Preparation and Characterization of κ-Carrageenan Modified with Maleic Anhydride and Its Application in Films
Abstract
:1. Introduction
2. Results and Discussion
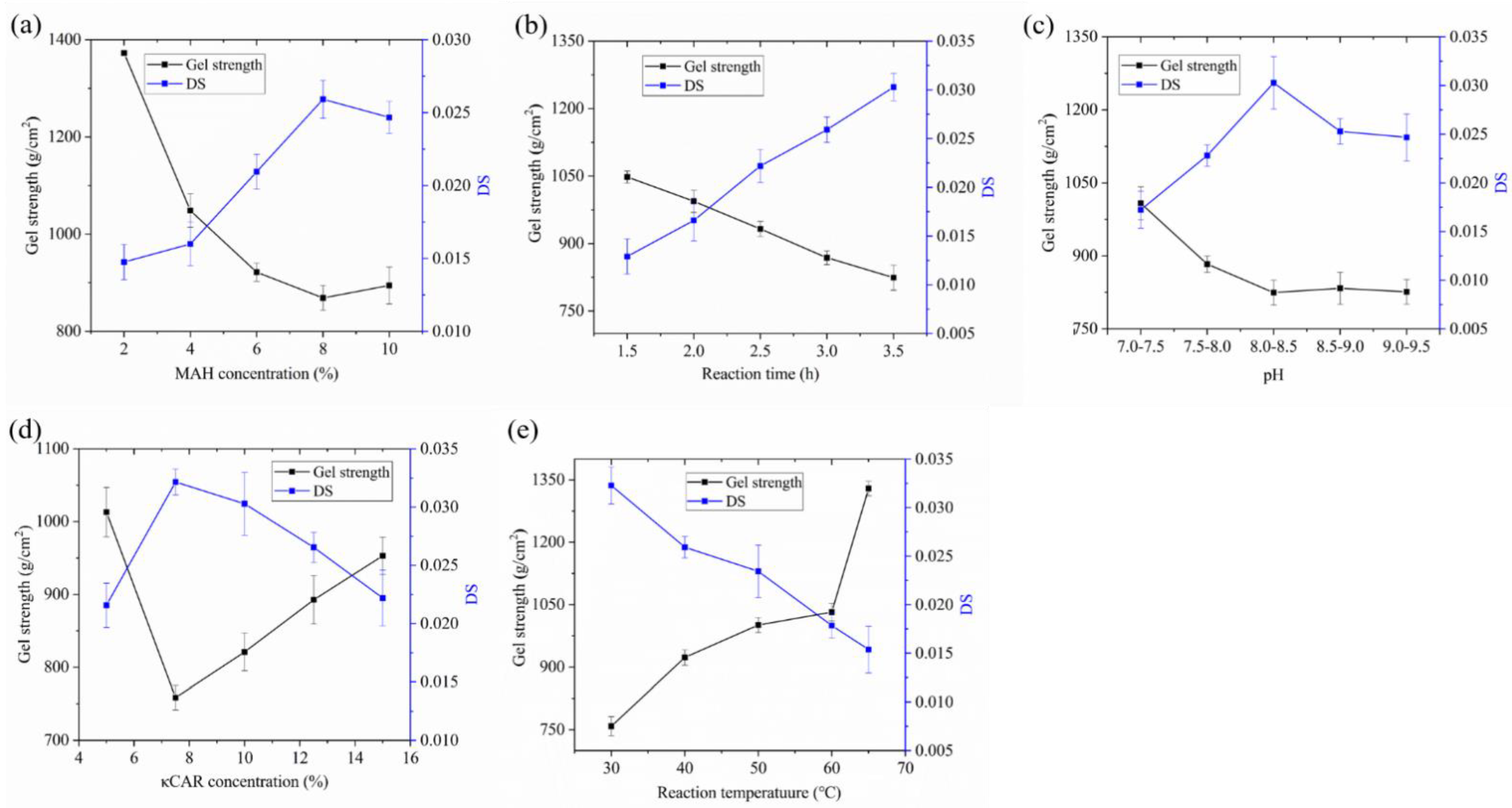
2.1. Optimization of Reaction Conditions for MC Synthesis
2.2. Characterization of MC
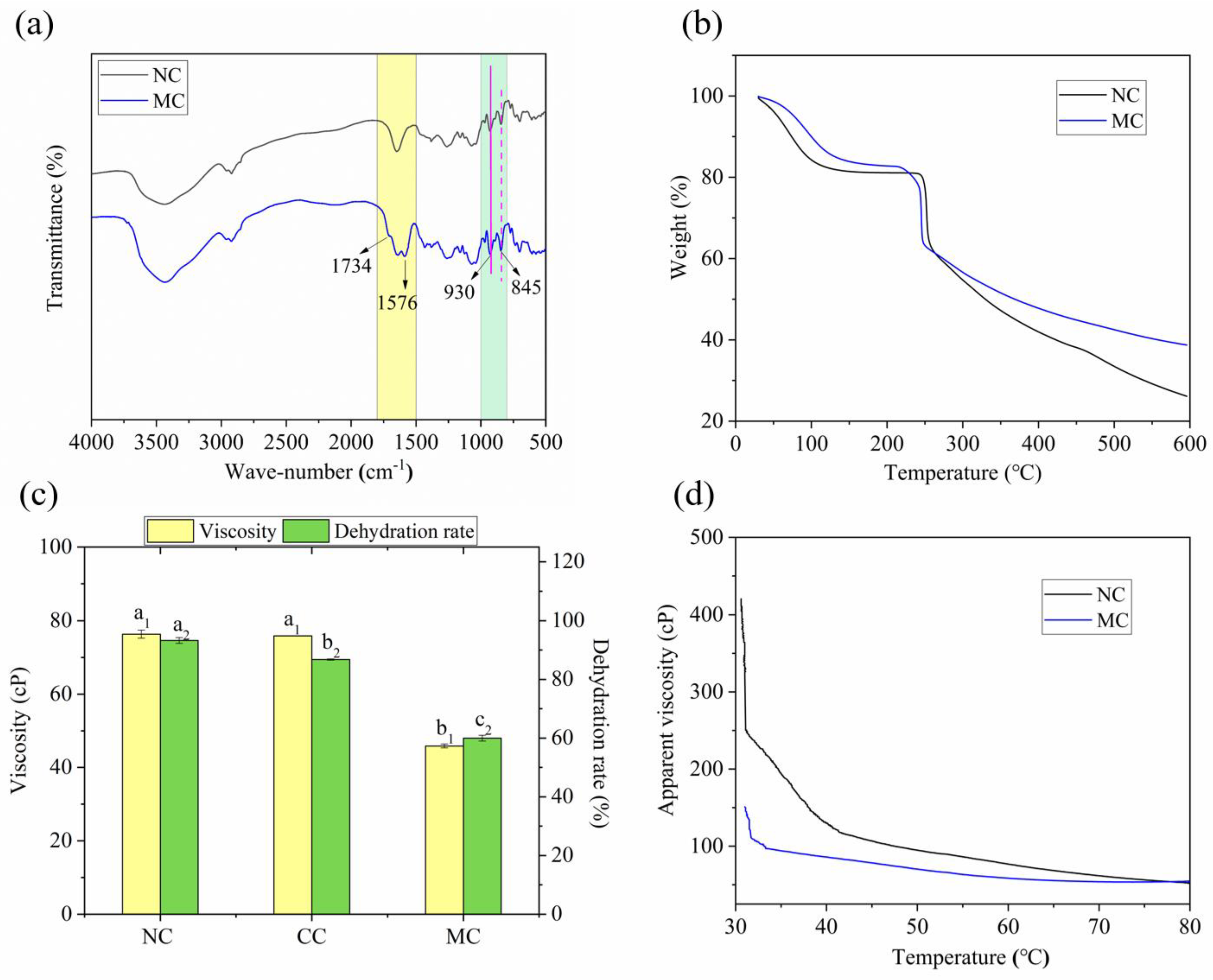
2.2.1. Fourier Transform Infrared (FT-IR) Analysis
2.2.2. Thermogravimetric Analysis (TGA)
2.3. Physicochemical Properties
2.3.1. Viscosity and Water Holding Capacity
2.3.2. Other Properties
2.4. Characterization and Properties of NC/MC Films
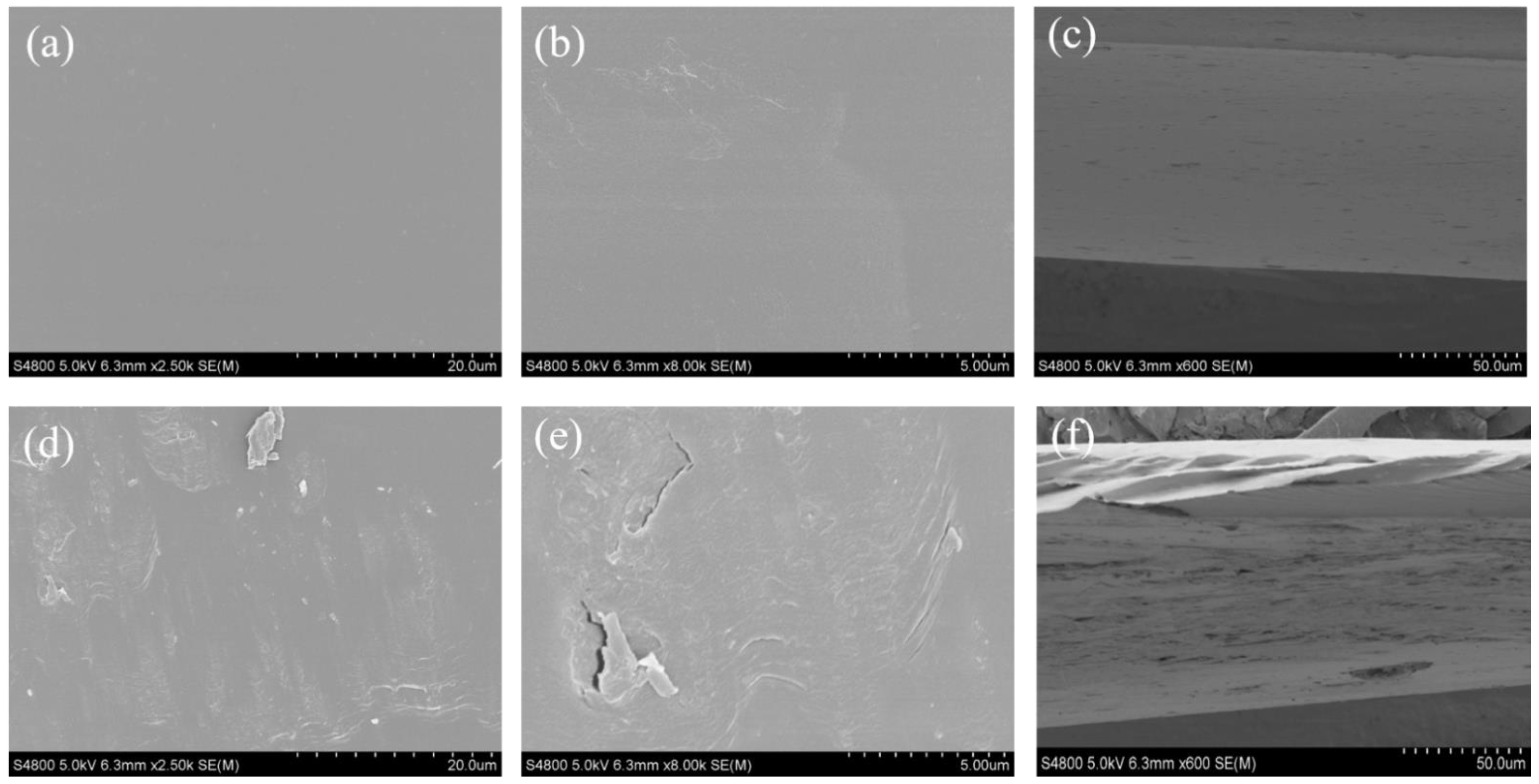
2.4.1. Morphology
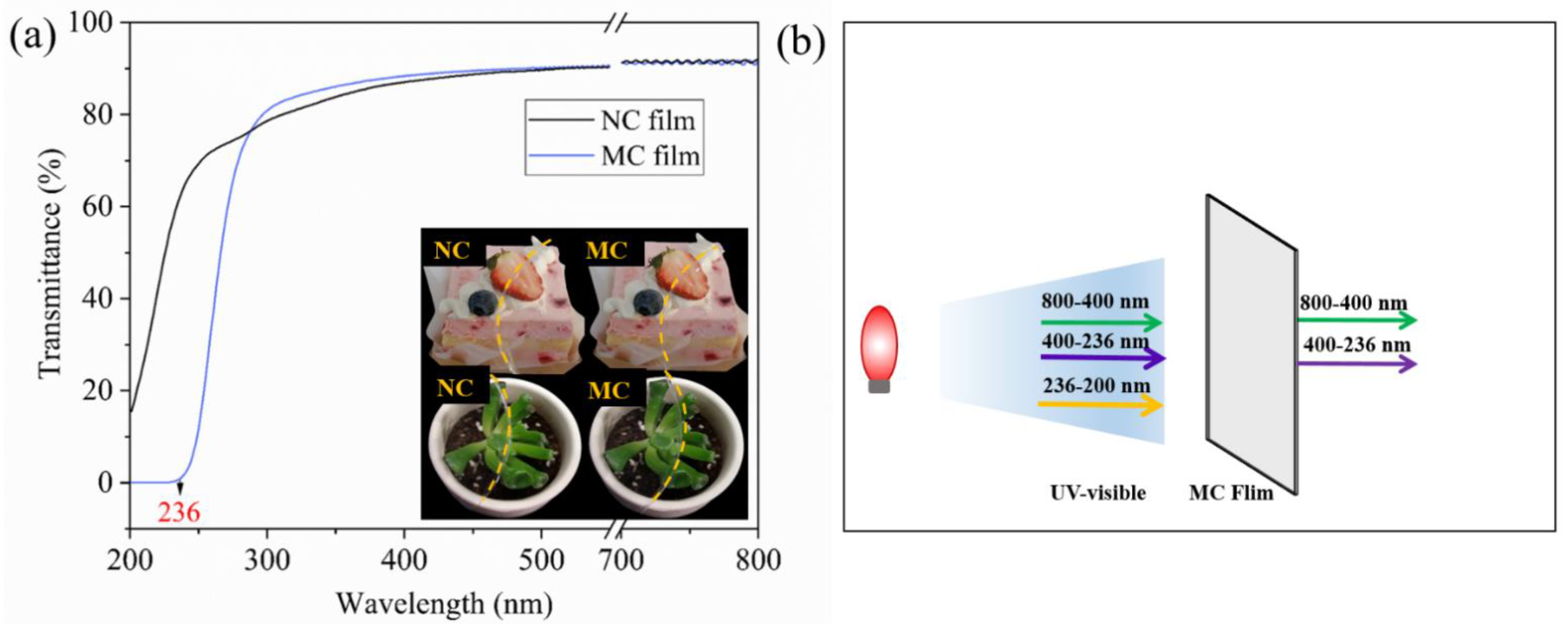
2.4.2. Optical Properties
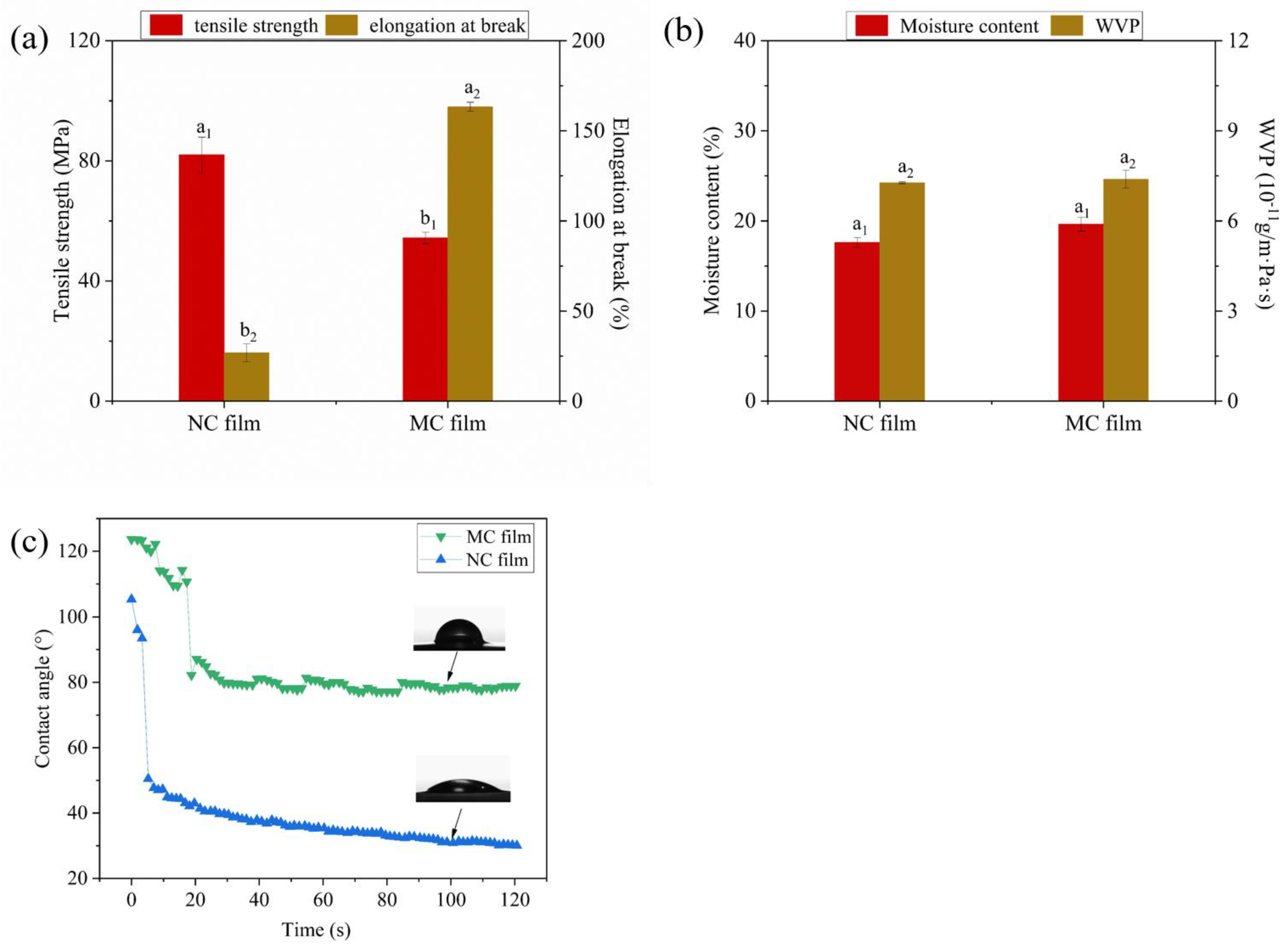
2.4.3. Mechanical and Water Affinity Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MC
3.3. Determination of DS
3.4. Characterization of MC
3.4.1. FT-IR
3.4.2. TGA
3.5. Determination of Physical Properties
3.6. Determination of Chemical Compositions
3.7. Preparation of NC/MC Films
3.8. Surface Morphology and Cross-Section of NC and MC Films
3.9. Performance Measurement for NC and MC Films
3.9.1. Thickness Measurement
3.9.2. Determination of Optical Properties
3.9.3. Determination of Mechanical Properties
3.9.4. Determination of Water Affinity Properties
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campo, V.L.; Kawano, D.F.; Silva, D.B.d.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Torres, M.D.; Chenlo, F.; Moreira, R. Structural features and water sorption isotherms of carrageenans: A prediction model for hybrid carrageenans. Carbohydr. Polym. 2018, 180, 72–80. [Google Scholar] [CrossRef]
- Torres, M.D.; Florez-Fernandez, N.; Dominguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [Green Version]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Fan, L.; Wang, L.; Gao, S.; Wu, P.; Li, M.; Xie, W.; Liu, S.; Wang, W. Synthesis, characterization and properties of carboxymethyl kappa carrageenan. Carbohydr. Polym. 2011, 86, 1167–1174. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Swedlund, P.J.; Hemar, Y. Physical and rheological properties of fish gelatin gel as influenced by κ-carrageenan. Food Biosci. 2017, 20, 88–95. [Google Scholar] [CrossRef]
