Antiviral Activity of Carrageenans and Processing Implications
Abstract
:1. Introduction
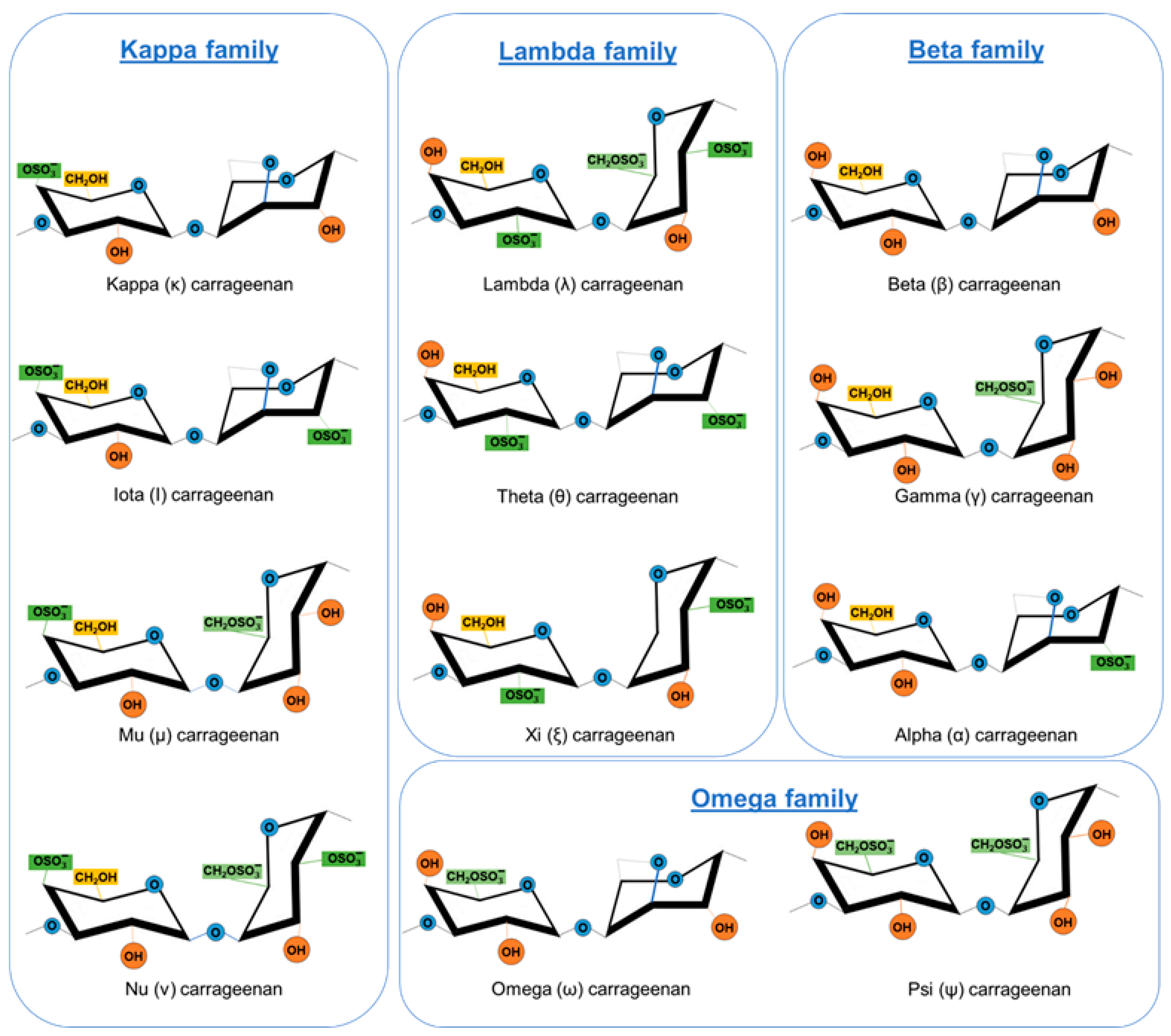
2. Carrageenans
3. Mechanisms of Antiviral Activity
3.1. Direct Virucidal Effect
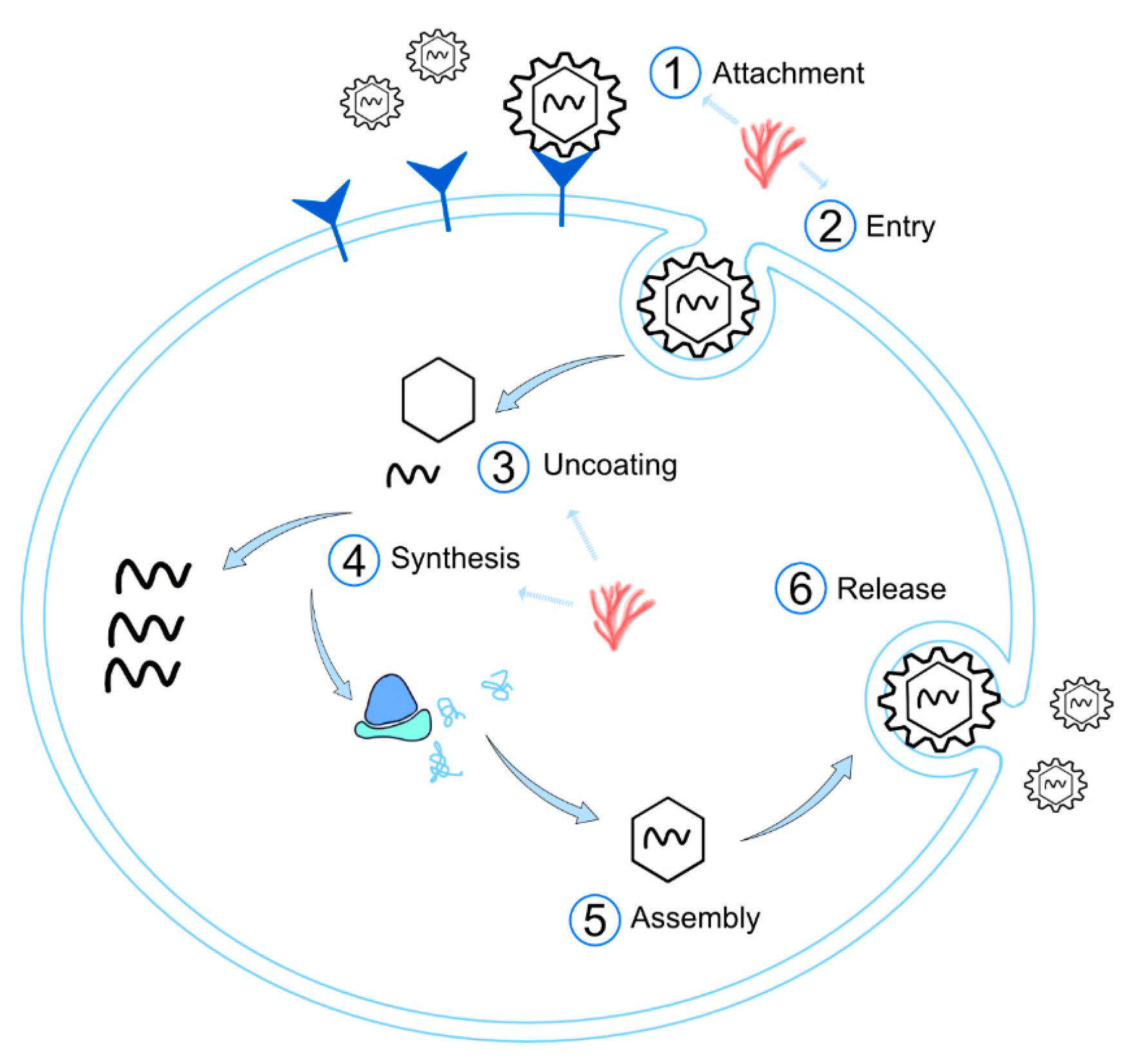
3.2. Effect on the Viral Replication
3.2.1. Inhibition of Viral Adsorption
3.2.2. Inhibition of Viral Internalization/Entry
3.2.3. Inhibition of Uncoating
3.2.4. Inhibition of Synthesis
3.3. Protective Effect on Cells
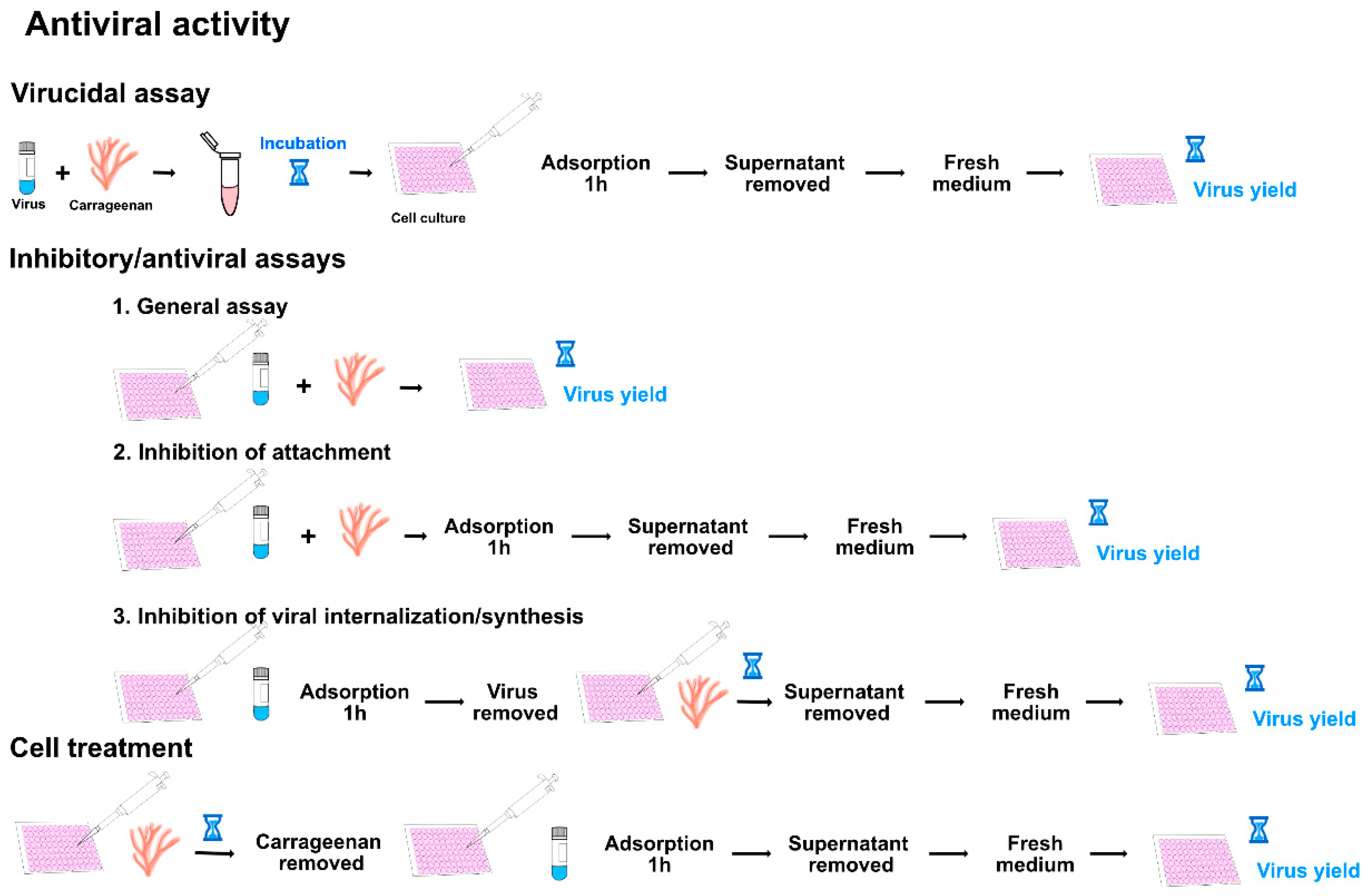
4. Determination of the Antiviral Activity
5. In Vivo Studies and Medical Applications
5.1. Animal Models
5.2. Medical Applications
5.3. Combination with Conventional Drugs
5.4. Patents Claiming the Use of Carrageenan
6. Factors Influencing Antiviral Properties
6.1. Type of Carrageenan
6.2. Sulfate Content
6.3. Molecular Weight
7. Processing
7.1. Extraction
7.2. Depolymerization
7.2.1. Mild Acid Hydrolysis
7.2.2. Subcritical Water Extraction
7.2.3. Enzymatic Depolymerization
7.2.4. Oxidative–Reductive Depolymerization
7.2.5. Hydrogen Peroxide (H2O2) Oxidation
7.2.6. Autohydrolysis
7.2.7. Gamma Irradiation
7.3. Fractionation and Purification of Carrageenan Oligosaccharides
7.4. Chemical Modifications
7.4.1. Acylation
7.4.2. Sulfation
7.4.3. Cyclization
8. Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, Q.; Guo, Q.; Xu, W.P.; Li, Z.; Zhao, T.T. Specific inhibitory effect of κ-carrageenan polysaccharide on swine pandemic 2009 H1N1 influenza virus. PLoS ONE 2015, 10, e0126577. [Google Scholar] [CrossRef]
- Hao, C.; Yu, G.; He, Y.; Xu, C.; Zhang, L.; Wang, W. Marine glycan–based antiviral agents in clinical or preclinical trials. Rev. Med. Virol. 2019, 29, e2043. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Brunet, C.; Noonan, D.M.; Albini, A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants 2020, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2021, 13, 100623. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.L.; Zhang, W.Z.; Ni, W.X.; Shao, J.W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carbohydr. Polym. 2021, 257, 117642. [Google Scholar] [CrossRef]
- Peñuela, A.; Bourgougnon, N.; Bedoux, G.; Robledo, D.; Madera-Santa, T.; Freile-Pelegrin, Y. Anti-Herpes simplex virus (HSV-1) activity and antioxidant capacity of carrageenan-rich enzymatic extracts from Solieria filiformis (Gigartinales, Rhodophyta). Int. J. Biol. Macromol. 2021, 168, 322–330. [Google Scholar]
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Klimyte, E.M.; Smith, S.E.; Oreste, P.; Lembo, D.; Dutch, R.E. Inhibition of Human Metapneumovirus Binding to Heparan Sulfate Blocks Infection in Human Lung Cells and Airway Tissues. J. Virol. 2016, 90, 9237–9250. [Google Scholar] [CrossRef] [Green Version]
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L.; et al. Prophylactic Antiviral Activity of Sulfated Glycomimetic Oligomers and Polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265. [Google Scholar] [CrossRef] [PubMed]
- Diogo, J.V.; Novo, S.G.; González, M.J.; Ciancia, M.; Bratanich, A.C. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res. Vet. Sci. 2015, 98, 142–144. [Google Scholar] [CrossRef]
- Abu-Galiyun, E.; Huleihel, M.; Levy-Ontman, O. Antiviral bioactivity of renewable polysaccharides against Varicella Zoster. Cell Cycle 2019, 18, 3540–3549. [Google Scholar] [CrossRef] [PubMed]
- Rojas Pérez, L.; Álvarez Vera, M.; Morier Díaz, L.F.; Valdés Iglesias, O.; del Barrio Alonso, G. Preliminary evaluation of the antiviral activity of Laurencia obtuse extract against herpesvirus and dengue virus. Rev. Cuba. Farm. 2016, 50, 106–116. [Google Scholar]
- Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application Prospect of Polysaccharides in the Development of Anti-Novel Coronavirus Drugs and Vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar]
- Ngo, D.H.; Kim, S.K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Praptyana, I.R.; Nurfiningsih; Ratnawati. Carrageenan: Nutraceutical and functional food as future food. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012068. [Google Scholar] [CrossRef]
- Bouhlal, R.; Haslin, C.; Chermann, J.C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef]
- Usov, A.I. Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocoll. 1998, 12, 301–308. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications. Curr. Pharm. Des. 2019, 25, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.H.; Chan, Y.L.; Tsai, L.W.; Li, T.L.; Wu, C.J. Prevention of human enterovirus 71 infection by kappa carrageenan. Antivir. Res. 2012, 95, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhu, Z.; Yu, P.; Zhang, X.; Dong, W.; Wang, X.; Chen, Y.; Liu, X. Inhibitory effect of iota-carrageenan on porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2019, 24, 261–270. [Google Scholar] [CrossRef]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. Biomed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 115, 77–82. [Google Scholar] [CrossRef]
- Pagarete, A.; Ramos, A.S.; Puntervoll, P.; Allen, M.J.; Verdelho, V. Antiviral Potential of Algal Metabolites—A Comprehensive Review. Mar. Drugs 2021, 19, 94. [Google Scholar] [CrossRef]
