Fucoidans and Bowel Health
Abstract
:1. Introduction
2. Influence of Fucoidans on Intestinal Bowel Function
2.1. Effect on “Non-Immune Cells”
2.2. Effect on “Immune Cells”
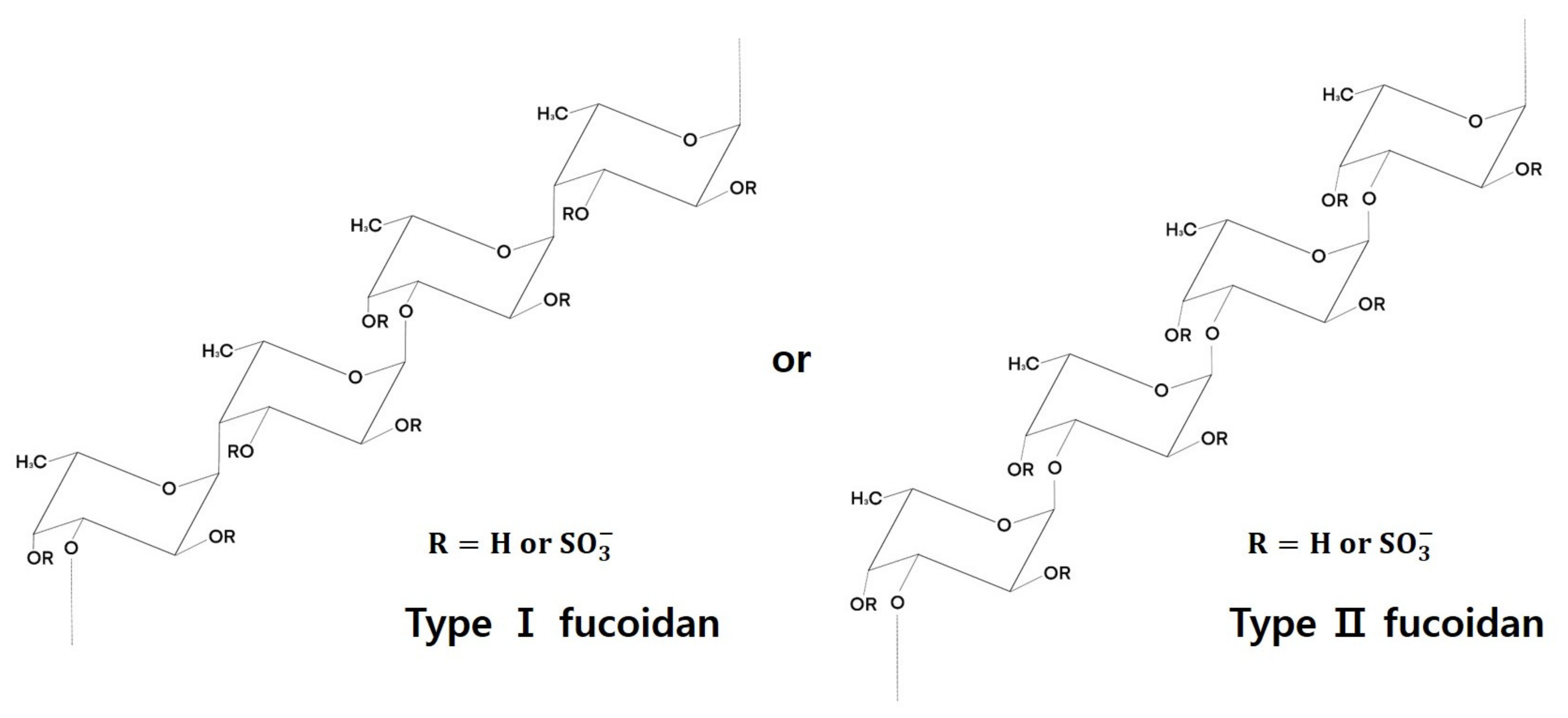
3. Fucoidan Structure
4. Functional Effects of Fucoidans
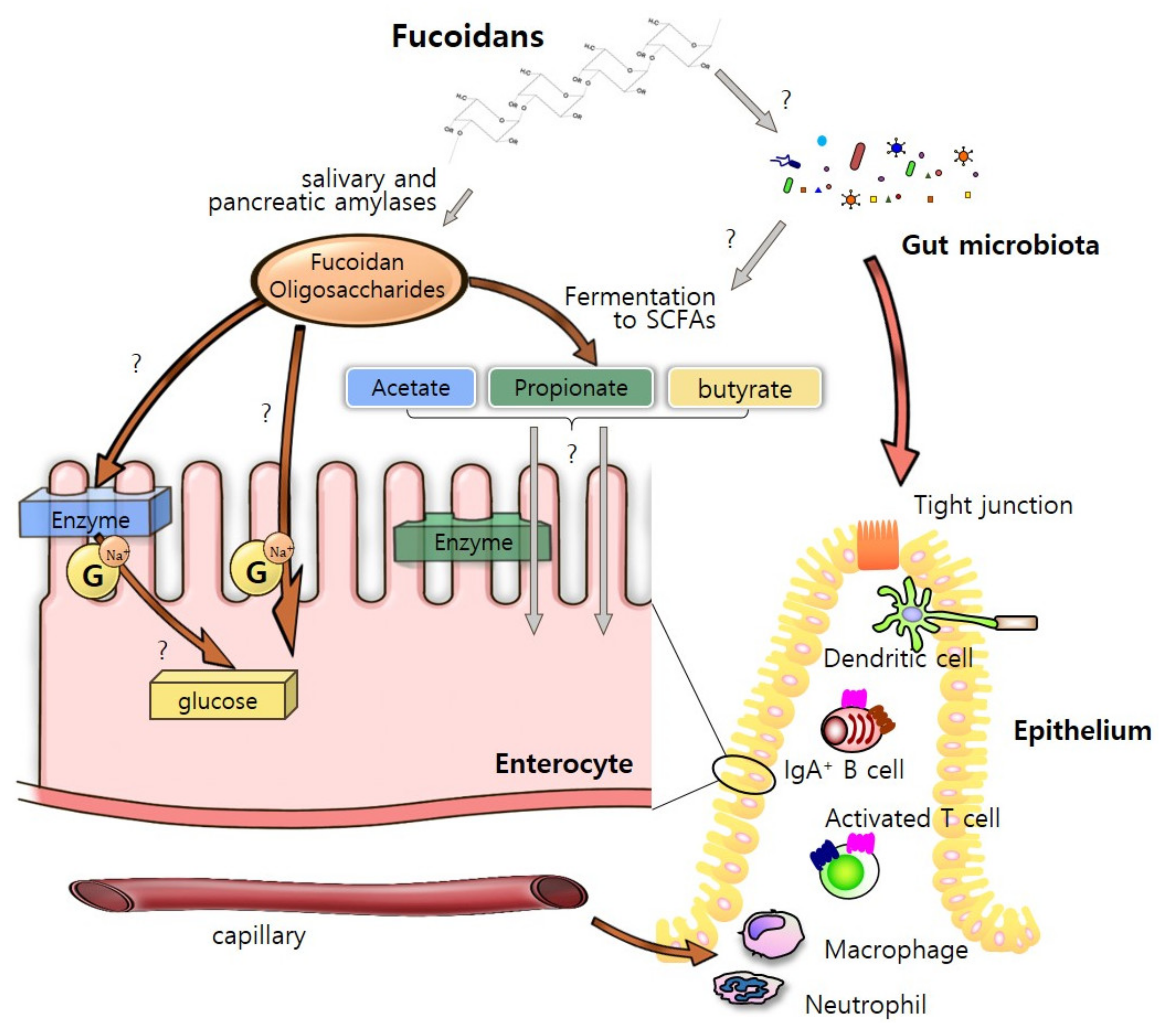
4.1. As an “Energy Sources”
4.2. As an “Immune Regulators”
5. “Fucoidan-Microbiota-Intestine” Axis
5.1. Steady-State Condition
5.2. Disease Condition
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Beitz, D.C. Physiological and metabolic systems important to animal growth: An overview. J. Anim. Sci. 1985, 61 (Suppl. S2), 1–20. [Google Scholar] [CrossRef]
- Owens, F.N.; Dubeski, P.; Hanson, C.F. Factors that alter the growth and development of ruminants. J. Anim. Sci. 1993, 71, 3138–3150. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Rodger, J.; Blache, D. Nutritional and environmental effects on reproduction in small ruminants. Reprod. Fertil. Dev. 2004, 16, 491–501. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Simpi, C.C.; Nagathan, C.V.; Karajgi, S.R.; Kalyane, N.V. Evaluation of marine brown algae Sargassum ilicifolium extract for analgesic and anti-inflammatory activity. Pharmacogn. Res. 2013, 5, 146–149. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive compounds: Natural defense against cancer? Biomolecules 2019, 9, 758. [Google Scholar] [CrossRef] [Green Version]
- Fitton, J.H. Therapies from fucoidan; Multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeguchi, M.; Saito, H.; Miki, Y.; Kimura, T. Effect of fucoidan dietary supplement on the chemotherapy treatment of patients with unresectable advanced gastric cancer. J. Cancer Ther. 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.L.; Geem, D.; Harusato, A.; Denning, T.L. Intestinal antigen-presenting cells: Key regulators of immune homeostasis and inflammation. Am. J. Pathol. 2015, 185, 1809–1819. [Google Scholar] [CrossRef]
- Lebedev, K.A.; Poniakina, I.D. Immunophysiology of epithelial cells and pattern-recognition receptors. Fiziol. Cheloveka 2006, 32, 114–126. [Google Scholar] [CrossRef]
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar] [CrossRef]
- Xue, M.; Ji, X.; Liang, H.; Liu, Y.; Wang, B.; Sun, L.; Li, W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018, 9, 1214–1223. [Google Scholar] [CrossRef]
- Ikeda-Ohtsubo, W.; Lopez Nadal, A.; Zaccaria, E.; Iha, M.; Kitazawa, H.; Kleerebezem, M.; Brugman, S. Intestinal microbiota and immune modulation in zebrafish by fucoidan from Okinawa mozuku (Cladosiphon okamuranus). Front. Nutr. 2020, 7, 67. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Magnusson, M.K.; Öhman, L. Mucosal immune system of the gastrointestinal tract: Maintaining balance between the good and the bad. Scand. J. Gastroenterol. 2017, 52, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef]