- Savoji, M.T.; Pourjavadi, A. Partially hydrolyzed kappa carrageenan—Polyacrylonitrile as a novel biopolymer-based superabsorbent hydrogel: Synthesis, characterization, and swelling behaviors. Polym. Eng. Sci. 2006, 46, 1778–1786. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Nanaki, S.G.; Kyzas, G.Z.; Koulouktsi, C.; Bikiaris, D.N.; Lambropoulou, D.A. Novel Isocyanate-Modified Carrageenan Polymer Materials: Preparation, Characterization and Application Adsorbent Materials of Pharmaceuticals. Polymers 2017, 9, 595. [Google Scholar] [CrossRef] [Green Version]
- Marshall, P.; Papadimitriou, V.C.; Papanastasiou, D.K.; Roberts, J.M.; Burkholder, J.B. UV and infrared absorption spectra and 248 nm photolysis of maleic anhydride (C4H2O3). J. Photochem. Photobiol. 2019, 382, 111953. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Yang, L.; Qiao, Z.; Tan, H.; Zhang, Y. Synthesis and characterization of maleic anhydride esterified corn starch by the dry method. Int. J. Biol. Macromol. 2013, 62, 241–247. [Google Scholar] [CrossRef]
- Jiang, Y.-P.; Guo, X.-K.; Tian, X.-F. Synthesis and NMR structural analysis of O-succinyl derivative of low-molecular-weight κ-carrageenan. Carbohydr. Polym. 2005, 61, 399–406. [Google Scholar] [CrossRef]
- Sumathra, M.; Rajan, M.; Amarnath Praphakar, R.; Marraiki, N.; Elgorban, A.M. In Vivo Assessment of a Hydroxyapatite/kappa-Carrageenan-Maleic Anhydride-Casein/Doxorubicin Composite-Coated Titanium Bone Implant. ACS Biomater. Sci. Eng. 2020, 6, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.A. Structure, Conformation, and Mechanism in the Formation of Polysaccharide Gels and Networks. Adv.Carbohydr. Chem. Biochem. 1969, 24, 267–332. [Google Scholar] [PubMed]
