Electrolysis as a Universal Approach for Isolation of Diverse Chitin Scaffolds from Selected Marine Demosponges †
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Biological Samples and Chemicals
4.2. Electrolysis Cell Setup
4.3. Isolation of Chitin Structures
4.3.1. Isolation of Chitinous Scaffold from A. archeri Demosponge
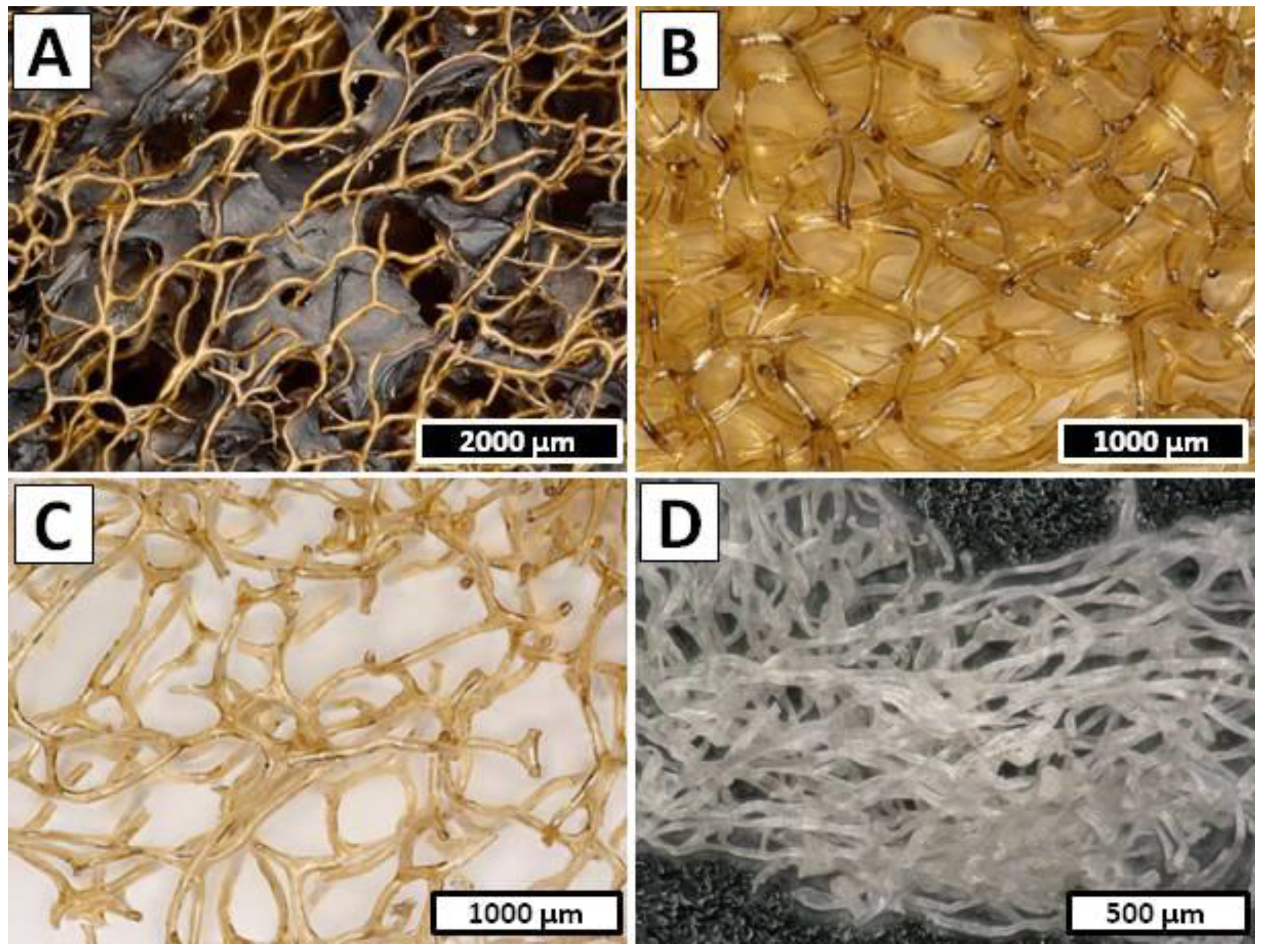
- Step 1—Decellularization was carried out in the cathode chamber for 1.5 h (12 V, 0.5 A) (Figure 10A) and during this the electro-alkali treatment of the pH of catholyte solution was established up to 12.0. Hence, the rapid dissolution of preswelled cells followed by the removal of soft tissues from the interlayer spaces and a partial depigmentation of the sponge skeleton were observed. The sample after treatment was composed of a gold color cell-free skeleton with the net-like 3D structure (Figure 2B).
- Step 2—Decalcification was performed in the anode chamber for 1.5 h (12 V, 0.5 A); during this, the electro-acidic pH treatment of the anolyte solution was established down to 1.5. Low pH was necessary to purify the sponge skeleton from the mineral calcium salts and acid-soluble pigments. After treatment, the remaining sample was in the form of a light yellow skeleton without significant structure deformation (Figure 2C).
- Step 3—The deproteinization/depigmentation/desilicification processes were carried out after the exchange of the electrolyte in the cathode chamber due to high content of impurities that remained after dissolution of the cells in the decellurarization step. The sample was placed in the cathode chamber for 2.5 h (16 V, 1.0 A) and electro-alkali treatment the pH of catholyte solution was established up to 12.5. Lack of the possible barriers such as sponge cells or layers of the mineral salts gave the catholyte solution free access to the chitinous skeleton which, along with extremely high pH, caused incomplete removal of pigments and residual proteins from the chitinous matrix. After treatment, the remaining sample in form of a colorless scaffold (Figure 2D) was extensively rinsed using distilled water up to neutral pH and stored in ethanol absolute (4 °C).
