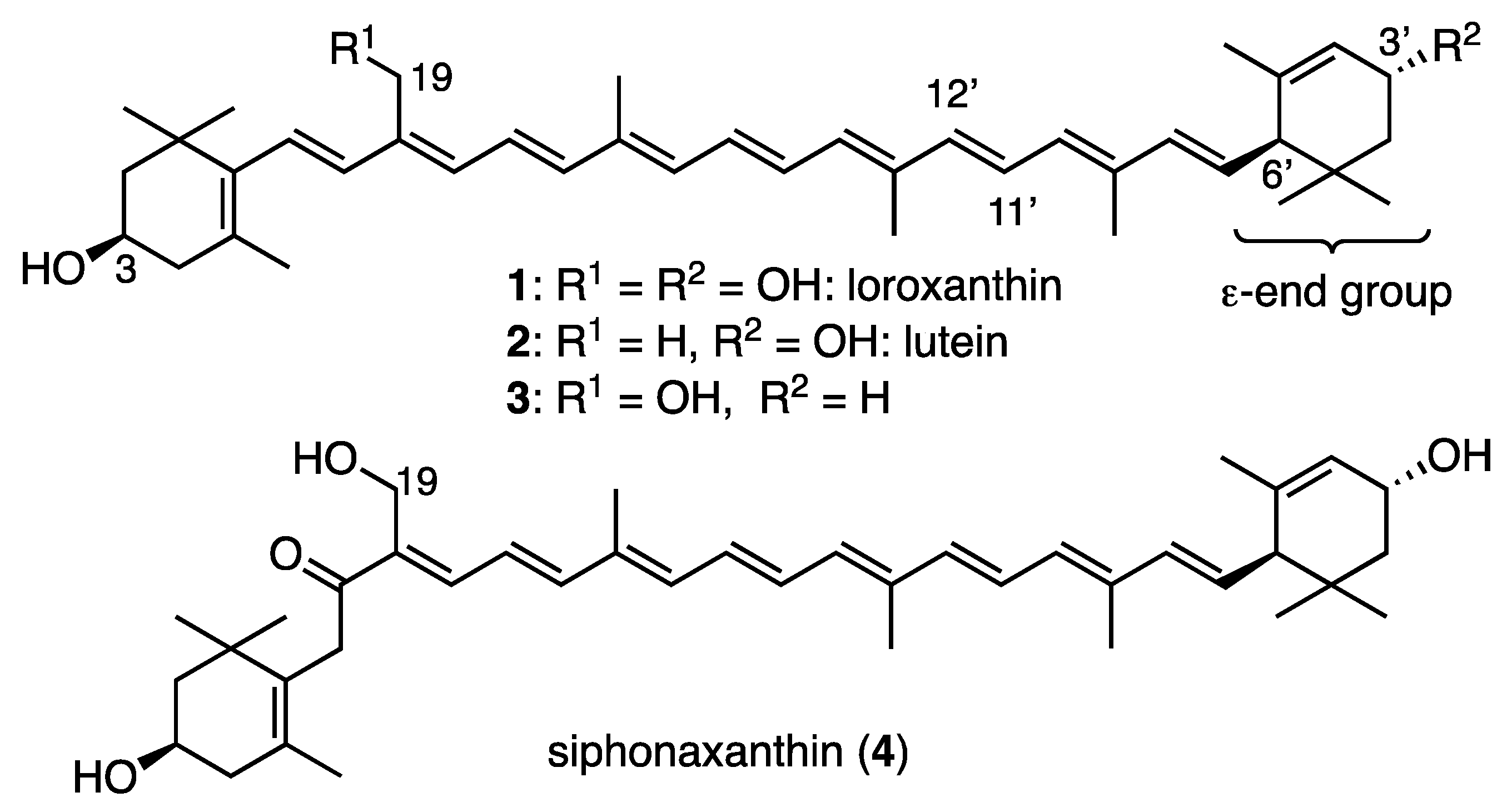
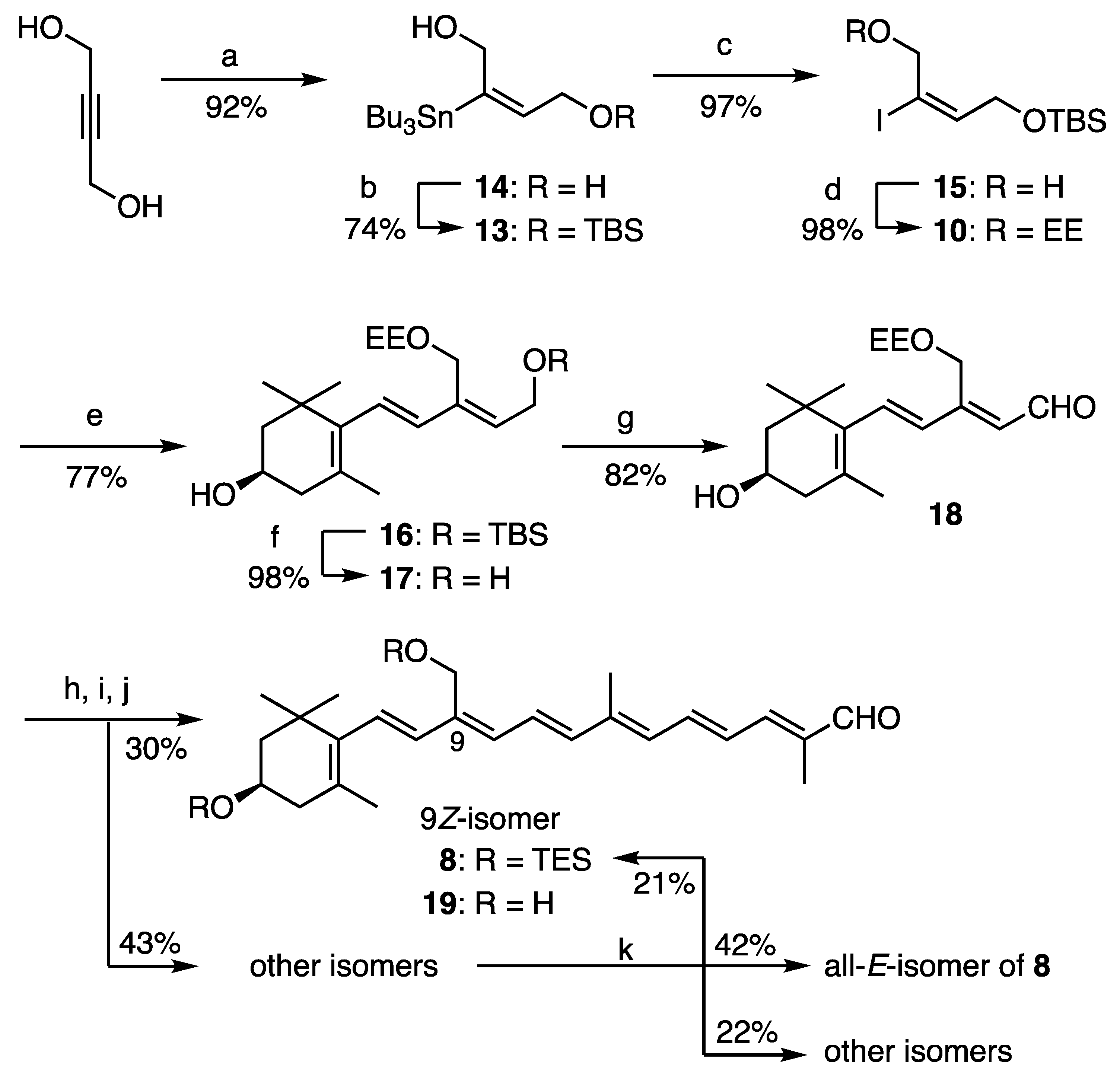
3.2. Synthesis of C25-Apocarotenal 8
(
E)-7-Iodo-2,2,3,3,10-pentamethyl-4,9,11-trioxa-3-silatridec-6-ene (
10). To a cooled (0 °C) solution of I
2 (3.79 g, 14.9 mmol) in dry CH
2Cl
2 (40 mL) was slowly added a solution of the alkenyl stananne
13 [
17] (7.00 g, 14.2 mmol) in dry CH
2Cl
2 (10 mL) and the reaction mixture was stirred at 0 °C for 10 min (min). 10% aq. Na
2S
2O
3 (20 mL) was added and the mixture was stirred at room temperature (rt) for 5 min. After CH
2Cl
2 was evaporated off, the mixture was diluted with AcOEt and washed brine, dried and evaporated. The residue was purified by flash CC [KF-SiO
2 (1:9), AcOEt-hexane, 15:85 to 2:8] to give the labile alkenyl iodide
15 (4.54 g, 97%) as a colorless oil: δ
H (300 MHz) 0.08 (6H, s, SiCH
3 × 2), 0.89 (9H, s,
tert-Bu), 2.52 (1H, t,
J 6.5, OH), 4.21 (2H, td,
J 0.5, 6.3, C
H2OTBS), 4.28 (2H, qd-like,
J 1, 6.6, C
H2OH), 6.45 (1H, tt,
J 1.5, 6.3, =CH); δ