- Zuo, T.; Li, X.; Chang, Y.; Duan, G.; Yu, L.; Zheng, R.; Xue, C.; Tang, Q. Dietary fucoidan of Acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food Funct. 2015, 6, 415–422. [Google Scholar] [CrossRef]
- Kishida, K.; Pearce, S.C.; Yu, S.; Gao, N.; Ferraris, R.P. Nutrient sensing by absorptive and secretory progenies of small intestinal stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G592–G605. [Google Scholar] [CrossRef] [Green Version]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.M.; Gibson, P.R.; Costa, R.J.S. Carbohydrate and protein intake during exertional heat stress ameliorates intestinal epithelial injury and small intestine permeability. Appl. Physiol. Nutr. Metab. 2017, 42, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, V.; Goddeeris, B.; Cox, E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 2005, 7, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Kuda, T.; Eda, M.; Yamakawa, H.; Takahashi, H.; Kimura, B. Protective effects of mekabu aqueous solution fermented by Lactobacillus plantarum Sanriku-SU7 on human enterocyte-like HT-29-luc cells and DSS-induced murine IBD model. Probiotics Antimicrob. Proteins 2017, 9, 48–55. [Google Scholar] [CrossRef]
- Lee, M.-C.; Huang, Y.-C. Soluble eggshell membrane protein-loaded chitosan/fucoidan nanoparticles for treatment of defective intestinal epithelial cells. Int. J. Biol. Macromol. 2019, 131, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tanaka, M.; Hashimoto, M.; Inoue, M.; Sasahara, T. The suppressive effect of Mekabu fucoidan on an attachment of Cryptosporidium parvum oocysts to the intestinal epithelial cells in neonatal mice. Life Sci. 2007, 80, 775–781. [Google Scholar] [CrossRef]
- Takahashi, M.; Takahashi, K.; Abe, S.; Yamada, K.; Suzuki, M.; Masahisa, M.; Endo, M.; Abe, K.; Inoue, R.; Hoshi, H. Improvement of psoriasis by alteration of the gut environment by oral administration of fucoidan from Cladosiphon Okamuranus. Mar. Drugs 2020, 18, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Mar. Drugs 2015, 13, 48–64. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Xue, M.; Liang, H.; Ji, X.; Liu, Y.; Ge, Y.; Hou, L.; Sun, T. Fucoidan prevent murine autoimmune diabetes via suppression TLR4-signaling pathways, regulation DC/Treg induced immune tolerance and improving gut microecology. Nutr. Metab. 2019, 16, 87. [Google Scholar] [CrossRef] [Green Version]
- Oomizu, S.; Yanase, Y.; Suzuki, H.; Kameyoshi, Y.; Hide, M. Fucoidan prevents Cε germline transcription and NFκB p52 translocation for IgE production in B cells. Biochem. Biophys. Res. Commun. 2006, 350, 501–507. [Google Scholar] [CrossRef]