4.3.2. Isolation of Chitinous Scaffold from I. basta Demosponge
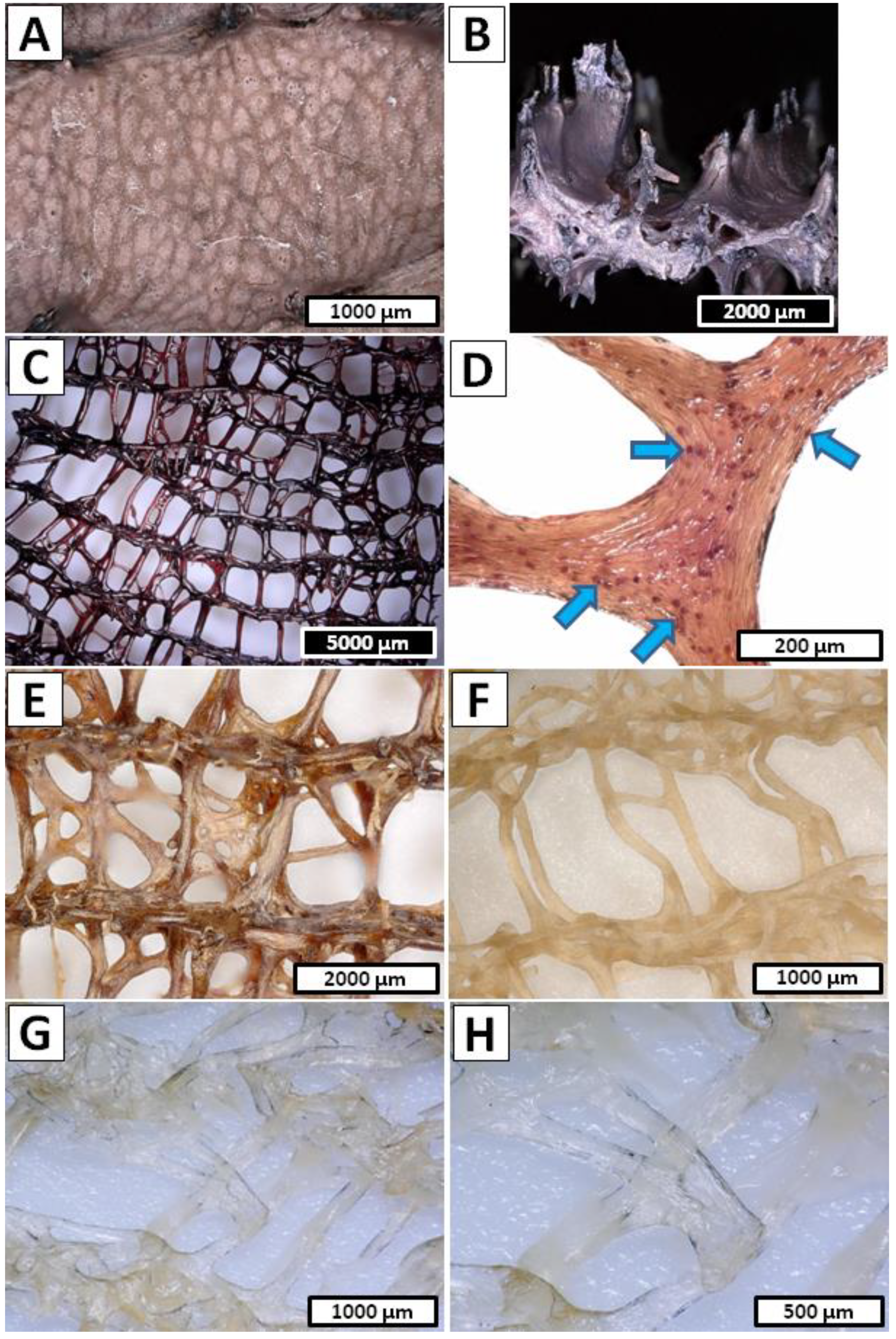
- Step 1—Decellularization of the I. basta was performed in the same way as in the A. archeri case (1.5 h in cathode chamber; 12 V, 0.5 A) and the post-treated sample was in the form of a rigid deep brown cell-free skeleton (Figure 4C,D).
- Step 2—Decalcification was carried out in the anode chamber for 0.5 h (12 V, 0.5 A) and during this, the electro-acidic treatment the pH of anolyte solution was established down to 1.5.
- Step 3—Deproteinization/depigmentation/desilicification was performed in the cathode chamber for 0.5 h (12 V, 0.5 A) and during this electro-alkali treatment of the pH of the catholyte solution was established up to 12.0.
- Step 2 and Step 3 cycle—Unique chemical composition of the I. basta demosponge skeleton [66,67] forced modification of the electrolysis-supported isolation method known from the earlier reports [52,53]. Due to the possible multilayered structure of proteins, pigments and biominerals within the I. basta skeleton, the decalcification and deproteinization/depigmentation/desilicification steps were repeated multiple times in order to ensure free access for the anolyte/catholyte to the layers soluble in corresponding pH conditions. Thus, a full 15 cycles consisting of the successive anolyte and catholyte treatment were performed (Figure 10B). During this process, the I. basta skeleton was gradually decolorized and softened until the colorless chitinous scaffold was obtained (Figure 4E–H). After treatment, the sample was extensively rinsed using distilled water up to neutral pH and stored in ethanol absolute (4 °C). Loss of the electrolyte caused by evaporation was compensated by refill of the anolyte and catholyte solutions every 5th cycle (5 h of treatment).
4.3.3. Isolation of Chitinous Scaffold from S. clavata Demosponge
- Step 1—Decalcification was carried out in the anode chamber for 1.0 h (12 V, 0.5 A) and during this electro-acidic treatment the pH of anolyte solution was established down to 1.5.
- Step 2—Deproteinization/depigmentation/desilicification was performed in the cathode chamber for 1.0 h (12 V, 0.5 A) and during this electro-alkali treatment the pH of catholyte solution was established up to 12.0.
