Collagen-Containing Fish Sidestream-Derived Protein Hydrolysates Support Skin Repair via Chemokine Induction
Abstract
:1. Introduction
2. Results
2.1. Chemical and Nutritional Analysis of Fish Sidestream-Derived Protein Hydrolysates
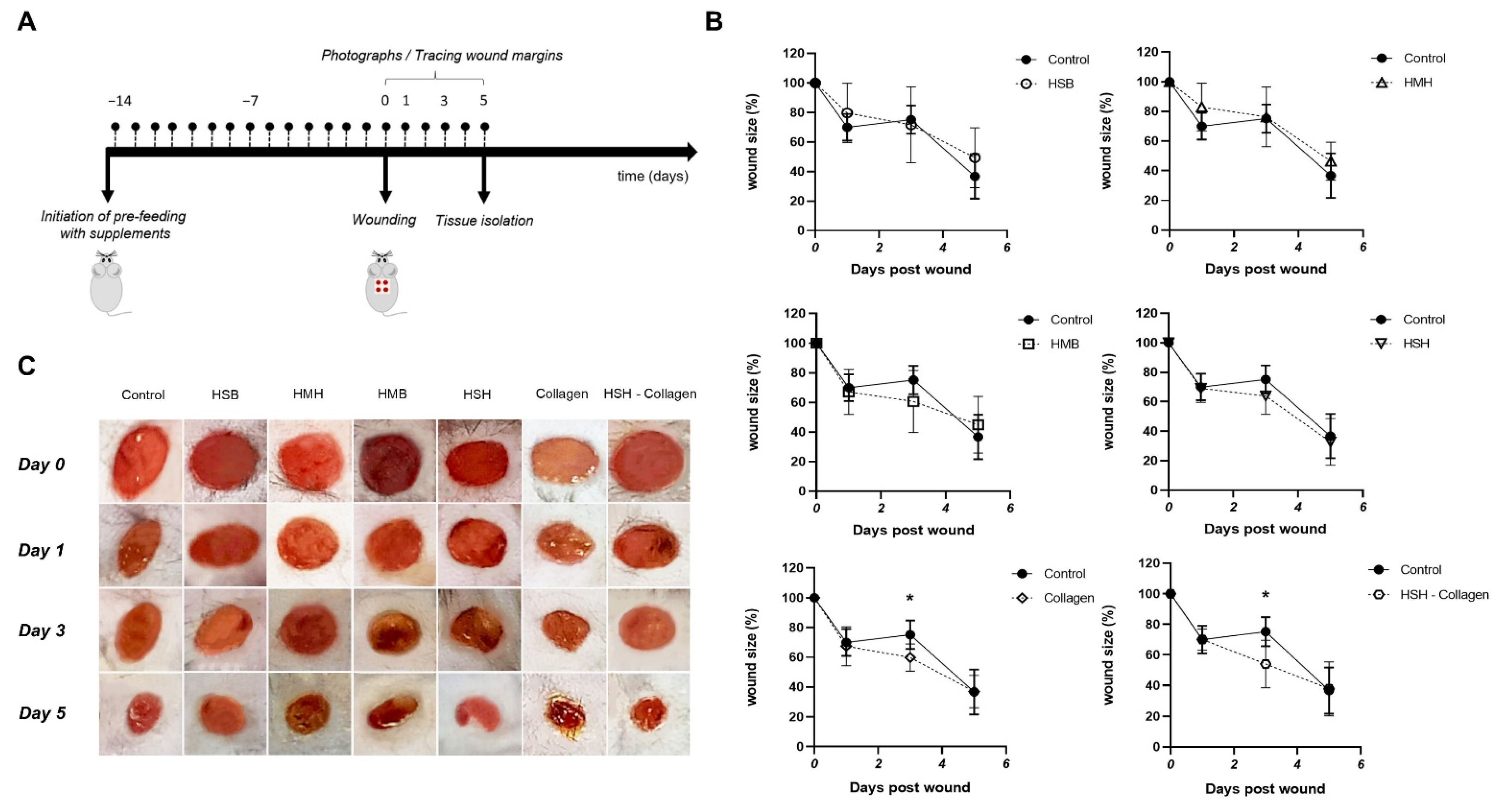
2.2. Accelerated Wound Healing Is Observed in Collagen and HSH-Collagen Fed Mice
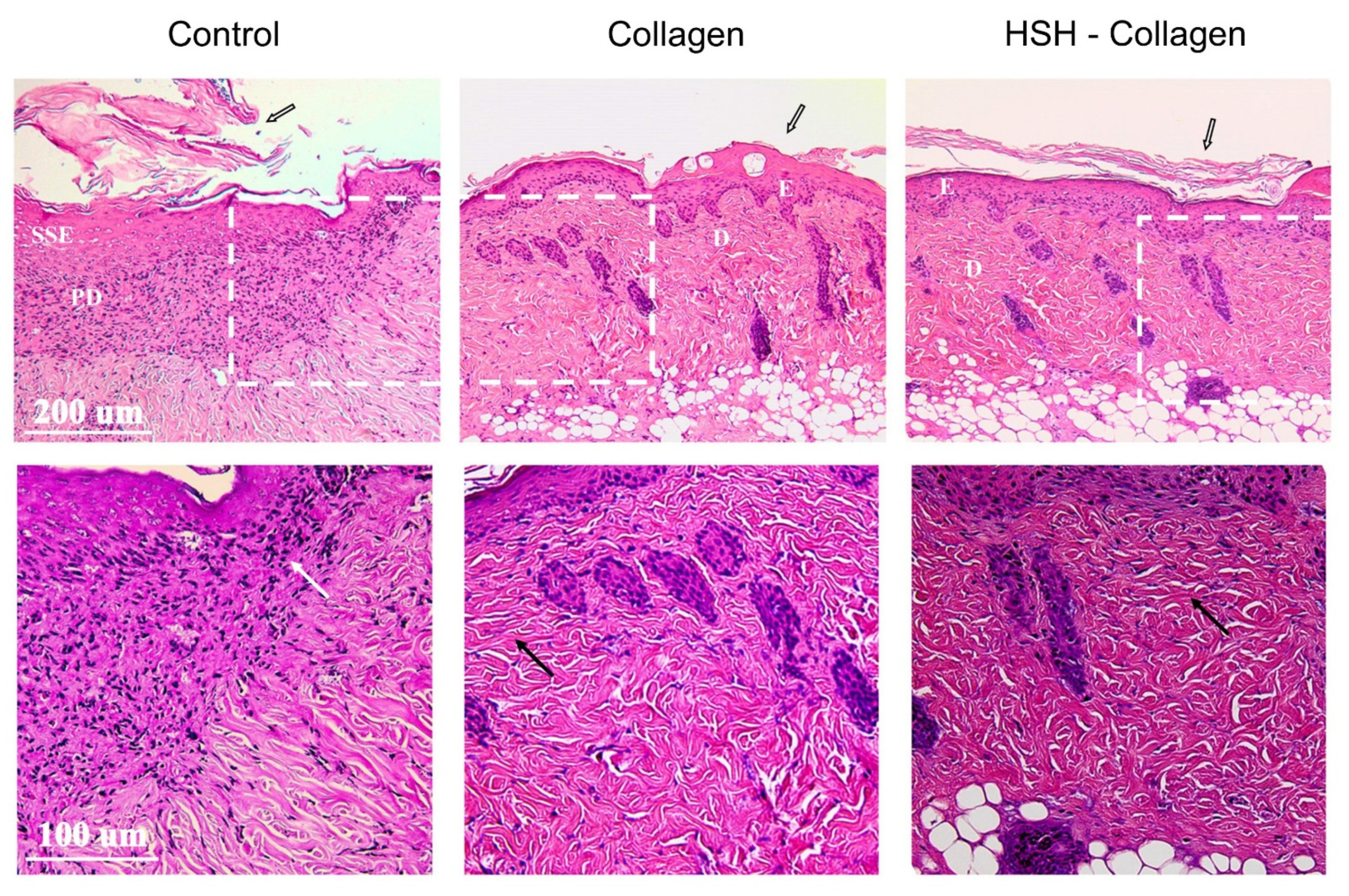
2.3. Collagen and HSH-Collagen Supplemented Diets Result in a More Mature Dermis after Wound Tissue Damage
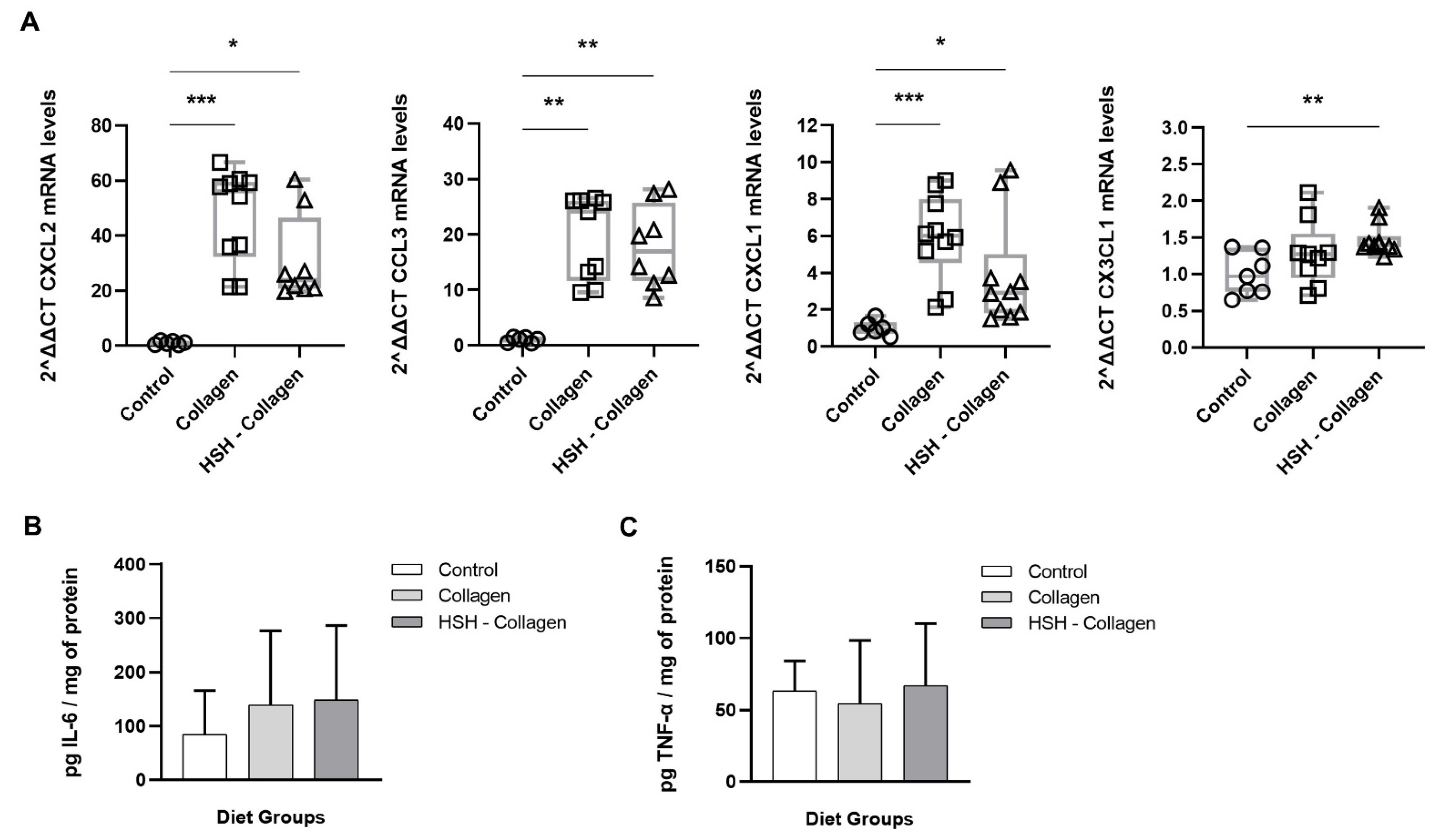
2.4. Collagen and HSH-Collagen Groups Show Increased Expression of Chemokines Involved in the Healing Process
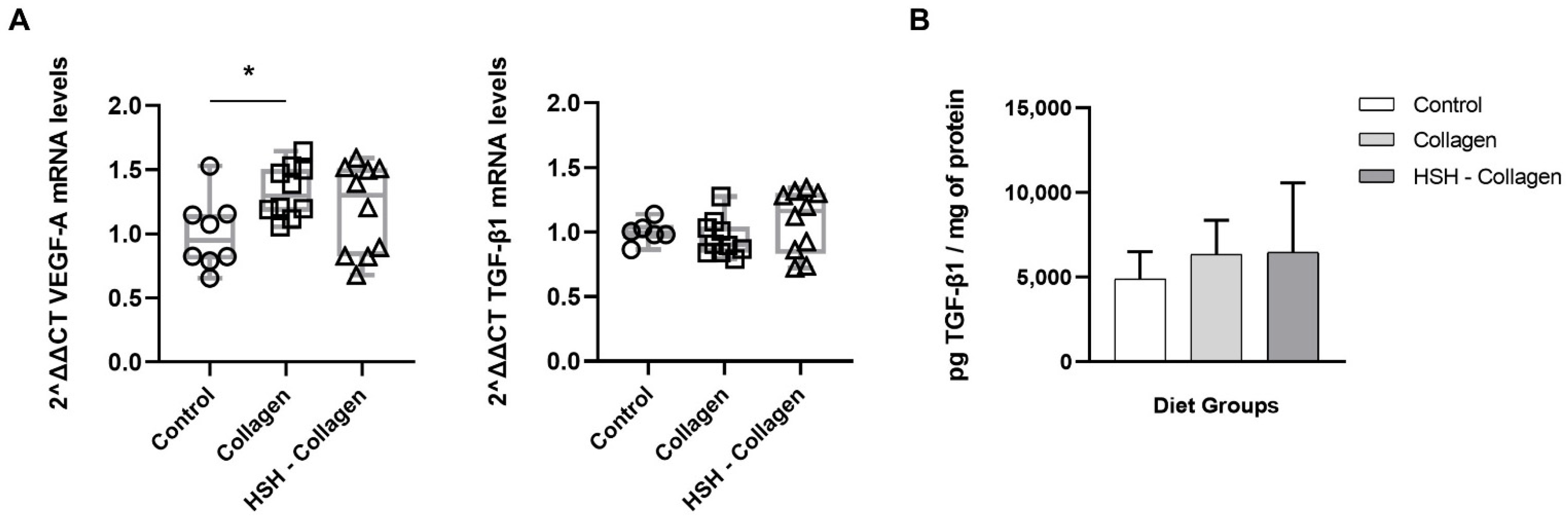
2.5. Collagen-Supplemented Diet Stimulates VEFG-A Expression at the Wound Site
2.6. Collagen Production Is Increased in HSH-Collagen Supplemented Group
3. Discussion
4. Materials and Methods
4.1. Preparation of Fish Sidestream-Derived Nutritional Supplements
4.2. Wounding
4.3. RNA Extraction and Real-Time PCR
4.4. Tissue Processing and Histological Analysis
4.5. Soluble Collagen Assay
4.6. ELISA
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. Engl. Ed. 2017, 20, 189–193. [Google Scholar] [CrossRef]
- MacLeod, A.S.; Mansbridge, J.N. The Innate Immune System in Acute and Chronic Wounds. Adv. Wound Care 2016, 5, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The role of chemokines in wound healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [Green Version]
- Rees, P.A.; Greaves, N.S.; Baguneid, M.; Bayat, A. Chemokines in Wound Healing and as Potential Therapeutic Targets for Reducing Cutaneous Scarring. Adv. Wound Care 2015, 4, 687–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojadinovic, O.; Pastar, I.; Vukelic, S.; Mahoney, M.G.; Brennan, D.; Krzyzanowska, A.; Golinko, M.; Brem, H.; Tomic-Canic, M. Deregulation of keratinocyte differentiation and activation: A hallmark of venous ulcers. J. Cell. Mol. Med. 2008, 12, 2675–2690. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, H.; Li, X.; Li, Y.; Chen, Z. Activation of mTORC1 in fibroblasts accelerates wound healing and induces fibrosis in mice. Wound Repair Regen. 2020, 28, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Rustad, K.C.; Akaishi, S.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Nelson, E.R.; Levi, K.; Paterno, J.; Vial, I.N.; et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 2012, 18, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Miao, M.; Niu, Y.; Xie, T.; Yuan, B.; Qing, C.; Lu, S. Diabetes-impaired wound healing and altered macrophage activation: A possible pathophysiologic correlation. Wound Repair Regen. 2012, 20, 203–213. [Google Scholar] [CrossRef]
- Whitney, J.A.D. Overview: Acute and chronic wounds. Nurs. Clin. N. Am. 2005, 40, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Ski. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, B.; Vadalà, M.; Laurino, C. Nutrition in wound healing: Investigation of the molecular mechanisms, a narrative review. J. Wound Care 2019, 28, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Wild, T.; Rahbarnia, A.; Kellner, M.; Sobotka, L.; Eberlein, T. Basics in nutrition and wound healing. Nutrition 2010, 26, 862–866. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Basiri, R.; Spicer, M.T.; Levenson, C.W.; Ormsbee, M.J.; Ledermann, T.; Arjmandi, B.H. Nutritional supplementation concurrent with nutrition education accelerates the wound healing process in patients with diabetic foot ulcers. Biomedicines 2020, 8, 263. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.A.; Araujo, T.A.; Avanzi, I.R.; Parisi, J.R.; de Andrade, A.L.M.; Rennó, A.C.M. Collagen from Marine Sources and Skin Wound Healing in Animal Experimental Studies: A Systematic Review. Mar. Biotechnol. 2021, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Nasri, M. Protein Hydrolysates and Biopeptides: Production, Biological Activities, and Applications in Foods and Health Benefits. A Review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Lorenzo, J.M.; Cantalapiedra, J.; Zapata, C.; Franco, J.M.; Franco, D. Aquaculture and by-products: Challenges and opportunities in the use of alternative protein sources and bioactive compounds. Adv. Food Nutr. Res. 2020, 92, 127–185. [Google Scholar] [CrossRef]
- Panyam, D.; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Technol. 1996, 7, 120–125. [Google Scholar] [CrossRef]
- Aspevik, T.; Thoresen, L.; Steinsholm, S.; Carlehög, M.; Kousoulaki, K. Sensory and Chemical Properties of Protein Hydrolysates Based on Mackerel (Scomber scombrus) and Salmon (Salmo salar) Side Stream Materials. J. Aquat. Food Prod. Technol. 2021, 30, 176–187. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Axarlis, K.; Aspevik, T.; Orfanakis, M.; Kolliniati, O.; Lapi, I.; Tzardi, M.; Dermitzaki, E.; Venihaki, M.; Kousoulaki, K.; et al. Fish Sidestream-Derived Protein Hydrolysates Suppress DSS-Induced Colitis by Modulating Intestinal Inflammation in Mice. Mar. Drugs 2021, 19, 312. [Google Scholar] [CrossRef]
- Stechmiller, J.K.; Childress, B.; Cowan, L. Arginine supplementation and wound healing. Nutr. Clin. Pract. 2005, 20, 52–61. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; San Lio, R.M.; Evola, G.; Agodi, A.; Basile, G. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashcroft, G.S.; Mills, S.J.; Lei, K.J.; Gibbons, L.; Jeong, M.J.; Taniguchi, M.; Burow, M.; Horan, M.A.; Wahl, S.M.; Nakayama, T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Investig. 