C (75 MHz) –5.33 (C × 2), 18.23, 25.78 (C × 3), 61.19, 66.61, 104.67, 141.50.
To a cooled (0 °C) solution of the compound 15 (4.54 g, 13.8 mmol) and ethyl vinyl ether (4.13 mL, 41.5 mmol) in dry CH2Cl2 (40 mL) was added PPTS (174 mg, 0.69 mmol) and the reaction mixture was stirred at rt for 40 min. Saturated aq. NaHCO3 (20 mL) was added and the mixture was stirred at rt for 5 min. After CH2Cl2 was evaporated off, the mixture was diluted with AcOEt and washed brine, dried and evaporated. The residue was purified by flash CC (SiO2, AcOEt-hexane, 1:9) to give the EE-protected alkenyl iodide 10 (5.42 g, 98%) as a colorless oil: δH (300 MHz) 0.07 (6H, s, SiCH3 × 2), 0.89 (9H, s, tert-Bu), 1.22 (3H, t, J 7.2, OCH2CH3), 1.36 (3H, d, J 5.4, CHCH3), 3.53 and 3.68 (each 1H, qd, 7.2, 9.3, OCH2CH3), 4.22 (4H, m, OCH2 × 2), 4.77 (1H, q, J 5.4, CHCH3), 6.51 (1H, tt, J 1.5, 6.3, =CH); δC (75 MHz) –5.30 (C × 2), 15.26, 18.23, 19.67, 25.80 (C × 3), 60.51, 61.08, 67.02, 98.52, 99.46, 144.03; HRMS (ESI) m/z calcd for C14H29O3INaSi [M + Na]+ 423.0823, found 428.0823.