- Gunaratne, A.; Corke, H. Influence of unmodified and modified cycloheptaamylose (β-cyclodextrin) on transition parameters of amylose–lipid complex and functional properties of starch. Carbohydr. Polym. 2007, 68, 226–234. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Sánchez-Rivera, M.M.; Núñez-Santiago, C.; Rodríguez-Ambriz, S.L.; Román-Gutierrez, A.D. Effect of the pearled in the isolation and the morphological, physicochemical and rheological characteristics of barley starch. Carbohydr. Polym. 2010, 81, 63–69. [Google Scholar] [CrossRef]
- Xiao, Q.; Weng, H.; Chen, G.; Xiao, A. Preparation and characterization of octenyl succinic anhydride modified agarose derivative. Food Chem. 2019, 279, 30–39. [Google Scholar] [CrossRef] [PubMed]
- de Lima Barizao, C.; Crepaldi, M.I.; Junior, O.O.S.; de Oliveira, A.C.; Martins, A.F.; Garcia, P.S.; Bonafe, E.G. Biodegradable films based on commercial kappa-carrageenan and cassava starch to achieve low production costs. Int. J. Biol. Macromol. 2020, 165, 582–590. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Meng, X.; Wang, Z.; Wu, S. Preparation and characterization of k-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation. Food Chem. 2021, 364, 130441. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A Review of Property Enhancement Techniques for Carrageenan-based Films and Coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef]
- Shahbazi, M.; Majzoobi, M.; Farahnaky, A. Physical modification of starch by high-pressure homogenization for improving functional properties of κ-carrageenan/starch blend film. Food Hydrocolloid. 2018, 85, 204–214. [Google Scholar] [CrossRef]
- Saedi, S.; Rhim, J.-W. Synthesis of Fe3O4@SiO2@PAMAM dendrimer@AgNP hybrid nanoparticles for the preparation of carrageenan-based functional nanocomposite film. Food Packag. Shelf. 2020, 24, 100473. [Google Scholar] [CrossRef]
- Zhuikova, Y.V.; Zhuikov, V.A.; Zubareva, A.A.; Akhmedova, S.A.; Sviridova, I.K.; Sergeeva, N.S.; Varlamov, V.P. Physicochemical and biological characteristics of chitosan/kappa-carrageenan thin layer-by-layer films for surface modification of nitinol. Micron. Technol. 2020, 138, 102922. [Google Scholar] [CrossRef]
- Hernández-Moreno, D.; de la Casa Resino, I.; Soler-Rodríguez, F. Maleic Anhydride. In Encyclopedia of Toxicology, 3rd ed.; Philip, W., Ed.; US National Library of Medicine: Bethesda, MD, USA, 2014; pp. 138–141. [Google Scholar]