- Junter, G.-A.; Karakasyan, C. Polysaccharides against viruses: Immunostimulatory properties and the delivery of antiviral vaccines and drugs. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Carrageenans as Broad-Spectrum Microbicides: Current Status and Challenges. Mar. Drugs 2020, 18, 435. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elaya Perumal, U.; Sundararaj, R. Algae: A potential source to prevent and cure the novel coronavirus—A review. Int. J. Emerg. Technol. 2020, 11, 479–483. [Google Scholar]
- Frediansyah, A. The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review. Clin. Epidemiol. Glob. Health 2021, 12, 100826. [Google Scholar] [CrossRef]
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydari, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.H.; et al. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Pujol, C.A.; Damonte, E.B.; Stortz, C.A. Partial and total C-6 oxidation of gelling carrageenans. Modulation of the antiviral activity with the anionic character. Carbohydr. Polym. 2015, 128, 199–206. [Google Scholar] [CrossRef] [PubMed]
- De S.F-Tischer, P.C.; Talarico, L.B.; Noseda, M.D.; Silvia, S.M.; Damonte, E.B.; Duarte, M.E.R. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr. Polym. 2006, 63, 459–465. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Falshaw, R.; Bixler, H.J.; Johndro, K. Structure and performance of commercial κ-2 carrageenan extracts. Part III. Structure analysis and performance in two dairy applications of extracts from the New Zealand red seaweed, Gigartina atropurpurea. Food Hydrocoll. 2003, 17, 129–139. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir. Res. 1999, 43, 93–102. [Google Scholar] [CrossRef]
- Pujol, C.A.; Scolaro, L.A.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiviral activity of a carrageenan from Gigartina skottsbergii against intraperitoneal murine herpes simplex virus infection. Planta Med. 2006, 72, 121–125. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.M.; Faria, P.C.S.; Scolaro, L.A.; Duarte, M.E.R.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Kido, Y.; Kobayashi, N.; Motoki, Y.; Neushul, M.; Yamamoto, N. Purification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob. Agents Chemother. 1987, 31, 1524–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Tian, D.; Zhou, M.; Xiao, W.; Zhang, Y.; Li, M.; Sui, B.; Wang, W.; Guan, H.; Chen, H.; et al. lambda;-carrageenan P32 is a potent inhibitor of rabies virus infection. PLoS ONE 2015, 10, e0140586. [Google Scholar] [CrossRef]
- Sanjivkumar, M.; Chandran, M.N.; Suganya, A.M.; Immanuel, G. Investigation on bio-properties and in-vivo antioxidant potential of carrageenans against alloxan induced oxidative stress in Wistar albino rats. Int. J. Biol. Macromol. 2020, 151, 650–662. [Google Scholar] [CrossRef]
- Weiner, M.L. Parameters and pitfalls to consider in the conduct of food additive research, Carrageenan as a case study. Food Chem. Toxicol. 2016, 87, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Manuhara, G.J.; Praseptiangga, D.; Riyanto, R.A. Extraction and Characterization of Refined K-carrageenan of Red Algae [Kappaphycus alvarezii (Doty ex P.C. Silva, 1996)] Originated from Karimun Jawa Islands. Aquat. Procedia 2016, 7, 106–111. [Google Scholar] [CrossRef]
- Pereira, L. A Review of the Nutrient Composition of Selected Edible Seaweeds; Pomin, V.H., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; ISBN 978-161470878-0. [Google Scholar]
- Pereira, L.; Van De Velde, F. Portuguese carrageenophytes: Carrageenan composition and geographic distribution of eight species (Gigartinales, Rhodophyta). Carbohydr. Polym. 2011, 84, 614–623. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. Population studies and carrageenan properties in eight Gigartinales (Rhodophyta) from Iberian Peninsula. Seaweeds Agric. Uses Biol. Antioxid. Agents 2013, 2013, 115–134. [Google Scholar]
- Briones, A.V.; Ambal, W.O.; Monroyo, E.C.; Villanueva, M.A.; Estrella, R.R.; Lanto, E. USP grade Lambda-Like carrageenan from Halymenia durvillaei bory de Saint Vincent. Philipp. J. Sci. 2000, 129, 15–17. [Google Scholar]
- Azevedo, G.; Torres, M.D.; Sousa-Pinto, I.; Hilliou, L. Effect of pre-extraction alkali treatment on the chemical structure and gelling properties of extracted hybrid carrageenan from Chondrus crispus and Ahnfeltiopsis devoniensis. Food Hydrocoll. 2015, 50, 150–158. [Google Scholar] [CrossRef]
- Tuvikene, R.; Truus, K.; Vaher, M.; Kailas, T.; Martin, G.; Kersen, P. Extraction and quantification of hybrid carrageenans from the biomass of the red algae Furcellaria lumbricalis and Coccotylus truncatus. Proc. Est. Acad. Sci. Chem 2006, 55, 40–53. [Google Scholar]
- Ponthier, E.; Domínguez, H.; Torres, M.D. The microwave assisted extraction sway on the features of antioxidant compounds and gelling biopolymers from Mastocarpus stellatus. Algal Res. 2020, 51, 102081. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018, 16, e05238. [Google Scholar] [PubMed]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Impact of counterions on the thermo-rheological features of hybrid carrageenan systems isolated from red seaweed Gigartina skottsbergii. Food Hydrocoll. 2018, 84, 321–329. [Google Scholar] [CrossRef]
