Uncommon Capnosane Diterpenes with Neuroprotective Potential from South China Sea Soft Coral Sarcophyton boettgeri
Abstract
1. Introduction
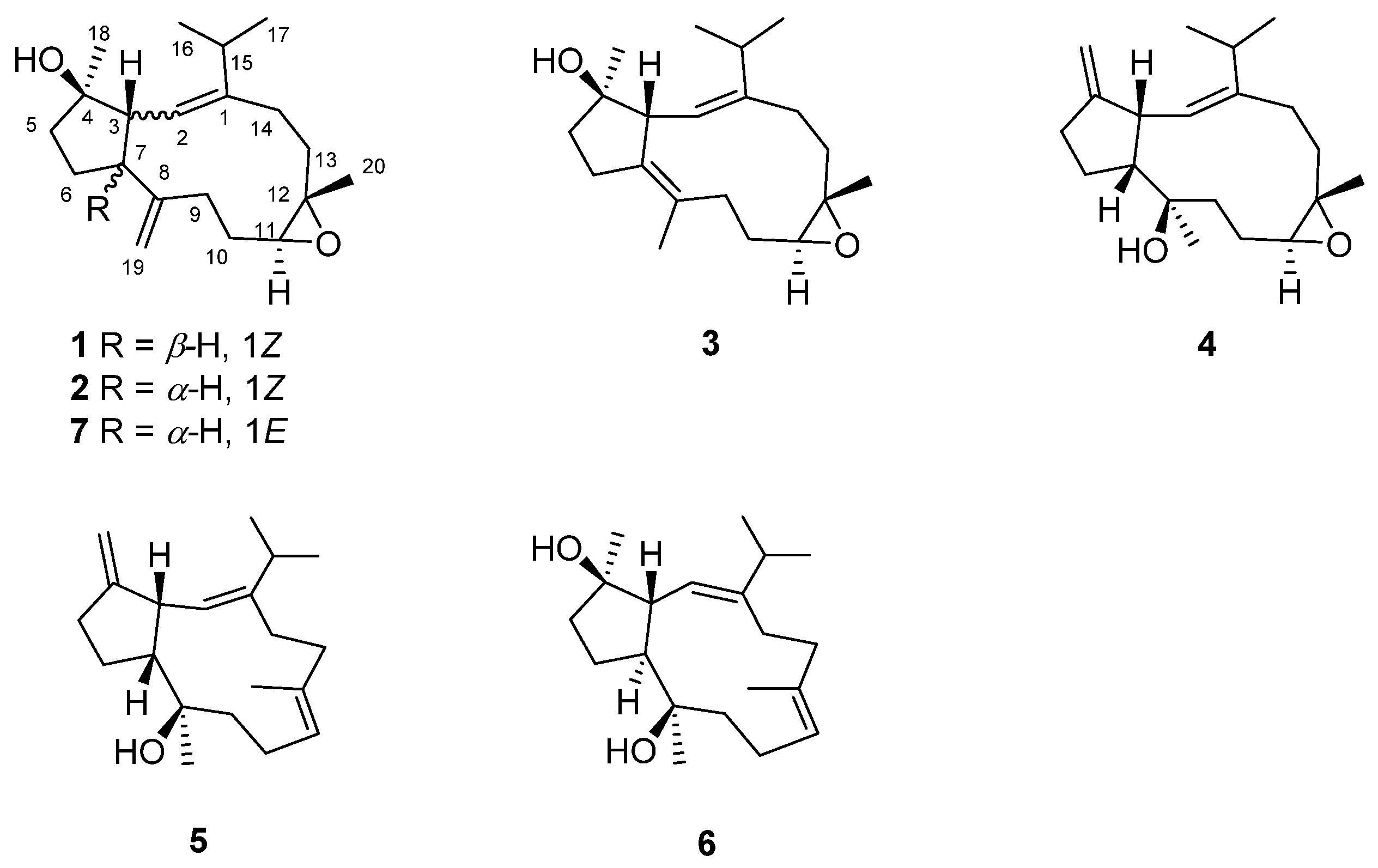
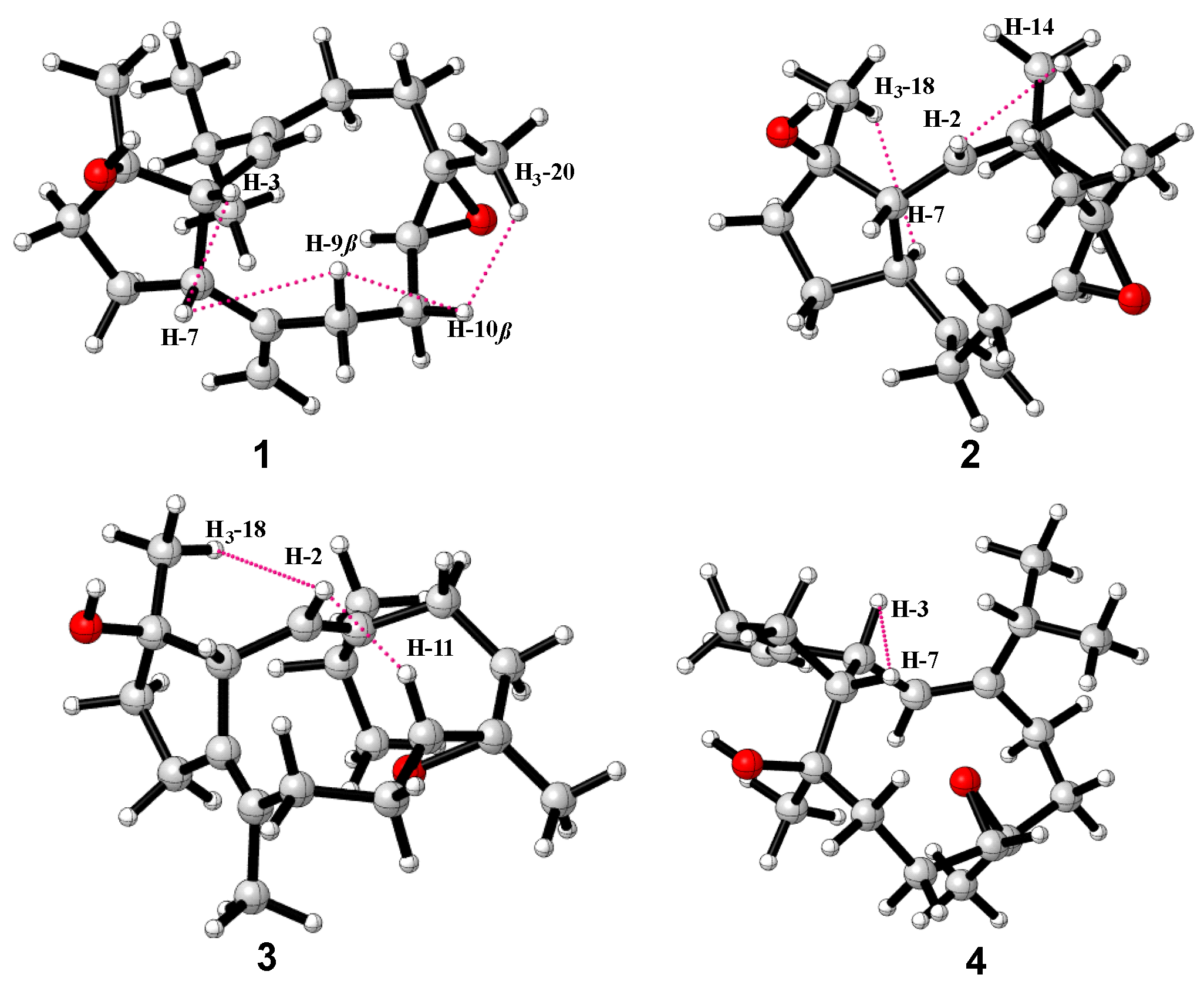
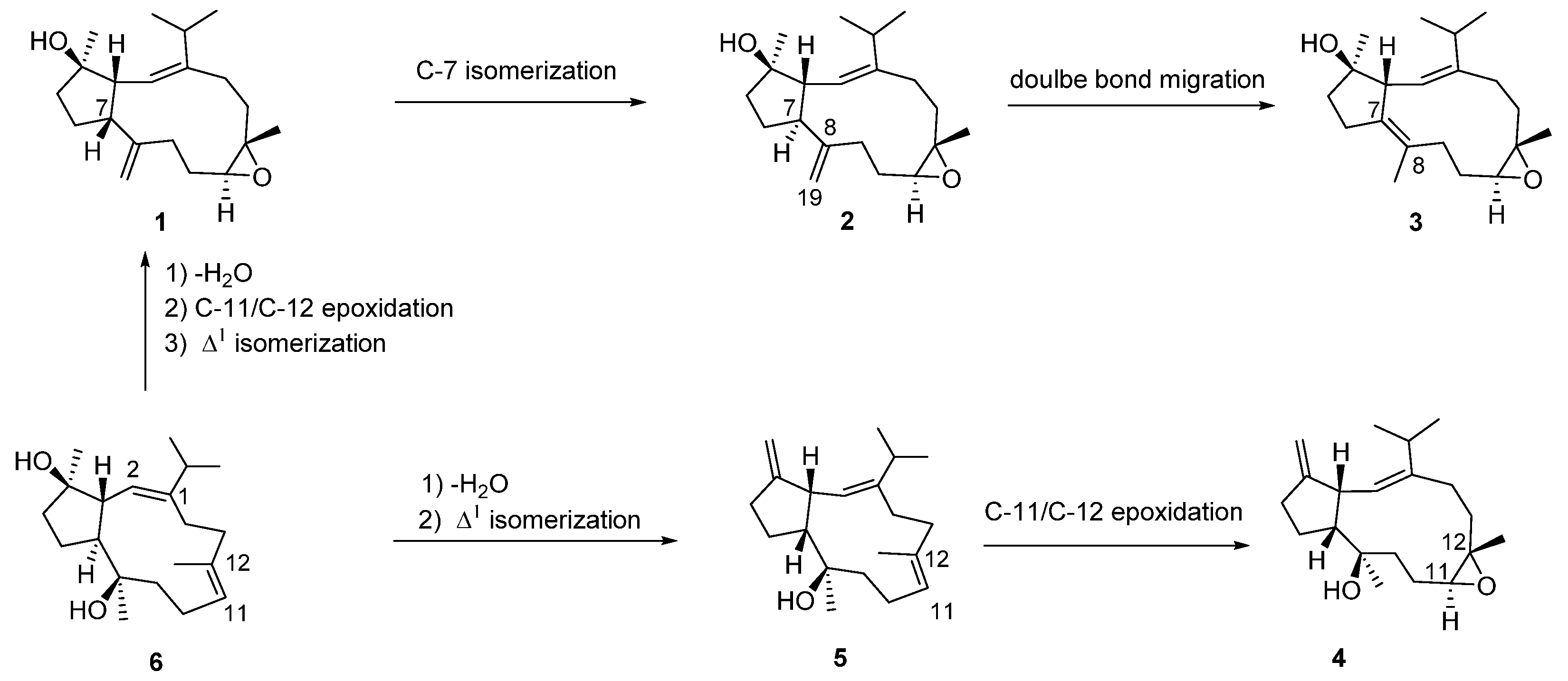
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of Compounds
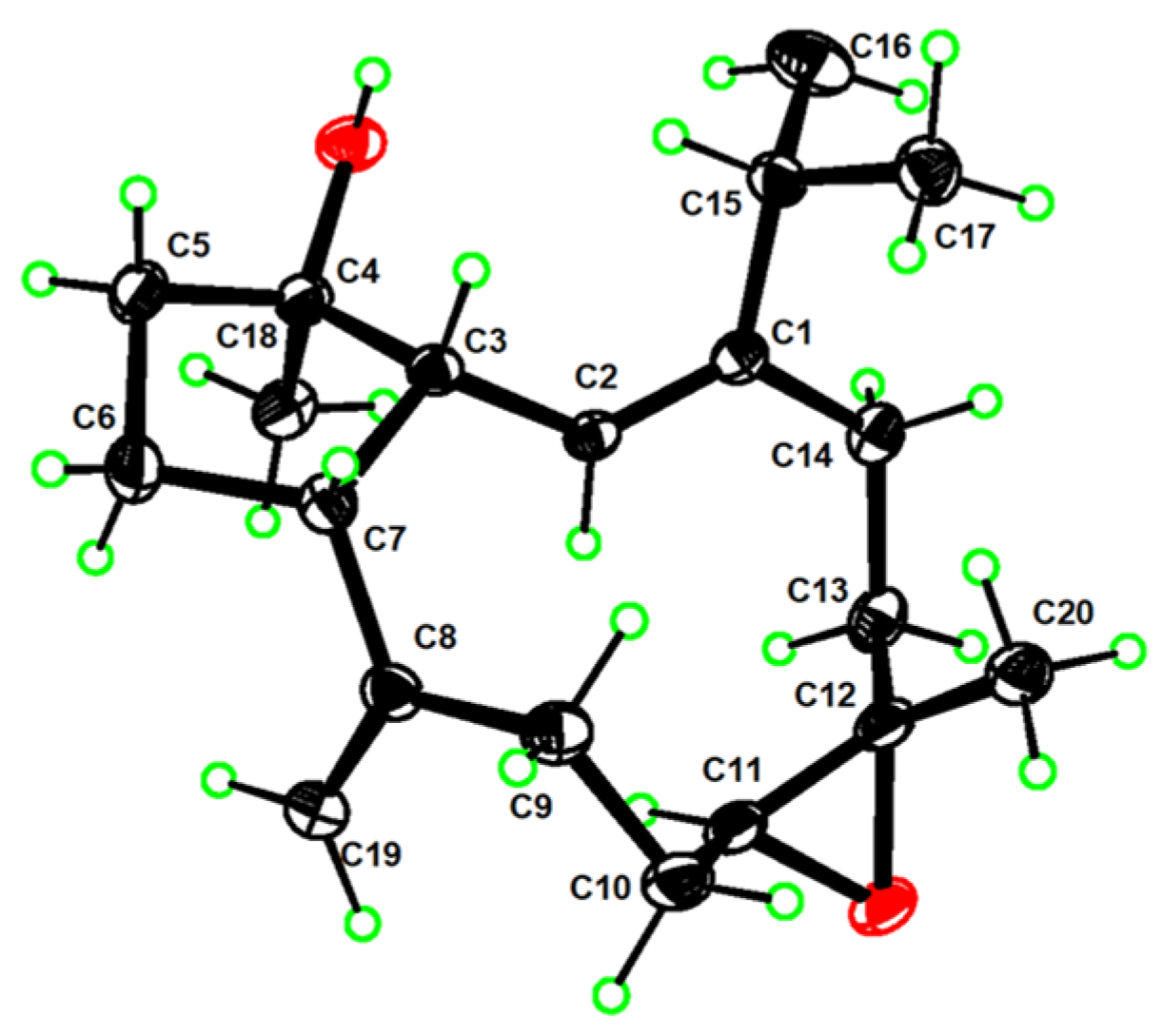
3.5. Crystal Structure Analysis of 1
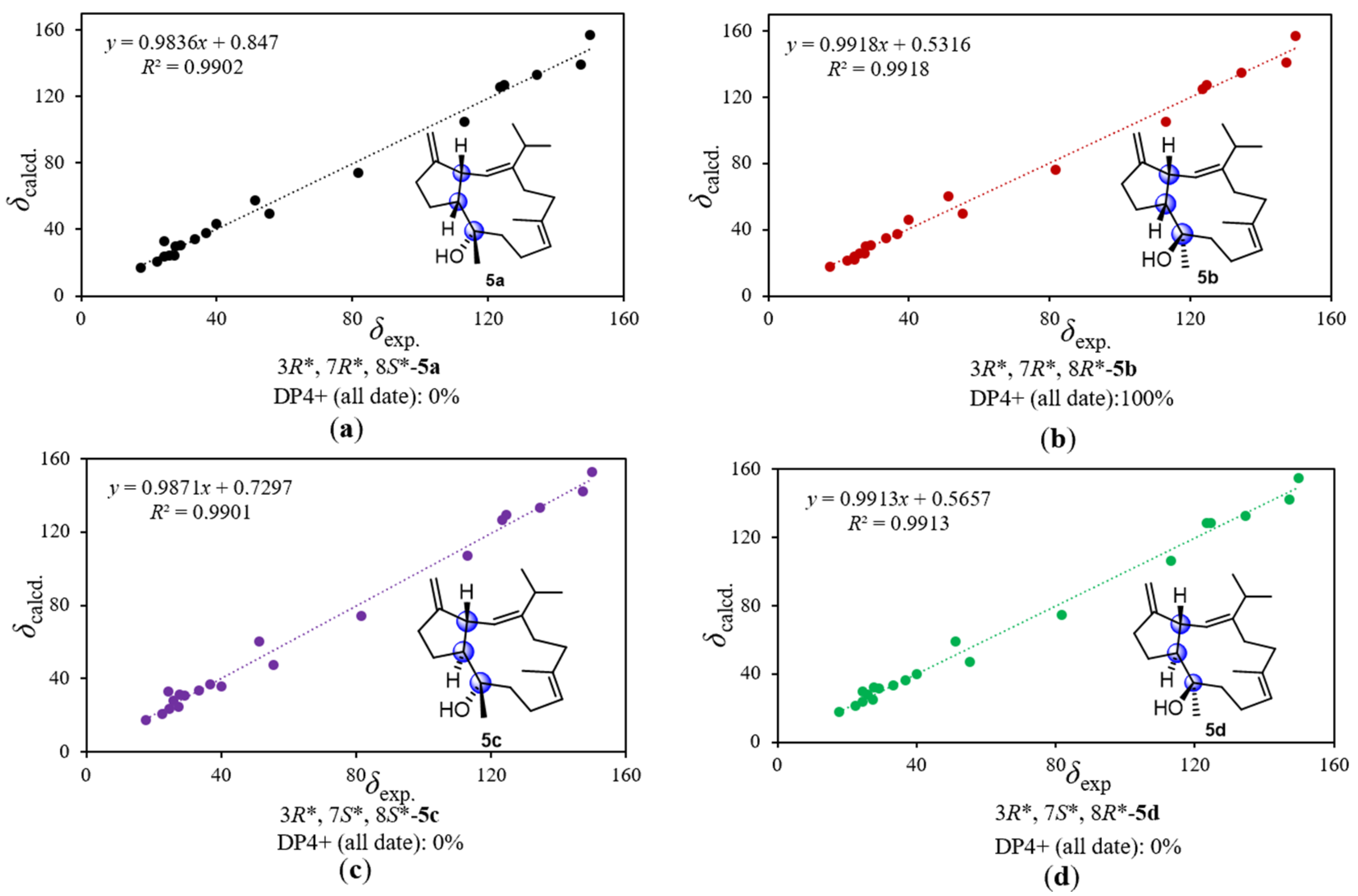
3.6. Computational Details
3.7. Cell Cultures
3.8. In Vitro Anti-Neuroinflammatory Activity Assay
3.9. Cell Viability Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Zhang, P.; Gong, D.; Zhang, Z. Chemical structures, biosynthesis, bioactivities, biocatalysis and semisynthesis of tobacco cembranoids: An overview. Ind. Crops Prod. 2016, 83, 66–80. [Google Scholar] [CrossRef]
- Li, B.; Shen, Y.-H.; He, Y.-R.; Zhang, W.-D. Chemical constituents and biological activities of Pinus species. Chem. Biodivers. 2013, 10, 2133–2160. [Google Scholar] [CrossRef]
- Liang, L.-F.; Guo, Y.-W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodivers. 2013, 10, 2161–2196. [Google Scholar] [CrossRef]
- Elkhawas, Y.A.; Elissawy, A.M.; Elnaggar, M.S.; Mostafa, N.M.; Al-Sayed, E.; Bishr, M.M.; Singab, A.N.B.; Salama, O.M. Chemical diversity in species belonging to soft coral genus Sacrophyton and its impact on biological activity: A review. Mar. Drugs 2020, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, Sinularia, and Lobophytum since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Alarif, W.M.; Alburae, N.A.; Algandaby, M.M. Alcyonium octocorals: Potential source of diverse bioactive terpenoids. Molecules 2019, 24, 1370. [Google Scholar] [CrossRef]
