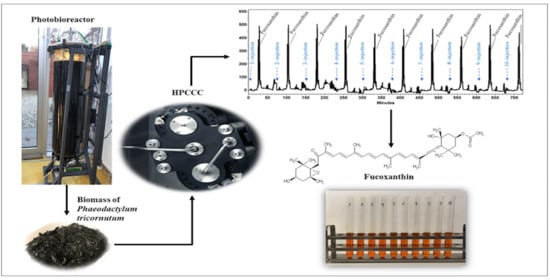
Production of Fucoxanthin from Phaeodactylum tricornutum Using High Performance Countercurrent Chromatography Retaining Its FOXO3 Nuclear Translocation-Inducing Effect
Abstract
1. Introduction
2. Results and Discussion
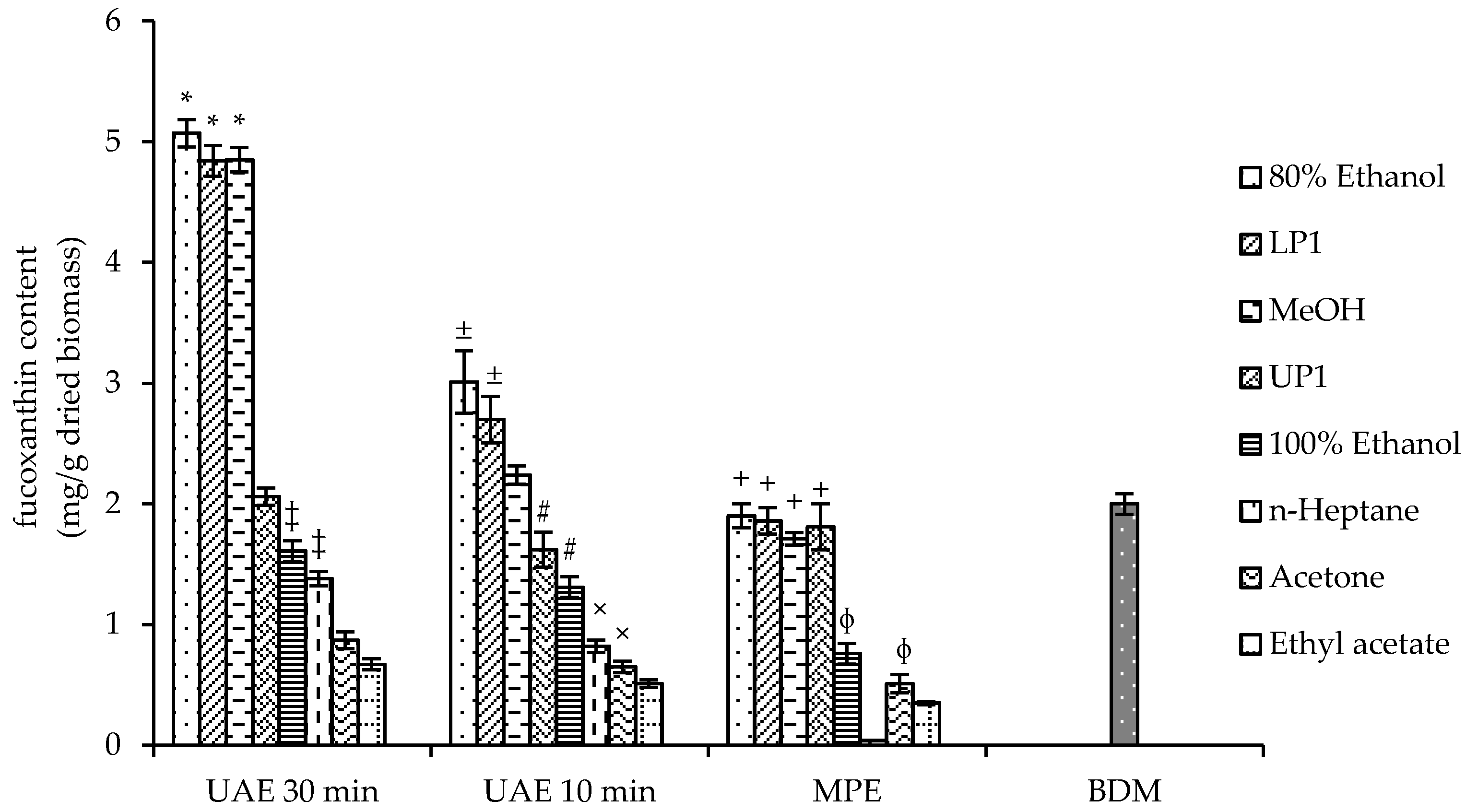
2.1. Extract Preparation
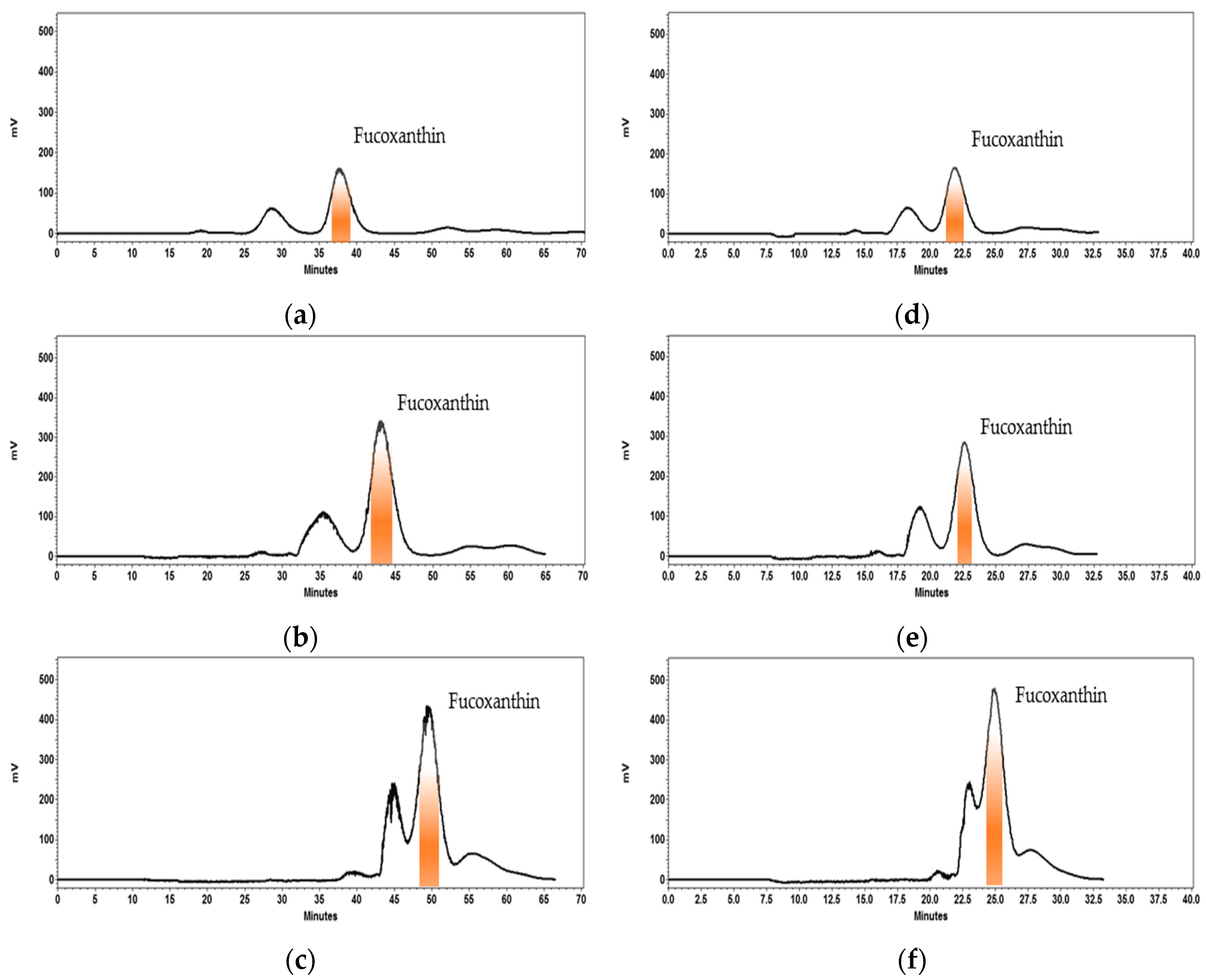
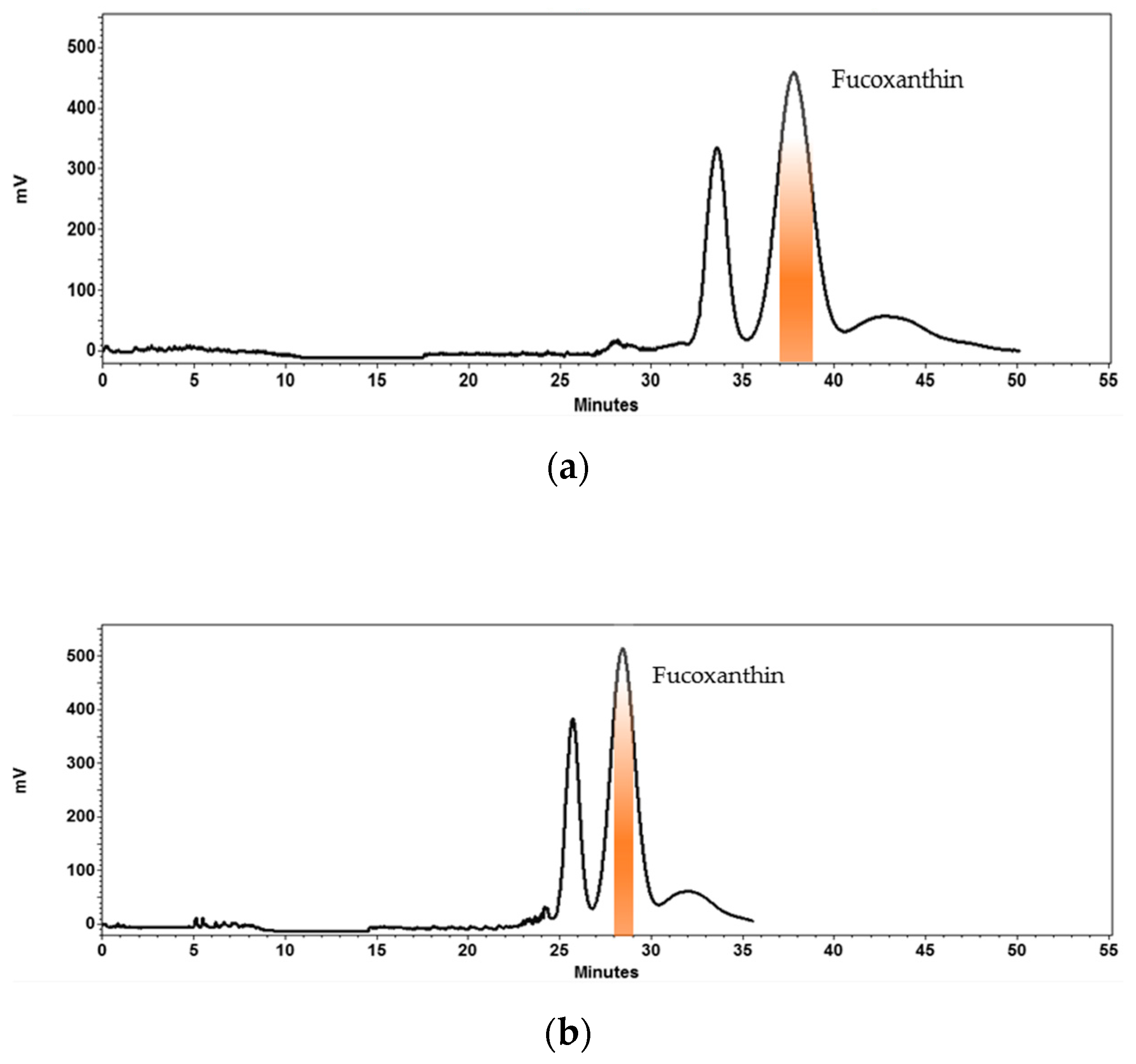
2.2. Development and Optimization of HPCCC Separation at Analytical Scale and Scale-Up to Semi-Prep Column
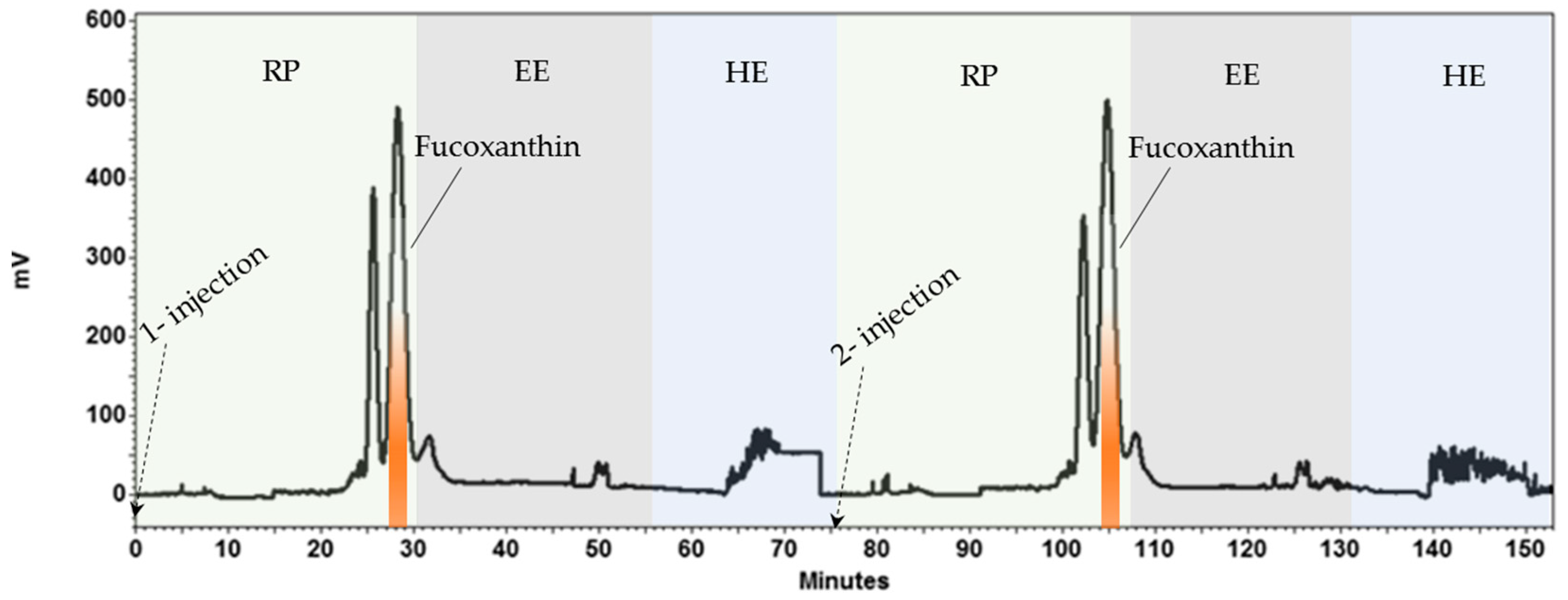
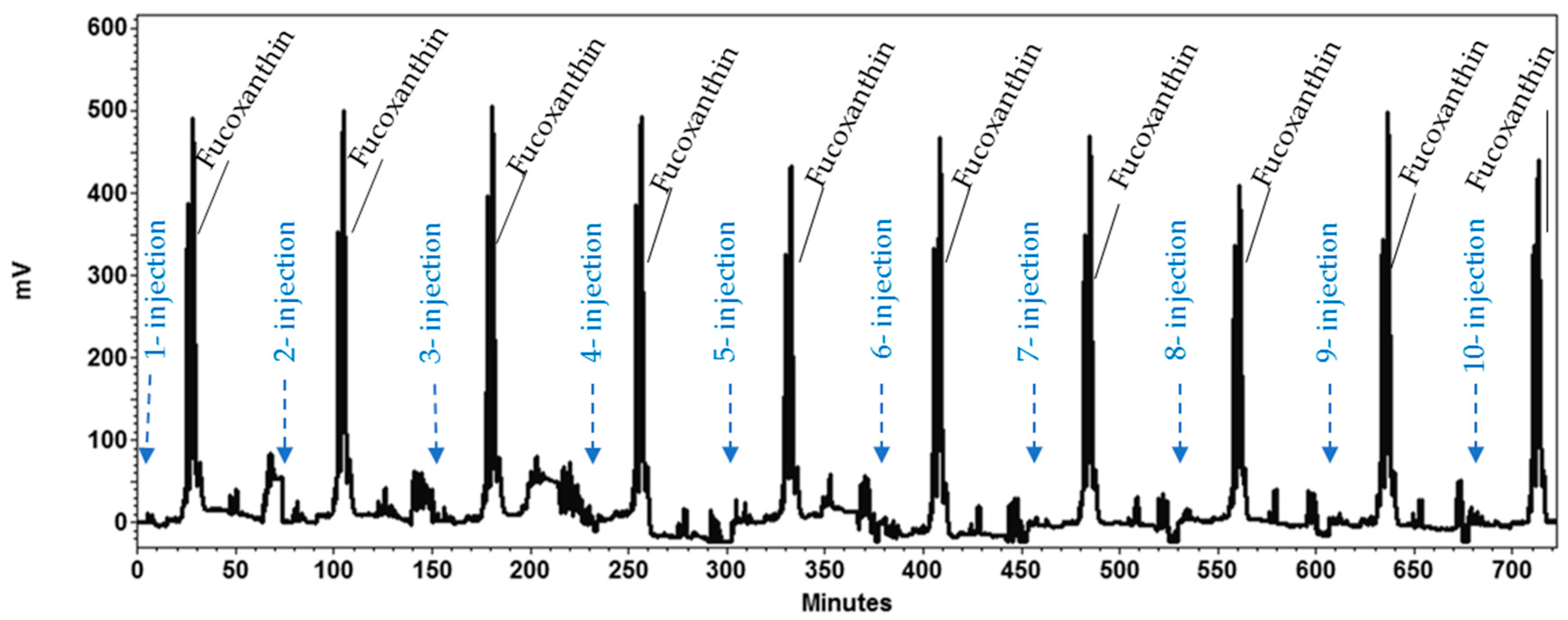
2.3. HPCCC Sequential Isolation of Fucoxanthin
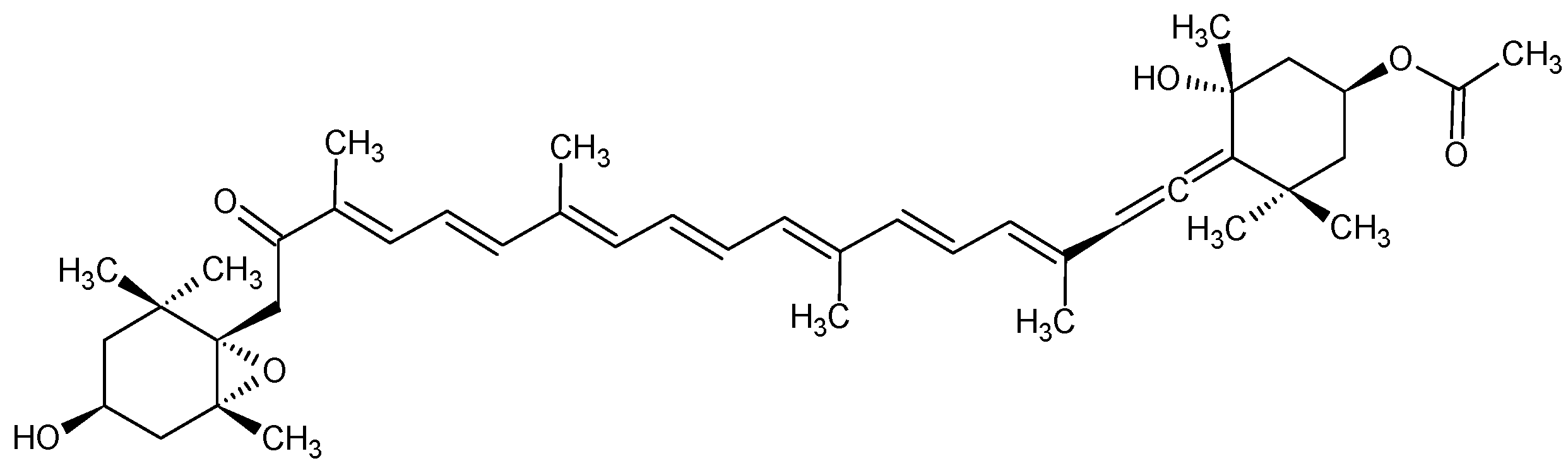
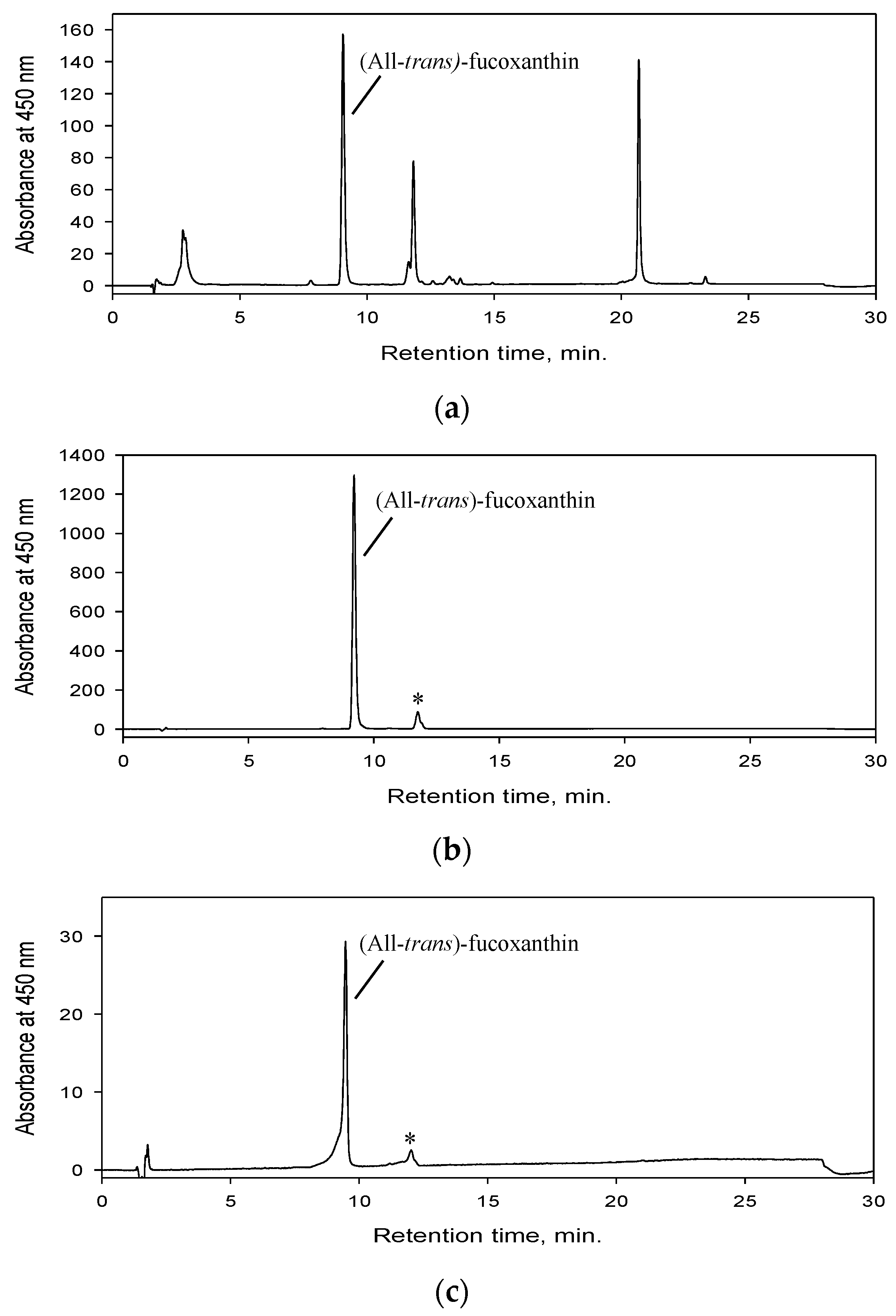
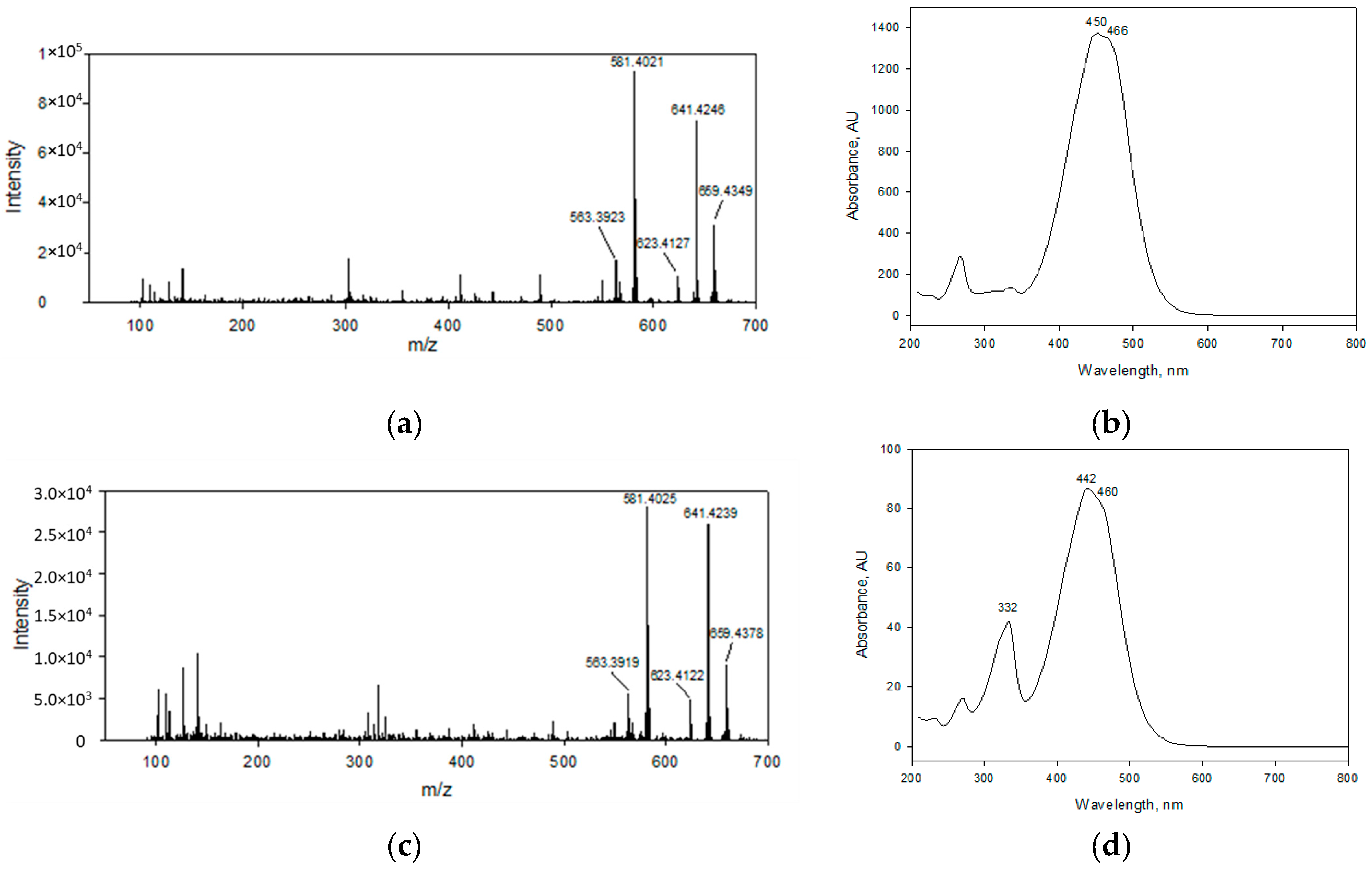
2.4. Identity Confirmation of The Isolated Target Compound
2.5. HPCCC Process Performance
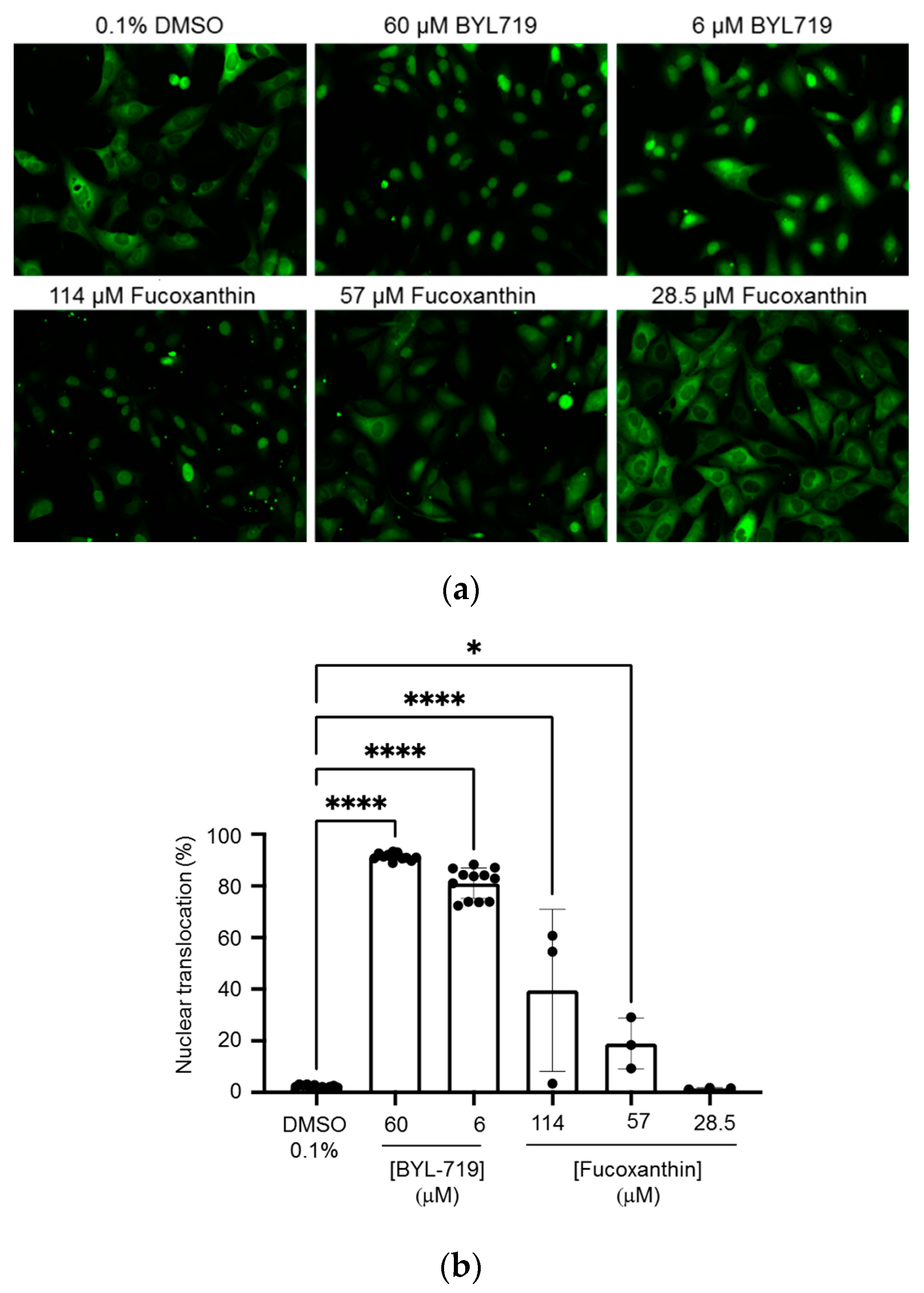
2.6. Induction of Nuclear Translocation of FOXO3 by Fucoxanthin
3. Materials and Methods
3.1. Biomass Production
3.2. Optimization of Biomass Extraction
3.3. High Performance Countercurrent Chromatography (HPCCC) Separation
3.3.1. HPCCC Equipment
3.3.2. Selection of the Suitable Biphasic Solvent System for HPCCC
3.3.3. HPCCC Separation Process
3.4. HPLC-DAD Analysis of Extract and Fractions
3.5. Confirmation of the Chemical Identity of the Purified Target Compound
3.6. High Performance Countercurrent Chromatography (HPCCC) Process Performance
3.7. Induction of Nuclear Translocation of FOXO3
3.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guler, B.A.; Deniz, I.; Demirel, Z.; Yesil-Celiktas, O.; Imamoglu, E. A novel subcritical fucoxanthin extraction with a biorefinery approach. Biochem. Eng. J. 2020, 153, 107403. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complement. Alternat. Med. 2015, 723515. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Kim, H.J.; Woo, M.N.; Lee, M.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol. J. 2010, 5, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.N.; Jeon, S.M.; Shin, Y.C.; Lee, M.K.; Kang, M.A.; Choi, M.S. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res. 2009, 53, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 1, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.K.; Jeon, J.Y. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.; Wang, G.; Chen, L.; et al. Fucoxanthin, a marine xanthophyll isolated from Conticribra weissflogii ND-8: Preventive anti-inflammatory effect in a mouse model of sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Karimian, H.; Khanabdali, R.; Razavi, M.; Firoozinia, M.; Zandi, K.; Kadir, H.A. Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci. World J. 2014, 768323. [Google Scholar] [CrossRef]
- Ishikawa, C.; Tafuku, S.; Kadekaru, T.; Sawada, S.; Tomita, M.; Okudaira, T.; Nakazato, T.; Toda, T.; Uchihara, J.N.; Taira, N.; et al. Antiadult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int. J. Cancer 2008, 123, 2702–2712. [Google Scholar] [CrossRef]
- Das, S.K.; Hashimoto, T.; Kanazawa, K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim. Biophys. 2008, 1780, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, P.; Hamada, M.; Takahashi, S.; Xing, G.; Liu, J.; Sugiura, N. Potential chemoprevention effect of dietary fucoxanthin on urinary bladder cancer EJ-1 cell line. Oncol. Rep. 2008, 20, 1099–1103. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kikuchi, M.; Kubodera, A.; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-diphenyl-2-picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int. 1997, 42, 361–370. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Mise, T.; Ueda, M.; Yasumoto, T. Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol. 2011, 3, 73–76. [Google Scholar]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative effects of beta-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef]
- Yang, G.; Jin, L.; Zheng, D.; Tang, X.; Yang, J.; Fan, L.; Xie, X. Fucoxanthin Alleviates Oxidative Stress through Akt/Sirt1/FoxO3α Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs. Mar. Drugs 2019, 17, 702. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, J.; Fang, C.; Cao, Q.; Xing, M.; Li, X.; Hou, J.; Ji, A.; Song, S. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Mar. Drugs 2020, 18, 634. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, D.; Xie, J. Diatoms as cell factories for high-value products: Chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- BGG World. Available online: https://bggworld.com/thinogentm-fucoxanthin/ (accessed on 10 June 2021).