- Hebar, A.; Koller, C.; Seifert, J.M.; Chabicovsky, M.; Bodenteich, A.; Bernkop-Schnürch, A.; Grassauer, A.; Prieschl-Grassauer, E. Non-clinical safety evaluation of intranasal iota-carrageenan. PLoS ONE 2015, 10, e0122911. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L.; Ferguson, H.E.; Thorsrud, B.A.; Nelson, K.G.; Blakemore, W.R.; Zeigler, B.; Cameron, M.J.; Brant, A.; Cochrane, L.; Pellerin, M.; et al. An infant formula toxicity and toxicokinetic feeding study on carrageenan in preweaning piglets with special attention to the immune system and gastrointestinal tract. Food Chem. Toxicol. 2015, 77, 120–131. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.; Kim, C.W.; Lee, H.R.; Kim, M. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 821. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Alarcon, B.; Carrasco, L. Polysaccharides as antiviral agents: Antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987, 31, 1388–1393. [Google Scholar] [CrossRef] [Green Version]
- Baba, M.; Snoeck, R.; Pauwels, R.; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlucci, M.J.; Pujol, C.A.; Ciancia, M.; Noseda, M.D.; Matulewicz, M.C.; Damonte, E.B.; Cerezo, A.S. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: Correlation between structure and biological activity. Int. J. Biol. Macromol. 1997, 20, 97–105. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zúñiga, E.A. Carrageenans from chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Gomaa, H.H.A.; Elshoubaky, G.A. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 34–42. [Google Scholar]
- Vissani, M.A.; Galdo Novo, S.; Ciancia, M.; Zabal, O.; Thiry, E.; Bratanich, A.; Barrandeguy, M. Effects of lambda-carrageenan on equid herpesvirus 3 in vitro. J. Equine Vet. Sci. 2016, 39, S61–S62. [Google Scholar] [CrossRef]
- Talarico, L.B.; Noseda, M.D.; Ducatti, D.R.B.; Duarte, M.E.R.; Damonte, E.B. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J. Gen. Virol. 2011, 92, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ogamo, A.; Saito, T.; Uchiyama, H.; Nakagawa, Y. Preparation of O-acylated low-molecular-weight carrageenans with potent anti-HIV activity and low anticoagulant effect. Carbohydr. Polym. 2000, 41, 115–120. [Google Scholar] [CrossRef]
- Pavliga, S.N.; Kompanets, G.G.; Tsygankov, V.Y. The Experimental Research (in vitro) of Carrageenans and Fucoidans to Decrease Activity of Hantavirus. Food Environ. Virol. 2016, 8, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Bansal, S.; Jonsson, C.B.; Taylor, S.L.; Manuel Figueroa, J.; Vanesa, A.; Palacios, C.; César Vega, J. Iota-carrageenan and Xylitol inhibit SARS-CoV-2 in cell culture. bioRxiv 2020, 1–17. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Mitra, D.; Mccandless, M.G.; Sharma, P. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020, 163, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Jayaraman, G. Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19). Carbohydr. Res. 2021, 505, 108326. [Google Scholar] [CrossRef]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, 0671–0680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T.; et al. Iota-carrageenan is a potent inhibitor of influenza a virus infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef]
- Talarico, L.B.; Damonte, E.B. Characterization of in vitro Dengue Virus Resistance to Carrageenan. J. Med. Virol. 2016, 88, 1120–1129. [Google Scholar] [CrossRef]
- Bourne, K.Z.; Bourne, N.; Reising, S.F.; Stanberry, L.R. Plant products as topical microbicide candidates: Assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 1999, 42, 219–226. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Hao, C.; Zhang, X.E.; Cui, Z.Q.; Guan, H.S. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antivir. Res. 2011, 92, 237–246. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Yu, G.L.; Li, C.X.; Hao, C.; Qi, X.; Zhang, L.J.; Guan, H.S. Preparation and anti-influenza A virus activity of κ-carrageenan oligosaccharide and its sulphated derivatives. Food Chem. 2012, 133, 880–888. [Google Scholar] [CrossRef]
- Raman, R.; Tharakaraman, K.; Sasisekharan, V.; Sasisekharan, R. Glycan–protein interactions in viral pathogenesis. Curr. Opin. Struct. Biol. 2016, 40, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Shanker, S.; Hu, L.; Ramani, S.; Atmar, R.L.; Estes, M.K.; Venkataram Prasad, B.V. Structural features of glycan recognition among viral pathogens. Curr. Opin. Struct. Biol. 2017, 44, 211–218. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, C.D.; Shukla, D. The importance of heparan sulfate in herpesvirus infection. Virol. Sin. 2008, 23, 383–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobhy, H. A comparative review of viral entry and attachment during large and giant dsDNA virus infections. Arch. Virol. 2017, 162, 3567–3585. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef]