- Nurrachma, M.Y.; Sakaraga, D.; Nugraha, A.Y.; Rahmawati, S.I.; Bayu, A.; Sukmarini, L.; Atikana, A.; Prasetyoputri, A.; Izzati, F.; Warsito, M.F.; et al. Cembranoids of soft corals: Recent updates and their biological activities. Nat. Prod. Bioprospect. 2021, 11, 243–306. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.-F.; Lin, X.-P.; Liu, J.; Peng, Y.; Yang, X.-W.; Liu, Y. Cembrane diterpenes chemistry and biological properties. Curr. Org. Chem. 2012, 16, 1512–1539. [Google Scholar] [CrossRef]
- Wu, M.-J.; Liu, J.; Wang, J.-R.; Zhang, J.; Wang, H.; Jiang, C.-S.; Guo, Y.-W. Sinucrassins A—K, casbane-type diterpenoids from the South China Sea soft coral Sinularia crassa. Chin. J. Chem. 2021, 39, 2367–2376. [Google Scholar] [CrossRef]
- Li, G.; Dickschat, J.S.; Guo, Y.-W. Diving into the world of marine 2,11-cyclized cembranoids: A summary of new compounds and their biological activities. Nat. Prod. Rep. 2020, 37, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-F.; Kurtán, T.; Mándi, A.; Gao, L.-X.; Li, J.; Zhang, W.; Guo, Y.-W. Sarsolenane and capnosane diterpenes from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller as PTP1B inhibitors. Eur. J. Org. Chem. 2014, 2014, 1841–1847. [Google Scholar] [CrossRef]
- Bu, Q.; Yang, M.; Yan, X.-Y.; Li, S.-W.; Ge, Z.-Y.; Zhang, L.; Yao, L.-G.; Guo, Y.-W.; Liang, L.-F. Mililatensols A–C, new records of sarsolenane and capnosane diterpenes from soft coral Sarcophyton mililatensis. Mar. Drugs 2022, 20, 566. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.-R.; Li, W.-S.; Nay, B.; Hu, P.; Zhang, H.-Y.; Wang, H.; Li, X.-W.; Guo, Y.-W. Sinunanolobatone A, an anti-inflammatory diterpenoid with bicyclo[13.1.0]pentadecane carbon scaffold, and related casbanes from the Sanya soft coral Sinularia nanolobata. Org. Lett. 2021, 23, 7575–7579. [Google Scholar] [CrossRef]
- Yang, M.; Li, X.-L.; Wang, J.-R.; Lei, X.; Tang, W.; Li, X.-W.; Sun, H.; Guo, Y.-W. Sarcomililate A, an unusual diterpenoid with tricyclo[11.3.0.02,16]hexadecane carbon skeleton, and its potential biogenetic precursors from the Hainan soft coral Sarcophyton mililatensis. J. Org. Chem. 2019, 84, 2568–2576. [Google Scholar] [CrossRef]