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Derwenskus, F.; Metz, F.; Gille, A.; Schmid-Staiger, U.; Briviba, K.; Schließmann, U.; Hirth, T. Pressurized extraction of unsaturated fatty acids and carotenoids from wet Chlorella vulgaris and Phaeodactylum tricornutum biomass using subcritical liquids. Glob. Bioenergy 2019, 11, 335–344. [Google Scholar] [CrossRef]
- Global Fucoxanthin (CAS 3351-86-8) Market 2020 by Manufacturers, Regions, Type and Application, Forecast to 2025. Available online: https://www.360researchreports.com/global-fucoxanthin-cas-3351-86-8-market-16507769 (accessed on 15 January 2021).
- Noviendri, D.; Jaswir, I.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Fucoxanthin extraction and fatty acid analysis of Sargassum bindery and S. dulicatum. J. Med. Plants Res. 2011, 5, 2405–2412. [Google Scholar]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Muhammad, T.; Miyashita, K. Isolation of fucoxanthin and fatty acids analysis of Padina australis and cytotoxic effect of fucoxanthin on human lung cancer (H1299) cell lines. Afr. J. Biotechnol. 2011, 10, 18855–18862. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Analysis of fucoxanthin content and purification of all-trans-fucoxanthin from Turbinaria turbinate and Sargassum plagyophyllum by SiO2 open column chromatography and reversed phase-HPLC. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1340–1354. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Gao, B.; Huang, L.; Zhang, C. An integrated biorefinery process: Stepwise extraction of fucoxanthin, eicosapentaenoic acid and chrysolaminarin from the same Phaeodactylum tricornutum biomass. Algal Res. 2018, 32, 193–200. [Google Scholar] [CrossRef]
- Sun, P.; Wong, C.C.; Li, Y.; He, Y.; Mao, X.; Wu, T.; Ren, Y.; Chen, F. A novel strategy for isolation and purification of fucoxanthinol and fucoxanthin from the diatom Nitzschia laevis. Food Chem. 2019, 30, 566–572. [Google Scholar] [CrossRef]
- Billakanti, M.J.; Catchpole, O.; Fenton, T.; Mitchell, K. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process. Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Partition behavior of fucoxanthin in ethanol-potassium phosphate two-phase systems. J. Chem. Technol. Biotechnol. 2014, 89, 1637–1645. [Google Scholar] [CrossRef]