- Shimizu, J.; Wada-Funada, U.; Mano, H.; Matahira, Y.; Kawaguchi, M.; Wada, M. Proportion of murine cytotoxic T cells is increased by high molecular-weight fucoidan extracted from Okinawa mozuku (Cladosiphon okamuranus). J. Health Sci. 2005, 51, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.; Tian, J.J.; Ko, W.S.; Shih, C.J.; Chiou, Y.L. Oligo-fucoidan improved unbalance the Th1/Th2 and Treg/Th17 ratios in asthmatic patients: An ex vivo study. Exp. Med. 2019, 17, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.-O.; Zhang, W.; Du, J.-Y.; Wong, K.-W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, Z.A.; Khoury-Hanold, W.; Lim, J.; Smillie, C.; Biton, M.; Reis, B.S.; Zwick, R.K.; Pope, S.D.; Israni-Winger, K.; Parsa, R.; et al. γδ T cells regulate the intestinal response to nutrient sensing. Science 2021, 371, eaba8310. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Badrinathan, S.; Shiju, T.M.; Sharon Christa, A.S.; Arya, R.; Pragasam, V. Purification and structural characterization of sulfated polysaccharide from Sargassum myriocystum and its efficacy in scavenging free radicals. Indian J. Pharm. Sci. 2012, 74, 549–555. [Google Scholar]
- Nagaoka, M.; Shibata, H.; Kimura-Takagi, I.; Hashimoto, S.; Kimura, K.; Makino, T.; Aiyama, R.; Ueyama, S.; Yokokura, T. Structural study of fucoidan from Cladosiphon okamuranus TOKIDA. Glycoconj. J. 1999, 16, 19–26. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifant’ev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef]
- Senthil, S.; Kumar, T.V.; Geetharamani, D.; Maruthupandi, T. Screening of seaweeds collected from southeast coastal area of India for α-amylase inhibitory activity, antioxidant activity and biocompatibility. Int. J. Pharm. Pharm. Sci. 2013, 5, 240–244. [Google Scholar]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Molecular weight and sulfate content modulate the inhibition of α-amylase by fucoidan relevant for type 2 diabetes management. PharmaNutrition 2015, 3, 108–114. [Google Scholar] [CrossRef]
- Shang, Q. Revisit the effects of fucoidan on gut microbiota in health and disease: What do we know and what do we need to know? Bioact. Carb. Diet. Fibre 2020, 23, 100221. [Google Scholar] [CrossRef]
- Pereira, M.S.; Mulloy, B.; Mourão, P.A.S. Structure and Anticoagulant Activity of Sulfated Fucans: Structure and anticoagulant activity of sulfated fucans: Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J. Biol. Chem. 1999, 274, 7656–7667. [Google Scholar]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Bakunina, I.Y.; Nedashkovskaya, O.I.; Alekseeva, S.A.; Ivanova, E.P.; Romanenko, L.A.; Gorshkova, N.M.; Isakov, V.V.; Zvyagintseva, T.N.; Mikhailov, V.V. Degradation of fucoidan by the marine proteobacterium Pseudoalteromonas citrea. Microbiology 2002, 71, 41–47. [Google Scholar] [CrossRef]