2003, 111, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Campbell, L.; Emmerson, E.; Davies, F.; Gilliver, S.C.; Krust, A.; Chambon, P.; Ashcroft, G.S.; Hardman, M.J. Estrogen promotes cutaneous wound healing via estrogen receptor β independent of its antiinflammatory activities. J. Exp. Med. 2010, 207, 1825–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Yang, Z.; Chen, Y.; Chen, Y.; Huang, Z.; You, B.; Peng, Y.; Chen, J. Estrogen accelerates cutaneous wound healing by promoting proliferation of epidermal keratinocytes via Erk/Akt signaling pathway. Cell. Physiol. Biochem. 2016, 38, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Fang, D.; Fang, J.; Ren, X.; Yang, X.; Wen, F.; Su, S.B. Impaired Wound Healing with Defective Expression of Chemokines and Recruitment of Myeloid Cells in TLR3-Deficient Mice. J. Immunol. 2011, 186, 3710–3717. [Google Scholar] [CrossRef] [Green Version]
- Badr, G.; Badr, B.M.; Mahmoud, M.H.; Mohany, M.; Rabah, D.M.; Garraud, O. Treatment of diabetic mice with undenatured whey protein accelerates the wound healing process by enhancing the expression of MIP-1α, MIP-2, KC, CX3CL1 and TGF-β in wounded tissue. BMC Immunol. 2012, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Scapini, P.; Morini, M.; Tecchio, C.; Minghelli, S.; Di Carlo, E.; Tanghetti, E.; Albini, A.; Lowell, C.; Berton, G.; Noonan, D.M.; et al. CXCL1/Macrophage Inflammatory Protein-2-Induced Angiogenesis In Vivo Is Mediated by Neutrophil-Derived Vascular Endothelial Growth Factor-A. J. Immunol. 2004, 172, 5034–5040. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Sun, Y.; Mu, X.; Guo, P.; Gao, F.; Zhang, J.; Zhu, Y.; Zhang, X.; Chen, L.; Ning, Z.; et al. Phospholipase Cε deficiency delays the early stage of cutaneous wound healing and attenuates scar formation in mice. Biochem. Biophys. Res. Commun. 2017, 484, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabete Med. 2006, 23, 594–608. [Google Scholar] [CrossRef]
- Kostarnoy, A.V.; Gancheva, P.G.; Logunov, D.Y.; Verkhovskaya, L.V.; Bobrov, M.A.; Scheblyakov, D.V.; Tukhvatulin, A.I.; Filippova, N.E.; Naroditsky, B.S.; Gintsburg, A.L. Topical bacterial lipopolysaccharide application affects inflammatory response and promotes wound healing. J. Interf. Cytokine Res. 2013, 33, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Kanno, E.; Kawakami, K.; Tanno, H.; Suzuki, A.; Sato, N.; Masaki, A.; Imamura, A.; Takagi, N.; Miura, T.; Yamamoto, H.; et al. Contribution of CARD9-mediated signalling to wound healing in skin. Exp. Dermatol. 2017, 26, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Lestinova, T.; Derrick, T.; Martin, O.; Dillon, R.J.; Volf, P.; Műller, I.; Bates, P.A.; Rogers, M.E. Leishmania proteophosphoglycans regurgitated from infected sand flies accelerate dermal wound repair and exacerbate leishmaniasis via insulin-like growth factor 1-dependent signalling. PLoS Pathog. 2018, 14, e1006794. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Gao, J.-L.; Murphy, P.M. Chemokine Receptor CX3CR1 Mediates Skin Wound Healing by Promoting Macrophage and Fibroblast Accumulation and Function. J. Immunol. 2008, 180, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Wetzler, C.; Kampfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: Prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Petreaca, M.L.; Do, D.; Dhall, S.; McLelland, D.; Serafino, A.; Lyubovitsky, J.; Schiller, N.; Martins-Green, M.M. LIGHT -/- mice wounds mimic human chronic ulcers. Wound Repair Regen. 2012, 20, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Bodnar, R.J. Chemokine Regulation of Angiogenesis During Wound Healing. Adv. Wound Care 2015, 4, 641–650. [Google Scholar] [CrossRef]