- Moad, G. Chemical modification of starch by reactive extrusion. Prog. Polym. Sci. 2011, 36, 218–237. [Google Scholar] [CrossRef]
- Pongsawatmanit, R.; Srijunthongsiri, S. Influence of xanthan gum on rheological properties and freeze–thaw stability of tapioca starch. J. Food Eng. 2008, 88, 137–143. [Google Scholar] [CrossRef]
- Maity, T.; Saxena, A.; Raju, P.S. Use of hydrocolloids as cryoprotectant for frozen foods. Crit. Rev. Food. Sci. Nutr. 2018, 58, 420–435. [Google Scholar] [CrossRef]
- Bao, J.; Xing, J.; Phillips, D.L.; Corke, H. Physical Properties of Octenyl Succinic Anhydride Modified Rice, Wheat, and Potato Starches. J. Agric. Food Chem. 2003, 51, 2283–2287. [Google Scholar] [CrossRef]
- Abiddin, N.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological and stability of octenyl succinic anhydride (OSA) modified sago starch. Food Hydrocolloid. 2017, 75, 138–146. [Google Scholar] [CrossRef]
- Bartz, J.; Goebel, J.T.; Giovanaz, M.A.; Zavareze Eda, R.; Schirmer, M.A.; Dias, A.R. Acetylation of barnyardgrass starch with acetic anhydride under iodine catalysis. Food Chem. 2015, 178, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Ruan, H.; Chen, Q.H.; Fu, M.L.; Xu, Q.; He, G.Q. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86. [Google Scholar]
- Simsek, S.; Ovando-Martinez, M.; Marefati, A.; Sj, M.; Rayner, M. Chemical composition, digestibility and emulsification properties of octenyl succinic esters of various starches. Food Res. Int. 2015, 75, 41–49. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Xiao, Q.; Cai, M.; Yang, Q.; Weng, H.; Xiao, A. Structure and physicochemical properties of amphiphilic agar modified with octenyl succinic anhydride. Carbohydr. Polym. 2021, 251, 117031. [Google Scholar] [CrossRef]
- Quintero-Castano, V.D.; Castellanos-Galeano, F.J.; Alvarez-Barreto, C.I.; Lucas-Aguirre, J.C.; Bello-Perez, L.A.; Rodriguez-Garcia, M.E. Starch from two unripe plantains and esterified with octenyl succinic anhydride (OSA): Partial characterization. Food Chem. 2020, 315, 126241. [Google Scholar] [CrossRef]