- Imbs, T.I.; Skriptsova, A.V.; Zvyagintseva, T.N. Antioxidant activity of fucose-containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Phycol. 2015, 27, 545–553. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.F.; Hussein, M.M.-D.; Salem, H.M. Some structural features of sargassan, a sulphated heteropolysaccharide from Sargassum linifolium. Carbohydr. Res. 1974, 33, 19–24. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Lobeer, A.; Zhang, W.; Irfath, M.; Su, P.; Edirisinghe, N.; Amaratunga, G. Comparative analysis of sugar and mineral content of Sargassum spp. collected from different coasts of Sri Lanka. J. Appl. Phycol. 2019, 31, 2643–2651. [Google Scholar] [CrossRef]
- Duarte, M.E.R.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 2001, 333, 281–293. [Google Scholar] [CrossRef]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef]
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.; Choi, J.-I.; Tong, H. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Sci. Nutr. 2020, 8, 5195–5205. [Google Scholar] [CrossRef]
- Usov, A.; Smirnova, G.; Bilan, M.; Shashkov, A. Polysaccharides of algae. 53. Brown alga Laminaria saccharina (l.) Lam. as a source of fucoidan. Russ. J. Bioorg. Chem. 1998, 24, 437–445. [Google Scholar]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.-C.; Vieira, R.P.; Mourão, P.A.S.; Mulloy, B. A sulfated α-l-fucan from sea cucumber. Carbohydr. Res. 1994, 255, 225–240. [Google Scholar] [CrossRef]
- Mourão, P.A.; Bastos, I.G. Highly acidic glycans from sea cucumbers. Isolation and fractionation of fucose-rich sulfated polysaccharides from the body wall of Ludwigothurea grisea. Eur. J. Biochem. 1987, 166, 639–645. [Google Scholar] [CrossRef]
- Ponce, N.M.; Pujol, C.A.; Damonte, E.B.; Flores, M.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Bilan, M.I.; Zakharova, A.N.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Polysaccharides of algae: 60. Fucoidan from the pacific brown alga Analipus japonicus (Harv.) winne (Ectocarpales, Scytosiphonaceae). Russ. J. Bioorg. Chem. 2007, 33, 38–46. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Sun, J.; Su, X.T.; Yu, Q.L.; Yu, Q.Y.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Sajina, K.A.; Sahu, N.P.; Varghese, T.; Jain, K.K. Fucoidan-rich Sargassum wightii extract supplemented with α-amylase improve growth and immune responses of Labeo rohita (Hamilton, 1822) fingerlings. J. Appl. Phycol. 2019, 31, 2469–2480. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, S.-K. Research and application of marine microbial enzymes: Status and prospects. Mar. Drugs 2010, 8, 1920–1934. [Google Scholar] [CrossRef] [Green Version]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan structure and Its impact on glucose metabolism: Implications for diabetes and cancer therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, Y.-S.; Wu, S.-J.; Mi, F.-L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172. [Google Scholar] [CrossRef]
