Quinoid Pigments of Sea Urchins Scaphechinus mirabilis and Strongylocentrotus intermedius: Biological Activity and Potential Applications
Abstract
1. Introduction
2. Results
2.1. Sea Urchins—S. mirabilis and S. intermedius
2.1.1. Flat Sea Urchin—S. mirabilis
2.1.2. Gray Sea Urchin—S. intermedius
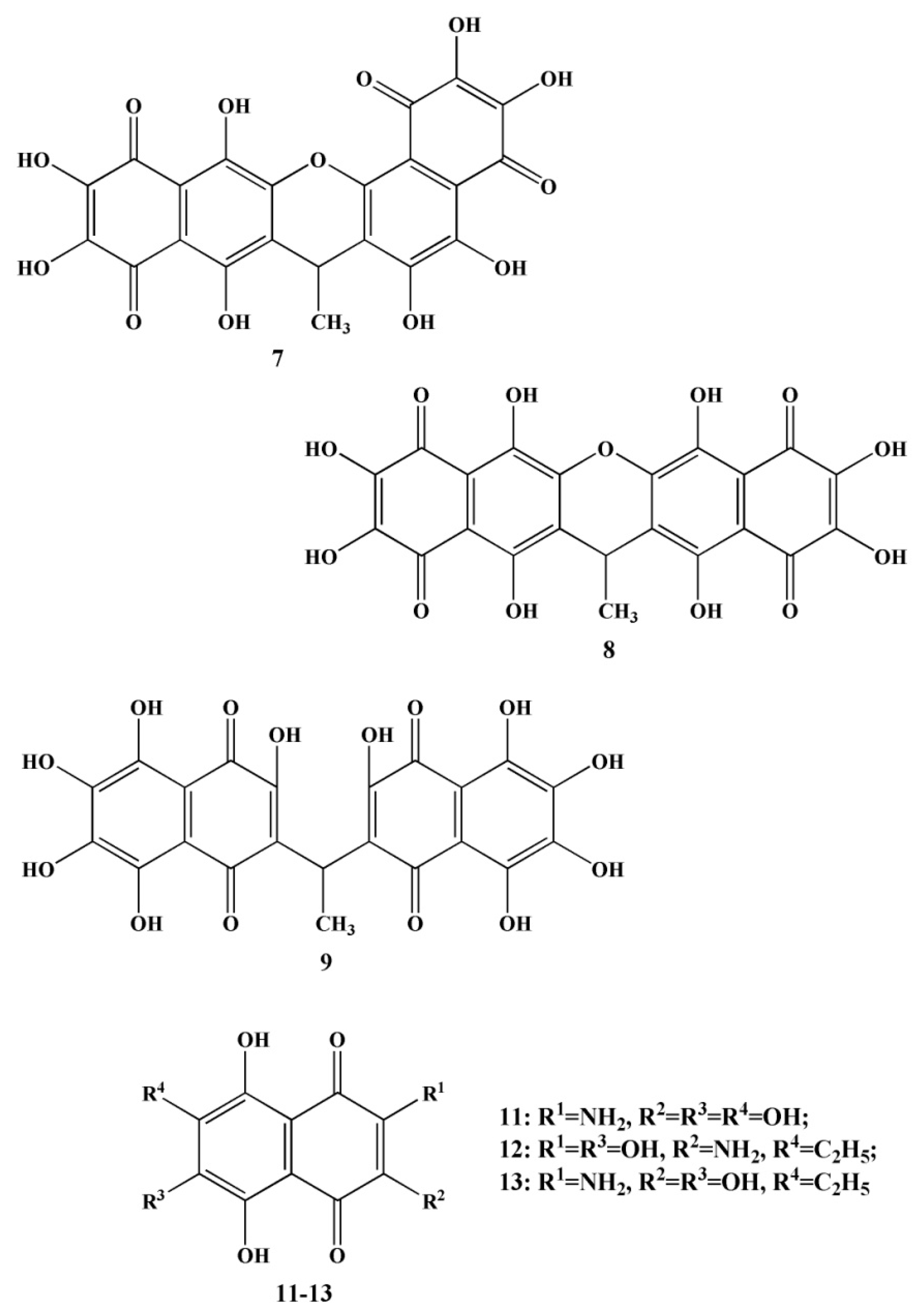
2.2. Naphthoquinoid Pigments of Sea Urchins
| Naphthoquinone Pigments | Family Species | |
|---|---|---|
| Strongylocentrotus intermedius | Scaphechinus mirabilis | |
| Content of Main Spinochromes, % of Pigment Sum | ||
| Echinochrome A | - | 89.1 ± 8.7 |
| Spinochrome A | 3.3 ± 0.3 | - |
| Spinochrome B | 4.0 ± 1.3 | - |
| Spinochrome C | 9.2 ± 1.5 | - |
| Spinochrome D | 16.5 ± 5.7 | 1.8 ± 0.9 |
| Spinochrome E | 3.1 ± 1.6 | - |
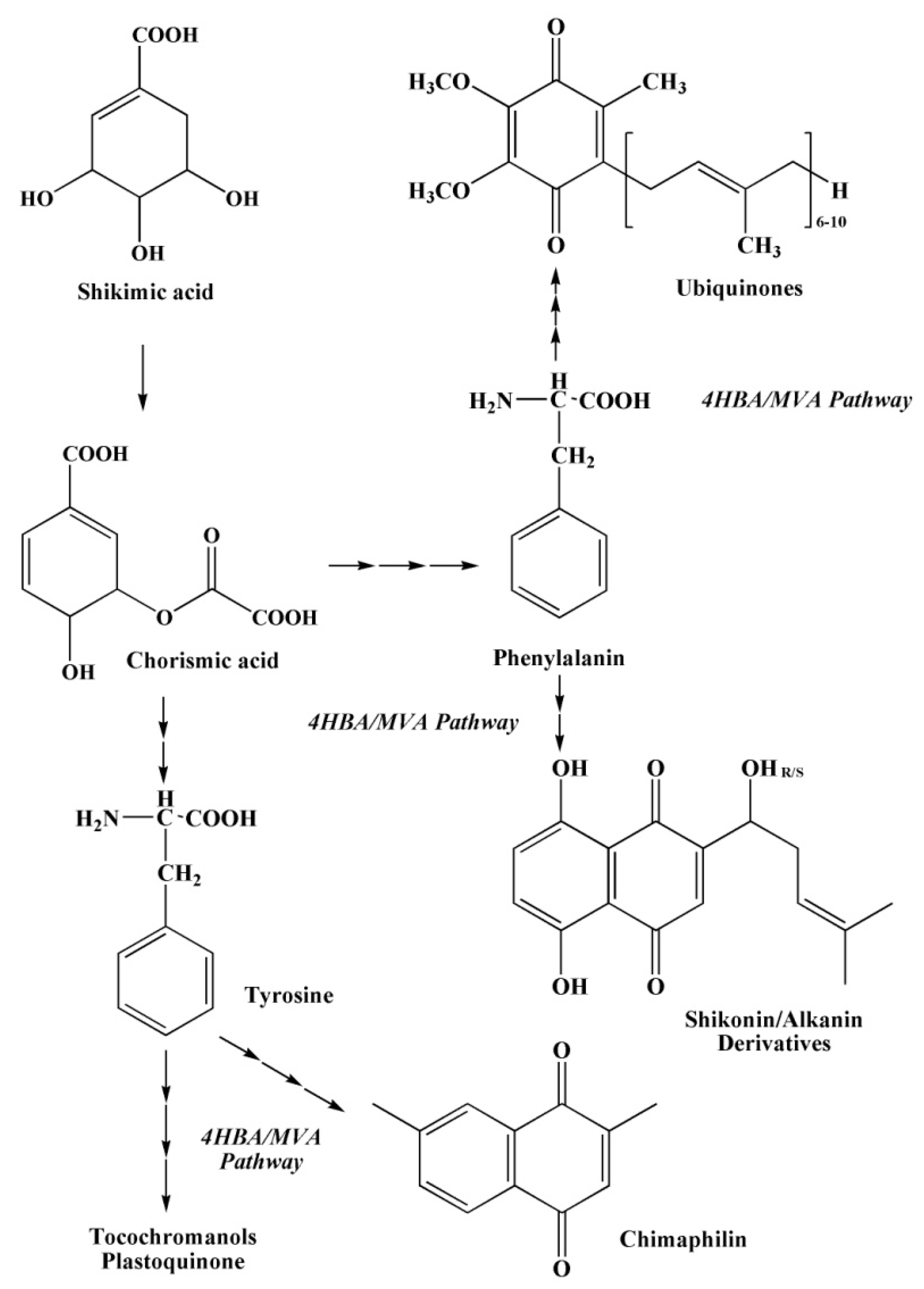
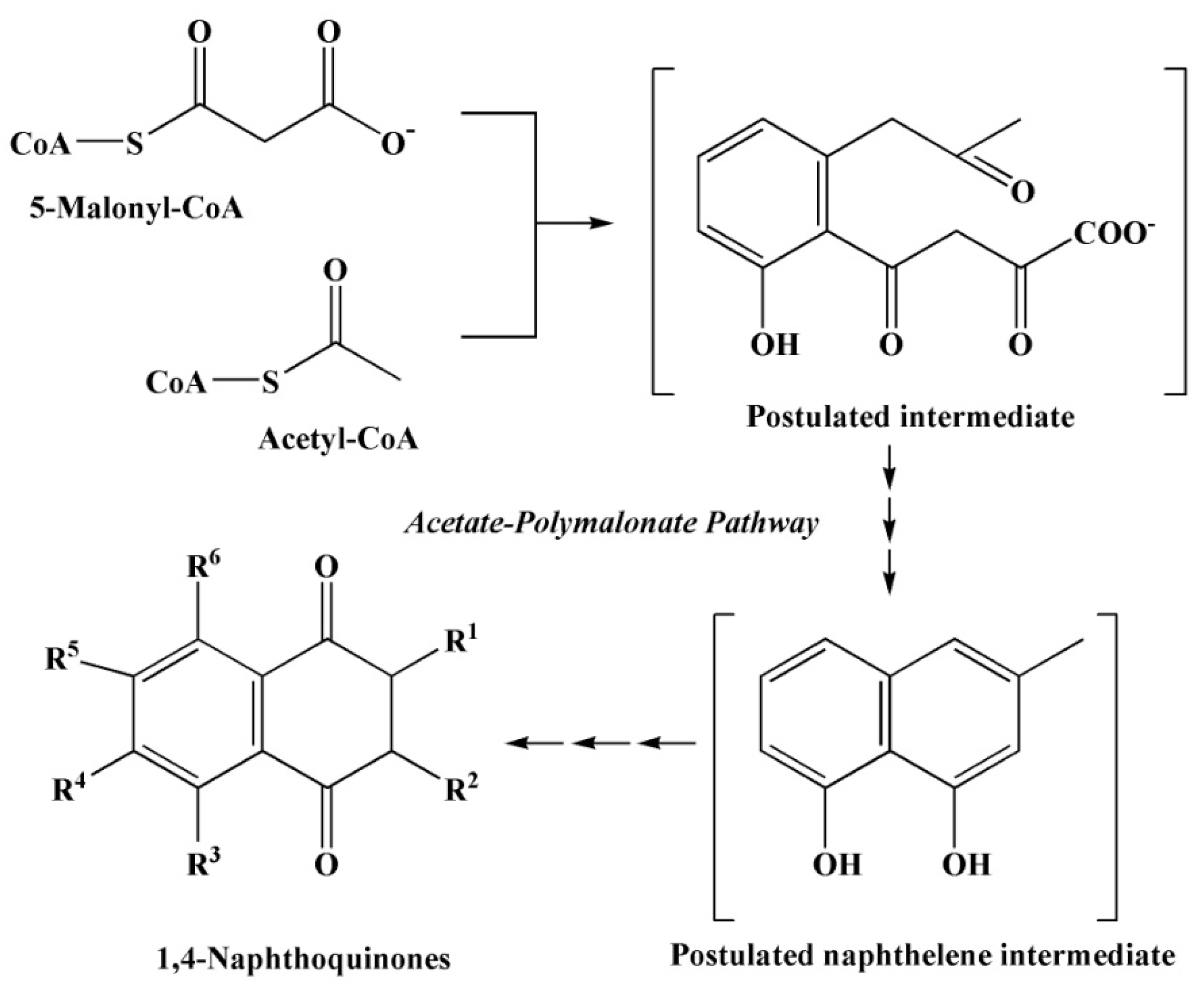
2.3. The Main Ways of Biosynthesis of Quinoid Pigment Compounds
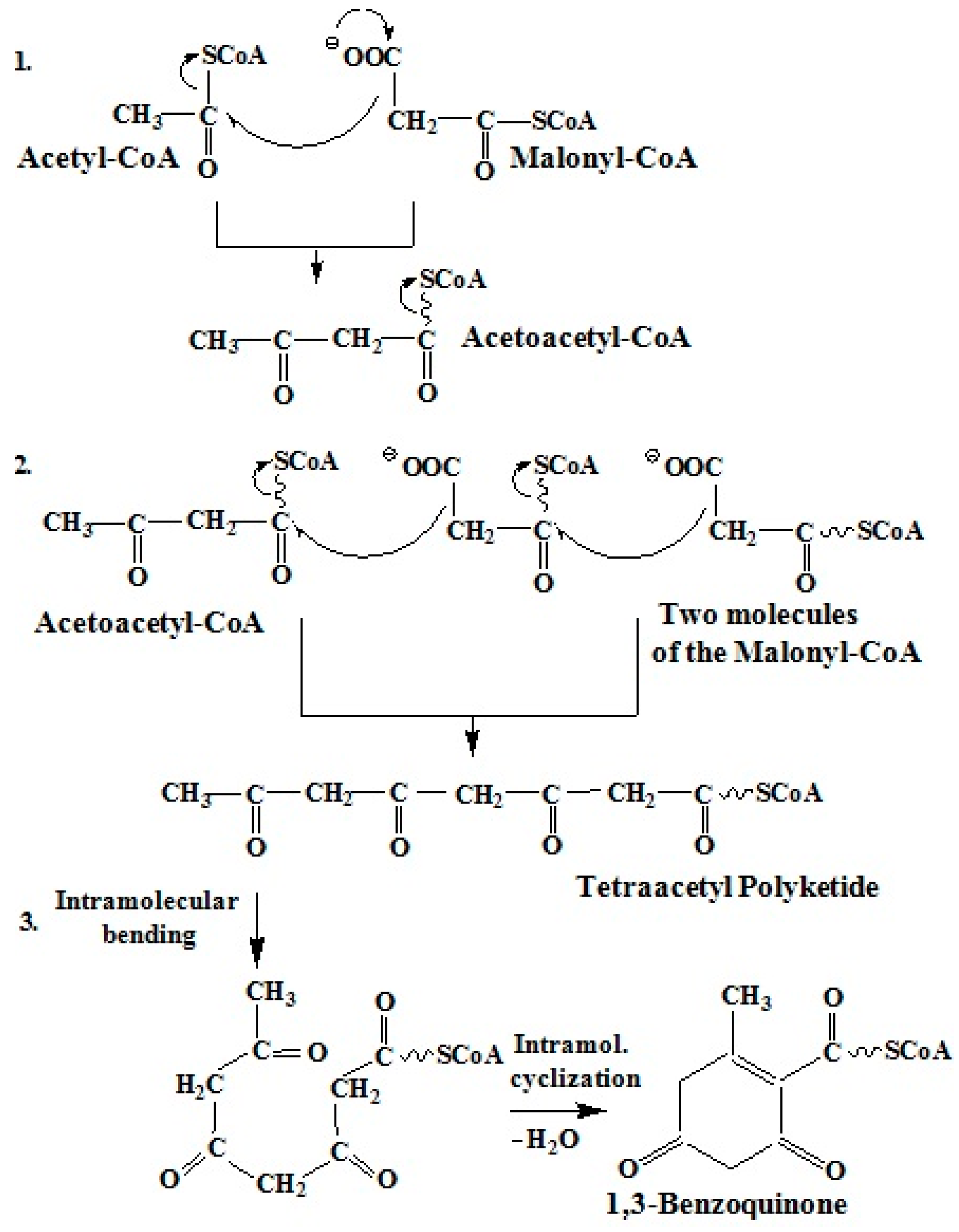
2.3.1. Polyketide Pathway of Quinone Biosynthesis
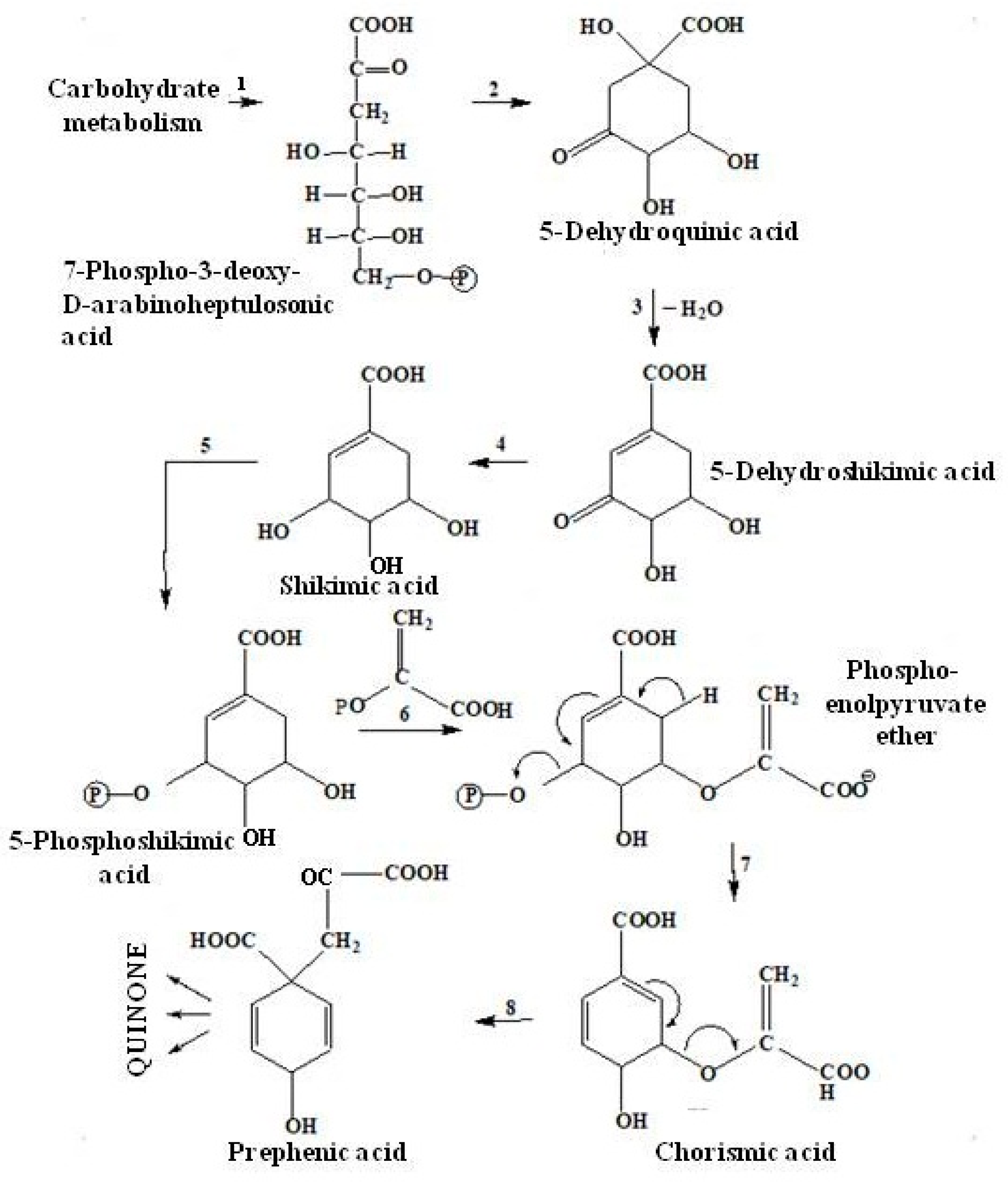
2.3.2. The Shikimate Pathway of Quinone Biosynthesis
2.3.3. The Mevalonate Pathway of Quinone Biosynthesis
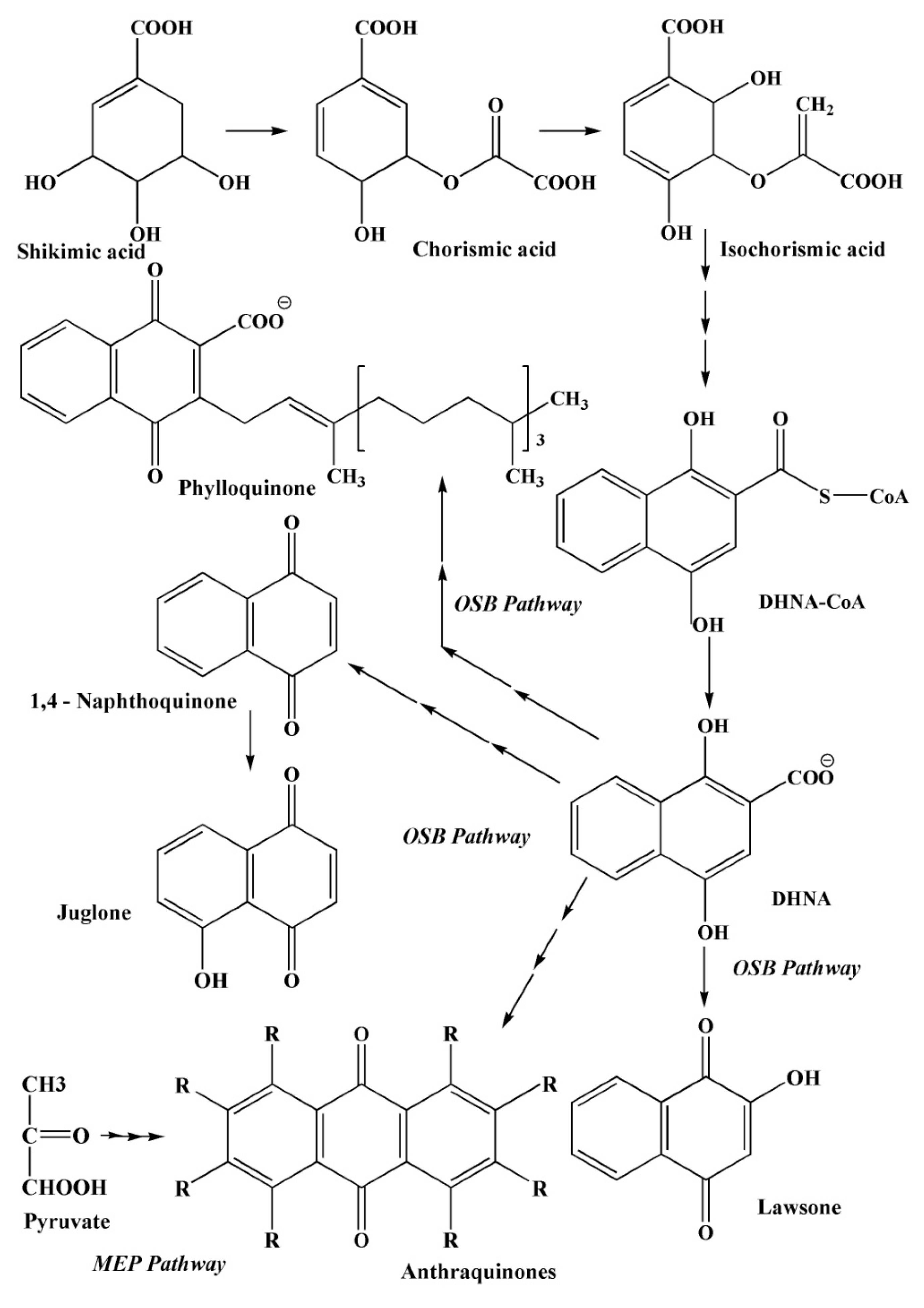
2.3.4. The Pathways of 1,4–Naphthoquinone Biosynthesis
2.4. Biological Activity of Naphthoquinoid Pigments of Sea Urchins
2.4.1. Anti-Oxidant Activity of Naphthoquinones
2.4.2. Anti-Bacterial Activity of Naphthoquinones
2.4.3. Anti-Viral Activity of Naphthoquinones
2.4.4. Anti-Inflammatory Activity of Naphthoquinones
2.4.5. Anti-Allergic Activity of Naphthoquinones
2.4.6. Cytotoxic Activity of Naphthoquinones
2.4.7. Study of Pharmacokinetic Properties of Naphthoquinones
2.4.8. The Prevention and Treatment of Cardiovascular Diseases, Disorders of Carbohydrate and Lipid Metabolism during Aging, Ophthalmological Substances
2.4.9. Naphthoquinones Are Analogues of Medicines
2.4.10. Using in Agriculture
2.4.11. Biotechnological Using
2.5. Food Supplements Based on Sea Urchins
2.5.1. Food Supplements Based on Freeze-Dried Sea Urchin Caviar
2.5.2. The Use of Food Supplements Based on Sea Urchin Caviar in the Treatment of Women during Menopause
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hou, Y.; Vasileva, E.A.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A.; Mishchenko, N.P. Naphthoquinones of the spinochrome class: Occurrence, isolation, biosynthesis and biomedical applications. RSC Adv. 2018, 8, 32637. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A. Spinochromes of Pacific Sea Urchins: Distribution and Bioactivity. Mar. Drugs 2020, 18, 40. [Google Scholar] [CrossRef]
- Peyregne, V.P.; Kar, S.; Ham, S.W.; Wang, M.; Wang, Z.; Carr, B.I. Novel hydroxyl naphthoquinones with potent Cdc25 antagonizing and growth inhibitory properties. Mol. Cancer Ther. 2005, 4, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.; Hoffman, I. Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 2000, 4, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; Nemoto, K.; Pestell, K.E.; Cooley, K.; Southwick, E.C.; Mitchell, D.A.; Furey, W.; Gussio, R.; Zaharevitz, D.W.; Joo, B.; et al. Identification of a Potent and Selective Pharmacophore for Cdc25 Dual Specificity Phosphatase Inhibitors. Mol. Pharmacol. 2002, 61, 720–728. [Google Scholar] [CrossRef]
- Levin, V.S.; Korobkov, V.A. Morskie ezhi Rossii. Biologiya, promysel, ispol’zovanie (Sea Urchins of Russia: Biology, Fishery and Use). Russ. J. Mar. Biol. 2006, 32, 206. [Google Scholar] [CrossRef]
- Vaughn, D.; Strathmann, R.R. Predators induce cloning in Echinoderm larvae. Science 2008, 319, 1503. [Google Scholar] [CrossRef] [PubMed]
- Bazhin, A.G.; Stepanov, V.G. Morskie ezhi semeistva Strongylocentrotidae morei Rossii. In Sea Urchins of the Family Strongylocentrotidae of the Seas of Russia; KamchatNIRO: Petropavlovsk-Kamchatskii, Russia, 2012; Volume 196. [Google Scholar]
- Evdokimov, V.V.; Matrosova, I.V. Reproductive biology of sea urchins Strongylocentrotus intermedius and Strongylocentrotus nudus. Pac. Med. J. 2012, 2, 105–110. [Google Scholar]
- Evdokimov, V.V.; Matrosova, I.V. Morphofunctional assessment of gametes and production capabilities of hydrobionts during their reproduction in mono- and polyculture. Izv. TINRO 2018, 192, 103–120. [Google Scholar] [CrossRef][Green Version]