- Wongprasert, K.; Rudtanatip, T.; Praiboon, J. Immunostimulatory activity of sulfated galactans isolated from the red seaweed Gracilaria fisheri and development of resistance against white spot syndrome virus (WSSV) in shrimp. Fish Shellfish. Immunol. 2014, 36, 52–60. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodriguez-Llamazares, S.; Abella, V.; Barral, L.; Bouza, R.; Farrag, Y.; Lago, F. Entrapment of chitosan, pectin or kappa-carrageenan within methacrylate based hydrogels: Effect on swelling and mechanical properties. Mat. Sci. Eng. C-Mater. 2019, 96, 583–590. [Google Scholar] [CrossRef]
- Meindrawan, B.; Suyatma, N.E.; Wardana, A.A.; Pamela, V.Y. Nanocomposite coating based on carrageenan and ZnO nanoparticles to maintain the storage quality of mango. Food Packag. Shelf. 2018, 18, 140–146. [Google Scholar] [CrossRef]
- Normand, V. Effect of sucrose on agarose gels mechanical behaviour. Carbohydr. Polym. 2003, 54, 83–95. [Google Scholar] [CrossRef]
- Sanchez-Rivera, M.M.; Almanza-Benitez, S.; Bello-Perez, L.A.; Mendez-Montealvo, G.; Nunez-Santiago, M.C.; Rodriguez-Ambriz, S.L.; Gutierrez-Meraz, F. Acetylation of banana (Musa paradisiaca L.) and corn (Zea mays L.) starches using a microwave heating procedure and iodine as catalyst: II. Rheological and structural studies. Carbohydr. Polym. 2013, 92, 1256–1261. [Google Scholar] [CrossRef]
- Ruiter, G.; Rudolph, B. Carrageenan biotechnology. Trends Food Sci. Technol. 1997, 8, 389–395. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Effect of octenylsuccinylation on physicochemical and functional properties of waxy maize and amaranth starches. Carbohydr. Polym. 2007, 68, 447–456. [Google Scholar] [CrossRef]
- Duckworth, M.; Yaphe, W. The structure of agar: Part I. Fractionation of a complex mixture of polysaccharides. Carbohydr. Res. 1971, 16, 189–197. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Liu, J.; Zhao, G. Effects of octenylsuccination on physical, mechanical and moisture-proof properties of stretchable sweet potato starch film. Food Hydrocolloid. 2015, 46, 226–232. [Google Scholar] [CrossRef]