- Craig, R.A.; Stoltz, B.M. Polycyclic furanobutenolide-derived cembranoid and norcembranoid natural products: Biosynthetic connections and synthetic efforts. Chem. Rev. 2017, 117, 7878–7909. [Google Scholar] [CrossRef]
- Li, Y.; Pattenden, G. Perspectives on the structural and biosynthetic interrelationships between oxygenated furanocembranoids and their polycyclic congeners found in corals. Nat. Prod. Rep. 2011, 28, 1269–1310. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chao, C.-H.; Huang, T.-Z.; Huang, C.-Y.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. Cembranoid-related metabolites and biological activities from the soft coral Sinularia flexibilis. Mar. Drugs 2018, 16, 278. [Google Scholar] [CrossRef]
- Yu, Q.-H.; Sura, M.B.; Wang, D.-W.; Huang, D.; Yan, Y.-M.; Jiao, Y.-B.; Lu, Q.; Cheng, Y.-X. Isolation of boswelliains A—E, cembrane-type diterpenoids from Boswellia papyifera, and an evaluation of their wound healing properties. Chin. J. Chem. 2021, 39, 2451–2459. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Zhang, Z. A review on bioactivities of tobacco cembranoid diterpenes. Biomolecules 2019, 9, 30. [Google Scholar] [CrossRef]
- Bogdanov, A.; Hertzer, C.; Kehraus, S.; Nietzer, S.; Rohde, S.; Schupp, P.J.; Wägele, H.; König, G.M. Secondary metabolome and its defensive role in the aeolidoidean Phyllodesmium longicirrum, (Gastropoda, Heterobranchia, Nudibranchia). Beilstein J. Org. Chem. 2017, 13, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Du, X.; Ren, X.; Li, X.; Li, H.; Fu, X.; Wei, X. Structural modifications and biological activities of natural α- and β-cembrenediol: A comprehensive review. Pharmaceuticals 2022, 15, 601. [Google Scholar] [CrossRef] [PubMed]
- Takamura, H.; Kikuchi, T.; Iwamoto, K.; Nakao, E.; Harada, N.; Otsu, T.; Endo, N.; Fukuda, Y.; Ohno, O.; Suenaga, K.; et al. Unified total synthesis, stereostructural elucidation, and biological evaluation of sarcophytonolides. J. Org. Chem. 2018, 83, 11028–11056. [Google Scholar] [CrossRef]