- Piccini, L.E.; Carro, A.C.; Quintana, V.M.; Damonte, E.B. Antibody-independent and dependent infection of human myeloid cells with dengue virus is inhibited by carrageenan. Virus Res. 2020, 290, 198150. [Google Scholar] [CrossRef]
- Hamasuna, R.; Eizuru, Y.; Minamishima, Y. Inhibition by iota-carrageenan of the spread of murine cytomegalovirus from the peritoneal cavity to the blood plasma. J. Gen. Virol. 1994, 75 Pt 1, 111–116. [Google Scholar] [CrossRef]
- Kim, M.; Yim, J.H.; Kim, S.Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; König-Schuster, M.; Koller, C.; Graf, C.; Graf, P.; Kirchoff, N.; Reutterer, B.; Seifert, J.M.; Unger, H.; Grassauer, A.; et al. The intranasal application of Zanamivir and carrageenan is synergistically active against influenza A virus in the murine model. PLoS ONE 2015, 10, e0128794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Chen, X.; Cheng, Z.; Liu, S.; Yu, H.; Wang, X.; Li, P. Degradation of polysaccharides from Grateloupia filicina and their antiviral activity to avian leucosis virus subgroup J. Mar. Drugs 2017, 15, 345. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Shao, B.; Nie, W.; Wei, X.W.; Li, Y.L.; Wang, B.L.; He, Z.Y.; Liang, X.; Ye, T.H.; Wei, Y.Q. Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015, 5, 11062. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Damonte, E.B. Herpes simplex virus type 1 variants arising after selection with an antiviral carrageenan: Lack of correlation between drug susceptibility and syn phenotype. J. Med. Virol. 2002, 68, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cantwell, C.A.; Johnson, P.F.; Pfarr, C.M.; Williams, S.C. Transcriptional activity of CCAAT/enhancer-binding proteins is controlled by a conserved inhibitory domain that is a target for sumoylation. J. Biol. Chem. 2002, 277, 38037–38044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Romero, J.A.; Abraham, C.J.; Rodriguez, A.; Kizima, L.; Jean-Pierre, N.; Menon, R.; Begay, O.; Seidor, S.; Ford, B.E.; Gil, P.I.; et al. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob. Agents Chemother. 2012, 56, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacharopoulos, V.R.; Phillips, D.M. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin. Diagn. Lab. Immunol. 1997, 4, 465–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marais, D.; Gawarecki, D.; Allan, B.; Ahmed, K.; Altini, L.; Cassim, N.; Gopolang, F.; Hoffman, M.; Ramjee, G.; Williamson, A.L. The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antivir. Ther. 2011, 16, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, M.; Enzenhofer, E.; Schneider, S.; Rauch, M.; Bodenteich, A.; Neumann, K.; Prieschl-Grassauer, E.; Grassauer, A.; Lion, T.; Mueller, C.A. Efficacy of a Carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir. Res. 2013, 14, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenighofer, M.; Lion, T.; Bodenteich, A.; Prieschl-Grassauer, E.; Grassauer, A.; Unger, H.; Mueller, C.A.; Fazekas, T. Carrageenan nasal spray in virus confirmed common cold: Individual patient data analysis of two randomized controlled trials. Multidiscip. Respir. Med. 2014, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Fazekas, T.; Eickhoff, P.; Pruckner, N.; Vollnhofer, G.; Fischmeister, G.; Diakos, C.; Rauch, M.; Verdianz, M.; Zoubek, A.; Gadner, H.; et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement. Altern. Med. 2012, 12, 147. [Google Scholar] [CrossRef] [Green Version]
- Skoler-Karpoff, S.; Ramjee, G.; Ahmed, K.; Altini, L.; Plagianos, M.G.; Friedland, B.; Govender, S.; De Kock, A.; Cassim, N.; Palanee, T.; et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1977–1987. [Google Scholar] [CrossRef]
- Eccles, R.; Meier, C.; Jawad, M.; Weinmüllner, R.; Grassauer, A.; Prieschl-Grassauer, E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: A randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir. Res. 2010, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Moakes, R.J.A.; Davies, S.P.; Stamataki, Z.; Grover, L.M. Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-COV-2. Adv. Mater. 2021, 33, 2008304. [Google Scholar] [CrossRef]
- Schütz, D.; Conzelmann, C.; Fois, G.; Groß, R.; Weil, T.; Wettstein, L.; Stenger, S.; Zelikin, A.; Hoffmann, T.K.; Frick, M.; et al. Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L750–L756. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the use of carrageenan matrices for the development of antiviral edible coatings of interest in berries. Food Hydrocoll. 2019, 92, 74–85. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Strasfeld, L.; Chou, S. Antiviral drug resistance: Mechanisms and clinical implications. Infect. Dis. Clin. N. Am. 2010, 24, 809–833. [Google Scholar] [CrossRef] [PubMed]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizima, L.; Rodríguez, A.; Kenney, J.; Derby, N.; Mizenina, O.; Menon, R.; Seidor, S.; Zhang, S.; Levendosky, K.; Jean-Pierre, N.; et al. A potent combination microbicide that targets SHIV-RT, HSV-2 and HPV. PLoS ONE 2014, 9, e94547. [Google Scholar] [CrossRef] [PubMed]