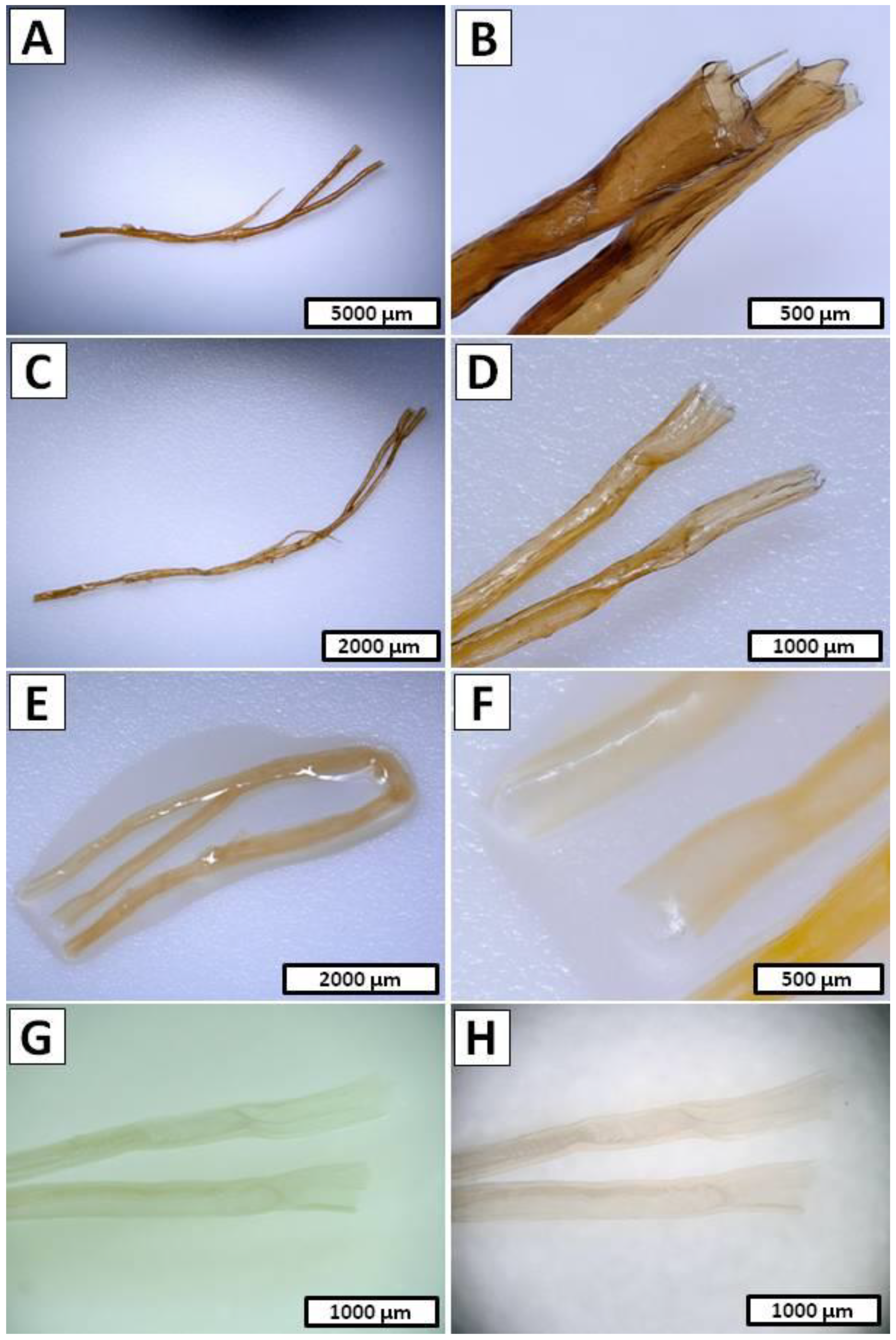
- Step 1 and Step 2 cycle—Due to similar reasons [68] in the I. basta case, the decalcification and deproteinization/depigmentation/desilicification steps were repeated multiple times. Hence, full 10 cycles consisting of the successive anolyte and catholyte treatment were performed (Figure 10C). During this process, the S. clavata skeletal fibers were gradually decolorized (Figure 5A–F) and had lost their mechanical rigidity till the colorless chitinous tubes were obtained (Figure 5G,H). Loss of the electrolyte caused by evaporation was compensated by the refill of the anolyte and catholyte solutions every 3 cycles (6 h of treatment). Obtained samples in the posttreatment were rinsed in distilled water up to a neutral pH and stored in ethanol absolute (4 °C).
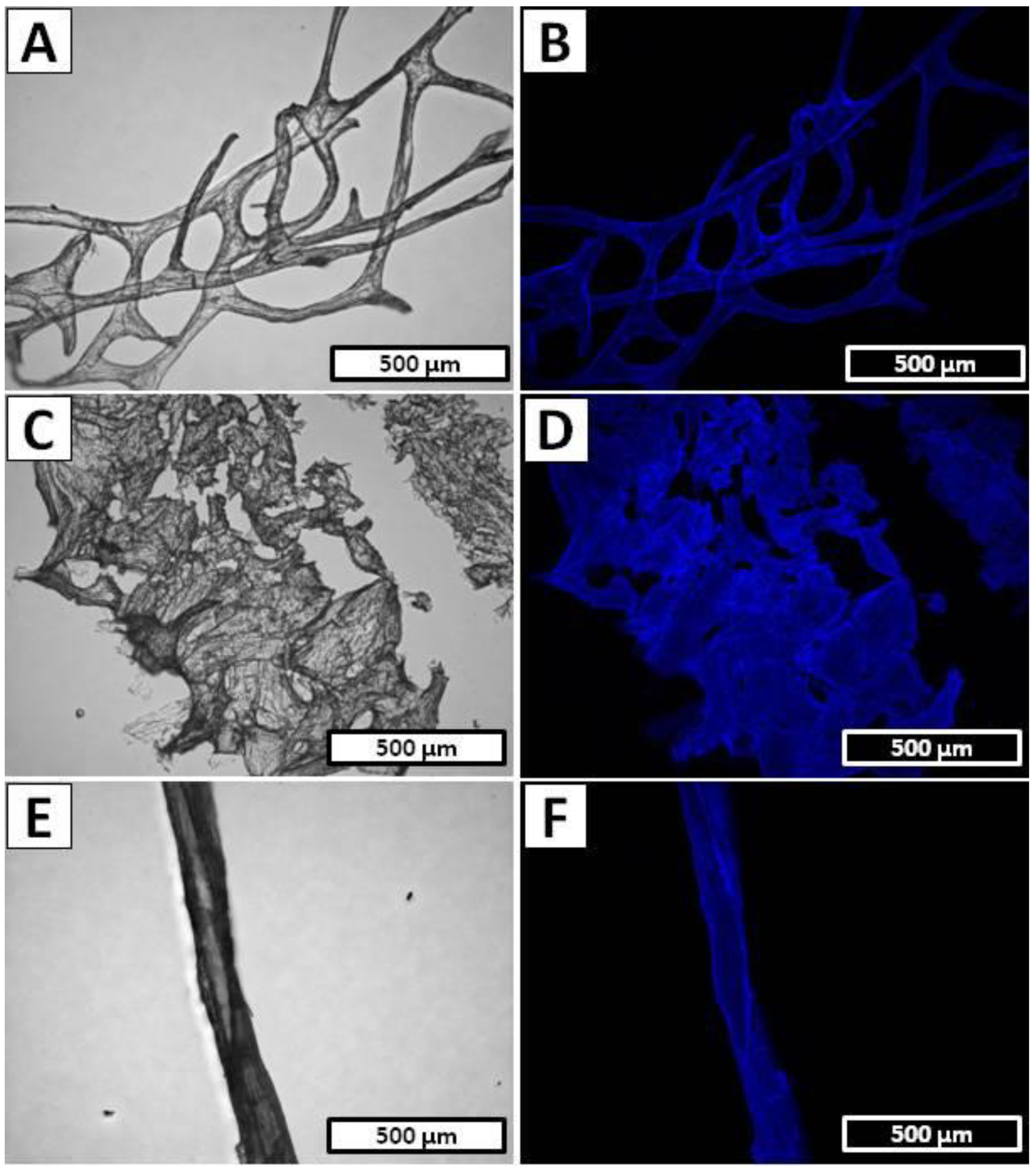
4.4. Light and Fluorescence Microscopy
4.5. Calcofluor White Staining
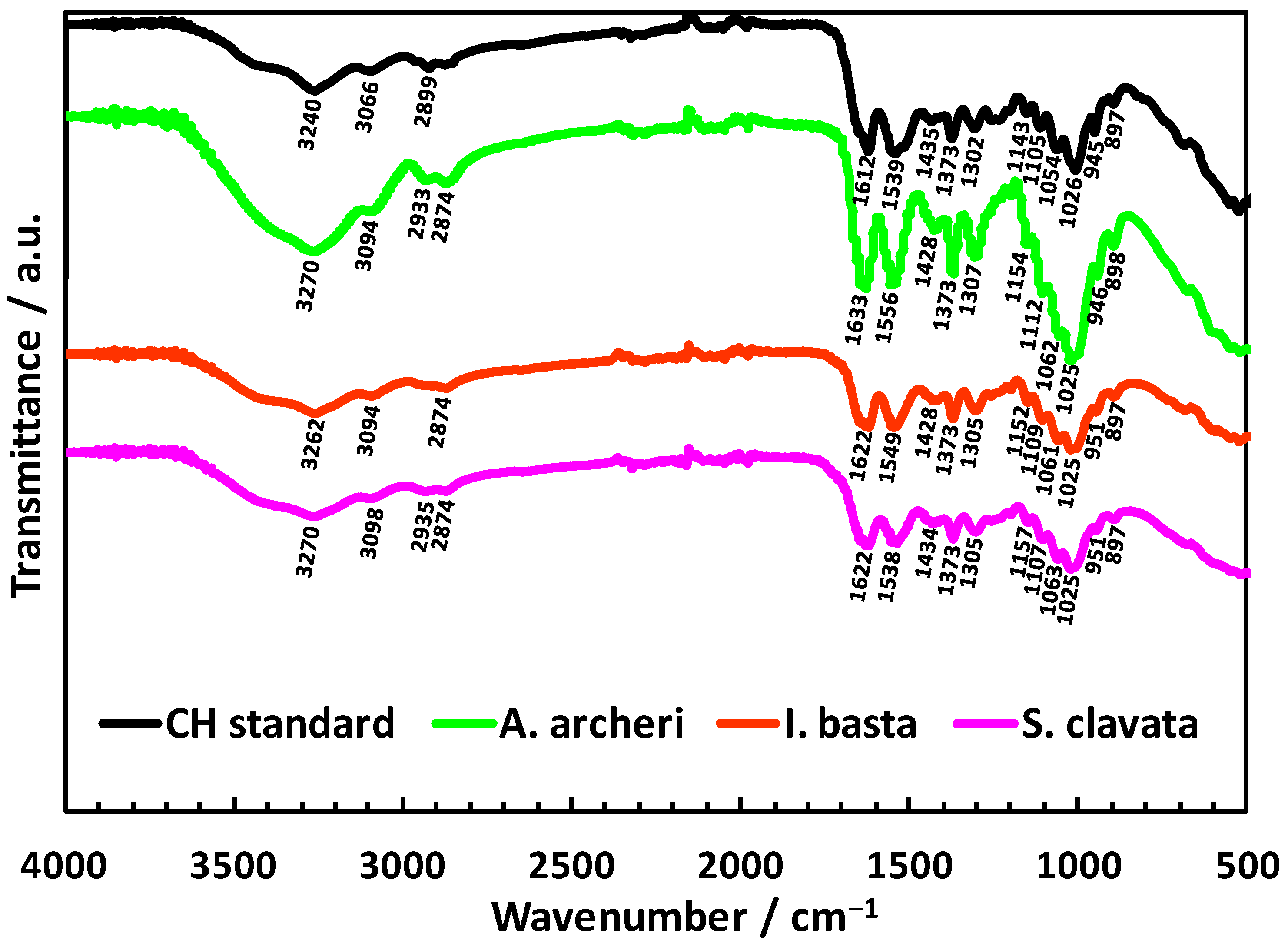
4.6. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
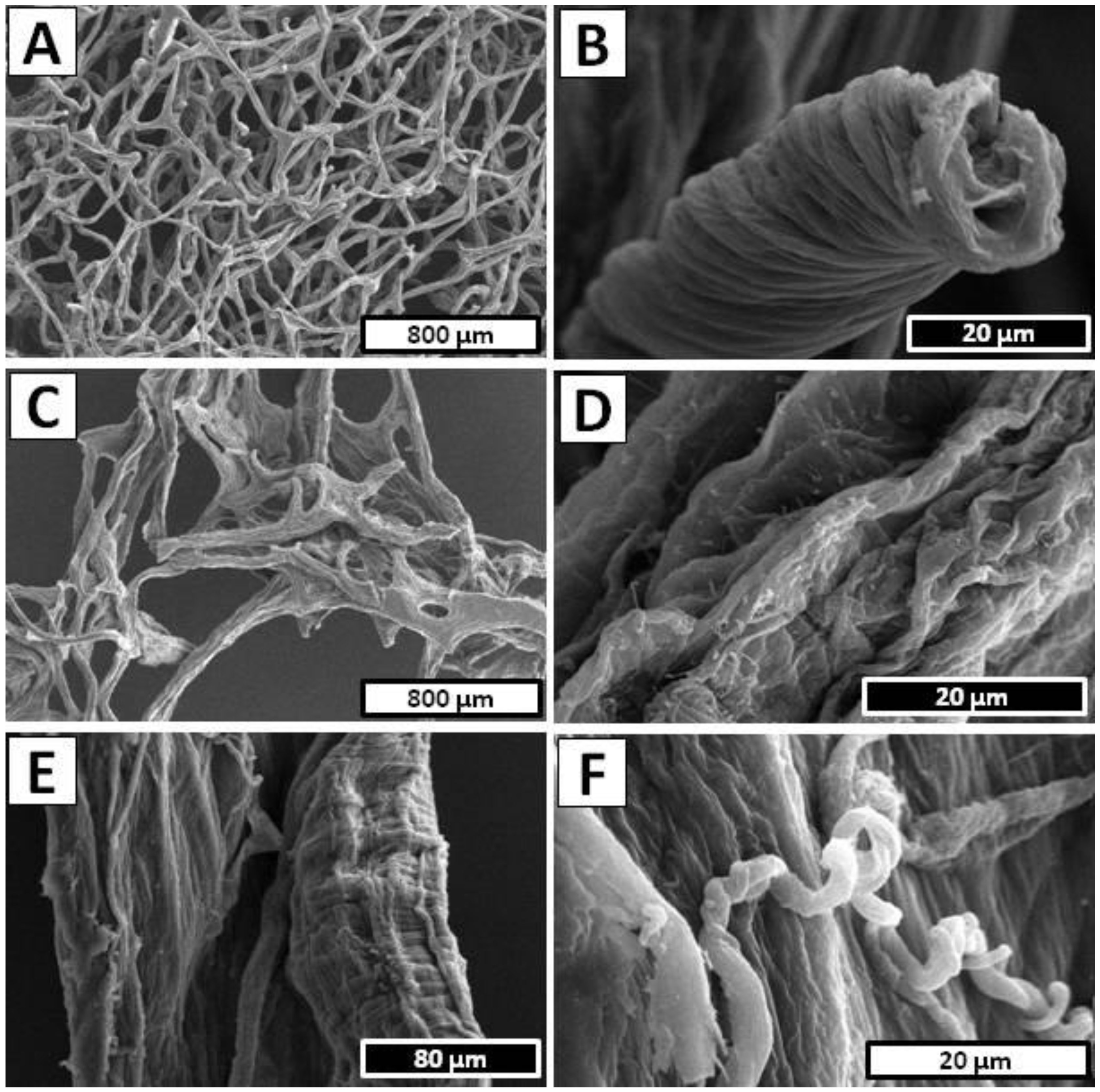
4.7. Scanning Electron Microscopy
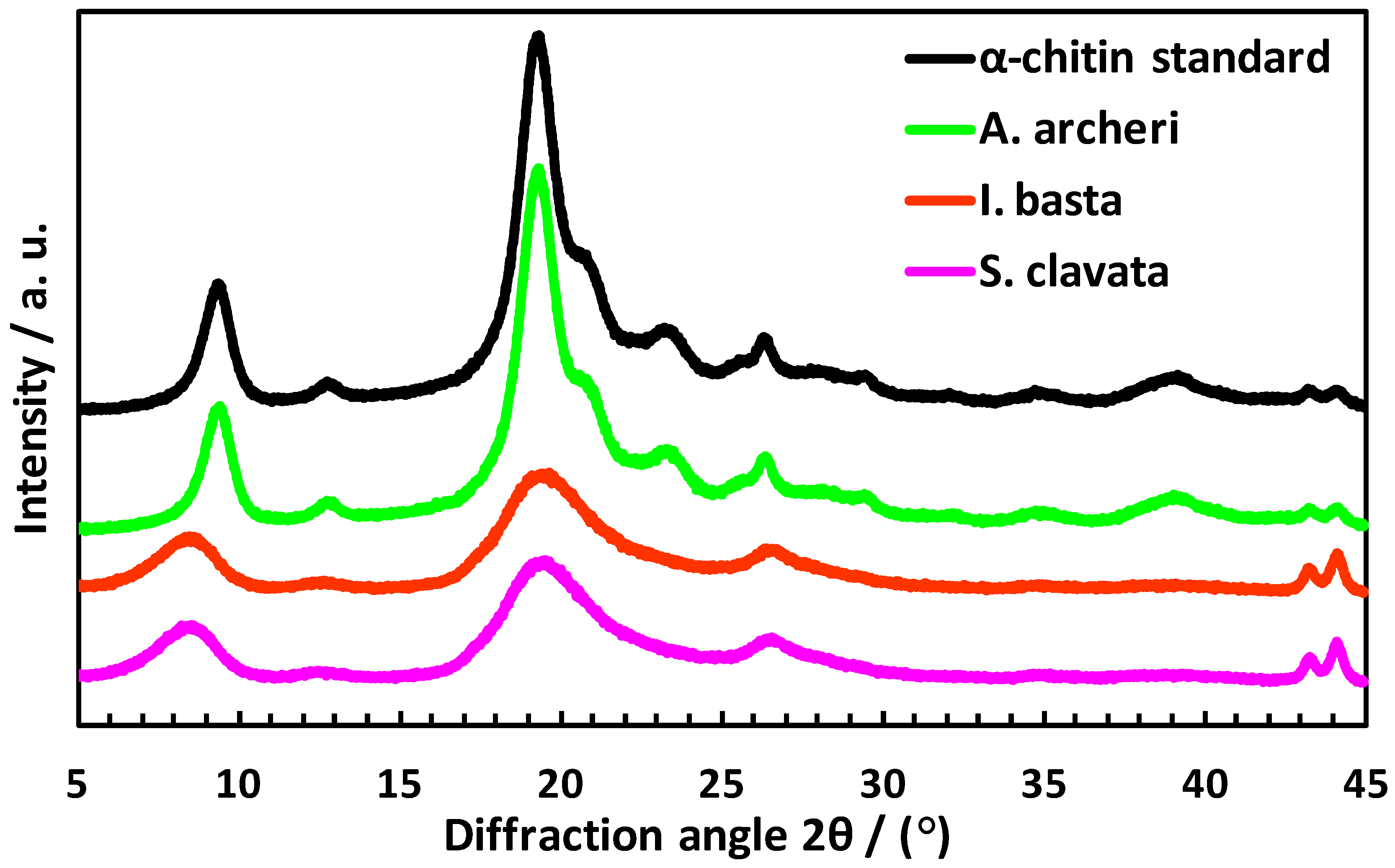
4.8. X-ray Diffraction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Petrášek, Z.; Pisera, A.; Simon, P.; Sivkov, V.N.; et al. Discovery of 505-Million-Year Old Chitin in the Basal Demosponge Vauxia Gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Zatoń, M.; Bazhenov, V.V.; Behm, T.; Ehrlich, A.; Stelling, A.L.; Hog, M.; Ehrlich, H. Identification of Chitin in 200-Million-Year-Old Gastropod Egg Capsules. Paleobiology 2014, 40, 529–540. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t Waste Seafood Waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in Chitin Analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, L.; Vafaei, A.; Rösner, J.; Merzendorfer, H. Chitin Prevalence and Function in Bacteria, Fungi and Protists. Adv. Exp. Med. Biol. 2019, 1142, 19–59. [Google Scholar] [CrossRef]
- Żółtowska, S.; Klinger, C.; Petrenko, I.; Wysokowski, M.; Joseph, Y.; Jesionowski, T.; Ehrlich, H. Methods of Isolating Chitin from Sponges (Porifera). In Chitin and Chitosan; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 35–59. ISBN 9781119450467. [Google Scholar]
- Arrouze, F.; Desbrieres, J.; Lidrissi Hassani, S.; Tolaimate, A. Investigation of β-Chitin Extracted from Cuttlefish: Comparison with Squid β-Chitin. Polym. Bull. 2020, 78, 7219–7239. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Schimpf, C.; Rafaja, D.; Brendler, E.; Viehweger, C.; Żółtowska-Aksamitowska, S.; Petrenko, I.; et al. Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena Versicolor Spider Molt Cuticle. Molecules 2019, 24, 3736. [Google Scholar] [CrossRef]
- Song, Y.-S.; Jo, Y.H.; Han, Y.S.; Jung, W.-J. Production of Chitin- and Chitosan-oligosaccharide Using the Edible Insect. Tenebrio Molitor. Entomol. Res. 2022, 52, 207–213. [Google Scholar] [CrossRef]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.-H. Chitin-Based Organic Networks: An Integral Part of Cell Wall Biosilica in the Diatom Thalassiosira Pseudonana. Angew. Chem. Int. Ed. Engl. 2009, 48, 9724–9727. [Google Scholar] [CrossRef]