- Zhadan, P.; Vashchenko, M.; Almyashova, T. The influence of environmental factors on the spawning of sea urchins. In «Marine Biological Research: Achievements and Prospects» with International Participation, Dedicated to the 145th Anniversary of the Sevastopol Biological Station, Proceedings of the All-Russian Scientific and Practical Conference, Sevastopol, Crimea, 19–23 September 2016; ECOSY-Hydrophysics Publ.: Sevastopol, Russia, 2016; Volume 1, pp. 170–173. Available online: https://marine-biology.ru/mbj/article/view/24 (accessed on 23 September 2022).
- Kashenko, S.D. Resistance of sea urchins Strongylocentrotus nudus and S. intermedius to experimental changes in the salinity of sea water. Russ. J. Mar. Biol. 2012, 38, 395–399. [Google Scholar]
- Chaliyenko, M.O. Features of group growth of the gray sea urchin (Strongylocentrotus intermedius) off the northwest coast of the Sea of Japan. Izv. TINRO 2018, 194, 3–17. [Google Scholar] [CrossRef]
- Chaliyenko, M.O.; Kulepanov, V.N.; Matveev, V.I. The influence of some environmental factors on the growth of the gray sea urchin (Strongylocentrotus intermedius) off the northwest coast of the Sea of Japan. Izv. TINRO 2018, 195, 111–127. [Google Scholar] [CrossRef]
- Chaliyenko, M.O.; Kalinina, M.V.; Kulepanov, V.N.; Matveev, V.I. Size and Age at Maturity of the Sea Urchin Strongylocentrotus intermedius along the Northwestern Coast of the Sea of Japan. Oceanology 2021, 61, 69–79. [Google Scholar] [CrossRef]
- Borisovets, E.E.; Bregman, Y.E.; Viktorovskaya, G.I.; Kalinina, M.V. Biology of the gray sea urchin Strongylocentrotus intermedius (A. Agassiz) off the northwestern coast of the Sea of Japan. I. Distribution and size composition of clusters. Izv. TINRO 2000, 127, 416–439. [Google Scholar]
- Pereira, D.M.; Valentão, P.; Andrade, P. Marine natural pigments: Chemistry, distribution and analysis. Dyes Pigment 2014, 111, 124–134. [Google Scholar] [CrossRef]
- Mohebbi, G.H.; Vaziri, A.; Nabipour, I. Sea urchin: Toxinology, bioactive compounds and its treatment management. Iran South Med. J. 2016, 19, 704–735. [Google Scholar] [CrossRef]
- Kuwahara, R.; Hatate, H.; Yuki, T.; Murata, H.; Tanaka, R.; Hama, Y. Antioxidant property of polyhydroxylated naphthoquinone pigments from shells of purple sea urchin Anthocidaris crassispina. LWT-Food Sci. Technol. 2009, 42, 1296–1300. [Google Scholar] [CrossRef]
- Shankarlal, S.; Prabu, K.; Natarajan, E. Antimicrobial and antioxidant activity of purple sea urchin shell (Salmacis virgulata L. Agassiz and Desor 1846). Am.-Euras. J. Sci. Res. 2011, 6, 178–181. [Google Scholar]
- MacMunn, C.A. Memoirs: On the chromatology of the blood of some invertebrates. J. Cell Sci. 1885, 25, 469–490. [Google Scholar] [CrossRef]
- Mishchenko, N.P.; Vasilyeva, E.A.; Fedoreev, S.A. Pentahydroxyethyl naphthoquinone from sea urchins: Structure and properties. Health Med. Ecol. Sci. 2014, 3, 41–42. [Google Scholar]
- Studt, L.; Wiemann, P.; Kleigrewe, K.; Humpf, H.U.; Tudzynski, B. Biosynthesis of fusarubins accounts for pigmentation of fusarium Fujikuroi perithecia. Appl. Environ. Microbiol. 2012, 78, 4468–4480. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.; Hughes, A.D.; Kelly, M.S.; Conner, S.; McDougall, G.J.G. Extraction and identification of antioxidant polyhydroxynaphthoquinone pigments from the sea urchin, Psammechinus miliaris. LWT-Food Sci. Technol. 2014, 59, 455–460. [Google Scholar] [CrossRef]
- Drozdov, A.L.; Artyukov, A.A.; Elkin, Y.N. Pigments of the flat sea urchin Scaphechinus mirabilis (Echinoidea, Clypeasteroida) in eggs and epidermis. Ontogenesis 2017, 48, 301–307. [Google Scholar] [CrossRef]
- Moore, R.E.; Singh, H.; Scheuer, P.J. A pyranonaphthazarin pigment from the sea urchin Echinothrix diadema. Tetrahedron Lett. 1968, 9, 4581–4583. [Google Scholar] [CrossRef]
- Koltsova, E.A.; Maksimov, O.B. Quinoid pigments of echinoderms. VIII. Pigments of sea urchins Diadema setosum and Diadema savignyi. Chem. Natur. Compd. 1981, 1, 115. [Google Scholar]
- Mathieson, J.W.; Thomson, R.H. Naturally occurring quinones. Part XVIII. New spinochromes from Diadema antillarum, Spatangus purpureus, and Temnopleurus toreumaticus. J. Chem. Soc. C 1971, 153–160. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Ivanova, S.A.; Shikov, A.N.; Makarov, V.G. Evaluation of free radical-scavenging activity of sea urchin pigments using HPTLC with post-chromatographic derivatization. Chromatographia 2013, 76, 1353–1358. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Krishtopina, A.S.; Makarov, V.G. Naphthoquinone pigments from sea urchins: Chemistry and pharmacology. Phytochem. Rev. 2018, 17, 509–534. [Google Scholar] [CrossRef]