- Derby, N.; Aravantinou, M.; Kenney, J.; Ugaonkar, S.R.; Wesenberg, A.; Wilk, J.; Kizima, L.; Rodriguez, A.; Zhang, S.; Mizenina, O.; et al. An intravaginal ring that releases three antiviral agents and a contraceptive blocks SHIV-RT infection, reduces HSV-2 shedding, and suppresses hormonal cycling in rhesus macaques. Drug Deliv. Transl. Res. 2017, 7, 840–858. [Google Scholar] [CrossRef] [Green Version]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 2018, 9, 3881. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Bernkop-Schnürch, A.; Egyed, A.; Koller, C.; Prieschl-Grassauer, E.; Morokutti-Kurz, M. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. Int. J. Gen. Med. 2018, 11, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Kim, Y.H.; Kwon, T.K.; Park, J.H.; Woo, J.S. Antiviral Composition Comprising a Carrageenan as an Active Ingredient. KR20160003964A, 12 January 2016. [Google Scholar]
- Kwon, T.-K.; Kim, Y.-I.; Park, J.-H.; Lim, H.-T. Antiviral Composition Comprising a Carrageenan as an Active Ingredient. KR20180096558A, 29 August 2018. [Google Scholar]
- Ungheri, D.; Verini, M.A.; Ganceff, L.; Mazué, G.; Carminati, P. Antiviral activity of nerve growth factor in vitro. Drugs Exp. Clin. Res. 1993, 19, 151–157. [Google Scholar]
- Enomoto, Y.; Fujii, M.; Furusho, T.; Yamamoto, N. Condom Coated with Acidic Polysaccharides. US5878747 (A), 9 March 1993. [Google Scholar]
- Grassauer, A.; Prieschl-Grassauer, E. Antiviral Composition Comprising a Sulfated Polysaccharide. PL2178533 (T3), 28 March 2013. [Google Scholar]
- Grassauer, A.; Prieschl-Grassauer, E.; Meier, C.; Pretsch, A. Use of iota-carrageenan for the prophylaxis or treatment of a rhinovirus infection. UA94781 (C2), 10 June 2011. [Google Scholar]
- Bodenteich, A.; Prieschl-Grassauer, E.; Morokutti-Kurz, M.; Grassauer, A.; Nakowitsch, S.; König-Schuster, M.; Koller, C.; Pilotaz, F. Composition Effective against Viral Conjunctivitis. MX2016009475A, 16 January 2017. [Google Scholar]
- Tremblay, M.E. Personal Lubricants Comprising LAMBDA-Carrageenan. US10688043B1, 23 June 2020. [Google Scholar]
- Tolcheez, Y.Z.; Kozlovsky, V.A. Antiviral Pharmaceutical Composition. RU2017125688, 29 September 2019. [Google Scholar]
- Vestweber, A.M.; Galan Sousa, J.; Unkauf, M.; Ploch, M. Composition Useful e.g., for Treating Inflammatory Diseases of Oral and Pharyngeal Cavity, Comprises Virustatic and/or Anti-Viral Polysaccharide, Surface Disinfectant, Optionally Acidic Glycosaminoglycan and Optionally Local Anesthetic. DE102013000700 (A1), 10 April 2014. [Google Scholar]
- Bull, C.; Wilson, J.E. Light-Activated Antimicrobial and Antiviral Materials. US5830526(A), 28 December 1994. [Google Scholar]
- Yu, G.; Yu, L.; Yuan, M.; Wang, F. Antimicrobial and Antiviral Oral Liquid for Regulating Metabolism and Enhancing Immunity. CN104606659A, 13 May 2015. [Google Scholar]
- Deng, D.; Xu, C.; Sun, P.; Wu, J.; Yan, C.; Hu, M.; Yan, N. Crystal structure of the human glucose transporter GLUT1. Nature 2014, 510, 121–125. [Google Scholar] [CrossRef]
- Grassauer, A.; Prieschl-Grassauer, E. Synergistic Antiviral Composition and Use Thereof 2014. NZ599839A, 28 February 2014. [Google Scholar]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S.; Ewart, S.H. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Yermak, I.; Khotimchenko, Y.S. Chemical properties, biological activities and applications of carrageenan from red algae. Recent Adv. Mar. Biotechnol. 2003, 9, 207–257. [Google Scholar]
- Yamada, T.; Ogamo, A.; Saito, T.; Watanabe, J.; Uchiyama, H.; Nakagawa, Y. Preparation and anti-HIV activity of low-molecular-weight carrageenans and their sulfated derivatives. Carbohydr. Polym. 1997, 32, 51–55. [Google Scholar] [CrossRef]
- Tang, F.; Chen, F.; Li, F. Preparation and potential in vivo anti-influenza virus activity of low molecular-weight κ-carrageenans and their derivatives. J. Appl. Polym. Sci. 2013, 127, 2110–2115. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barral-Martínez, M.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Tailoring hybrid carrageenans from Mastocarpus stellatus red seaweed using microwave hydrodiffusion and gravity. Carbohydr. Polym. 2020, 248, 116830. [Google Scholar] [CrossRef] [PubMed]
- Ratnawati, R.; Indriyani, N. Ultrasound-Assisted Ultra-Mild-Acid Hydrolisis of K-Carrageenan. Reaktor 2017, 17, 191–196. [Google Scholar] [CrossRef]
- Ratnawati, R.; Prasetyaningrum, A.; Wardhani, D.H. Kinetics and thermodynamics of ultrasound-assisted depolymerization of Κ-carrageenan. Bull. Chem. React. Eng. Catal. 2016, 11, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.M.; Alotaibi, B.S.; EL-Sheekh, M.M. Therapeutic uses of red macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Abad, L.V.; Kudo, H.; Saiki, S.; Nagasawa, N.; Tamada, M.; Katsumura, Y.; Aranilla, C.T.; Relleve, L.S.; De La Rosa, A.M. Radiation degradation studies of carrageenans. Carbohydr. Polym. 2009, 78, 100–106. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G.; Zhao, X.; Jiao, G.; Ren, S.; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J. 2009, 276, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Hjerdez, T.; Smidsrød, O.; Christensen, B.E. The influence of the conformational state of κ- and ι-carrageenan on the rate of acid hydrolysis. Carbohydr. Res. 1996, 288, 175–187. [Google Scholar]