(1R)-4-[(1E,3Z)-5-tert-Butyldimethylsilyloxy-3-(1-ethoxyethoxymethyl)penta-1,3-dien-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol (16). To a degassed solution of the C11-alkenyl stananne 9 (910 mg, 2.00 mmol) and the alkenyl iodide 10 (960 mg, 2.40 mmol) in DMF (20 mL) were added CsF (608 mg, 4.00 mmol) and Pd(PPh3)4 (232 mg, 0.20 mmol) and CuI (76 mg, 0.40 mmol). After being stirred at 45 °C for 1.5 h (h), the mixture was diluted with water and extracted with AcOEt. The organic layer was washed with brine, dried and evaporated to give the residue, which was purified by flash CC [KF-SiO2 (1:9), AcOEt-hexane, 3:7] to give the coupling product 16 (673 mg, 77%) as a pale yellow oil: [α]D22 −64.6 (c 1.00, MeOH); νmax/cm−1 3606 and 3459 (OH); δH (300 MHz) 0.09 (6H, s, SiCH3 × 2), 0.91 (9H, s, tert-Bu), 1.05 (6H, s, gem-CH3), 1.22 (3H, t, J 7.2, OCH2CH3), 1.32 (3H, d, J 5.4, CHCH3), 1.46 (1H, t, J 12, 2-Hax), 1.71 (3H, br s, 5-CH3), 1.76 (1H, ddd, J 2, 3.5, 12, 2-Heq), 2.02 (1H, br dd, J 9.5, 16.5, 4-Hax), 2.36 (1H, br dd, J 6, 16.5, 4-Heq), 3.51 and 3.63 (each 1H, qd, 7.2, 9.3, OCH2CH3), 3.99 (1H, m, 3-H), 4.21 and 4.30 (each 1H, d, J 11.4, CH2OEE), 4.40 (2H, d, J 6.5, CH2OTBS), 4.73 (1H, q, J 5.4, CHCH3), 5.73 (1H, t, J 6.5, 10-H), 5.95 (1H, d, J 16, 8-H), 6.23 (1H, br d, J 16, 7-H); δC (75 MHz) –5.18 (C × 2), 15.31, 18.36, 19.68, 21.44, 25.94 (C × 3), 28.54 and 28.56 (split), 30.12, 37.02, 42.31, 48.27, 59.72, 59.91, 60.06, 65.01, 98.59, 125.87, 126.59 and 126.62 (split), 134.04, 134.70, 135.14 and 135.17 (split), 137.47; HRMS (ESI) m/z calcd for C25H46O4NaSi [M + Na]+ 461.3058, found 461.3062.
(1R)-4-[(1E,3Z)-3-(1-ethoxyethoxymethyl)-5-hydroxypenta-1,3-dien-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol (17). To a cooled (0 °C) solution of the compound 16 (1.90 g, 4.33 mmol) in dry THF (17 mL) was added TBAF (1.0 M in THF; 5.85 mL, 5.85 mmol) and the mixture was stirred at rt for 1 h. After being quenched by addition of saturated aq. NH4Cl, the mixture was extracted with AcOEt. The extracts were washed with brine, dried and evaporated to give the residue, which was purified by flash CC (SiO2, acetone-hexane, 1:2 to 2:3) to provide the alcohol 17 (1.38 g, 98%) as a pale brown oil: [α]D23 −83.8 (c 1.02, MeOH); νmax/cm−1 3608 and 3430 (OH); δH (300 MHz) 1.04 and 1.05 (each 3H, s, gem-CH3), 1.23 (3H, t, J 7.2, OCH2CH3), 1.34 (3H, d, J 5.5, CHCH3), 1.46 (1H, t, J 12, 2-Hax), 1.70 (3H, br s, 5-CH3), 1.76 (1H, ddd, J 2, 3.5, 12, 2-Heq), 2.02 (1H, br dd, J 9.5, 17, 4-Hax), 2.36 (1H, br dd, J 5.5, 17, 4-Heq), 3.55 and 3.63 (each 1H, qd, 7.2, 9.3, OCH2CH3), 3.98 (1H, m, 3-H), 4.22 and 4.33 (each 1H, dd, J 7, 13, CH2OH), 4.35 (2H, s, CH2OEE), 4.80 (1H, q, J 5.4, CHCH3), 5.91 (1H, t, J 7, 10-H), 5.96 (1H, d, J 16, 8-H), 6.29 (1H, br d, J 16, 7-H); δC (75 MHz) 15.15, 19.42, 21.40, 28.51 and 28.55 (split), 30.10, 36.98 and 37.01 (split), 42.23, 48.13, 58.35, 59.15, 59.60, 64.92, 97.82 and 97.87 (split), 126.11 and 126.14 (split), 127.53, 132.69, 134.96 and 135.02 (split), 136.84, 137.35; HRMS (ESI) m/z calcd for C19H32O4Na [M + Na]+ 347.2193, found 347.2196.