- He, C.; Xuan, J.; Rao, P.; Xie, P.-P.; Hong, X.; Lin, X.; Ding, H. Total syntheses of (+)-sarcophytin, (+)-chatancin, (−)-3-oxochatancin, and (−)-pavidolide B: A divergent approach. Angew. Chem. Int. Ed. 2019, 58, 5100–5104. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nakano, E. Stereochemical course of the transannular cyclization, in chloroform, of epoxycembranoids derived from the geometrical isomers of (14S)-14-hydroxy-1,3,7,11-cembratetraenes. J. Org. Chem. 1990, 55, 1947–1951. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Capnosane-type cembranoids from the soft coral Sarcophyton trocheliophorum with antibacterial effects. Tetrahedron 2014, 70, 8703–8713. [Google Scholar] [CrossRef]
- Chen, W.-T.; Yao, L.-G.; Li, X.-W.; Guo, Y.-W. Sarcophytrols A–C, new capnosane diterpenoids from the South China Sea soft coral Sarcophyton trocheliophorum. Tetrahedron Lett. 2015, 56, 1348–1352. [Google Scholar] [CrossRef]
- Zhang, M.; Long, K.-H.; Huang, S.-H.; Shi, K.-L.; Mak, T.C.W. A novel diterpenolide from the soft coral Sarcophyton solidun. J. Nat. Prod. 1992, 55, 1672–1675. [Google Scholar] [CrossRef]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Sarcophyolides B–E, new cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2013, 11, 3186–3196. [Google Scholar] [CrossRef]
- Shen, S.; Zhu, H.-J.; Chen, D.-W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.-H. Pavidolides A–E, new cembranoids from the soft coral Sinularia pavida. Tetrahedron Lett. 2012, 53, 5759–5762. [Google Scholar] [CrossRef]