- Hong, Y.; Lange-Asschenfeldt, B.; Velasco, P.; Hirakawa, S.; Kunstfeld, R.; Brown, L.F.; Bohlen, P.; Senger, D.R.; Detmar, M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the α1β1 and α2β1 integrins. FASEB J. 2004, 18, 1111–1113. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Erikss, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [Green Version]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S.C.M. Marine-derived polymeric materials and biomimetics: An overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Bafiti, P.; Kikionis, S.; Laskou, M.; Roussis, V.; Ioannou, E.; Kampranis, S.C.; Tsatsanis, C. Disulfides from the Brown Alga Dictyopteris membranacea Suppress M1 Macrophage Activation by Inducing AKT and Suppressing MAPK/ERK Signaling Pathways. Mar. Drugs 2020, 18, 527. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and related diterpenes from the red alga Laurencia inhibit inflammatory bowel disease in mice by suppressing M1 and promoting M2-like macrophage responses. Mar. Drugs 2019, 17, 97. [Google Scholar] [CrossRef] [Green Version]
- Chandika, P.; Ko, S.C.; Jung, W.K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P.E.S.; Tsatsanis, C.; Domínguez, R.; Zhang, W.; Barba, F.J.; Lorenzo, J.M. Evaluation of the protein and bioactive compound bioaccessibility/bioavailability and cytotoxicity of the extracts obtained from aquaculture and fisheries by-products. Adv. Food Nutr. Res. 2020, 92, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Pilcher, B.K.; Dumin, J.A.; Sudbeck, B.D.; Krane, S.M.; Welgus, G.; Parks, W.C.; Pilcher, B.K.; Dumin, J.A.; Sudbeck, B.D.; Krane, S.M.; et