- Roh, M.K.; Uddin, M.S.; Chun, B.S. Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using supercritical carbon dioxide with co-solvent. Biotechnol Bioprocess. Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Shang, Y.F.; Kim, S.M.; Lee, W.J.; Um, B.H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) setchell. J. Biosci Bioeng. 2011, 111, 237–241. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Barranco, A.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Development of new green processes for the recovery of bioactives from Phaeodactylum tricornutum. Food Res. Int. 2017, 99, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Destandau, E.; Elfakir, C. New advances in countercurrent chromatography and centrifugal partition chromatography: Focus on coupling strategy. Anal. Bioanal. Chem. 2014, 406, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Bárcenas-Pérez, D.; Lukeš, M.; Hrouzek, P.; Kubáč, D.; Kopecký, J.; Kaštánek, P.; Cheel, J. A biorefinery approach to obtain docosahexaenoic acid and docosapentaenoic acid n-6 from Schizochytrium using high performance countercurrent chromatography. Algal Res. 2021, 55, 102241. [Google Scholar] [CrossRef]
- Cheel, J.; Urajová, P.; Hájek, J.; Hrouzek, P.; Kuzma, M.; Bouju, E.; Faure, K.; Kopecký, J. Separation of cyclic lipopeptide puwainaphycins from cyanobacteria by countercurrent chromatography combined with polymeric resins and HPLC. Anal. Bioanal. Chem. 2017, 409, 917–930. [Google Scholar] [CrossRef]
- Cheel, J.; Hájek, J.; Kuzma, M.; Saurav, K.; Smýkalová, I.; Ondráčková, E.; Urajová, P.; Vu, D.L.; Faure, K.; Kopecký, J.; et al. Application of HPCCC combined with polymeric resins and HPLC for the separation of cyclic lipopeptides muscotoxins A–C and their antimicrobial activity. Molecules 2018, 23, 2653. [Google Scholar] [CrossRef]
- Fábryová, T.; Cheel, J.; Kubac, D.; Hrouzek, P.; Vu, D.L.; Tůmová, L.; Kopecký, J. Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC). Algal Res. 2019, 41, 101574. [Google Scholar] [CrossRef]
- Fábryová, T.; Tůmová, L.; da Silva, D.C.; Pereira, D.M.; Andrade, P.B.; Valentão, P.; Hrouzek, P.; Kopecký, J.; Cheel, J. Isolation of astaxanthin monoesters from the microalgae Haematococcus pluvialis by high performance countercurrent chromatography (HPCCC) combined with high performance liquid chromatography (HPLC). Algal Res. 2020, 49, 101947. [Google Scholar] [CrossRef]