- Connors, M.J.; Ehrlich, H.; Hog, M.; Godeffroy, C.; Araya, S.; Kallai, I.; Gazit, D.; Boyce, M.; Ortiz, C. Three-Dimensional Structure of the Shell Plate Assembly of the Chiton Tonicella Marmorea and Its Biomechanical Consequences. J. Struct. Biol. 2012, 177, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Bavestrello, G.; Kurek, D.; Paasch, S.; Brunner, E.; Born, R.; Galli, R.; Stelling, A.L.; Sivkov, V.N.; Petrova, O.V.; et al. Isolation and Identification of Chitin in the Black Coral Parantipathes Larix (Anthozoa: Cnidaria). Int. J. Biol. Macromol. 2012, 51, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and Identification of Chitin in Three-Dimensional Skeleton of Aplysina Fistularis Marine Sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef]
- Ehrlich, H.; Kaluzhnaya, O.V.; Brunner, E.; Tsurkan, M.V.; Ereskovsky, A.; Ilan, M.; Tabachnick, K.R.; Bazhenov, V.V.; Paasch, S.; Kammer, M.; et al. Identification and First Insights into the Structure and Biosynthesis of Chitin from the Freshwater Sponge Spongilla Lacustris. J. Struct. Biol. 2013, 183, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On Chemistry of γ-Chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guan, K.; Bornstein, S.R.; Czaczyk, K.; Pokrovsky, O.; et al. Spider Chitin. The Biomimetic Potential and Applications of Caribena Versicolor Tubular Chitin. Carbohydr. Polym. 2019, 226, 115301. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, X.; Miao, J.; Leng, K. Chitin from Antarctic Krill Shell: Eco-Preparation, Detection, and Characterization. Int. J. Biol. Macromol. 2020, 164, 4125–4137. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Sharma, C.P. Production, Properties and Applications of Fibres from Chitin and Chitosan. Trends Biomater. Artif. Organs 2018, 32, 23–82. [Google Scholar]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the Structural Diversity of Chitins as a Versatile Biomaterial. Philos. Trans. A Math. Phys. Eng. Sci. 2021, 379, 20200331. [Google Scholar] [CrossRef]
- Ehrlich, H.; Maldonado, M.; Spindler, K.-D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First Evidence of Chitin as a Component of the Skeletal Fibers of Marine Sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella Labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Chitin of Poriferan Origin as a Unique Biological Material. In Blue Biotechnology: Production and Use of Marine Molecules; Barre, L., Bates, S., Eds.; Wiley-VCH, Verlag: Weinheim, Germany, 2018; Volume 2, pp. 821–854. [Google Scholar]
- Choi, A.H. Marine-Derived Biomaterials for Tissue Engineering Applications, 1st ed.; Ben-Nissan, B., Ed.; Springer: Singapore, 2020; ISBN 9789811388576. [Google Scholar]
- Zdarta, J.; Machałowski, T.; Degórska, O.; Bachosz, K.; Fursov, A.; Ehrlich, H.; Ivanenko, V.N.; Jesionowski, T. 3D Chitin Scaffolds from the Marine Demosponge Aplysina Archeri as a Support for Laccase Immobilization and Its Use in the Removal of Pharmaceuticals. Biomolecules 2020, 10, 646. [Google Scholar] [CrossRef]
- Tsurkan, D.; Wysokowski, M.; Petrenko, I.; Voronkina, A.; Khrunyk, Y.; Fursov, A.; Ehrlich, H. Modern Scaffolding Strategies Based on Naturally Pre-Fabricated 3D Biomaterials of Poriferan Origin. Appl. Phys. A Mater. Sci. Process. 2020, 126, 382. [Google Scholar] [CrossRef]