- Li, J.; Huan, X.-J.; Wu, M.-J.; Chen, Z.-H.; Chen, B.; Miao, Z.-H.; Guo, Y.-W.; Li, X.-W. Chemical constituents from the South China sea soft coral Sinularia humilis. Nat. Prod. Res. 2022, 36, 3324–3330. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Geng, Z.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Cembranoids from the soft coral Sinularia rigida with antifouling activities. J. Agric. Food Chem. 2013, 61, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhu, Z.-D.; Gu, Y.-C.; Li, J.; Zhu, W.-L.; Guo, Y.-W. Further new diterpenoids as PTP1B inhibitors from the Xisha soft coral Sinularia polydactyla. Mar. Drugs 2018, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Li, X.; Han, X.; Wang, Z.; Li, G. A new capnosane-type diterpenoid from the South China sea soft coral Lobophytum pauciflorum. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.-W.; Yao, L.-G.; Wu, B.; Guo, Y.-W. Three new capnosane-type diterpenoids from the South China Sea soft coral Lobophytum sp. Fitoterapia 2019, 133, 70–74. [Google Scholar] [CrossRef]
- Tseng, W.-R.; Ahmed, F.A.; Huang, C.-Y.; Tsai, Y.-Y.; Tai, C.-J.; Orfali, S.R.; Hwang, T.-L.; Wang, Y.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive capnosanes and cembranes from the soft coral Klyxum flaccidum. Mar. Drugs 2019, 17, 461. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Gulbis, J.M.; Mackay, M.F.; Willis, R.H. Studies of Australian soft corals. XXXVIII. Structure determination of several diterpenes derived from a Cespitularia species (Coelenterata, Octocorallia, Xeniidae). Aust. J. Chem. 1986, 39, 803–812. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Pietra, F. Novel cembranolides (coralloidolide D and E) and a 3,7-cyclized cembranolide (coralloidolide C) from the mediterranean coral Alcyonium coralloides. Helv. Chim. Acta 1989, 72, 1590–1596. [Google Scholar] [CrossRef]
- Sun, P.; Yu, Q.; Li, J.; Riccio, R.; Lauro, G.; Bifulco, G.; Kurtán, T.; Mándi, A.; Tang, H.; Li, T.-J.; et al. Bissubvilides A and B, cembrane–capnosane heterodimers from the soft coral Sarcophyton subviride. J. Nat. Prod. 2016, 79, 2552–2558. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Wu, M.