- Cazon, P.; Vazquez, M.; Velazquez, G. Cellulose-glycerol-polyvinyl alcohol composite films for food packaging: Evaluation of water adsorption, mechanical properties, light-barrier properties and transparency. Carbohydr. Polym. 2018, 195, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.A.; Almendárez, B.E.G.; Reyes, A.A.; Pastrana, D.M.R.; López, G.F.G.; Belloso, O.M.; González, C.R. Design and Characterization of Corn Starch Edible Films Including Beeswax and Natural Antimicrobials. Food Bioprocess Technol. 2016, 10, 103–114. [Google Scholar] [CrossRef]
- Lu, J.; Fang, J.; Li, J.; Zhu, L. Engineering highly transparent UV-shielding films with disassembled polydopamine oligomers as light adsorber. Appl. Sur. Sci. 2021, 550, 149284. [Google Scholar] [CrossRef]
- Omar-Aziz, M.; Khodaiyan, F.; Yarmand, M.S.; Mousavi, M.; Gharaghani, M.; Kennedy, J.F.; Hosseini, S.S. Combined effects of octenylsuccination and beeswax on pullulan films: Water-resistant and mechanical properties. Carbohydr. Polym. 2021, 255, 117471. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Van der Meeren, P.; Hosseini, S.M.H. Study on hydrophobic modification of basil seed gum-based (BSG) films by octenyl succinate anhydride (OSA). Carbohydr. Polym. 2019, 219, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Huang, Q.; Luo, F.-x.; Fu, X.; Jiang, H.; Jane, J.-l. Effects of octenylsuccinylation on the structure and properties of high-amylose maize starch. Carbohydr. Polym. 2011, 84, 1276–1281. [Google Scholar] [CrossRef]
- Li, Y.T.; Wang, R.S.; Liang, R.H.; Chen, J.; He, X.H.; Chen, R.Y.; Liu, W.; Liu, C.M. Dynamic high-pressure microfluidization assisting octenyl succinic anhydride modification of rice starch. Carbohydr. Polym. 2018, 193, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-K.; Lim, P.-E.; Phang, S.-M.; Namasivayam, P.; Ho, C.