rajkumar, E. Chitosan-terephthaldehyde hydrogels—Effect of concentration of cross-linker on structural, swelling, thermal and antimicrobial properties. Materialia 2021, 16, 101082. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H.; Su, W.-W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Deshaies, M.; Lamari, N.; Ng, C.K.Y.; Ward, P.; Doohan, F.M. The impact of chitosan on the early metabolomic response of wheat to infection by Fusarium graminearum. BMC Plant Biol. 2022, 22, 73. [Google Scholar] [CrossRef]
- Galván Márquez, I.; Akuaku, J.; Cruz, I.; Cheetham, J.; Golshani, A.; Smith, M.L. Disruption of protein synthesis as antifungal mode of action by chitosan. Int. J. Food Microbiol. 2013, 164, 108–112. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.A.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Maldonado, P.D.; Rivero-Cruz, I.; Mata, R.; Pedraza-Chaverri, J. Antioxidant activity of A-type proanthocyanidins from Geranium niveum (Geraniaceae). J. Agric. Food Chem. 2005, 53, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Y.; Xing, R.E.; Liu, S.; Zhong, Z.M.; Ji, X.; Wang, L.; Li, P.C. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr. Res. 2007, 342, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Tan, W.Q.; Luan, F.; Yin, X.L.; Dong, F.; Li, Q.; Guo, Z.Y. Synthesis of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate for Antifungal and Antibacterial Activities. Polymers 2018, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Haile, A. Multifunctional properties of cotton fabric treated with chitosan and carboxymethyl chitosan. Carbohydr. Polym. 2007, 69, 164–171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).