-J.; Tang, W.; Wang, J.-R.; Gu, Y.-C.; Wang, H.; Li, X.-W.; Guo, Y.-W. Sinueretone A, a diterpenoid with unprecedented tricyclo[12.1.0.05,9]pentadecane carbon scaffold from the South China Sea soft coral Sinularia erecta. J. Org. Chem. 2021, 86, 10975–10981. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Li, W.-S.; Zhang, Z.-Y.; Luo, H.; Wang, J.-R.; Zhang, H.-Y.; Zeng, Z.-R.; Chen, B.; Li, X.-W.; Guo, Y.-W. Sinusiaetone A, an anti-inflammatory norditerpenoid with a bicyclo[11.3.0]hexadecane nucleus from the Hainan soft coral Sinularia siaesensis. Org. Lett. 2021, 23, 5621–5625. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, S.-W.; Xu, H.; Wang, H.; Hu, P.; Zhang, H.; Luo, C.; Chen, K.-X.; Nay, B.; Guo, Y.-W.; et al. Complex polypropionates from a South China Sea photosynthetic mollusk: Isolation and biomimetic synthesis highlighting novel rearrangements. Angew. Chem. Int. Ed. 2020, 59, 12105–12112. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-W.; Cuadrado, C.; Yao, L.-G.; Daranas, A.H.; Guo, Y.-W. Quantum mechanical–NMR-aided configuration and conformation of two unreported macrocycles isolated from the soft coral Lobophytum sp.: Energy calculations versus coupling constants. Org. Lett. 2020, 22, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.-Z.; Yao, L.-G.; Zhang, Z.-Y.; Wang, J.-R.; Wang, H.; Guo, Y.-W. Polyoxygenated cembranoids from the Hainan soft coral Lobophytum crassum. Tetrahedron 2021, 90, 132204. [Google Scholar] [CrossRef]
- Li, X.-L.; Ru, T.; Navarro-Vázquez, A.; Lindemann, P.; Nazaré, M.; Li, X.-W.; Guo, Y.-W.; Sun, H. Weizhouochrones: Gorgonian-derived symmetric dimers and their structure elucidation using anisotropic NMR combined with DP4+ probability and CASE-3D. J. Nat. Prod. 2022, 85, 1730–1737. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Bittner, T.; Jung, C.K.E.; Burgold, S.; Page, R.M.; Mitteregger, G.; Haass, C.; LaFerla, F.M.; Kretzschmar, H.; Herms, J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2010, 13, 411–413. [Google Scholar] [CrossRef]