al. The Activity of Collagenase-1 Is Required for Keratinocyte Migration on a Type I Collagen Matrix Stable. J. Cell Biol. 2017, 137, 1445–1457. Available online: http://www.jstor.org/stable/1618093 (accessed on 5 July 2021). [CrossRef]
- Pilchcr, B.K.; Sudbeck, B.D.; Dumin, J.A.; Welgus, H.G.; Parks, W.C. Collagenase-1 and collagen in epidermal repair. Arch. Dermatol. Res. Suppl. 1998, 290, S37–S46. [Google Scholar] [CrossRef]
- Peng, X.; Xu, J.; Tian, Y.; Liu, W.; Peng, B. Marine fish peptides (Collagen peptides) compound intake promotes wound healing in rats after cesarean section. Food Nutr. Res. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Liang, R.; Zhao, M.; Zhang, Z.; Li, Y. Oral administration of marine collagen peptides prepared from chum salmon (Oncorhynchus keta) improves wound healing following cesarean section in rats. Food Nutr. Res. 2015, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, M.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of skin gelatin isolated from chum salmon (Oncorhynchus keta) enhances wound healing in diabetic rats. Mar. Drugs 2011, 9, 696–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Supplement | Raw Material |
|---|---|
| HMB | Mackerel Backbones |
| HMH | Mackerel Heads |
| HSB | Salmon Backbones |
| HSH | Salmon Heads |
| Collagen | Flounder Skin Collagen |
| HSH-Collagen | 50% Salmon Heads + 50% Collagen |
| Soy (control) | Soy protein |
| Gene | Sequence |
|---|---|
| B-actin | F: 5′-CATTGCTGACAGGATGCAGAAGG-3′ & R: 5′-TGCTGGAAGGTGGACAGTGAGG-3′ |
| CXCL1 | F: 5′-CCCAAACCGAAGTCATAGCCA-3′ & R: 5′-CTCCGTTACTTGGGGACACC-3′ |
| CXCL2 | F: 5′-CGCCCAGACAGAAGTCATAGCCAC-3′ & R: 5′-CGTTGAGGGACAGCAGCCCAG-3′ |
| CCL3 | F: 5′-GAAGGATACAAGCAGCAGCG-3′ & R: 5′-TTCTCTTAGTCAGGAAAATGACACC-3′ |