- Utkina, N.K.; Shchedrin, A.P.; Maksimov, O.B. On the new binaphtoquinone from Strongylocentrotus intermedius. Chem. Nat. Compd. 1976, 4, 439–441. [Google Scholar]
- Koltsova, E.A.; Denisenko, V.A.; Maksimov, O.B. Quinoid pigments of echinoderms. V. Pigments of the sea urchin Strongylocentrotus droebachiensis. Chem. Nat. Compd. 1978, 4, 438–441. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Fedoreyev, S.A.; Pokhilo, N.D.; Anufriev, V.P.; Denisenko, A.V.A.; Glazunov, V.P. Echinamines A and B, first aminated hydroxynaphthazarins from the sea urchin Scaphechinus mirabilis. J. Nat. Prod. 2005, 68, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Yakubovskaya, A.Y.; Pokhilo, N.D.; Mishchenko, N.P.; Anufriev, V.F. Spinazarin and ethylspinazarin—Pigments of the sea urchin Scaphechinus mirabilis. Russ. Chem. Bull. 2007, 56, 819–822. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Qin, L.; Zhu, B.-W.; Wang, X.-D.; Tan, H.; Yang, J.-F.; Li, D.-M.; Dong, X.-P.; Wu, H.-T.; Sun, L.-M.; et al. Extraction and antioxidant property of polyhydroxylated naphthoquinone pigments from spines of purple sea urchin Strongylocentrotus nudus. Food Chem. 2011, 129, 1591–1597. [Google Scholar] [CrossRef]
- Vasileva, E.A.; Mishchenko, N.P.; Tran, V.T.T.; Vo, H.M.N.; Fedoreyev, S.A. Spinochrome Identification and Quantification in Pacific Sea Urchin Shells, Coelomic Fluid and Eggs Using HPLC-DAD-MS. Mar. Drugs 2021, 19, 21. [Google Scholar] [CrossRef]
- Ageenko, N.V.; Kiselev, K.V.; Dmitrenok, P.S.; Odintsova, N.A. Pigment Cell Differentiation in Sea Urchin Blastula-Derived Primary Cell Cultures. Mar. Drugs 2014, 12, 3874–3891. [Google Scholar] [CrossRef]
- Kovalev, N.N.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Kostetsky, E.Y.; Besednova, N.N. Sea Urchins: Biomedical Aspects of Practical Application; Dalnauka: Vladivostok, Russia, 2016; p. 128. [Google Scholar]
- Shikov, A.N.; Ossipov, V.; Karonen, M.; Pozharitskaya, O.; Krishtopina, A.S.; Makarov, V.G. Comparative stability of dimeric and monomeric pigments extracted from sea urchin Strongylocentrotus droebachiensis. Nat. Prod. Res. 2017, 31, 1747–1751. [Google Scholar] [CrossRef]
- Widhalma, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinones natural products produced by horticultural plants. Hortic. Res. 2016, 3, 16046. [Google Scholar] [CrossRef]
- Vasileva, E.A.; Mishchenko, N.P.; Fedoreev, S.A. Diversity of polyhydroxynaphthoquinone pigments in north pacific sea urchins. Chem. Biodivers. 2017, 14, e1700182. [Google Scholar] [CrossRef]
- Britton, G. The Biochemistry of Natural Pigments; Cambridge University Press: Cambridge, UK, 1986; pp. 1–366. [Google Scholar]
- Chan, Y.A.; Podevels, A.M.; Kevany, B.M.; Thomas, M.G. Biosynthesis of Polyketide Synthase Extender Units. Nat. Prod. Rep. 2009, 26, 90–114. [Google Scholar] [CrossRef]
- Piel, J. Biosynthesis of polyketides by trans-AT-polyketide synthases. Nat. Prod. Rep. 2010, 27, 996–1047. [Google Scholar] [CrossRef]
- Helfrich, E.J.N.; Piel, J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 2016, 33, 231–316. [Google Scholar] [CrossRef] [PubMed]
- Till, M.; Race, P.R. The Assembly Line Enzymology of Polyketide Biosynthesis. Race Methods Mol. Biol. 2016, 1401, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Keatinge-Clay, A.T. Polyketide Synthase Modules Redefined. Angew. Chem. Int. Ed. 2017, 18, 4658–4660. [Google Scholar] [CrossRef] [PubMed]
- Calestani, C.; Wessel, G.M. These Colors Don’t Run: Regulation of Pigment-Biosynthesis in Echinoderms. Results Probl. Cell Differ. 2018, 65, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Garcion, C.; Lohmann, A.; Lamodière, E.; Catinot, J.; Buchala, A.; Doermann, P.; Métraux, J.-P. Characterization and biological function of the isocorismate synthase 2 gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef]
- Gross, J.; Cho, W.K.; Lezhneva, L.; Falk, J.; Krupinska, K.; Shinozaki, K.; Seki, M.; Herrmann, R.G.; Meurer, J. A plant locus essential for phylloquinone (vitamin K1) biosynthesis originated from a fusion of four eubacterial genes. J. Biol. Chem. 2006, 281, 17189–17196. [Google Scholar] [CrossRef]