- Sun, T.; Xue, M.; Yang, J.; Pei, Z.; Zhang, N.; Qin, K.; Liang, H. Metabolic regulation mechanism of fucoidan via intestinal microecology in diseases. J. Sci. Food Agric. 2021, 101, 4456–4463. [Google Scholar] [CrossRef]
- Cox, A.J.; Cripps, A.W.; Taylor, P.A.; Fitton, J.H.; West, N.P. Fucoidan supplementation restores fecal lysozyme concentrations in high-performance athletes: A pilot study. Mar. Drugs 2020, 18, 412. [Google Scholar] [CrossRef]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan extracts ameliorate acute colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahgoub, H.A.; El-Adl, M.A.M.; Ghanem, H.M.; Martyniuk, C.J. The effect of fucoidan or potassium permanganate on growth performance, intestinal pathology, and antioxidant status in Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2020, 46, 2109–2131. [Google Scholar] [CrossRef]
- O’Doherty, J.V.; Dillon, S.; Figat, S.; Callan, J.J.; Sweeney, T. The effects of lactose inclusion and seaweed extract derived from Laminaria spp. on performance, digestibility of diet components and microbial populations in newly weaned pigs. Anim. Feed Sci. Technol. 2010, 157, 173–180. [Google Scholar] [CrossRef]
- Wang, L.; Ai, C.; Wen, C.; Qin, Y.; Liu, Z.; Wang, L.; Gong, Y.; Su, C.; Wang, Z.; Song, S. Fucoidan isolated from Ascophyllum nodosum alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Funct. 2020, 11, 5595–5606. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P. Is the transformation of fucoidans in human body possible? Int. J. Biol. Macromol. 2020, 142, 778–781. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and tissue distribution of fucoidan from Fucus vesiculosus after oral administration to rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of marine-derived drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Sasirekha, R.; Sivasubramanian, S.; Babu, M.D.; Jeganathan, K.; Thirumurugan, R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharmacother. 2019, 109, 1181–1195. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Kuo, T.-H. O-carboxymethyl chitosan/fucoidan nanoparticles increase cellular curcumin uptake. Food Hydrocoll. 2016, 53, 261–269. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Maruya, R.; Koikeda, T.; Nakano, T. Effects of Undaria pinnatifida (wakame) on the human intestinal environment. Funct. Foods Health Dis. 2018, 8, 488–504. [Google Scholar] [CrossRef]