- Nováková, M.; Fábryová, T.; Vokurková, D.; Dolečková, I.; Kopecký, J.; Hrouzek, P.; Tůmová, L.; Cheel, J. Separation of the glycosylated carotenoid myxoxanthophyll from Synechocystis salina by HPCCC and evaluation of its antioxidant, tyrosinase inhibitory and immune-stimulating properties. Separations 2020, 7, 73. [Google Scholar] [CrossRef]
- Fábryová, T.; Kubáč, D.; Kuzma, M.; Hrouzek, P.; Kopecký, J.; Tůmová, L.; Cheel, J. High-performance countercurrent chromatography for lutein production from a chlorophyll-deficient strain of the microalgae Parachlorella kessleri HY1. J. Appl. Phycol. 2021. [Google Scholar] [CrossRef]
- Sutherland, I.; Thickitt, C.; Douillet, N.; Freebairn, K.; Johns, D.; Mountain, C.; Wood, P.; Edwards, N.; Rooke, D.; Harris, G.; et al. Scalable technology for the extraction of pharmaceutics: Outcomes from a 3 year collaborative industry/academia research programme. J. Chromatogr. A 2013, 1282, 84–94. [Google Scholar] [CrossRef][Green Version]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Kim, S.M.; Shang, Y.F.; Um, B.H. A preparative method for isolation of fucoxanthin from Eisenia bicyclis by centrifugal partition chromatography. Phytochem Anal. 2011, 22, 322–329. [Google Scholar] [CrossRef]
- Gonçalves de Oliveira-Júnior, R.; Grougnet, R.; Bodet, P.E.; Bonnet, A.; Nicolau, E.; Jebali, A.; Rumin, J.; Picot, L. Updated pigment composition of Tisochrysis lutea and purification of fucoxanthin using centrifugal partition chromatography coupled to flash chromatography for the chemosensitization of melanoma cells. Algal Res. 2020, 51, 102035. [Google Scholar] [CrossRef]
- Cheel, J.; Minceva, M.; Urajová, P.; Aslam, R.; Hrouzek, P.; Kopecký, J. Separation of Aeruginosin -865 from cultivated soil cyanobacterium (Nostoc sp.) by centrifugal partitition chromatography combined with gel permeation chromatography. Nat. Prod. Commun. 2015, 10, 1719–1722. [Google Scholar] [CrossRef]
- Ito, Y.; Conway, W.D. Experimental observations of the hydrodynamic behavior of solvent systems in high-speed counter-current chromatography. III. Effects of physical properties of the solvent systems and operating temperature on the distribution of two-phase solvent systems. J. Chromatogr. A 1984, 301, 405–414. [Google Scholar] [CrossRef]