- Mustafa, N.R.; Kim, H.K.; Choi, Y.H.; Erkelens, C.; Lefeber, A.W.; Spijksma, G.; Van Der Heijden, R.; Verpoorte, R. Biosynthesis of salicylic acid in fungus elicited Catharanthus roseus cells. Phytochemistry 2009, 70, 532–539. [Google Scholar] [CrossRef]
- Muljono, R.A.B.; Scheffer, J.J.C.; Verpoorte, R. Isochorismate is an intermediate in 2,3-dihydroxybenzoic acid biosynthesis in Catharanthus roseus cell cultures. Plant Physiol. Biochem. 2002, 40, 231–234. [Google Scholar] [CrossRef]
- Bartsch, M.; Bednarek, P.; Vivancos, P.D.; Schneider, B.; von Roepenack-Lahaye, E.; Foyer, C.H.; Kombrink, E.; Scheel, D.; Parker, J.E. Accumulation of isochorismate-derived 2,3-dihydroxybenzoic 3-O-β-D-xyloside in Arabidopsis resistance to pathogens and ageing of leaves. J. Biol. Chem. 2010, 285, 25654–25665. [Google Scholar] [CrossRef]
- Cox, G.B.; Gibson, F. Biosynthesis of vitamin K and ubiquinone relation to the shikimic acid pathway in Escherichia coli. Biochim. Biophys. Acta 1964, 93, 204–206. [Google Scholar] [CrossRef]
- Whistance, G.R.; Threlfall, D.R.; Goodwin, T.W. Incorporation of [G-14C]shikimate and [U-14C]para-hydroxybenzoate into phytoquinones and chromanols. Biochem. Biophys. Res. Commun. 1966, 23, 849–853. [Google Scholar] [CrossRef]
- Chen, D.; Bohm, B.A. Naphthoquinone biosynthesis in higher plants: I. Studies on 2-hydroxy-l,4-naphthoquinone in Impatiens balsamina L. Can. J. Biochem. 1966, 44, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Zenk, M.H.; Leistner, E. On the mode of incorporation of shikimic acid into 2-hydroxy-1,4-naphthoquinone (lawsone). Z. Naturforsch. B. 1967, 22, 460. [Google Scholar] [CrossRef]
- Leistner, E.; Zenk, M.H. Zur Biogenese von 5-Hydroxy-1,4-naphthochinon (Juglon) in Juglans regia L. Z. Naturforsch. B. 1968, 23, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.M. The roles of alanine, aspartate and glutamate in lawsone biosynthesis in Impatiens balsamina. Tetrahedron Lett. 1969, 10, 4777–4780. [Google Scholar] [CrossRef]
- Grotzinger, E.; Campbell, I.M. The role of 2-ketoglutarate in lawsone biosynthesis in Impatiens balsamina. Tetrahedron Lett. 1972, 13, 4685–4686. [Google Scholar] [CrossRef]
- Kim, H.U.; Van Oostende, C.; Basset, G.J.C.; Browse, J. The AAE14 gene encodes the Arabidopsis o-succinylbenzoyl-CoA ligase that is essential for phylloquinone synthesis and photosystem-I function. Plant J. 2008, 54, 272–283. [Google Scholar] [CrossRef]
- Meganathan, R.; Bentley, R. Thiamine pyrophosphate requirement for o-succinylbenzoic acid synthesis in Escherichia coli and evidence for an intermediate. J. Bacteriol. 1983, 153, 739–746. [Google Scholar] [CrossRef]
- Palmer, D.R.J.; Garrett, J.B.; Sharma, V.; Meganathan, R.; Babbitt, A.P.C.; Gerlt, J.A. Unexpected divergence of enzyme function and sequence: ‘N-acylamino acid racemase’ is o-succinylbenzoate synthase. Biochemistry 1999, 38, 4252–4258. [Google Scholar] [CrossRef]
- Kwon, O.; Bhattacharyya, D.K.; Meganathan, R. Menaquinone (vitamin K2) biosynthesis: Overexpression, purification, and properties of o-succinylbenzoylcoenzyme A synthetase from Escherichia coli. J. Bacteriol. 1996, 178, 6778–6781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Truglio, J.J.; Theis, K.; Feng, Y.; Gajda, R.; Machutta, C.; Tonge, P.; Kisker, C. Crystal Structure of Mycobacterium tuberculosis MenB, a key enzyme in vitamin K2 biosynthesis. J. Biol. Chem. 2003, 278, 42352–42360. [Google Scholar] [CrossRef] [PubMed]
- Salaque, A.; Barbier, M.; Lederer, E. On the biosynthesis of echinochrome A by the sea urchin Arbacia pustulosa. Bull. Soc. Chim. Biol. 1967, 49, 841–848. [Google Scholar]
- Calestani, C.; Rast, J.P.; Davidson, E.H. Isolation of pigment cell specific genes in the sea urchin embryo by differential macroarray screening. Development 2003, 130, 4587–4596. [Google Scholar] [CrossRef] [PubMed]
- Ageenko, N.V.; Kiselev, K.V.; Odintsova, N.A. Expression of Pigment Cell-Specific Genes in the Ontogenesis of the Sea UrchinStrongylocentrotus intermedius. Evid.-Based Complement. Altern. Med. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, T.; Barbieri, E.S.; Gazquez, A.; Avaro, M. Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome. Mar. Drugs 2021, 19, 267. [Google Scholar] [CrossRef]
- Kim, H.; Vasileva, E.; Mishchenko, N.; Fedoreyev, S.; Han, J. Multifaceted Clinical Effects of Echinochrome. Mar. Drugs 2021, 19, 412. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef]