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in Modern Marine Biomaterials Research. Mar. Drugs 2020, 18, 589. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and Chitosan in Selected Biomedical Applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine Biomaterials: Biomimetic and Pharmacological Potential of Cultivated Aplysina Aerophoba Marine Demosponge. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110566. [Google Scholar] [CrossRef]
- van den Broek, L.A.M.; Boeriu, C.G. Chitin and Chitosan: Properties and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2019; p. 510. [Google Scholar]
- Thomas, S.; Pius, A.; Gopi, S. (Eds.) Handbook of Chitin and Chitosan: Volume 1: Preparation and Properties; Elsevier Science Publishing: Philadelphia, PA, USA, 2020; ISBN 9780128179703. [Google Scholar]
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.L.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O.J. Nanochitin: Chemistry, Structure, Assembly, and Applications. Chem. Rev. 2022, 122, 11604–11674. [Google Scholar] [CrossRef]
- Kertmen, A.; Ehrlich, H. Patentology of Chitinous Biomaterials. Part I: Chitin. Carbohydr. Polym. 2022, 282, 119102. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme Biomimetic Approach for Developing Novel Chitin-GeO2 Nanocomposites with Photoluminescent Properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Ehrlich, H. (Ed.) Extreme Biomimetics; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319453385. [Google Scholar]
- Wysokowski, M.; Motylenko, M.; Rafaja, D.; Koltsov, I.; Stöcker, H.; Szalaty, T.J.; Bazhenov, V.V.; Stelling, A.L.; Beyer, J.; Heitmann, J.; et al. Extreme Biomimetic Approach for Synthesis of Nanocrystalline Chitin-(Ti,Zr)O2 Multiphase Composites. Mater. Chem. Phys. 2017, 188, 115–124. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of Poriferan Origin and the Bioelectrometallurgy of Copper/Copper Oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Machałowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Ziętek, J.; Pantović, S.; Voronkina, A.; Kovalchuk, V.; et al. 3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo. Mar. Drugs 2020, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Wertz, J.-L. Chitin and Chitosans in the Bioeconomy, 1st ed.; Taylor & Francis: London, UK, 2022; ISBN 9781032128481. [Google Scholar]
- Said Al Hoqani, H.A.; Al-Shaqsi, N.; Hossain, M.A.; Al Sibani, M.A. Isolation and Optimization of the Method for Industrial Production of Chitin and Chitosan from Omani Shrimp Shell. Carbohydr. Res. 2020, 492, 108001. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Ehrlich, H.; Simon, P.; Carrillo-Cabrera, W.; Bazhenov, V.V.; Botting, J.P.; Ilan, M.; Ereskovsky, A.V.; Muricy, G.; Worch, H.; Mensch, A.; et al. Insights into Chemistry of Biological Materials: Newly Discovered Silica-Aragonite-Chitin Biocomposites in Demosponges. Chem. Mater. 2010, 22, 1462–1471. [Google Scholar] [CrossRef]
- Ehrlich, H.; Koutsoukos, P.G.; Demadis, K.D.; Pokrovsky, O.S. Principles of Demineralization: Modern Strategies for the Isolation of Organic Frameworks. Part I. Common Definitions and History. Micron 2008, 39, 1062–1091. [Google Scholar] [CrossRef]