- Wei, P.; Yang, Y.; Li, T.; Ding, Q.; Sun, H. A engineered Bifidobacterium longum secreting a bioative penetratin-glucagon-like peptide 1 fusion protein enhances glucagon-like peptide 1 absorption in the intestine. J. Microbiol. Biotechnol. 2014, 24, 10. [Google Scholar]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, T.Y.; Kim, Y.; Lee, S.H.; Kim, S.; Kang, S.W.; Yang, J.Y.; Baek, I.J.; Sung, Y.H.; Park, Y.Y.; et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104 Pt A, 1185–1193. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.H.; Nam, T.J. Fucoidan downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. Oncol. Rep. 2018, 39, 1516–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Liu, F.; Li, C.; Li, S.; Wu, H.; Guo, B.; Gu, J.; Wang, L. Fucoidan suppresses the gastric cancer cell malignant phenotype and production of TGF-beta1 via CLEC-2. Glycobiology 2020, 30, 301–311. [Google Scholar] [CrossRef] [PubMed]
| Cells | Fucoidan Sources | Fucoidan Concentration Ranges | Positive/Negative Controls | Results | References |
|---|---|---|---|---|---|
| Caco-2 | Sargassum cinereum | 0–1000 μg/mL | fluorouracil (a standard drug, 4 μg/mL) as positive control intact cells as a negative control | increase in ROS production and augment mitochondrial membrane permeability decrease in rate of viable cells with increasing fucoidan concentrations enhance ROS induced apoptosis | [25] |
| Human enterocyte-like HT-29-luc cells | Undaria pinnatifida | 0–11.1 mg/mL | intact cells as a negative control DSS-induced group with H2O2 treatment as a negative control | higher superoxide anion (O2−) radical scavenging capacities increased survival rate of the cells under H2O2 toxicity | [26] |
| Caco-2 | Laminaria japonica | 1.0 mg/mL | intact cells as a negative control | reductions in NO, TNF-α and IL-6 productions. decreased intestinal paracellular permeability of fluorescein isothiocyanate-dextran. | [27] |
| IECs | Undaria pinnatifida | 0–100 μg/mL | unstimulated mice as a negative control infected mice by Cryptosporidium parvum as a positive control | reduction in binding of Cryptosporidium parvum oocysts to the cells | [28] |
| Intestinal macrophages | Cladosiphon okamuranus | 0.1–2.0 mg/mL | rats fed on standard chow as a negative control | identified ED1 (macrophage marker) positive cells internalizing fucoidans | [30] |
| RAW 264.7 cells | Fucus vesiculosus | 0–200 μg/mL | intact cells as a negative control | reductions in NO, PGE2, TNF-α and IL-1β productions recruitment of macrophages and neutrophils | [31] |
| Bone marrow-derived DCs | Fucus vesiculosus | 0–600 mg/kg | unstimulated NOD mice as a negative control | lower expression levels of MHCII and CD86 | [32] |
| Spleen-derived B cells | Cladosiphon novae-caledonias Kylin and Fucus vesiculosus | 0–100 μg/mL | intact cells as a negative control | decrease in NF-κB p52 in B cells, stimulated murine B cell proliferation for 48 h incubation after co-stimulation with interleukin IL-4 and anti-CD40 antibodies | [33] |
| Spleen-derived cytotoxic T cells | Okinawa mozuku | 0–3 × 105 g/moL | A mice group fed control diet | increase in CD8+ T cells decrease in the ratio of CD4+/CD8+ T cells | [34] |