| No. | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| δH mult. (J, Hz) | δC | δH mult. (J, Hz) | δC | δH mult. (J, Hz) | δC | δH mult. (J, Hz) | δC | δH mult. (J, Hz) | δC | |
| 1 | - | 143.6 | - | 143.2 | - | 139.8 | - | 149.2 | 147.3 | |
| 2 | 4.92 d (10.8) | 120.9 | 4.82 dd (9.6, 2.4) | 124.8 | 4.81 d (9.6) | 126.4 | 4.91 d (10.2) | 122.5 | 4.88 d (10.2) | 123.4 |
| 3 | 3.20 t (10.8) | 50.3 | 2.80 dd (11.4, 9.6) | 56.7 | 3.36 d (9.6) | 52.4 | 2.43 m | 54.7 | 2.39 overlap | 55.4 |
| 4 | - | 82.0 | - | 80.8 | - | 82.6 | - | 148.2 | - | 149.9 |
| 5 | 1.82 m | 40.6 | 1.94 m 1.75 m | 40.8 | 1.70 m 1.63 m | 39.2 | 1.67 m | 25.9 | 1.82 m 2.17 m | 27.8 |
| 6 | 1.87 m | 26.7 | 1.56 overlap 1.96 overlap | 32.2 | 2.48 m 2.37 m | 29.2 | 2.57 t (13.2) 1.67 m | 25.6 | 1.62 m | 25.9 |
| 7 | 2.88 m | 47.0 | 2.19 m | 55.4 | - | 140.3 | 2.66 m | 52.3 | 2.39 overlap | 51.2 |
| 8 | - | 149.1 | 152.8 | - | 127.6 | - | 81.6 | - | 81.6 | |
| 9 | 2.23 m 1.88 m | 32.5 | 2.51 m 1.99 m | 35.2 | 2.32 m 1.95 m | 31.0 | 1.75 m | 40.0 | 1.76 m 1.69 m | 40.0 |
| 10 | 1.34 m 2.36 m | 24.9 | 1.56 overlap 1.96 overlap | 31.2 | 1.23 m 2.04 m | 23.7 | 2.35 m 1.35 m | 28.2 | 2.27 m 2.17 m | 27.4 |
| 11 | 3.15 dd (10.8, 3.3) | 67.0 | 2.89 dd (9.6, 1.5) | 67.9 | 2.84 dd (11.4, 2.4) | 66.5 | 2.88 dd (9.6, 4.5) | 60.5 | 5.08 t (10.5) | 124.6 |
| 12 | - | 59.3 | - | 61.0 | - | 59.9 | - | 60.6 | 134.5 | |
| 13 | 1.48 m 2.12 m | 33.8 | 2.11 m 1.56 overlap | 34.9 | 1.48 td (13.5, 4.8) 2.11 m | 35.3 | 2.06 m | 37.2 | 2.06 m 1.79 m | 36.8 |
| 14 | 2.21 m | 25.3 | 2.23 m 2.05 m | 23.8 | 2.04 m 2.24 m | 23.8 | 1.94 m | 24.7 | 2.43 m 1.94 m | 29.2 |
| 15 | 3.10 m | 28.9 | 2.95 m | 29.8 | 3.03 m | 29.6 | 2.15 m | 32.7 | 2.20 m | 33.4 |
| 16 | 1.01 d (6.9) | 20.8 | 0.96 d (6.9) | 20.6 | 1.05 d (6.9) | 20.0 | 1.00 d (6.9) | 24.5 | 1.03 d (6.9) | 22.4 |