(2Z,4E)-3-(1-ethoxyethoxymethyl)-5-[(R)-4-hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl]penta-2,4-dienal (18). MnO2 (2.5 g) was added to a stirred solution of the alcohol 17 (490 mg, 1.51 mmol) in Et2O (15 mL) at rt. After being stirred at rt for 1.5 h, the mixture was filtered through a pad of Celite and the filtrate was concentrated. The resulting mixture was purified by flash CC (SiO2, acetone-hexane, 1:2) to give the aldehyde 18 (400 mg, 82%) as an yellow oil: [α]D25 −75.7 (c 1.00, MeOH); νmax/cm−1 3606 and 3462 (OH), 1663 (conj. CO), 1609 (C=C); δH (300 MHz) 1.09 (6H, br s, gem-CH3), 1.22 (3H, t, J 7.2, OCH2CH3), 1.37 (3H, d, J 5.4, CHCH3), 1.49 (1H, t, J 12, 2-Hax), 1.75 (3H, br s, 5-CH3), 1.78 (1H, ddd, J 2, 3.5, 12, 2-Heq), 2.06 (1H, br dd, J 9.5, 17.5, 4-Hax), 2.41 (1H, br dd, J 5.5, 17.5, 4-Heq), 3.51 and 3.62 (each 1H, qd, J 7, 9, OCH2CH3), 4.00 (1H, m, 3-H), 4.67 (2H, s, CH2OEE), 4.80 (1H, q, J 5.4, CHCH3), 6.01 (1H, d, J 8, 10-H), 6.13 (1H, d, J 16, 8-H), 6.80 (1H, br d, J 16, 7-H), 10.18 (1H, d, J 8, CHO); δC (75 MHz) 15.26, 19.62, 21.57, 28.69, 30.12, 37.07, 42.51, 48.21, 59.15, 60.57, 64.72, 99.13, 129.40, 129.86, 133.48, 135.79, 136.96, 153.14, 191.55; HRMS (ESI) m/z calcd for C19H30O4Na [M + Na]+ 345.2036, found 345.2039.
2,7-Dimethyl-11-triethylsilyloxymethyl-13-[(
R)-2,6,6-trimethyl-4-triethylsilyloxycyclohex-1-en-1-yl]trideca-2,4,6,8,10,12-hexaenal (
8). An acidic solution (1.5 mL) prepared from
p-TsOH (500 mg) and H
3PO
4 (725 mg) in MeOH (40 mL) and methyl orthoformate (1.90 mL, 17.4 mmol) was added to a solution of the C
10-phosphonium chloride
11 [
15] (2.58 g, 5.78 mmol) in THF (2 mL) and MeOH (20 mL). The reaction mixture was stirred at rt for 2 h and neutralized with NaOMe (28% in MeOH) until just before the red color of an ylide appeared to give a solution of the Wittig salt
12. To this solution were added a solution of the aldehyde
18 (790 mg, 2.45 mmol) in CH
2Cl
2 (5 mL) and NaOMe (28% in MeOH; 1.67 mL, 8.65 mmol) at rt. After being stirred at rt for 30 min, the mixture was poured into saturated aq. NH
4Cl and extracted with AcOEt. The extracts were washed with brine and concentrated. The resulting mixture were dissolved in THF (25 mL) and MeOH (5 mL) and 5% aq. HCl was added to it. After being stirred at rt for 20 min, the mixture was diluted with AcOEt and washed with brine, dried and evaporated. The resulting residue was purified by flash CC (SiO
2, MeOH-acetone-CH
2Cl
2, 2:20:80) to provide the isomeric mixture of the dihydroxy apocarotenal
19, a part of which was purified by preparative HPLC (COSMOSIL 5SL-II 2 × 25 cm (Nacalai tesque, Kyoto, Japan); MeOH-AcOEt-hexane, 1.5:30:60) to provide the pure 9
Z-apocarotenal of
19 as orange foam.
A solution of the above isomeric mixture of 19, Et3N (1.76 mL, 12.2 mmol) and dimethylaminopyridine (DMAP) (19 mg, 0.16 mmol) in dry CH2Cl2 (30 mL) was added TESCl (1.27 mL, 7.6 mmol) at 0 °C and the mixture was stirred at rt for 20 min. After addition of saturated aq NaHCO3 (5 mL), CH2Cl2 was evaporated off and the resulting mixture was diluted with AcOEt and washed with brine, dried and evaporated. The residue was purified by flash CC (SiO2, AcOEt-hexane, 1:4) and then preparative HPLC (COSMOSIL 5SL-II 2 × 25 cm (Nacalai tesque, Kyoto, Japan); AcOEt-hexane, 6:94) to provide the 9Z-isomer of di-TES-apocarotenal 8 (445 mg, 30% from 18) and the other isomeric mixture of 8 (642 mg, 43% from 18) as an orange foam, respectively.
To a solution of the latter isomeric mixture (642 mg) of 8 in MeCN (40 mL) was added to a solution of PdCl2(MeCN)2 (13 mg), Et3N (7 μL) in MeCN (8.8 mL) and water (1.2 mL). After being stirred at rt for 3.5 h, the mixture was concentrated and purified by flash CC (SiO2, AcOEt-hexane, 1:4) and then preparative HPLC (COSMOSIL 5SL-II 2 × 25 cm (Nacalai tesque, Kyoto, Japan); AcOEt-hexane, 6:94) to provide the 9Z-isomer of apocarotenal 8 (136 mg, 21%) and the all-E-isomer of 8 (271 mg, 42%).