- Berthod, A.; Maryutina, T.; Spivakov, B.; Shpigun, O.; Sutherland, I. Countercurrent chromatography in analytical chemistry (IUPAC Technical Report). Pure Appl. Chem. 2009, 81, 355–387. [Google Scholar] [CrossRef]
- Wood, P.; Ignatova, S.; Janaway, L.; Keay, D.; Hawes, D.; Garrard, I.; Sutherland, I.A. Counter-current chromatography separation scaled up from an analytical column to a production column. J. Chromatogr. A. 2007, 1151, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.A. Liquid stationary phase retention and resolution in hydrodynamic CCC. In Comprehensive Analytical Chemistry; Berthod, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2002; Volume 38, pp. 159–176. [Google Scholar]
- Crupi, P.; Toci, A.T.; Mangini, S.; Wrubl, F.; Rodolfi, L.; Tredici, M.R.; Coletta, A.; Antonacci, D. Determination of fucoxanthin isomers in microalgae (Isochrysis sp.) by high-performance liquid chromatography coupled with diode-array detector multistage mass spectrometry coupled with positive electrospray ionization. Rapid Commun. Mass Spectrom. 2013, 15, 1027–1035. [Google Scholar] [CrossRef]
- Haugan, J.A.; Englert, G.; Glinz, E.; Liaaen-Jensen, S. Algal Carotenoids. 48. Structural Assignments of Geometrical Isomers of Fucoxanthin. Acta Chem. Scand. 1992, 46, 389–395. [Google Scholar] [CrossRef]
- DeAmicis, C.; Edwards, N.A.; Giles, M.B.; Harris, G.H.; Hewitson, P.; Janaway, L.; Ignatova, S. Comparison of preparative reversed phase liquid chromatography and countercurrent chromatography for the kilogram scale purification of crude spinetoram insecticide. J. Chromatogr. A 2011, 1218, 6122–6127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ignatova, S.; Liang, Q.; Wu Jun, F.; Sutherland, I.; Wang, Y.; Luo, G. Rapid and high-throughput purification of salvianolic acid B from Salvia miltiorrhiza Bunge by high-performance counter-current chromatography. J. Chromatogr. A 2009, 18, 3869–3873. [Google Scholar] [CrossRef] [PubMed]
- Barradas, M.; Link, W.; Megias, D.; Fernandez-Marcos, P.J. High-Throughput Image-Based Screening to Identify Chemical Compounds Capable of Activating FOXO. Methods Mol. Biol. 2019, 1890, 151–161. [Google Scholar] [CrossRef]