- Krivoshapko, O.N.; Popov, A.M.; Artyukov, A.A.; Kostetsky, E.Y. Peculiarities of the corrective effects of polar lipids and bioantioxidants from sea hydrobionts in impairments of lipid and carbohydrate metabolism. Biochem. Suppl. Ser. B Biomed. Chem. 2011, 5, 152–157. [Google Scholar] [CrossRef]
- Qin, L.; Zhu, B.-W.; Zhou, D.-Y.; Wu, H.-T.; Tan, H.; Yang, J.-F.; Li, D.-M.; Dong, X.-P.; Murata, Y. Preparation and antioxidant activityof enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT-Food Technol. 2011, 44, 1113–1118. [Google Scholar] [CrossRef]
- Gomes, A.R.; Freitas, A.; Rocha-Santos, T.; Duarte, A. Bioactive compounds derived from echinoderms. RSC Adv. 2014, 4, 29365–29382. [Google Scholar] [CrossRef]
- Skulachev, V.P. Cytochrome c in the apoptotic and antioxidant cascades. FEBS Lett. 1998, 423, 275–280. [Google Scholar] [CrossRef]
- Zenkov, N.K.; Lapkin, V.Z.; Menshikova, E.B. Oxidative Stress: Biochemical and Pathophysiological Aspects; MAIK Nauka Interperiodica: Moscow, Russia, 2001; p. 343. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. The antioxidants of human extracellular fluids. Arch. Biochem. Biophys. 1990, 280, 1–8. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals, reactive oxygen species and human disease: A critical evaluation with special reference to atherosclerosis. Br. J. Exp. Pathol. 1989, 70, 737–757. [Google Scholar]
- Peskin, A.B. Interaction of active oxygen with DNA. Biochemistry 1997, 62, 1571–1578. [Google Scholar]
- Lankin, V.Z.; Tikhase, A.K.; Belenkov, Y.N. Antioxidants in the complex therapy of atherosclerosis: Pro et contra. Kardiologiia 2004, 44, 72–81. [Google Scholar]
- Starke-Reed, P.E.; Oliver, C.N. Protein oxidation and proteolysis during aging and oxidative stress. Arch. Biochem. Biophys. 1989, 275, 559–567. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Toyokuni, S. Reactive oxygen speciesinduced molecular damage and its application in pathology. Pathol. Int. 1999, 49, 91–102. [Google Scholar] [CrossRef]
- Kanungo, M. Biochemistry of Ageing; Academic Press Inc., Ltd.: London, UK, 1980. [Google Scholar]
- Skulachev, V.P. Alternative functions of cellular respiration. Soros Educ. J. 1998, 8, 2–7. [Google Scholar]
- Harman, D. Free radical theory of aging/Increasing the functional life span. Ann. N. Y. Acad. Sci. 1994, 717, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Menshchikova, E.B.; Lankin, V.Z.; Zenkov, N.K.; Bondar, I.A.; Krugovykh, N.F.; Trufakin, V.A. Oxidative Stress. Prooxidants and Antioxidants; Slovo: Moscow, Russia, 2006; p. 556. [Google Scholar]
- Danilenko, A.O. Regulation of Free Radical Processes and Apoptosis under Oxidative Stress. Ph.D. Thesis, Southern Federal University, Rostov-on-Don, Russia, 29 March 2012. [Google Scholar]
- Dayronas, Z.V.; Zilfikarov, I.N. Natural Naphthoquinones: Prospects for Medical Use; Marhotin P.Y.: Schyolkovo, Russia, 2011; p. 252. ISBN 978-5-904456-90-0. [Google Scholar]
- Lyakhovich, V.V.; Vavilin, V.A.; Zenkov, N.K.; Menshikova, E.B. Activated oxygen metabolites in monooxydase reactions. Bull. SO RAMS 2005, 4, 7–12. [Google Scholar]
- Gulyaeva, L.F.; Vavilin, V.A.; Lyakhovich, V.V. Enzymes of xenobiotic biotransformation in chemical carcinogenesis. Ecology 2000, 85, 7. [Google Scholar]
- Mamelona, J.; Saint-Louis, R.; Pelletier, E. Nutritional composition and antioxidant properties of Atlantic sea cucumber and green urchin hydrolysates. Jnt. J. Food Sci. Technol. 2010, 45, 147–154. [Google Scholar] [CrossRef]
- Mamelona, J.; Saint-Louis, R.; Pelletier, E. Producing high antioxidant activity extracts from echinoderm by products using pressured liquid extraction. Biotechnology 2010, 9, 523–528. [Google Scholar] [CrossRef][Green Version]
- Sheean, P.D.; Hodges, L.D.; Kalafatis, N.; Wright, P.F.; Wynne, P.M.; Whitehouse, M.W.; Macrides, T.A. Bioactivity of extracts from gonadal tissue of the edible Australian purple sea urchin Heliocidaris erythrogramma. J. Sci. Food Agric. 2007, 87, 694–701. [Google Scholar] [CrossRef]
- Bandaranayake, W.W. The nature and role of pigments of marine invertebrates. Nat. Prod. Rep. 2006, 23, 223–255. [Google Scholar] [CrossRef]
- Garama, D.; Bremer, P.; Carne, A. Extraction and analysis of carotinoids from the New Zealand sea urchin Evechinus chloroticus gonads. Acta Biochim. Pol. 2012, 59, 83–85. [Google Scholar] [CrossRef]