9Z-Isomer of 19: λmax(EtOH)/nm 422; νmax/cm−1 3608 and 3454 (OH), 1658 (conj. CO), 1610, 1597 and 1548 (C=C); δH (500 MHz) 1.087 and 1.089 (each 3H, s, gem-CH3), 1.49 (1H, t, J 12, 2-Hax), 1.76 (3H, br s, 5- CH3), 1.78 (1H, ddd, J 2, 3.5, 12, 2-Heq), 1.89 (3H, br s, 13′-CH3), 2.04 (3H, br s, 13-CH3), 2.06 (1H, br dd, J 10, 17, 4-Hax), 2.40 (1H, br dd, J 5.5, 17, 4-Heq), 4.01 (1H, m, 3-H), 4.57 (2H, s, CH2OH), 6.07 (1H, d, J 16, 8-H), 6.26 (1H, d, J 12, 10-H), 6.34 (1H, br d, J 11.5, 14-H), 6.41 (1H, br d, J 16, 7-H), 6.45 (1H, d, J 15, 12-H), 6.71 (1H, dd, J 12, 14.5, 15′-H), 6.86 (1H, dd, J 12, 15, 11-H), 6.96 (1H, br d, J 11.5, 14′-H), 7.02 (1H, dd, J 12, 14.5, 15-H), 9.46 (1H, s, CHO); δC (125 MHz) 9.64 (13′-CH3), 13.11 (13-CH3), 21.68 (5-CH3), 28.77 and 30.31 (gem-CH3), 37.15 (C1), 42.57 (C4), 48.40 (C2), 57.41 (CH2OH), 64.99 (C3), 126.11 (C11), 127.06 (C5), 127.68 (C7), 128.09 (C15′), 132.07 (C14), 132.73 (C10), 135.25 (C8), 137.33 (C13′), 137.37 (C15), 137.57 (C6), 138.90 (C12), 138.96 (C9), 141.15 (C13), 148.61 (C14′), 194.48 (CHO); HRMS (ESI) m/z calcd for C25H35O3 [M+H]+ 383.2586, found 383.2587.
9Z-Isomer of 8: λmax(EtOH)/nm 420; νmax/cm−1 1661 (conj. CO), 1610, 1596 and 1548 (C=C); δH (500 MHz) 0.62 and 0.66 (each 6H, q, J 8, SiCH2 × 6), 0.981 and 0.986 (each 9H, t, J 8, SiCH2CH3 × 6), 1.06 and 1.07 (each 3H, s, gem-CH3), 1.51 (1H, t, J 12, 2-Hax), 1.67 (1H, ddd, J 2, 3.5, 12, 2-Heq), 1.73 (3H, br s, 5-CH3), 1.88 (3H, br s, 13′-CH3), 2.03 (3H, br s, 13-CH3), 2.10 (1H, br dd, J 10, 17, 4-Hax), 2.25 (1H, br dd, J 5, 17, 4-Heq), 3.95 (1H, m, 3-H), 4.54 (2H, s, CH2O), 6.04 (1H, d, J 16, 8-H), 6.21 (1H, d, J 12, 10-H), 6.32 (1H, br d, J 11.5, 14-H), 6.39 (1H, br d, J 16, 7-H), 6.40 (1H, d, J 15, 12-H), 6.70 (1H, dd, J 11.5, 14, 15′-H), 6.91 (1H, dd, J 12, 15, 11-H), 6.96 (1H, br d, J 11.5, 14′-H), 7.02 (1H, dd, J 11.5, 14, 15-H), 9.46 (1H, s, CHO); δC (125 MHz) 4.50 (C × 3), 4.93 (C × 3), 6.84 (C × 3), 6.87 (C × 3), 9.60, 12.95, 21.63, 28.62, 30.19, 37.14, 43.22, 48.93, 57.81, 65.31, 127.11, 127.27, 127.68, 128.11, 131.49, 131.67, 135.46, 137.06, 137.52, 137.60, 137.86, 139.62, 141.43, 148.75, 194.46; HRMS (ESI) m/z calcd for C37H63O3Si2 [M + H]+ 611.4310, found 611.4319.
all-E-Isomer of 8: λmax(EtOH)/nm 417; νmax/cm−1 1660 (conj. CO), 1610, 1602 and 1555 (C=C); δH (500 MHz) 0.63 and 0.66 (each 6H, q, J 8, SiCH2 × 6), 0.987 and 0.991 (each 9H, t, J 8, SiCH2CH3 × 6), 1.06 and 1.08 (each 3H, s, gem-CH3), 1.53 (1H, t, J 12, 2-Hax), 1.69 (1H, ddd, J 2, 3.5, 12, 2-Heq), 1.76 (3H, br s, 5-CH3), 1.89 (3H, br s, 13′-CH3), 2.04 (3H, br s, 13-CH3), 2.11 (1H, br dd, J 9.5, 17, 4-Hax), 2.28 (1H, br dd, J 5.5, 17, 4-Heq), 3.97 (1H, m, 3-H), 4.42 (2H, s, CH2O), 6.21 (1H, br d, J 16, 7-H), 6.32 (1H, br d, J 12, 14-H), 6.35 (1H, br d, J 12, 10-H), 6.41 (1H, d, J 15, 12-H), 6.50 (1H, d, J 16, 8-H), 6.69 (1H, dd, J 12, 14.5, 15′-H), 6.87 (1H, dd, J 12, 15, 11-H), 6.96 (1H, br d, J 12, 14′-H), 7.03 (1H, dd, J 11.5, 14, 15-H), 9.46 (1H, s, CHO); δC (125 MHz) 4.50 (C × 3), 4.93 (C × 3), 6.83 (C × 3), 6.87 (C × 3), 9.58, 13.10, 21.70, 28.62, 30.22, 37.04, 43.13, 48.78, 63.81, 65.27, 126.18, 126.99, 127.36, 127.50, 127.95, 128.18, 131.26, 136.97, 137.41, 137.60, 137.81, 138.31, 141.46, 148.81, 194.43; HRMS (ESI) m/z calcd for C37H63O3Si2 [M+H]+ 611.4310, found 611.4320.