-L. Agar properties of Gracilaria species (Gracilariaceae, Rhodophyta) collected from different natural habitats in Malaysia. Reg. Stud. Mar. Sci. 2016, 7, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Yarnpakdee, S.; Benjakul, S.; Kingwascharapong, P. Physico-chemical and gel properties of agar from Gracilaria tenuistipitata from the lake of Songkhla, Thailand. Food Hydrocolloid. 2015, 51, 217–226. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, C.; Li, H.; Dong, X.; Zhang, X. Studies on the physicochemical properties, gelling behavior and drug release performance of agar/kappa-carrageenan mixed hydrogels. Int. J. Biol. Macromol. 2020, 154, 878–887. [Google Scholar] [CrossRef]
- An, D.; Xiao, Q.; Zhang, C.; Cai, M.; Zhang, Y.; Weng, H.; Chen, F.; Xiao, A. Preparation, characterization, and application of high-whiteness agar bleached with hydrogen peroxide. Food Hydrocolloid. 2021, 113, 106520. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of kappa-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Liu, X.; Luan, J.; Zhang, X. Characterization of physicochemical properties of carboxymethyl agar. Carbohydr. Polym. 2014, 111, 449–455. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Influence of alkali treatment on agar from Gracilaria cornea from Yucatán, México. J. Appl. Phycol. 1997, 9, 533. [Google Scholar]
- Saedi, S.; Shokri, M.; Roy, S.; Rhim, J.-W. Silver loaded aminosilane modified halloysite for the preparation of carrageenan-based functional films. Appl. Clay Sci. 2021, 211, 106170. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Hao, G.; Weng, W.; Osako, K.; Tanaka, M. Effects of α1/α2 ratios and drying temperatures on the properties of gelatin films prepared from tilapia (Tilapia zillii) skins. Food Hydrocolloid. 2016, 52, 573–580. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Guerrero, P.; de la Caba, K. Characterization of agar/soy protein biocomposite films: Effect of agar on the extruded pellets and compression moulded films. Carbohydr. Polym. 2016, 151, 408–416. [Google Scholar] [CrossRef]
- Gennadios, A.; Brandenburg, A.H.; Park, J.W.; Weller, C.L.; Testin, R.F. Water vapor permeability of wheat gluten and soy protein isolate films. Ind. Crop. Prod. 1994, 2, 189–195. [Google Scholar] [CrossRef]