| CX3CL1 | F: 5′-CTACTAGGAGCTGCGACACG-3′ & R: 5′-TGTCGTCTCCAGGACAATGG-3′ |
| VEGF-A | F: 5′-GTACCTCCACCATGCCAAGT-3′ & R: 5′-ACTCCAGGGCTTCATCGTTA-3′ |
| TGF-β1 | F: 5′-GACACACAGTACAGCAAGGTCC-3′ & R: 5′-CGACCCACGTAGTAGACGATG-3′ |
| Collagen1a1 | F: 5′-GCTGCACGAGTCACACCG-3′ & R: 5′-GAGGGAACCAGATTGGGGTG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapi, I.; Kolliniati, O.; Aspevik, T.; Deiktakis, E.E.; Axarlis, K.; Daskalaki, M.G.; Dermitzaki, E.; Tzardi, M.; Kampranis, S.C.; Marsni, Z.E.; et al. Collagen-Containing Fish Sidestream-Derived Protein Hydrolysates Support Skin Repair via Chemokine Induction. Mar. Drugs 2021, 19, 396. https://doi.org/10.3390/md19070396
Lapi I, Kolliniati O, Aspevik T, Deiktakis EE, Axarlis K, Daskalaki MG, Dermitzaki E, Tzardi M, Kampranis SC, Marsni ZE, et al. Collagen-Containing Fish Sidestream-Derived Protein Hydrolysates Support Skin Repair via Chemokine Induction. Marine Drugs. 2021; 19(7):396. https://doi.org/10.3390/md19070396
Chicago/Turabian StyleLapi, Ioanna, Ourania Kolliniati, Tone Aspevik, Eleftherios E. Deiktakis, Konstantinos Axarlis, Maria G. Daskalaki, Eirini Dermitzaki, Maria Tzardi, Sotirios C. Kampranis, Zouhir El Marsni, and et al. 2021. "Collagen-Containing Fish Sidestream-Derived Protein Hydrolysates Support Skin Repair via Chemokine Induction" Marine Drugs 19, no. 7: 396. https://doi.org/10.3390/md19070396
APA StyleLapi, I., Kolliniati, O., Aspevik, T., Deiktakis, E. E., Axarlis, K., Daskalaki, M. G., Dermitzaki, E., Tzardi, M., Kampranis, S. C., Marsni, Z. E., Kousoulaki, K. C., Tsatsanis, C., & Venihaki, M. (2021). Collagen-Containing Fish Sidestream-Derived Protein Hydrolysates Support Skin Repair via Chemokine Induction. Marine Drugs, 19(7), 396. https://doi.org/10.3390/md19070396