- Lebedev, A.B.; Ivanova, M.B.; Krasnovid, N.I.; Koltsova, E.A. Acidity and interaction with superoxide anion radical of echinochrome and its structural analogs. Vopr. Med. Chem. 1999, 45, 123–128. [Google Scholar]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G. Potential of green sea urchins as a source of medicinal preparations. Planta Med. 2015, 81, 46. [Google Scholar] [CrossRef]
- Deveci, R.; Şener, E.; İzzetoğlu, S. Morphological and ultrastructural characterization of sea urchin immune cells. J. Morphol. 2015, 276, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Matranga, V.; Toia, G.; Bonaventura, R.; Muller, W.E. Cellular and biochemical responses to environmental and experimentally induced stress in sea urchin coelomocytes. Cell Stress Chaperones 2000, 5, 113–120. [Google Scholar] [CrossRef]
- Matranga, V.; Pinsino, A.; Celi, M.; Natoli, A.; Bonaventura, R.; Schröder, H.C.; Müller, W.E.G. Monitoring Chemical and Physical Stress Using Sea Urchin Immune Cells. In Echinodermata; Springer: Berlin/Heidelberg, Germany, 2005; Volume 39, pp. 85–110. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Levitskaya, E.L.; Tikhonova, E.V. Antioxidant properties, autoxidation and mutagenic activity of echinochrome A in comparison with its esterified analog. Biochemistry 2001, 66, 1089–1098. [Google Scholar] [CrossRef]
- Li, D.-M.; Zhou, D.-Y.; Zhu, B.-W.; Miao, L.; Qin, L.; Dong, X.-P.; Wang, X.-D.; Murata, Y. Extraction, structural characterization and antioxidant activity of polyhydroxylated 1,4-naphthoquinone pigments from spines of sea urchin Glyptocidaris crenularis and Strongylocentrotus intermedius. Eur. Food. Res. Technol. 2013, 237, 331–339. [Google Scholar] [CrossRef]
- Brasseur, L.; Hennebert, E.; Fievez, L.; Caulier, G.; Bureau, F.; Tafforeau, L.; Flammang, P.; Gerbaux, P.; Eeckhaut, I. The Roles of Spinochromes in Four Shallow Water Tropical Sea Urchins and Their Potential as Bioactive Pharmacological Agents. Mar. Drugs 2017, 15, 179. [Google Scholar] [CrossRef]
- Kiselev, K.; Ageenko, N.; Kurilenko, V. Involvement of the cell-specific pigment genes pks and sult in bacterial defense response of sea urchins Strongyocentrotus intermedius. Dis. Aquat. Org. 2013, 103, 121–132. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef]
- Lennikov, A.; Kitaichi, N.; Noda, K.; Mizuuchi, K.; Ando, R.; Dong, Z.; Fukuhara, J.; Kinoshita, S.; Namba, K.; Ohno, S.; et al. Amelioration of Endotoxin-Induced Uveitis Treated with the Sea Urchin Pigment Echinochrome in Rats. Mol. Vis. 2014, 20, 171–177. Available online: http://www.molvis.org/molvis/v20/171 (accessed on 23 September 2022).
- Pozharitskaya, O.N.; Shikov, A.N.; Makarova, M.N.; Ivanova, S.A.; Kosman, V.M.; Makarov, V.G.; Bazgier, V.; Berka, K.; Otyepka, M.; Ulrichová, J. Antiallergic effects of pigments isolated from green sea urchin (Strongylocentrotus droebachiensis) shells. Planta Med. 2013, 79, 1698–1704. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, E.K.; Bae, E.A.; Han, M.J. Metabolism of rhaponticin and chrysophanol 8-o-β-D-glucopyranoside from the rhizome of Rheum undulatum by human intestinal bacteria and their anti-allergic actions. Biol. Pharm. Bull. 2000, 23, 830–833. [Google Scholar] [CrossRef]
- Oku, H.; Kato, T.; Ishiguro, K. Antipruritic effects of 1,4-naphthoquinones and related compounds. Biol. Pharm. Bull. 2002, 25, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Kuo, S.C.; Lin, W.C. Pharmacodynamic and pharmacokinetic studies of anthraquinone 2-carboxylic acid on passive cutaneous anaphylaxis in rats. Res. Commun. Mol. Pathol. Pharmacol. 1999, 105, 97–103. [Google Scholar] [PubMed]
- Popov, A.M. Biological Activity and Mechanisms of Action of Secondary Metabolites of Terrestrial Plants and Marine Invertebrates. Ph.D. Dissertation, G.B. Elyakov Pacific Institute of Bioorganic Chemistry Far Eastern Branch of the Russian Academy of Sciences, Vladivostok, Russia, 2003. [Google Scholar]
- Shikov, A.N.; Elena VFlisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef] [PubMed]
- Stavitskaya, T.V.; Egorov, E.A.; Kadyrova, M.K. Features of Ocular Pharmacokinetics and Pharmacodynamics of the Drug Histochrome; All-Russian School of Ophthalmologists: Moscow, Russia, 2004; pp. 289–293. [Google Scholar]