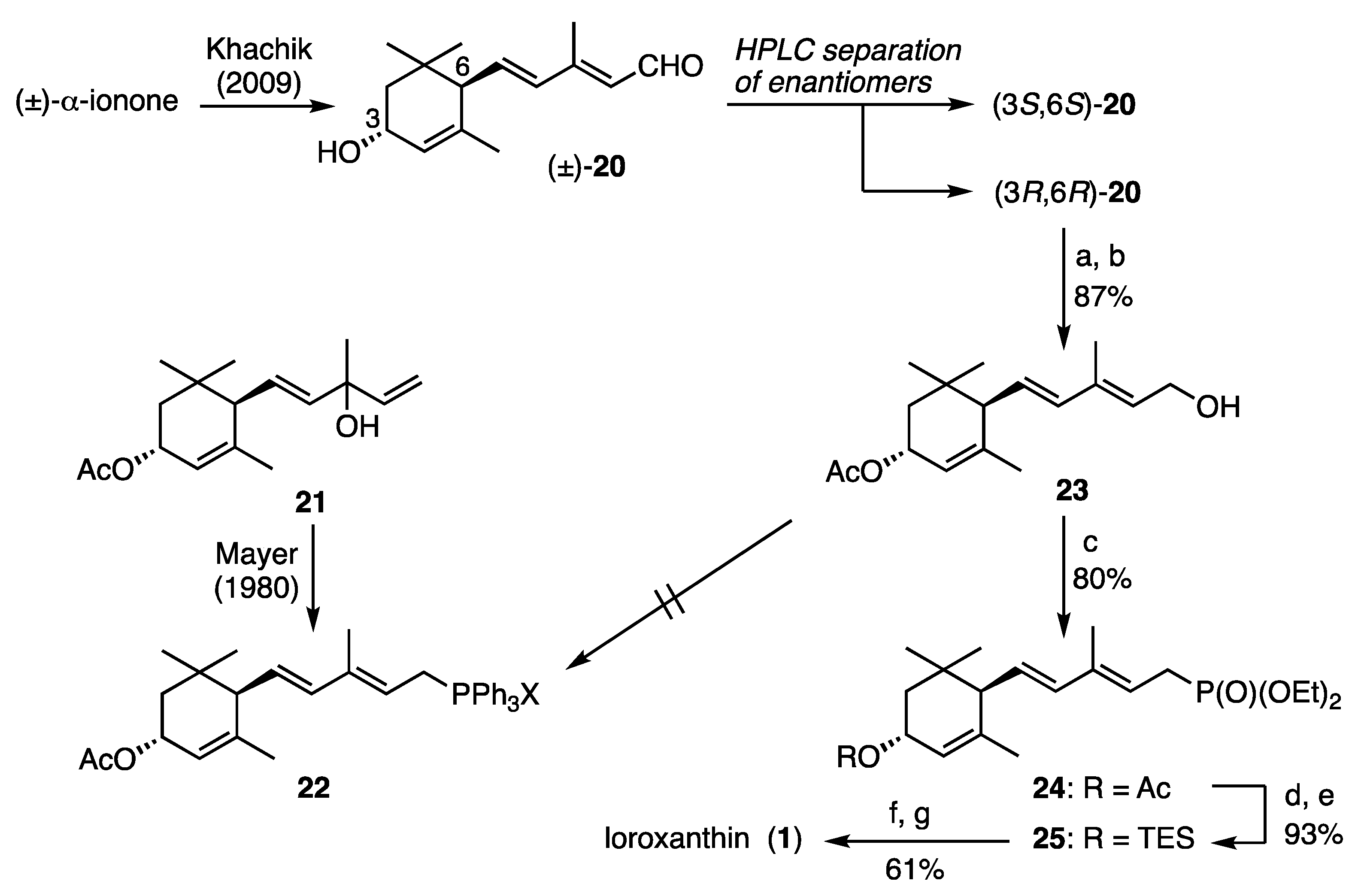
3.3. Synthesis of C15-Phosphonate 25
(3
R,6
R)-3-Hydroxy C
15-aldehyde
20. According to the Khachik’s method, racemic 3-hydroxy C
15-aldehyde
20 was synthesized starting from commercially available α-ionone and its enantiomers were directly separated using a semi-preparative chiral HPLC column [CHIRALPAK IF 2.0 × 25 cm (Daicel, Tokyo, Japan)] as shown in
Figure 2b. Spectral data of the resulting enantiomers were identical with those reported [
13].