- Hjerde, T.; Smidsrød, O.; Stokke, B.T.; Christensen, B.E. Acid hydrolysis of κ- and ι-carrageenan in the disordered and ordered conformations: Characterization of partially hydrolyzed samples and single-stranded oligomers released from the ordered structures. Macromolecules 1998, 31, 1842–1851. [Google Scholar] [CrossRef]
- Laporte, D.; Vera, J.; Chandía, N.P.; Zúñiga, E.A.; Matsuhiro, B.; Moenne, A. Structurally unrelated algal oligosaccharides differentially stimulate growth and defense against tobacco mosaic virus in tobacco plants. J. Appl. Phycol. 2007, 19, 79–88. [Google Scholar] [CrossRef]
- Mendes, G.S.; Duarte, M.E.R.; Colodi, F.G.; Noseda, M.D.; Ferreira, L.G.; Berté, S.D.; Cavalcanti, J.F.; Santos, N.; Romanos, M.T.V. Structure and anti-metapneumovirus activity of sulfated galactans from the red seaweed Cryptonemia seminervis. Carbohydr. Polym. 2014, 101, 313–323. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.P.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Zhou, G.; Yao, W.; Wang, C. Kinetics of microwave degradation of λ-carrageenan from Chondrus ocellatus. Carbohydr. Polym. 2006, 64, 73–77. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Bouanati, T.; Colson, E.; Moins, S.; Cabrera, J.C.; Eeckhaut, I.; Raquez, J.M.; Gerbaux, P. Microwave-assisted depolymerization of carrageenans from Kappaphycus alvarezii and Eucheuma spinosum: Controlled and green production of oligosaccharides from the algae biomass. Algal Res. 2020, 51, 102054. [Google Scholar] [CrossRef]
- Sun, H.; Gao, L.; Xue, C.; Mao, X. Marine-polysaccharide degrading enzymes: Status and prospects. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2767–2796. [Google Scholar] [CrossRef]
- Haijin, M.; Peng, W.; Bing, L.; Hui, L. Preparation of carrageenan sulfated oligosaccharide with antivirus activity. CN101463372A, 24 June 2009. [Google Scholar]
- Hong, Q.; Xiao, A.; Li, J.; Ji, H.; Zhong, X.; Chen, C.; Xiao, Q.; Ni, H.; Cai, H.; Jiang, Z. Kappa-carrageenase, gene encoding same and application of kappa-Carrageenase. CN107603994B, 29 December 2020. [Google Scholar]
- Yao, Z.; Wu, H.; Zhang, S.; Du, Y. Enzymatic preparation of κ-carrageenan oligosaccharides and their anti-angiogenic activity. Carbohydr. Polym. 2014, 101, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Saluri, K.; Tuvikene, R. Anticoagulant and antioxidant activity of lambda- and theta-carrageenans of different molecular weights. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100243. [Google Scholar] [CrossRef]
- Abad, L.V.; Kudo, H.; Saiki, S.; Nagasawa, N.; Tamada, M.; Fu, H.; Muroya, Y.; Lin, M.; Katsumura, Y.; Relleve, L.S.; et al. Radiolysis studies of aqueous κ-carrageenan. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1607–1612. [Google Scholar] [CrossRef]
- Relleve, L.; Nagasawa, N.; Luan, L.Q.; Yagi, T.; Aranilla, C.; Abad, L.; Kume, T.; Yoshii, F.; Dela Rosa, A. Degradation of carrageenan by radiation. Polym. Degrad. Stab. 2005, 87, 403–410. [Google Scholar] [CrossRef]
- Abad, L.V.; Relleve, L.S.; Racadio, C.D.T.; Aranilla, C.T.; De la Rosa, A.M. Antioxidant activity potential of gamma irradiated carrageenan. Appl. Radiat. Isot. 2013, 79, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bontpart, T.; Cheynier, V.; Ageorges, A.; Terrier, N. BAHD or SCPL acyltransferase? What a dilemma for acylation in the world of plant phenolic compounds. New Phytol. 2015, 208, 695–707. [Google Scholar] [CrossRef]
- Alseekh, S.; Perez de Souza, L.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.J.; Sun, P.L.; Zhang, F.M.; Linhardt, R.J.; Zhang, A.Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Viana, A.G.; Noseda, M.D.; Duarte, M.E.R.; Cerezo, A.S. Alkali modification of carrageenans. Part V. the iota-nu hybrid carrageenan from Eucheuma denticulatum and its cyclization to iota-carrageenan. Carbohydr. Polym. 2004, 58, 455–460. [Google Scholar] [CrossRef]
- Genicot-Joncour, S.; Poinas, A.; Richard, O.; Potin, P.; Rudolph, B.; Kloareg, B.; Helbert, W. The cyclization of the 3,6-anhydro-galactose ring of i-carrageenan is catalyzed by two D-galactose-2,6-sulfurylases in the red alga Chondrus crispus. Plant Physiol. 2009, 151, 1609–1616. [Google Scholar] [CrossRef] [Green Version]