- Zanella, F.; Rosado, A.; Garcia, B.; Carnero, A.; Link, W. Using multiplexed regulation of luciferase activity and GFP translocation to screen for FOXO modulators. BMC Cell Biol. 2009, 10, 14. [Google Scholar] [CrossRef]
- Zanella, F.; Rosado, A.; García, B.; Carnero, A.; Link, W. Chemical genetic analysis of FOXO nuclear-cytoplasmic shuttling by using image-based cell screening. Chembiochem 2008, 22, 2229–2237. [Google Scholar] [CrossRef]
- Furet, P.; Guagnano, V.; Fairhurst, R.A.; Imbach-Weese, P.; Bruce, I.; Knapp, M.; Fritsch, C.; Blasco, F.; Blanz, J.; Aichholz, R.; et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 2013, 23, 3741–3748. [Google Scholar] [CrossRef]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine Carotenoid Fucoxanthin Possesses Anti-Metastasis Activity: Molecular Evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Starr, R.C.; Zeikus, J.A. UTEX–The Culture Collection of Algae at the University of Texas at Austin 1993 List of Cultures. J. Phycol. 1993, 29, 106. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
| Solvent Systems | Composition | Relative Proportions of Solvents (v/v/v/v) | Phase Volume Ratio (UP/LP) | Settling Time (s) | Density Difference (LP−UP, g/mL) | Partition Coefficient (K) of Fucoxanthin |
|---|---|---|---|---|---|---|
| 1 | n-Hep–EtoAc–EtOH–H2O | 5/5/6/3 | 0.58 | 17 | 0.1284 | 0.515 |
| 2 | n-Hep–EtoAc–EtOH–H2O | 5/5/7/3 | 0.43 | 15 | 0.1171 | 0.314 |
| 3 | n-Hep–EtoAc–EtOH–H2O | 5/5/8/3 | 0.38 | 18 | 0.1311 | 0.205 |
| 4 | n-Hep–EtoAc–EtOH–H2O | 5/5/6/4 | 0.59 | 18 | 0.1301 | 0.897 |
| 5 | n-Hep–EtoAc–EtOH–H2O | 5/5/6/5 | 0.59 | 20 | 0.1497 | 1.942 |
| 6 | n-Hep–EtoAc–EtOH–H2O | 5/5/5/3 | 0.80 | 20 | 0.1232 | 0.590 |
| Optimization Experiments | Flow Rate (mL/min) | Sf at The Hydrodynamic Equilibrium in HPCCC (%) | Loading Per Injection (mg) | Peak Resolution (1/2) | Sf at The End of The HPCCC Separation Run (%) | Peak Purity (%) |
|---|---|---|---|---|---|---|
| a | 0.5 | 56.25 | 20 | 2.9 | 52.08 | 98 |
| b | 0.5 | 56.25 | 40 | 2.1 | 32.25 | 97 |
| c | 0.5 | 56.25 | 60 | 1.7 | 10.41 | 70 |
| d | 1.0 | 50 | 20 | 2.0 | 29.16 | 96 |
| e | 1.0 | 50 | 40 | 1.8 | 20.83 | 94 |
| f | 1.0 | 50 | 60 | 1.4 | 4.16 | 55 |
| Equipment | Column Volume | Throughput | Throughput |