| Physicochemical Properties | NC | CC | MC/DS = 0.015 | MC/DS = 0.032 |
|---|---|---|---|---|
| Gel strength (g/cm2) | 1441 ± 20 a | 1432 ± 19 a | 1329 ± 17 b | 759 ± 23 c |
| 3,6-AG content (%) | 22.5 ± 0.3 a | 22.4 ± 0.1 a | 18.8 ± 0.1 b | 17.1 ± 1.3 b |
| Sulfate content (%) | 25.7 ± 1.7 a | 23.2 ± 3.6 a | 22.5 ± 1.7 a | 22.1 ± 1.1 a |
| Transparency (%) | 81.9 ± 0.1 d | 86.9 ± 0.1 c | 87.6 ± 0.0 b | 91.6 ± 0.1 a |
| Whiteness (%) | 57.3 ± 0.2 b | 54.3 ± 0.2 d | 58.5 ± 0.2 a | 56.9 ± 0.1 c |
| Dissolving temperature (°C) | 96.6 ± 0.1 a | 95.1 ± 2.6 a | 93.1 ± 2.6 a | 93.1 ± 0.1 a |
| Gelling temperature (°C) | 40.9 ± 0.2 a | 39.4 ± 0.9 b | 35.8 ± 0.4 c | 33.3 ± 0.4 d |
| Melting temperature (°C) | 55.7 ± 0.1 a | 54.6 ± 0.4 b | 54.0 ± 0.9 b | 50.4 ± 0.0 c |
| L | a | b | ∆E | |
|---|---|---|---|---|
| NC film | 89.29 ± 0.22 a | −0.92 ± 0.06 a | 2.74 ± 0.08 b | 2.89 ± 0.20 a |
| MC film | 89.51 ± 0.25 a | −1.04 ± 0.05 a | 3.18 ± 0.02 a | 2.94 ± 0.21 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Chen, F.-Q.; Chen, S.; Xiao, Q.; Weng, H.-F.; Yang, Q.-M.; Xiao, A.-F. Preparation and Characterization of κ-Carrageenan Modified with Maleic Anhydride and Its Application in Films. Mar. Drugs 2021, 19, 486. https://doi.org/10.3390/md19090486
Zhou Y, Chen F-Q, Chen S, Xiao Q, Weng H-F, Yang Q-M, Xiao A-F. Preparation and Characterization of κ-Carrageenan Modified with Maleic Anhydride and Its Application in Films. Marine Drugs. 2021; 19(9):486. https://doi.org/10.3390/md19090486
Chicago/Turabian StyleZhou, Yuan, Fu-Quan Chen, Si Chen, Qiong Xiao, Hui-Fen Weng, Qiu-Ming Yang, and An-Feng Xiao. 2021. "Preparation and Characterization of κ-Carrageenan Modified with Maleic Anhydride and Its Application in Films" Marine Drugs 19, no. 9: 486. https://doi.org/10.3390/md19090486
APA StyleZhou, Y., Chen, F.-Q., Chen, S., Xiao, Q., Weng, H.-F., Yang, Q.-M., & Xiao, A.-F. (2021). Preparation and Characterization of κ-Carrageenan Modified with Maleic Anhydride and Its Application in Films. Marine Drugs, 19(9), 486. https://doi.org/10.3390/md19090486