- Ciancia, M.; Matulewicz, M.C.; Tuvikene, R. Structural Diversity in Galactans From Red Seaweeds and Its Influence on Rheological Properties. Front. Plant Sci. 2020, 11, 1392. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; García-Silva, I.; González-Ortega, O.; Sandoval-Vargas, J.M.; Malla, A.; Vimolmangkang, S. The Potential of Algal Biotechnology to Produce Antiviral Compounds and Biopharmaceuticals. Molecules 2020, 25, 4049. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Torres, M.D.; Flórez-Fernández, N.; Diego-González, L.; Simón-Vázquez, R.; Rodríguez-Argüelles, M.C.; Domínguez, H. Eco-friendly extraction of Mastocarpus stellatus carrageenan for the synthesis of gold nanoparticles with improved biological activity. Int. J. Biol. Macromol. 2021, 183, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful Approaches for a Red Seaweed Biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñuela, A.; Robledo, D.; Bourgougnon, N.; Bedoux, G.; Hernández-Núñez, E.; Freile-Pelegrín, Y. Environmentally friendly valorization of Solieria filiformis (Gigartinales, rhodophyta) from IMTA using a biorefinery concept. Mar. Drugs 2018, 16, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Carrageenan Type | Source (Content, % as Dry Weight) | Reference |
|---|---|---|
| kappa | Kappaphycus alvarezii (34.3%) | [49] |
| iota | Eucheuma denticulatum (35.5–39.7%) | [50] |
| Calliblepharis jubata (20–40.4%) | [51,52] | |
| lambda | Halymenia durvillaei (29.1%) | [53] |
| kappa/iota | Mastocarpus papillatus (5.4%) | [50] |
| Sarcothalia crispate (5.4–16.7%) | [50] | |
| Chondracanthus chamissoi (13.5%) | [50] | |
| Eucheuma isiforme (20.4%) | [50] | |
| Kappaphycus alvarezii (30.4–75.6%) | [50] | |
| Chondrus crispus (33.8–60%) | [50,52,54] | |
| Ahnfeltiopsis devoniensis (7.5–30%) | [51,54] | |
| Gymnogongrus crenulatus (12%) | [51] | |
| Chondracanthus teedei (30%) | [51] | |
| Gigartina pistillata (38–58.5%) | [51,52] | |
| Coccotylus trancatus (10%) | [55] | |
| Calliblepharis jubata (28.4%) | [52] | |
| Mastocarpus stellatus (20–45%) | [56] | |
| Gigartina skotbbergi (44.3–50.2%) | [57,58] | |
| Gymnogongrus crenulatus (23.3%) | [52] | |
| kappa/beta | Betaphycus gelatinum (71%) | [50] |
| Furcellaria lumbricalis (69%) | [55] | |
| xi/theta | Chondracanthus chamissoi (24.6%) | [50] |
| Chondracanthus teedei (58%) | [52] | |
| xi/lambda | Gigartina pistillata (57%) | [52] |
| kappa/theta/xi | Chondracanthus acicularis (43%) | [51] |
| Carrageenan Type | Chemical Characteristics | Inhibitory Conc. 50 for Virus and Cell Line (EC50, ED50, IC50) | Reference |
| ι-carrageenan (commercial) | MW = 500 kDa | HSV-1Vero = 2 µg/mL ASFVero = 10 µg/mL; ASFDX Sulf 5000 = 20 µg/mL EMCVero = 10 µg/mL; EMCDX Sulf 5000 = 5 µg/mL SFVVero = 10 µg/mL; SFVDX Sulf 5000 ≥ 200 µg/mL VSVVero > 200 µg/mL; VSVDX Sulf 5000 ≥ 200 µg/mL VacciniaHeLa 10 µg/mL Polio type 1HeLa > 200 µg/mL AdV5HeLa > 200 µg/mL | [62] |
| ι-carrageenan (commercial) | Purity > 95% MW > 100 kDa | IAVMDCK = 0.04–0.20 μg/mL (MOI = 0.01) | [78] |
| ι-carrageenan (commercial) | DENV-2Vero = 0.39 μg/mL | [79] | |
| ι-carrageenan (commercial) | DENV1Vero = 40.7 μg/mL DENV2Vero = 0.4 μg/mL DENV2Vero = 0.40 μg/mL DENV2HepG2 = 0.14 μg/mL DENV3Vero = 4.1 μg/mL DENV3Vero = 1.1 μg/mL DENV3HepG2 = 0.63 μg/mL DENV4Vero = 8.2 μg/mL | [8] | |
| ι-carrageenan | HPVHeLa = 1–10 μg/mL | [77] | |
| ι-carrageenan | IAVMDCK = 0.04 µg/mL | [78] | |
| λ-carrageenan (commercial) | DENV1Vero > 50 μg/mL DENV2Vero = 0.15 μg/mL DENV3Vero = 2.0 μg/mL DENV4Vero = 4.2 μg/mL DENV2Vero = 0.22μg/mL DENV2HepG2 = 0.14 μg/mL DENV3Vero = 0.60 μg/mL DENV3HepG2 = 0.63 μg/mL | [8] | |
| λ-carrageenan (commercial) | HSV-1PRK = 1.6 µg/mL HSV-2PRK = 1.5 µg/mL CMVHeLa = 0.3 µg/mL | [63] | |
| λ-carrageenan | HsV-2HeLa ≤ 7.0 mg/mL | [80] | |
| λ-carrageenan | 4 kDa | RABVHEK-293T = 15.9 μg/mL RABVSK-N-SH = 19.9 μg/mL RABVNA = 22.1 μg/mL RABVBSR = 57.7 μg/mL | [46] |
| κ-carrageenan (commercial) | EEV 71Vero > 10 µg/mL | [24] | |
| κ-carrageenan (commercial) | IAVMDCK = 89.6 μg/mL Ribavirin = 8.3 μg/mL | [1] | |
| κ-carrageenan (commercial) | Purity > 95% MW > 100 kDa | IAVMDCK = 2.70 μg/mL (MOI = 0.01) IAVMDCK = 0.30 μg/mL (MOI = 0.01) | [78] |
| κ-carrageenan (commercial) | DENV1Vero > 50 μg/mL DENV2Vero = 1.8 μg/mL DENV3Vero = 6.3 μg/mL DENV4Vero > 50 μg/mL | [8] | |
| κ-carrageenan | CO-1, ∼2 kDa CO-2, ∼3 kDa CO-3, ∼5 kDa | IAVMDCK = 32.1 (MOI = 1.0), 42.8 (MOI = 1.0) IAVMDCK = 239 (MOI = 1.0) IAVMDCK = 519 (MOI = 1.0) | [81] |
| κ-carrageenan (commercial) | KCO-2 kDa, 10.5% sulf KCO-1 kDa, 10.0% sulf KCO-4 kDa, 9.5% sulf KCO-2 kDa, 30.0% sulf KCO-S 2 kDa, 20.0% sulf KCO-DS-2 kDa, 4.0% sulf | IAVMDCK = 128.3 μM IAVMDCK = 14.9 μM IAVMDCK = 141.8 μM IAVMDCK = 23.8 μM IAVMDCK = 41.7 μM IAVMDCK = 137.5 μM | [82] |
| Carrageenan Source | Extraction, Purification Method | Chemical Characteristics | Inhibitory Concentration 50, Virus, CG Type, Cells = μg/mL or μM | Reference |
|---|---|---|---|---|
| Acanthophora specifira | E: hot W, EtOH pptn | λ-carrageenan | HSV-1Vero = 80.5; RVFV = 75.8 | [67] |
| Chondrus armatus Tichocarpus crinitus | E: Pseudoalteromonas carrageenovora, 0.2% in 0.1M NaCl + 10 μg enzyme/mL, 37 °C, 24 h, F; FD; GPC (Bio-gel P6) | k- carrageenan, 2.2 kDa κ/β-carrageenan, 4.3 kDa | NA * | [108] |
| Cryptonemia crenulata | E1: W, LSR 1.5, 25 °C, 5 h (C1) E2: 0.025M NaH2PO4, 80 °C, 6 h (C2) F: KCl frtn, 1.0 M KCl (C2S3) AEC: DEAE-Sephacel, W, 0.25 M NaCl | κ/ι/ν-carrageenan C1: Carb:Sulf:prot = 55.2:33.1:3.6% C2S3: Carb:Sulf:prot = 61.0:28.3:0.5% | HSV-1C1,Vero = 0.5; HSV-2 = 1.1 HSV-1C2S3,Vero = 0.5; HSV-2 = 1.9 HSV-1Heparin,Vero = 3.0; HSV-2 = 1.8 HSV-1DS8000,Vero = 2.8; HSV-2 = 2.5 | [44] |