|---|---|---|---|
| Spectrum | 134 mL | 0.189 g/h | 7.56 g/week a |
| Midi | 980 mL | 1.382 g/h | 55.28 g/week a |
| Maxi | 4.6 L | 6.488 g/h | 155.71 g/week b |
| NSMS | 8.820 L | 12.440 g/h | 298.56 g/week b |
| Maxi | 18 L | 25.388 g/h | 609.31 g/week b |
| HPCCC Process | Purity (%) | Pt (g/h) | Pe (g/h) | Er (L/g) | Ge (g2 h−1 L −1) |
|---|---|---|---|---|---|
| Method in this paper | 97.0 | 0.189 | 0.003 | 106.578 | 0.000028 |
| Method A1 [50] | 94.8 | 12.195 | 0.000405 | 900.6024 | 0.0000004496 |
| Method A2 [50] | 90.2 | 0.732 | 0.0005 | 685.7798 | 0.000000775 |
| Method A3 [50] | 90.4 | 7.317 | 0.0000976 | 3737.500 | 0.0000000261 |
| Method B [52] | 99.0 | 0.222 | 0.0026 | 261.797 | 0.00001010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárcenas-Pérez, D.; Střížek, A.; Hrouzek, P.; Kopecký, J.; Barradas, M.; Sierra-Ramirez, A.; Fernandez-Marcos, P.J.; Cheel, J. Production of Fucoxanthin from Phaeodactylum tricornutum Using High Performance Countercurrent Chromatography Retaining Its FOXO3 Nuclear Translocation-Inducing Effect. Mar. Drugs 2021, 19, 517. https://doi.org/10.3390/md19090517
Bárcenas-Pérez D, Střížek A, Hrouzek P, Kopecký J, Barradas M, Sierra-Ramirez A, Fernandez-Marcos PJ, Cheel J. Production of Fucoxanthin from Phaeodactylum tricornutum Using High Performance Countercurrent Chromatography Retaining Its FOXO3 Nuclear Translocation-Inducing Effect. Marine Drugs. 2021; 19(9):517. https://doi.org/10.3390/md19090517
Chicago/Turabian StyleBárcenas-Pérez, Daniela, Antonín Střížek, Pavel Hrouzek, Jiří Kopecký, Marta Barradas, Arantzazu Sierra-Ramirez, Pablo J. Fernandez-Marcos, and José Cheel. 2021. "Production of Fucoxanthin from Phaeodactylum tricornutum Using High Performance Countercurrent Chromatography Retaining Its FOXO3 Nuclear Translocation-Inducing Effect" Marine Drugs 19, no. 9: 517. https://doi.org/10.3390/md19090517
APA StyleBárcenas-Pérez, D., Střížek, A., Hrouzek, P., Kopecký, J., Barradas, M., Sierra-Ramirez, A., Fernandez-Marcos, P. J., & Cheel, J. (2021). Production of Fucoxanthin from Phaeodactylum tricornutum Using High Performance Countercurrent Chromatography Retaining Its FOXO3 Nuclear Translocation-Inducing Effect. Marine Drugs, 19(9), 517. https://doi.org/10.3390/md19090517