- Żółtowska-Aksamitowska, S.; Shaala, L.; Youssef, D.; Elhady, S.; Tsurkan, M.; Petrenko, I.; Wysokowski, M.; Tabachnick, K.; Meissner, H.; Ivanenko, V.; et al. First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale Euplectellioides Case. Mar. Drugs 2018, 16, 68. [Google Scholar] [CrossRef]
- Borić, M.; Vicente, F.A.; Jurković, D.L.; Novak, U.; Likozar, B. Chitin Isolation from Crustacean Waste Using a Hybrid Demineralization/DBD Plasma Process. Carbohydr. Polym. 2020, 246, 116648. [Google Scholar] [CrossRef]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Jesionowski, T. Isolation of Chitin from Aplysina Aerophoba Using a Microwave Approach. Prog. Chem. Appl. Chitin Deriv. 2019, XXIV, 61–74. [Google Scholar] [CrossRef]
- Ehrlich, H.; Martinović, R.; Joksimović, D.; Petrenko, I.; Schiaparelli, S.; Wysokowski, M.; Tsurkan, D.; Stelling, A.L.; Springer, A.; Gelinsky, M.; et al. Conchixes: Organic Scaffolds Which Resemble the Size and Shapes of Mollusks Shells, Their Isolation and Potential Multifunctional Applications. Appl. Phys. A Mater. Sci. Process. 2020, 126, 562. [Google Scholar] [CrossRef]
- Petrenko, I.; Khrunyk, Y.; Voronkina, A.; Kovalchuk, V.; Fursov, A.; Ivanenko, V. Poriferan Chitin: 3D Scaffolds from Nano- to Macroscale. A Review. Lett. Appl. Nano Bio. Sci. 2020, 9, 1004–1014. [Google Scholar] [CrossRef]
- Kuprina, E.E.; Timofeeva, K.G.; Vodolazhskaya, S.V. Electrochemical preparation of chitin materials. Russ. J. Appl. Chem. 2002, 75, 822–828. [Google Scholar] [CrossRef]
- Nowacki, K.; Stępniak, I.; Machałowski, T.; Wysokowski, M.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Langer, E.; Richter, A.; Ziętek, J.; et al. Electrochemical Method for Isolation of Chitinous 3D Scaffolds from Cultivated Aplysina Aerophoba Marine Demosponge and Its Biomimetic Application. Appl. Phys. A Mater. Sci. Process. 2020, 126, 368. [Google Scholar] [CrossRef]
- Nowacki, K.; Stępniak, I.; Langer, E.; Tsurkan, M.; Wysokowski, M.; Petrenko, I.; Khrunyk, Y.; Fursov, A.; Bo, M.; Bavestrello, G.; et al. Electrochemical Approach for Isolation of Chitin from the Skeleton of the Black Coral Cirrhipathes Sp. (Antipatharia). Mar. Drugs 2020, 18, 297. [Google Scholar] [CrossRef]
- Atwell, H.V.; Fuwa, T. The Electrolytic Production of Acid and Alkali from Sodium Sulfate Solutions. Ind. Eng. Chem. 1923, 15, 617–620. [Google Scholar] [CrossRef]
- Pletcher, D.; Walsh, F.C. Industrial Electrochemistry; Springer Netherlands: Dordrecht, The Netherlands, 1993; ISBN 9780751401486. [Google Scholar]
- Strathmann, H.; Giorno, L.; Drioli, E. Overview of Ion-Exchange Membrane Processes. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–22. ISBN 9780444502360. [Google Scholar]
- Savari, S.; Sachdeva, S.; Kumar, A. Electrolysis of Sodium Chloride Using Composite Poly(Styrene-Co-Divinylbenzene) Cation Exchange Membranes. J. Memb. Sci. 2008, 310, 246–261. [Google Scholar] [CrossRef]
- Zeppilli, M.; Lai, A.; Villano, M.; Majone, M. Anion vs Cation Exchange Membrane Strongly Affect Mechanisms and Yield of CO2 Fixation in a Microbial Electrolysis Cell. Chem. Eng. J. 2016, 304, 10–19. [Google Scholar] [CrossRef]