(1R,4R)-4-[(1E,3E)-5-hydroxy-3-methylpenta-1,3-dien-1-yl]-3,5,5-trimethylcyclohex-2-en-1-yl acetate (23). Ac2O (1.22 mL, 12.9 mmol) was added dropwise to a stirred solution of the (3R,6R)-aldehyde 20 (1.01 g, 4.3 mmol) in dry CH2Cl2 (18 mL), Et3N (3 mL, 21.5 mmol) and DMAP (0.05 g, 0.43 mmol) at rt for 15 min. The resulting mixture was poured into saturated aq. NaHCO3 and extracted with AcOEt and washed with brine. The organic layer was dried and evaporated to give the crude aldehyde, which was dissolved in MeOH (16 mL) and NaBH4 (0.165 g) was added to it at 0 °C. After being stirred at 0 °C for 10 min, the reaction was quenched by addition of saturated aq. NH4Cl. The resulting mixture was evaporated and the mixture was extracted with AcOEt and washed with brine, dried and evaporated to give a residue, which was purified flash CC (acetone-hexane, 3:7) to provide the allylic alcohol 23 (1.05 g, 87 % from 20) as a pale yellow viscous oil: [α]24D +335.4 (c 1.08, CHCl3); νmax/cm−1 3610 and 3447 (OH), 1720 (CO), 1623 and 1645 (C=C); δH (300 MHz) 0.87 and 0.99 (each 3H, s, gem-CH3), 1.45 (1H, dd, J 5.5, 14, 2-H), 1.64 (3H, br s, 5-CH3), 1.78 (3H, br s, 9-CH3), 1.83 (1H, dd, J 5.5, 14, 2-H), 2.04 (3H, s, CH3COO), 2.38 (1H, br d, J 9.5, 6-H), 4.28 (2H, d, J 7, 11-H2), 5.32 (1H, m, 3-H), 5.41 (1H, dd, J 9.5, 15.5, 7-H), 5.49 (1H, m, 4-H), 5.62 (1H, br t, J 7, 10-H), 6.08 (1H, d, J 15.5, 8-H); δC (75 MHz) 12.71, 21.41, 22.85, 25.13, 28.86, 33.23, 39.45, 54.55, 59.19, 68.80, 119.98, 128.54, 128.77, 135.90, 136.75, 140.21, 170.92; HRMS (ESI) m/z calcd for C17H26O3Na [M + Na]+ 301.1774, found 301.1774.
(1R,4R)-4-[(1E,3E)-5-diethoxyphosphoryl-3-methylpenta-1,3-dien-1-yl]-3,5,5-trimethylcyclohex-2-en-1-yl acetate (24). To a stirred suspension of ZnI2 (1.69 g, 5.29 mmol) in dry THF (2.6 mL) was added dropwise P(OEt)3 (1.84 mL, 10.6 mmol) and a solution of the alcohol 23 (0.98 g, 3.52 mmol) in dry THF (5.0 mL) at rt. After being stirred at 85 °C for 30 min, the resulting mixture was diluted with H2O and AcOEt. The resulting mixture was filtered through a pad of Celite and the resulting mixture was poured into saturated aq. NaHCO3 and extracted with AcOEt. The extracts were washed with brine, dried and evaporated to give a residue, which was purified by flash CC (AcOEt-hexane, 3:7) to provide phosphonate 24 (1.13 g, 81%) as a colorless oil: [α]24D +251.1 (c 0.93, CHCl3); νmax/cm−1 1720 (CO); δH (300 MHz) 0.86 and 0.99 (each 3H, s, gem-CH3), 1.31 (6H, t, J 7, OCH2CH3 × 2), 1.45 (1H, dd, J 5.5, 13.5, 2-H), 1.63 (3H, br s, 5-CH3), 1.77 (3H, dd, J 1, 4, 9-CH3), 1.83 (1H, dd, J 6, 13.5, 2-H), 2.04 (3H, s, CH3COO), 2.36 (1H, br d, J 9.5, 6-H), 2.76 (2H, dd, J 8, 23, CH2P), 4.04–4.17 (4H, m, OCH2 × 2), 5.31 (1H, m, 3-H), 5.35 (1H, ddd, J 2, 9.5, 15.5, 7-H), 6.09 (1H, dd, J 1, 15.5, 8-H); δC (75 MHz) 12.71 (Jcp 2.3), 16.36 (Jcp 6.3, C × 2), 21.38, 22.83, 25.12, 26.74 (Jcp 139.4), 28.85, 33.19 (Jcp 1.7), 39.43, 54.51, 61.85 (Jcp 6.8, C × 2), 68.75, 118.40 (Jcp 11.9), 119.93, 127.53 (Jcp 4.0), 136.65 (Jcp 5.1), 137.38 (Jcp 14.9), 140.24, 170.81; HRMS (ESI) m/z calcd for C21H35O5NaP [M + Na]+ 421.2114, found 421.2114.
Diethyl {(2E,4E)-3-methyl-5-[(1R,4R)-2,6,6-trimethyl-4-triethylsilyloxycyclohex-2-en-1-yl]penta-2,4-dien-1-yl}phosphonate (25). To a solution of phosphonate 24 (0.83 g, 2.09 mmol) was added MeOH (25 mL), and NaOMe (28% NaOMe, 1.6 mL, 8.36 mmol) at rt and the mixture was stirred at rt for 30 min. The resulting mixture was poured into saturated aq. NH4Cl and extracted with AcOEt. The extracts were washed with brine, dried and evaporated to give the crude alcohol. After TESCl (0.45 mL, 2.68 mmol) was added dropwise to a stirred solution of the crude alcohol (0.796 g, 2.23 mmol) in dry CH2Cl2 (9 mL), Et3N (0.93 mL, 6.69 mmol) and DMAP (13.6 mg, 0.11 mmol) at 0 °C. After being stirred at 0 °C for 15 min. The resulting mixture was evaporated and the mixture was extracted with AcOEt and washed with brine, dried and evaporated to give a residue, which was purified flash CC (acetone-hexane, 3:7) to provide TES-protected phosphonate 25 (0.915 g, 93% from 24) as a colorless oil: [α]24D +137.5 (c 1.00, CHCl3); δH (300 MHz) 0.62 (6H, q, J 7.5, SiCH2CH3 × 3), 0.82 and 0.94 (each 3H, s, gem-CH3), 0.98 (9H, t, J 7.5, SiCH2CH3 × 3), 1.31 (6H, t, J 7 Hz, OCH2CH3 × 2), 1.39 (1H, dd, J 8, 13, 2-H), 1.57 (3H, br s, 5-CH3), 1.70 (1H, dd, J 6, 13, 2-H), 1.77 (3H, br d, J 4, 9-CH3), 2.38 (1H, br d, J 10, 6-H), 2.70 (2H, dd, J 8, 23, CH2P), 4.04–4.16 (4H, m, OCH2 × 2), 4.25 (1H, m, 3-H), 5.35 (1H, ddd, J 2, 10, 15.5, 7-H), 5.43 (1H, br s, 4-H), 6.08 (1H, d, J 15.5, 8-H); δC (75 MHz) 4.87 (C × 3), 6.84 (C × 3), 12.75 (Jcp 2.3), 16.39 (Jcp 6.2, C × 2), 22.76, 23.36, 26.77 (Jcp 138.9), 29.53, 34.16 (Jcp 1.1), 45.66, 54.46, 61.86 (Jcp 6.8, C × 2), 66.05, 117.99 (Jcp 12.0), 125.69, 128.27 (Jcp 4.0), 136.48, 136.73 (Jcp 5.1), 137.49 (Jcp 14.3); HRMS (ESI) m/z calcd for C25H47O4NaPSi [M + Na]+ 493.2874, found 493.2865.
