Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats
Abstract
:1. Introduction
2. Results
2.1. Liver Function Findings
2.2. Immune Responses Findings
2.3. Serum Interleukin Findings
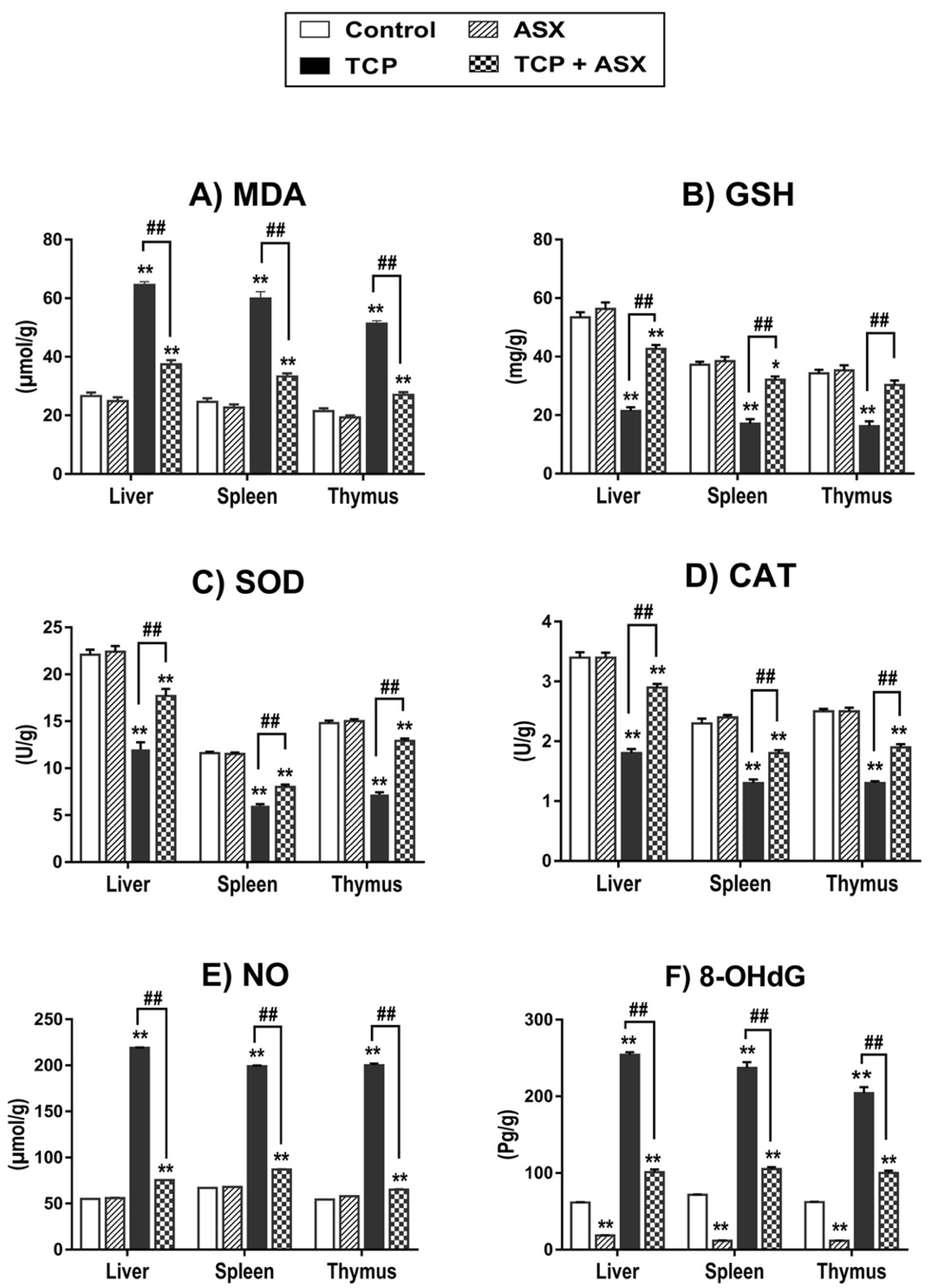