- Park, S.-G.; Chae, K.-J.; Lee, M. A Sulfonated Poly(Arylene Ether Sulfone)/Polyimide Nanofiber Composite Proton Exchange Membrane for Microbial Electrolysis Cell Application under the Coexistence of Diverse Competitive Cations and Protons. J. Memb. Sci. 2017, 540, 165–173. [Google Scholar] [CrossRef]
- Ito, H.; Kawaguchi, N.; Someya, S.; Munakata, T. Pressurized Operation of Anion Exchange Membrane Water Electrolysis. Electrochim. Acta 2019, 297, 188–196. [Google Scholar] [CrossRef]
- Salvatore, D.A.; Weekes, D.M.; He, J.; Dettelbach, K.E.; Li, Y.C.; Mallouk, T.E.; Berlinguette, C.P. Electrolysis of Gaseous CO2 to CO in a Flow Cell with a Bipolar Membrane. ACS Energy Lett. 2018, 3, 149–154. [Google Scholar] [CrossRef]
- Pisarska, B.; Wicher, I.; Dylewski, R. Studies on the Parameters for Membrane-Electrolysis Conversion of Sodium Sulfate Solutions. Przemysł Chem. 2004, 83, 186–190. [Google Scholar]
- Holze, S.; Jörissen, J.; Fischer, C.; Kalvelage, H. Hydrogen Consuming Anodes for Energy Saving in Sodium Sulphate Electrolysis. Chem. Eng. Technol. 1994, 17, 382–389. [Google Scholar] [CrossRef]
- Jorissen, J.; Simmrock, K.H. The Behaviour of Ion Exchange Membranes in Electrolysis and Electrodialysis of Sodium Sulphate. J. Appl. Electrochem. 1991, 21, 869–876. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-Dimensional Chitin-Based Scaffolds from Verongida Sponges (Demospongiae: Porifera). Part I. Isolation and Identification of Chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-Based Scaffolds Are an Integral Part of the Skeleton of the Marine Demosponge Ianthella Basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef]
- Kertmen, A.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Petrova, O.; Sivkov, V.; Nekipelov, S.; Fursov, A.; Stelling, A.L.; Heimler, K.; et al. Calcite Nanotuned Chitinous Skeletons of Giant Ianthella Basta Marine Demosponge. Int. J. Mol. Sci. 2021, 22, 12588. [Google Scholar] [CrossRef]
- Ehrlich, H.; Bazhenov, V.V.; Debitus, C.; de Voogd, N.; Galli, R.; Tsurkan, M.V.; Wysokowski, M.; Meissner, H.; Bulut, E.; Kaya, M.; et al. Isolation and Identification of Chitin from Heavy Mineralized Skeleton of Suberea Clavata (Verongida: Demospongiae: Porifera) Marine Demosponge. Int. J. Biol. Macromol. 2017, 104, 1706–1712. [Google Scholar] [CrossRef]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina Archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel Chitin Scaffolds Derived from Marine Sponge Ianthella Basta for Tissue Engineering Approaches Based on Human Mesenchymal Stromal Cells: Biocompatibility and Cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Moriou, C.; Lacroix, D.; Petek, S.; El-Demerdash, A.; Trepos, R.; Leu, T.M.; Florean, C.; Diederich, M.; Hellio, C.; Debitus, C.; et al. Bioactive Bromotyrosine Derivatives from the Pacific Marine Sponge Suberea Clavata (Pulitzer-Finali, 1982). Mar. Drugs 2021, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.T.T.; Thung, D.C.; Trang, D.T.; Yen, P.Y.; Cuc, N.T.; Dung, D.T.; Tai, B.H.; Nhiem, N.X.; Kiem, P.V. Chemical constituents of marine sponge Ianthella basta (Pallas, 1766). Vietnam. J. Chem. 2022, 60, 238–244. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Wessling, D.; Jobling, M.; Avery, V.M.; Davis, R.A.; Feng, Y.; Hooper, J.N.A.; Quinn, R.J. Clavatadines C-E, Guanidine Alkaloids from the Australian Sponge Suberea Clavata. J. Nat. Prod. 2009, 72, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D Chitinous Scaffolds Derived from Cultivated Marine Demosponge Aplysina Aerophoba for Tissue Engineering Approaches Based on Human Mesenchymal Stromal Cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Beil, S.; Schamberger, A.; Naumann, W.; Machill, S.; van Pée, K.-H. Determination of the Degree of N-Acetylation (DA) of Chitin and Chitosan in the Presence of Water by First Derivative ATR FTIR Spectroscopy. Carbohydr. Polym. 2012, 87, 117–122. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin Characterization by SEM, FTIR, XRD, And13C Cross Polarization/Mass Angle Spinning NMR: Chitin Characterization. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Montroni, D.; Marzec, B.; Valle, F.; Nudelman, F.; Falini, G. Β-Chitin Nanofibril Self-Assembly in Aqueous Environments. Biomacromolecules 2019, 20, 2421–2429. [Google Scholar] [CrossRef]
- Montroni, D.; Fermani, S.; Morellato, K.; Torri, G.; Naggi, A.; Cristofolini, L.; Falini, G. β-Chitin Samples with Similar Microfibril Arrangement Change Mechanical Properties Varying the Degree of Acetylation. Carbohydr. Polym. 2019, 207, 26–33. [Google Scholar] [CrossRef]
- Steck, E.; Burkhardt, M.; Ehrlich, H.; Richter, W. Discrimination between Cells of Murine and Human Origin in Xenotransplants by Species Specific Genomic in Situ Hybridization. Xenotransplantation 2010, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-Dimensional Chitin-Based Scaffolds from Verongida Sponges (Demospongiae: Porifera). Part II: Biomimetic Potential and Applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.; Gryshkov, O.; Rogulska, O.; Lode, A.; Petrenko, A.Y.; Gelinsky, M.; Glasmacher, B.; Ehrlich, H. Chitinous Scaffolds from Marine Sponges for Tissue Engineering. In Springer Series in Biomaterials Science and Engineering; Springer Singapore: Singapore, 2019; pp. 285–307. ISBN 9789811388545. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacki, K.; Galiński, M.; Fursov, A.; Voronkina, A.; Meissner, H.; Petrenko, I.; Stelling, A.L.; Ehrlich, H. Electrolysis as a Universal Approach for Isolation of Diverse Chitin Scaffolds from Selected Marine Demosponges. Mar. Drugs 2022, 20, 665. https://doi.org/10.3390/md20110665
Nowacki K, Galiński M, Fursov A, Voronkina A, Meissner H, Petrenko I, Stelling AL, Ehrlich H. Electrolysis as a Universal Approach for Isolation of Diverse Chitin Scaffolds from Selected Marine Demosponges. Marine Drugs. 2022; 20(11):665. https://doi.org/10.3390/md20110665
Chicago/Turabian StyleNowacki, Krzysztof, Maciej Galiński, Andriy Fursov, Alona Voronkina, Heike Meissner, Iaroslav Petrenko, Allison L. Stelling, and Hermann Ehrlich. 2022. "Electrolysis as a Universal Approach for Isolation of Diverse Chitin Scaffolds from Selected Marine Demosponges" Marine Drugs 20, no. 11: 665. https://doi.org/10.3390/md20110665
APA StyleNowacki, K., Galiński, M., Fursov, A., Voronkina, A., Meissner, H., Petrenko, I., Stelling, A. L., & Ehrlich, H. (2022). Electrolysis as a Universal Approach for Isolation of Diverse Chitin Scaffolds from Selected Marine Demosponges. Marine Drugs, 20(11), 665. https://doi.org/10.3390/md20110665