3.4. Synthesis of Loroxanthin (1)
To a solution of the TES-protected apocarotenal 8 (245 mg, 0.40 mmol) and the TES-protected phosphonate 25 (226 mg, 0.48 mmol) in dry THF (15 mL) were added NaN(TMS)2 (1.0 M in THF; 0.96 mL, 0.96 mmol) at −40 °C. After being stirred at −40 °C for 30 min, the mixture was poured into saturated aq. NH4Cl and extracted with AcOEt. The organic layer was washed with brine, dried and evaporated to give the residue was purified by flash CC (AcOEt-hexane, 8:92) to give the condensed product (247 mg, 67%) as a red viscous oil. This was dissolved in dry THF (10 mL) and TBAF (1.0 M in THF; 1.04 mL, 1.04 mmol) was added to it at rt. After being stirred at rt for 15 min, the mixture was poured into saturated aq. NH4Cl and extracted with AcOEt. The organic layer was washed with brine, dried and evaporated to give the residue was purified by flash CC (MeOH-CH2Cl2-acetone, 2:85:15 to 3:80:15) to give loroxanthin (1) (143 mg, 61% from apocarotenal 8) as red solids: λmax(EtOH)/nm 268, 423sh, 447, 476; δH (500 MHz) 0.85 and 1.00 (6H, s, 1′-gem-CH3), 1.08 (6H, s, 1-gem-CH3), 1.37 (1H, dd, J 6.5, 13, 2′-H), 1.48 (1H, t, J 12, 2-Hax), 1.63 (3H, br s, 5′-CH3), 1.75 (3H, br s, 5-CH3),1.77 (1H, overlapped, 2-Heq), 1.84 (1H, dd, J 6, 13, 2′-H), 1.91 (3H, br s, 9′-CH3), 1.97 (6H, br s, 13-CH3 and 13′-CH3), 2.05 (1H, br dd, J 9, 17, 4-Hax), 2.39 (1H, br dd, J 5.5, 17, 4-Heq), 2.41 (1H, br d, J 10, 6′-H), 4.00 (1H, m, 3-H), 4.25 (1H, m, 3′-H), 4.55 (2H, br s, 9-CH2), 5.44 (1H, dd, J 10, 15, 7′-H), 5.55 (1H, br s, 4′-H), 6.05 (1H, d, J 16, 8-H), 6.14 (1H, br d, J 12, 10′-H), 6.14 (1H, d, J 15, 8′-H), 6.24 (1H, d, J 11.5, 10-H), 6.26 (1H, br d, J 10, 14′-H), 6.29 (1H, br d, J 10, 14-H), 6.34 (1H, br d, J 16, 7-H), 6.36 (1H, d, J 14.5, 12′-H), 6.43 (1H, d, J 14.5, 12-H), 6.62 (1H, dd, J 12, 14.5, 11′-H), 6.61–6.66 (2H, m, 15-H and 15′-H), 6.71 (1H, dd, J 11.5, 14.5, 11-H); δC (125 MHz) 12.82 (13-CH3 and 13′-CH3), 13.10 (9′-CH3), 21.66 (5-CH3), 22.85 (5′-CH3), 24.25 (1′-CH3), 28.74 (1-CH3), 29.49 (1′-CH3), 30.29 (1-CH3), 34.02 (C1′), 37.13 (C1), 42.54 (C4), 44.63 (C2′), 48.39 (C2), 54.95 (C6′), 57.40 (9-CH2), 65.02 (C3), 65.90 (C3′), 123.47 (C11), 124.50 (C4′), 125.07 (C11′), 126.52 (C7 or C12′), 126.62 (C5), 128.85 (C7′), 129.83 (C15 or C15′), 130,74 and 130.76 (C10′ and C15 or C15′), 132.41 (C14′), 133.31 (C10), 133.76 (C14), 135.26 (C9′), 135.46 (C8), 136.04 (C13), 136.90 (C13′), 137.24 (C9), 137.46 (C7 or C12′), 137.65 (C6), 137.69 (C8′), 137.93 (C5′), 139.86 (C12); HRMS (APCI) m/z calcd for C40H55O3 [M − H]− 583.4157, found 583.4151.