| Cryptonemia crenulata | E: W, 25 °C (C1); 80 ºC (C2, C3) P: KCl gradient pptn. (C1S, C2S) C2S on DEAE-Sephacel (C2S-1-4) C2S-2 on DEAE-Sephacel (C2S-2d) | κ/ι/ν-carrageenan | HSV-1C3,Vero = 1.0 HSV-1C1S,Vero = 0.8 HSV-1C2-S3,Vero = 0.5 HSV-12S-2d,Vero = 1.0 | [44] |
| Gigartina atropurpurea Ts | E: 0.05 M NaHCO3, LSR 60, 90 °C, 2 h F; D; FD | λ-carrageenan | HSV-1k-C,HFF = 36; HSV-2 = 1 HSV-1L-C,HFF = 1.5; HSV-2 = 36 HSV-1ACV,HFF = 0.3; HSV-2 = 0.4 | [23] |
| Gigartina skottsbergii Ts | E: W, room temp, 3 vol EtOH pptn F: 0.60–0.70 M KCl (T1) Cyclization: NaBH4, room temp, 24 h; 3 M NaOH, 80 °C. F: 0.2 M KCl (soluble fract, T1c); D, FD | λ-carrageenan | HSV-1T1,Vero = 0.4; HSV-1PH = 0.8 HSV-1DS8000,Vero = 1.8; HSV-1PH = 0.8 | [42] |
| Gigartina skottsbergii Ts | E: W, room temp, KCl pptn (0.65 M) | λ-carrageenan | BoHV-1MDBK = 1.37; SuHV-1 = 73.54 | [13] |
| Gigartina skottsbergii, Cs | E: W, room temp; EtOH pptn F: 0.3 M KCl pptn (C1); soluble 0.2 M (C3) | C1: κ/ι-carrageenan C3: μ/ν-carrageenan | HSV-1k,i-C,Vero = 3.2; HSV-1PH = 3.3 HSV-1m,n-C,Vero = 0.9; HSV-1PH = 0.8 HSV-1DS8000,Vero = 1.8; HSV-1PH = 0.8 | [42,64] |
| Gigartina skottsbergii Cs | E: W, room temp.; 3 vol. isopropanol pptn.; 0.01–0.10 M KCI pptn; FD | κ/ι-carrageenan; 75–124 kDa Sulf:3,6-AnGal:β-Gal 2S = 33:43:38% | HSV-1Vero = 3.2–4.1; HSV-2 = 1.6–2.3 HSV-1DS8000,Vero = 1.0; HSV-2 = 2.1 HSV-1Heparin,Vero = 1.3; HSV-2 = 2.1 | [64] |
| Gymnogongrus griffithsiae | E: W, 100 °C, 2 h (G3: crude extract) P: GC: 2 M KCl (G3S); 1.2 M KCl (G3d); 2 M NaCl (G3S-6); 1M NaCl (G2S-2d) | κ/ι-carrageenan | HSV-1G3,Vero = 0.6 HSV-1G3S,Vero = 2.8 HSV-1G3d,Vero = 1.0 HSV-1G3S-6,Vero = 2.0 HSV-1G2S-2d,Vero = 1.0 | [44] |
| Gymnogongrus griffithsiae | E1, E2: W, 25 °C, LSR 3, 16 h, 2 stg; D, FD. Residue, W, 100 °C, 2 h, D, FD (G3) F: KCl frtn, 1.2 M KCl (G3d) AEC: DEAE-Sephacel, W, 0.25 M NaCl | ι/ν/κ-carrageenan, 845 kDa G3: Carb:Sulf:prot = 55.2:33.1:3.6% G3d: Carb:Sulf:prot = 52.0:29.4:1.7% ι- (70%), υ- (17%) and κ- (13%) | HSV-1G3,Vero = 1.1; HSV-2 = 1.2 HSV-1G3d,Vero = 1.0; HSV-2 = 1.0 HSV-1Heparin,Vero = 3.0; HSV-2 = 1.8 HSV-1DS8000,Vero = 2.8; HSV-2 = 2.5 | [44] |
| Hypnea musciformis | E: hot W, 0.125 M KCl pptn, D, FD (KC) Full/partial oxidation: primary alcohol, 5 h, NaClO, NaBr; N, D (OKC) | κ-carrageenan, Sigma–Aldrich, 215 kDa KC: Gal:GalA:AnGal:SO4 = 1.0:0.0:1.0:1.2 OKC: 163 kDa, Gal:GalA:AnGal:SO4 = 0.33:0.67:0.81:1.08 | HSV-1KC,Vero = 13.8; HSV-2 = 11.0 HSV-1OKC,Vero = 1.7; HSV-2 = 0.98 | [38] |
| Hypnea musciformis | E: hot W, 0.125 M KCl pptn, D, FD (KC) Full/partial oxidation: primary alcohol, 5 h, NaClO, NaBr; N, D (OKC) | ι-carrageenan, Sigma–Aldrich, 460 kDa IC: Gal:GalA:AnGal:SO4 = 0.97:0.03:1.0:2.0 OIC: 324 kDa, Gal:GalA:AnGal:SO4 = 0.33:0.67:0.81:1.1 | HSV-1KC,Vero = 0.67; HSV-2 = 0.43 HSV-1OKC,Vero = 0.40; HSV-2 = 0.40 | [38] |
| Meristiella gelidium | E: W, 25 °C (E1); 100 °C (E2) F: KCl pptn (E2F) | ι/κ/ν-carrageenan E2F: 957 kDa, Carb:sulf:prot = 43:29:9% | HSV-2E1,Vero = 0.06; C6/36 DENV-2HT = 0.79 HSV-2E2,Vero = 0.05; C6/36 DENV-2HT = 0.14 HSV-2E2F,Vero = 0.04; C6/36 DENV-2HT = 0.21 HSV-2Heparin,Vero = 0.6; C6/36 DENV-2HT = 1.9 HSV-2DS8000,Vero = 0.6; C6/36 DENV-2HT = 0.9 | [39] |
| Kappaphycus alvarezii | E: 0.1 N HCl; LSR 100, 60 °C, 4 h + 37 °C, 24 h neutral. (0.1M NaOH); EtOH pptn F; FD; GPC (Bio-gel P6) | κ-carrageenan, Sigma, 1.2–3.0 kDa | NA * | [108] |
| Plocamium cartilagineum | E: 0.05 M NaHCO3, LSR 60, 90 °C, 2 h F; D; FD | Complex sulfated galactan | HSV-1k-C,HFF = 5.4; HSV-2 = 36 | [23] |
| Schizymenia pacifica | E: 20% citrate-phosphate, 4 °C, 16 h P: DEAE-cellulose chromatography, eluted with NaCI, and Sepharose CL-4B | λ-carrageenan; 2 × 103 kDa Gal:Sulf:3,6-AG = 73:20:0.65 | HIVMT-4 = 9.5 × 103 IU/mL | [45] |
| Solieria chordalis | CE: W, room temp, 12 h, 1% KOH (E1); 85 °C, 3 h (E2) F: EtOH pptn + sodium acetate MAE1: 90 °C, MAE2: 105 °C; 25 min | ι-carrageenan CE: Carb:Sulf:prot = 15.4:5.0:3.0 MAE1: Carb:Sulf:prot = 13.5:5.1:2.1 MAE2: Carb:Sulf:prot = 13.5:4.7:2.3 | HSV-1CE = 0.1; Acyclovir = 0.2 HSV-1MAE = 0.3–0.5; Acyclovir = 0.5 | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral Activity of Carrageenans and Processing Implications. Mar. Drugs 2021, 19, 437. https://doi.org/10.3390/md19080437
Álvarez-Viñas M, Souto S, Flórez-Fernández N, Torres MD, Bandín I, Domínguez H. Antiviral Activity of Carrageenans and Processing Implications. Marine Drugs. 2021; 19(8):437. https://doi.org/10.3390/md19080437
Chicago/Turabian StyleÁlvarez-Viñas, Milena, Sandra Souto, Noelia Flórez-Fernández, Maria Dolores Torres, Isabel Bandín, and Herminia Domínguez. 2021. "Antiviral Activity of Carrageenans and Processing Implications" Marine Drugs 19, no. 8: 437. https://doi.org/10.3390/md19080437
APA StyleÁlvarez-Viñas, M., Souto, S., Flórez-Fernández, N., Torres, M. D., Bandín, I., & Domínguez, H. (2021). Antiviral Activity of Carrageenans and Processing Implications. Marine Drugs, 19(8), 437. https://doi.org/10.3390/md19080437