| Blood-derived T cells | Laminaria Japonica | 0–500 µg/mL | intact cells as a negative control | larger Th1 and Treg populations increase in IL-10 production | [35] |
| Spleen-derived DCs and T cells | Fucus vesiculosus | 0–10 mg/kg | intact cells as a negative control | increases in CD40, CD80, CD86, IL-6, IL-12 and TNF-α in spleen DCs promoted the generation of IFN-γ-producing Th1 and Tc1 cells in an IL-12-dependent manner up-regulation of MHC class I and II on spleen cDCs and strongly prompted the proliferation of CD4 and CD8 T cells | [36] |
| Human mononuclear U937 cells | Fucus vesiculosus L. | 0–0.25 μg/mL | intact cells (no stimulation with LPS) as a negative control control cells stimulated with LPS (1 μg/mL) as a negative control SB203580 (p38 MAPK Inhibitor, 1.88 μg/mL) as positive control | higher inhibition of MAPK p38 than those of SB203580 a greater inhibition of the COX-2 enzyme with a higher selectivity index than the synthetic non-steroidal anti-inflammatory drug indomethacin higher amount of fucoidan increased the thromboplastin and thrombin time | [37] |
| Brown Seaweed spp. | Order | Fucose Residue | Backbone | Molecular Weight (kDa) | Weight Ratio of Basic Sugars | References |
|---|---|---|---|---|---|---|
| Fucus vesiculosus | Fucales | 2-O-sulfated fucose 2,3-di-O-sulfated fucose | α(1→3) and α(1→4) linked fucose | 20–200 | Fuc:Gal:Man = 1.00:<0.02:<0.02 | [49,50] |
| Ascophyllum nodosum | Fucales | 2-O-sulfated fucose 2,3-di-O-sulfated fucose | α(1→3) and α(1→4) linked fucose | 417 | Fuc:Xyl:Gal = 31.5:2:3 | [50] |
| Fucus evanescens | Fucales | 2-O-sulfated fucose 2,4-di-O-sulfated fucose | α(1→3) and α(1→4) linked fucose | 620 | Fuc:Glc:Gal:Man:Xyl:Rha = 81:3:4:2:8:2 | [51,52] |
| Fucus serratus | Fucales | 2-O-sulfated fucose | α(1→3) and α(1→4) linked fucose | 1705 | Fuc:Xyl:Gal:Man:Glc = 54.8:4.0:2.6:1.4:0.6 | [42] |
| Sargassum linifolium | Fucales | residues of D-galactose, D-xylose, and L-fucose with sulfate attached to some galactose and fucose residues | a backbone chain of D-glucuronic acid and D-mannose residues | - | Fuc:Xyl:Gal = 1.04:1.00:1.12 | [53,54] |
| Sargassum stenophyllum | Fucales | 2,4-di-O-sulfated fucose | α(1→3) and α(1→4) linked fucose | - | Fuc:Xyl:Man:Gal = 67.8:16.1:1.2:13.6 | [55] |
| Sargassum fusiforme | Fucales | complicated glycosyl linkages, including terminal, 1,3-, 1,4-, 1,2,3-, and 1,3,4-linked Fucp, largely due to sulfate substitution at different positions | α(1→2) linked α-d-Manp and α(1→4) linked β-d-GlcpA | 224 | Fuc:Xyl:Man:Gal:Rha:Glc:Fru = 28.8:3.9:6.0:12.3: 2.3:1.0:12.3 | [56,57] |
| Chorda filum | Laminariales | 2,4-di-O-sulfated fucose 4-O-sulfated fucose | α(1→2) and α(1→3) linked fucose | 10–1000 | Fuc:Xyl:Man:Glc:Gal = 1.00:0.14:0.15:0.40:0.10 | [44] |