- Talalaeva, O.S.; Mishchenko, N.P.; Briukhanov, V.M.; Zverev, I.F.; Lampatov, V.V.; Dvornikova, L.G. Identification of histochrome metabolism products in urine for studying drug pharmacokinetics. Eksp. Klin. Farmakol. 2014, 77, 29–32. [Google Scholar]
- Zakirova, A.N.; Ivanova, M.V.; Golubiatnikov, V.B.; Mishchenko, N.P.; Kol’tsova, E.A.; Fedoreev, S.A.; Krasnovid, N.J.; Lebedev, A.V. Pharmacokinetics and clinical efficacy of histochrome in patients with acute myocardial infarction. Eksp. Klin. Farmakol. 1997, 60, 21–24. [Google Scholar]
- Bergström, C.A.S.; Holm, R.; Jørgensen, S.A.; Andersson, S.B.E.; Artursson, P.; Beato, S.; Borde, A.; Box, K.; Brewster, M.; Dressman, J.; et al. Early pharmaceutical profiling to predict oral drug absorption: Current status and unmet needs. Eur. J. Pharm. Sci. 2014, 57, 173–199. [Google Scholar]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Elyakov, G.B.; Maksimov, O.B.; Mishchenko, N.P. The Drug «Histochrome» for the Treatment of Inflammatory Diseases of the Retina and Cornea of the Eye. Russian Patent No. 2134107, 10 August 1999. [Google Scholar]
- Elyakov, G.B.; Maksimov, O.B.; Mishchenko, N.P. The Drug «Histochrome» for the Treatment of Acute Myocardial Infarction and Cardiac Ischemia. Russian Patent No. 2137472, 20 September 1999. [Google Scholar]
- Mishchenko, N.P.; Fedoreev, S.A.; Bagirova, V.L. Histochrome: A new original domestic drug. J. Pharm. Chem. 2003, 37, 48–52. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Kozlov, V.K.; Lebedko, O.A.; Morozova, N.V. The use of echinochrome A in the anti-relapse therapy of chronic inflammatory lung diseases in children with malformations of the respiratory system. Depart. Physiol. Pathol. Respir. 2011, 39, 52–55. [Google Scholar]
- Elkin, Y.N.; Cherednichenko, A.I.; Koltsova, E.A.; Artyukov, A.A. Mass-spectrometry with electron capture ionization of echinochrome A. Mass-Spectrum. 2011, 8, 147–149. [Google Scholar]
- Artyukov, A.A. Development of Biotechnological Bases for Obtaining Some Biologically Active Substances from Oceanic Raw Materials. Ph.D. Dissertation, G.B. Elyakov Pacific Institute of Bioorganic Chemistry Far Eastern Branch of the Russian Academy of Sciences, Vladivostok, Russia, 2012. [Google Scholar]
- Zakirova, A.N.; Lebedev, A.V.; Kukharchuk, V.V.; Mishchenko, N.P.; Fedoreev, S.A. The antioxidant histochrome: Its effect on lipid peroxidation and the blood rheological properties in patients with unstable stenocardia. Ter. Arkh. 1996, 68, 12–14. [Google Scholar] [PubMed]
- Buĭmov, G.A.; Maksimov, I.V.; Perchatkin, V.A.; Repin, A.N.; Afanas’Ev, S.A.; Markov, V.A.; Karpov, R.S. Effect of the bioantioxidant histochrome on myocardial injury in reperfusion therapy on patients with myocardial infarction. Ter. Arkh. 2002, 74, 12–16. [Google Scholar]
- Tsybulsky, A.; Popov, A.; Artyukov, A.; Krivoshapko, O.; Kozlovskaya, E.; Bogdanovich, L.; Krijanovsky, S.; Blinov, Y. The effects of preparation «Histochrome» in biochemical parameters of blood for patients with cardiopathologies. Biomed. Khim. 2013, 60, 115–124. [Google Scholar] [CrossRef][Green Version]
- Guseva, M.R.; Beslaneyeva, M.B. Clinical rationale for the efficiency of using the Russian antioxidant agent Histochrome. Ann. Ophthalmol. 2010, 126, 37–40. [Google Scholar]
- Egorov, E.A.; Alekhina, V.A.; Volobueva, T.M.; Fedoreev, S.A.; Mishchenko, N.P.; Kol’tsova, E.A. Histochrome, a new antioxidant, in the treatment of ocular disease. Ann. Ophthalmol. 1999, 115, 34–35. [Google Scholar]
- Odintsova, N.A.; Boroda, A.V. Cryopreservation of cells and larvae of marine organisms. Russ. J. Mar. Biol. 2012, 38, 101–111. [Google Scholar] [CrossRef]
- Rodda, D.J.; Chew, J.-L.; Lim, L.-H.; Loh, Y.-H.; Wang, B.; Ng, H.-H.; Robson, P. Transcriptional Regulation of Nanog by OCT4 and SOX2. J. Biol. Chem. 2005, 280, 24731–24737. [Google Scholar] [CrossRef]
- Odintsova, N.A. Stem Cells of Marine Invertebrates: Regulation of Proliferation and Induction of Differentiation in vitro. Cell Tiss. Biol. 2009, 3, 403–408. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Odintsova, N.A.; Plotnikov, S.V.; Kiselev, K.V.; Zacharov, E.V.; Zhuravlev, Y.N. Gal4–gene dependent alterations of embryo development and cell growth in primary culture of sea urchins. Mar. Biotechnol. 2002, 4, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Kiselev, K.V.; Yakovlev, K.V.; Zhuravlev, Y.N.; Gontcharov, A.A.; Odintsova, N.A. Agrobacterium-mediated transformation of sea urchin embryos. Biotechnol. J. 2006, 1, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, N.A.; Kiselev, K.V.; Bulgakov, V.P.; Koltsova, E.A.; Yakovlev, K.V. The effect of gal4 transcription activator on the growth and development of embryos and embryonic cells in primary cultures of the sand dollar Scaphechinus mirabilis. Russ. J. Biol. Developmt. 2003, 34, 217–222. [Google Scholar] [CrossRef]