| 17 | 0.99 d (6.9) | 21.3 | 0.98 d (6.9) | 21.4 | 0.98 d (6.9) | 20.5 | 1.02 d (6.6) | 22.5 | 1.05 d (6.9) | 24.5 |
| 18 | 1.09 s | 24.6 | 1.14 s | 26.3 | 1.23 s | 24.9 | 4.96 s 4.89 s | 110.1 | 4.86 s 4.79 s | 113.0 |
| 19 | 5.20 s 5.11 s | 108.6 | 4.92 s 4.96 s | 110.8 | 1.67 s | 18.3 | 1.12 s | 25.0 | 1.08 s | 24.4 |
| 20 | 1.14 s | 15.4 | 1.09 s | 16.3 | 1.18 s | 16.1 | 1.38 s | 16.6 | 1.62 s | 17.6 |
| Compounds | NO Production 1 (% of LPS Group) ± SEM | |
|---|---|---|
| 20 μM | 40 μM | |
| 1 | 92.97 ± 5.44 | 81.07 ± 4.04 * |
| 2 | 87.00 ± 3.16 * | 73.73 ± 3.36 *** |
| 3 | 82.63 ± 3.86 * | 67.33 ± 4.11 *** |
| 4 | 72.00 ± 0.81 *** | 39.03 ± 2.31 *** |
| 5 | 53.57 ± 1.24 *** | 13.30 ± 3.21 *** |
| 6 | 87.60 ± 2.55 ** | 71.00 ± 1.86 *** |
| Resveratrol (20 μM) | 49.93 ± 6.66 ** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.-Q.; Chen, J.; Wu, M.-J.; Zhang, H.-Y.; Liang, L.-F.; Guo, Y.-W. Uncommon Capnosane Diterpenes with Neuroprotective Potential from South China Sea Soft Coral Sarcophyton boettgeri. Mar. Drugs 2022, 20, 602. https://doi.org/10.3390/md20100602
Du Y-Q, Chen J, Wu M-J, Zhang H-Y, Liang L-F, Guo Y-W. Uncommon Capnosane Diterpenes with Neuroprotective Potential from South China Sea Soft Coral Sarcophyton boettgeri. Marine Drugs. 2022; 20(10):602. https://doi.org/10.3390/md20100602
Chicago/Turabian StyleDu, Ye-Qing, Jing Chen, Meng-Jun Wu, Hai-Yan Zhang, Lin-Fu Liang, and Yue-Wei Guo. 2022. "Uncommon Capnosane Diterpenes with Neuroprotective Potential from South China Sea Soft Coral Sarcophyton boettgeri" Marine Drugs 20, no. 10: 602. https://doi.org/10.3390/md20100602
APA StyleDu, Y.-Q., Chen, J., Wu, M.-J., Zhang, H.-Y., Liang, L.-F., & Guo, Y.-W. (2022). Uncommon Capnosane Diterpenes with Neuroprotective Potential from South China Sea Soft Coral Sarcophyton boettgeri. Marine Drugs, 20(10), 602. https://doi.org/10.3390/md20100602