| Laminaria saccharina | Laminariales | 2,4-di-O-sulfated fucose 4-O-sulfated fucose | α(1→2) and α(1→3) linked fucose | - | - | [58] |
| Lessonia vadosa | Laminariales | 2,4-di-O-sulfated fucose 4-O-sulfated fucose | α(1→3) linked fucose | 320 | Fuc:Sulfate = 1.0:1.12 | [59] |
| Ludwigothurea grisea | Echinodermata | 2-O-sulfated fucose 2,4-di-O-sulfated fucose | α(1→3) linked fucose | 40 | Fuc:Gal:Glu:Sulfate = 13.9:1.0:0.5:13.9 | [60,61] |
| Cladosiphon okamuranus | Chordariales | 4-O-sulfated fucose a portion of the fucose residues is O-acetylated at C-5 | α(1→2) and α(1→3) linked fucose | 75 | Fuc:Glu:Sulfate = 6.1:1.0:2.9 | [41] |
| Adenocystis utricularis | Ectocarpales | 4-O-sulfated fucose | mainly composed of 3-linked α-l-fucopyranosyl backbone | 6.5–19 | Fuc:Man:Glc:Gal = 74:2:1:22 | [62] |
| Analipus japonicas | Ectocarpales | 2-O-sulfated fucose 2,4-di-O-sulfated fucose | mainly α(1→3) linked fucose | - | Fuc:Sulfate = 3:2 | [63] |
| Species | Fucoidan Sources | Dose | Tissues | Results | References |
|---|---|---|---|---|---|
| Human | Fucus vesiculosus and Undaria pinnatifida | 1 g/d | feces | increase in fecal lysozyme | [71] |
| C57BL/6 mice | Fucus vesiculosus | 5 mg/mL | colon, spleen, and feces | reduced diarrhea and fecal blood loss lower in colon and spleen weight decreases in IL-1α, IL-1β, IL-10, MIP-1α, MIP-1β, G-CSF and GM-CSF in the colon tissue | [72] |
| Newly weaned pig | Laminaria spp. | 240 ppm | colon | increased intestinal villous height and the ratio of villus height to crypt depth | [73] |
| Nile tilapia | Fucus vesiculosus | 0.1% 0.2%, 0.4%, or 0.8% in basal diet | intestine | improved WG and SGR increases in organosomatic index in the intestine higher IEL and IEC counts | [74] |
| Newly weaned pig | Laminaria spp. | 2.8 g/kg | feces | increased the coefficient of total tract apparent digestibility decreased counts of Escherichia coli | [75] |
| C57BL/6J mice | Ascophyllum nodosum | 400 mg/kg | colon | decreases in TNF-α, IL-1β, IL-6, and IL-10 | [76] |
| C57BL/6 mice | Fucus vesiculosus | 400 mg/kg | Colon and feces | reduction in diarrhea and fecal blood decreased the infiltration of inflammatory cells and the expression levels of TNF-α and IL-1β in the colon tissue | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-Y.; Lim, S.Y. Fucoidans and Bowel Health. Mar. Drugs 2021, 19, 436. https://doi.org/10.3390/md19080436
Yang J-Y, Lim SY. Fucoidans and Bowel Health. Marine Drugs. 2021; 19(8):436. https://doi.org/10.3390/md19080436
Chicago/Turabian StyleYang, Jin-Young, and Sun Young Lim. 2021. "Fucoidans and Bowel Health" Marine Drugs 19, no. 8: 436. https://doi.org/10.3390/md19080436
APA StyleYang, J.-Y., & Lim, S. Y. (2021). Fucoidans and Bowel Health. Marine Drugs, 19(8), 436. https://doi.org/10.3390/md19080436