| № | Name of Naphthoquinone Pigment | Structural Formula |
|---|---|---|
| 1 | Echinochrome A | 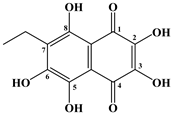 |
| 2 | Spinochrome A | 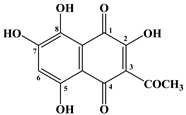 |
| 3 | Spinochrome B |  |
| 4 | Spinochrome C | 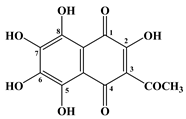 |
| 5 | Spinochrome D | 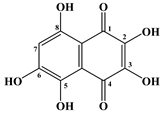 |
| 6 | Spinochrome E | 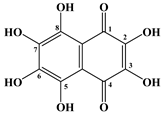 |
| Naphthoquinone Pigments | Cell Culture Medium | ||
|---|---|---|---|
| SW | CFn | CFreg | |
| Fresh Biomass of Cells (mg/g) | |||
| Strongylocentrotus intermedius | |||
| Spinochrome E | 0.024 ± 0.004 | 0.013 ± 0.002 | 0.015 ± 0.002 |
| Spinochrome D | 0.052 ± 0.006 | 0.044 ± 0.005 | 0.062 ± 0.007 |
| Scaphechinus mirabilis | |||
| Spinochrome E | 0.021 ± 0.003 | 0.062 ± 0.007 | 0.054 ± 0.006 |
| Echinochrome A | 0.250 ± 0.026 | 0.640± 0.061 | 0.540 ± 0.055 |
| № | Naphthoquinone Pigments of Sea Urchin | Naphthoquinone Pigments of Terrestrial Plants |
|---|---|---|
| 1 | Echinochrome A | Lawsone |
| 2 | Spinochrome A (Spinochrome B3; Spinochrome M; Spinochrome M1; Spinochrome Aka2; Spinochrome P) | Lapachol |
| 3 | Spinochrome B (Spinochrome B1; Spinochrome M2;Spinochrome N; Spinochrome P1) | Alizarin |
| 4 | Spinochrome C (Spinochrome B2; Spinochrome F; Spinochrome F1; Spinochrome M3; Spinone A; Isoechinochrome) | Shikonins |
| 5 | Spinochrome D (Spinochrome Aka; Spinochrome Aka1) | Alkannins |
| 6 | Spinochrome E | Chimaphilins |
| 7 | Spinochrome G | Plumbagin |
| 8 | Spinochrome S | 2-methoxy-1,4-naphthoquinone |
| 9 | 2-Hydroxy-3-acetylnaphthazarin | Droserone |
| 10 | 2,3,7-trihydroxy-6-ethyljuglone | Juglone |
| 11 | 2-hydroxy-6-ethyljuglone | 7-Methyljuglone |
| 12 | Naphthopurpurin | Anthrasesamones |
| 13 | 6-Ethyl-2-hydroxynaphthazarin | |
| 14 | 6-Acetyl-2,7-dihydroxyjuglone | |
| 15 | 6-Acetyl-2-hydroxynaphthazarin | |
| 16 | Mompain | |
| 17 | Ethylmompain | |
| 18 | 6-Ethyl-3,7-dihydroxy-2-methoxynaphthazarin | |
| 19 | 6-Ethyl-2,7-dihydroxy-3-methoxynaphthazarin | |
| 20 | 3-Acetyl-2,7-dihydroxy-6-methylnaphthazarin | |
| 21 | Echinamine A | |
| 22 | Echinamine B | |
| 23 | Aminopentahydroxynaphthoquinone | |
| 24 | Spinamine E | |
| 25 | Spinazarin | |
| 26 | Ethylspinazarin | |
| 27 | Tetrahydroxydimethoxynaphthoquinone | |
| 28 | Namakochrome | |
| 29 | Ethylidene-6,6′-bis(2,3,7-trihydroxynaphthazarin) | |
| 30 | Ethylidene-3,3′-bis(2,6,7-trihydroxynaphthazarin). | |
| 31 | Anhydroethylidene-6,6′,-bis(2,3,7-trihydroxynaphthazarin) | |
| 32 | Anhydroethylidene-3,3′-bis(2,6,7-trihydroxynaphthazarin) | |
| 33 | Mirabiquinone A | |
| 34 | Pyranonaphthazarin | |
| 35 | Acetylaminotrihydroxynaphthoquinone | |
| 36 | Spinochrome dimer | |
| 37 | Spinochrome B sulfate derivative | |
| 38 | Spinochrome E sulfate derivative | |
| 39 | Spinochrome A—Iso 2 | |
| 40 | Spinochrome D—Iso 1 | |
| 41 | Spinochrome D—Iso 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageenko, N.V.; Kiselev, K.V.; Odintsova, N.A. Quinoid Pigments of Sea Urchins Scaphechinus mirabilis and Strongylocentrotus intermedius: Biological Activity and Potential Applications. Mar. Drugs 2022, 20, 611. https://doi.org/10.3390/md20100611
Ageenko NV, Kiselev KV, Odintsova NA. Quinoid Pigments of Sea Urchins Scaphechinus mirabilis and Strongylocentrotus intermedius: Biological Activity and Potential Applications. Marine Drugs. 2022; 20(10):611. https://doi.org/10.3390/md20100611
Chicago/Turabian StyleAgeenko, Natalya V., Konstantin V. Kiselev, and Nelly A. Odintsova. 2022. "Quinoid Pigments of Sea Urchins Scaphechinus mirabilis and Strongylocentrotus intermedius: Biological Activity and Potential Applications" Marine Drugs 20, no. 10: 611. https://doi.org/10.3390/md20100611
APA StyleAgeenko, N. V., Kiselev, K. V., & Odintsova, N. A. (2022). Quinoid Pigments of Sea Urchins Scaphechinus mirabilis and Strongylocentrotus intermedius: Biological Activity and Potential Applications. Marine Drugs, 20(10), 611. https://doi.org/10.3390/md20100611