2.4. Oxidant/Antioxidant Biomarkers Findings in Liver, Spleen, and Thymus
2.5. NO Level Findings in Liver, Spleen, and Thymus
2.6. 8-OHdG Findings in Liver, Spleen, and Thymus
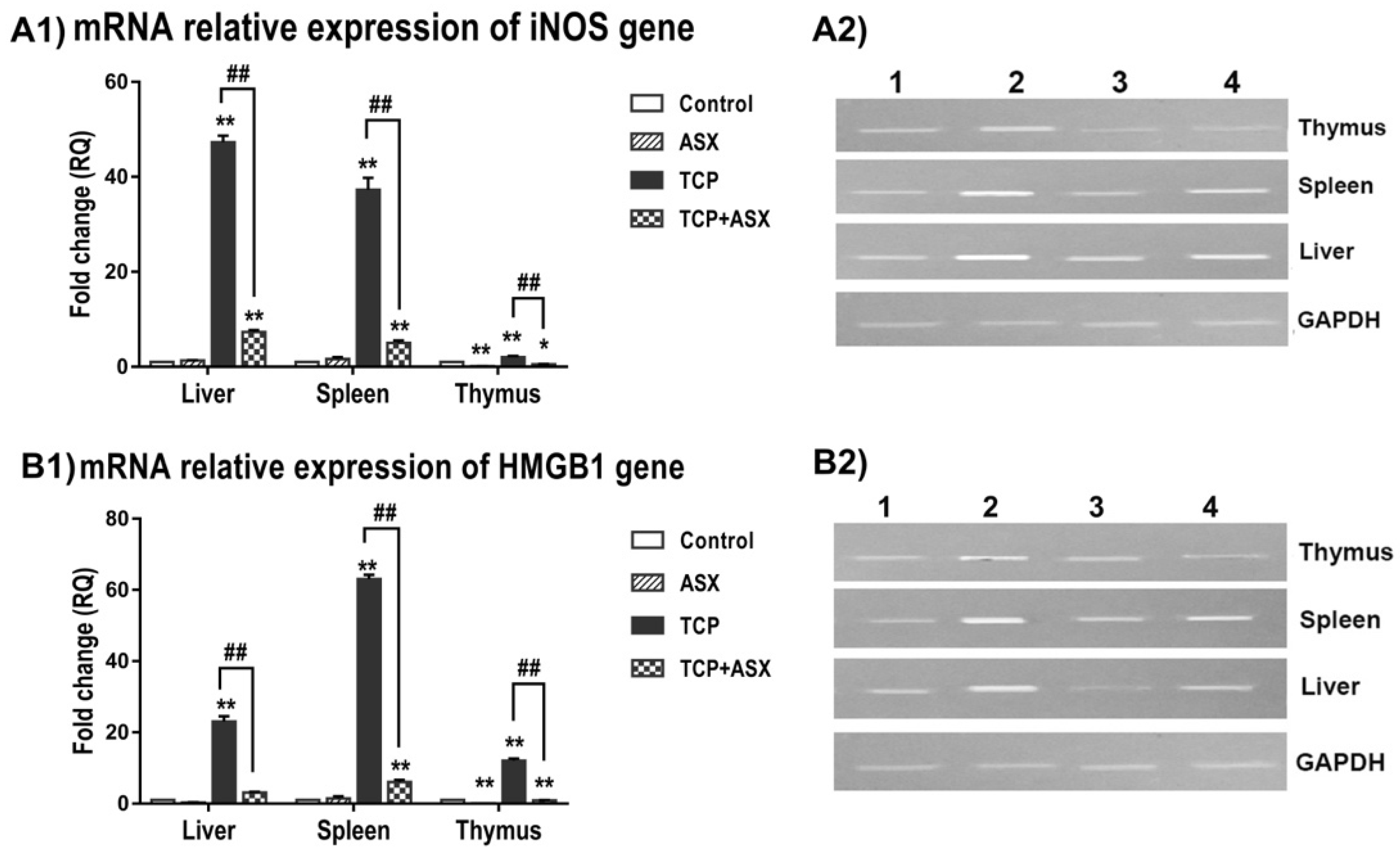
2.7. iNOS and HMGB1 Gene Expression in Liver, Spleen, and Thymus
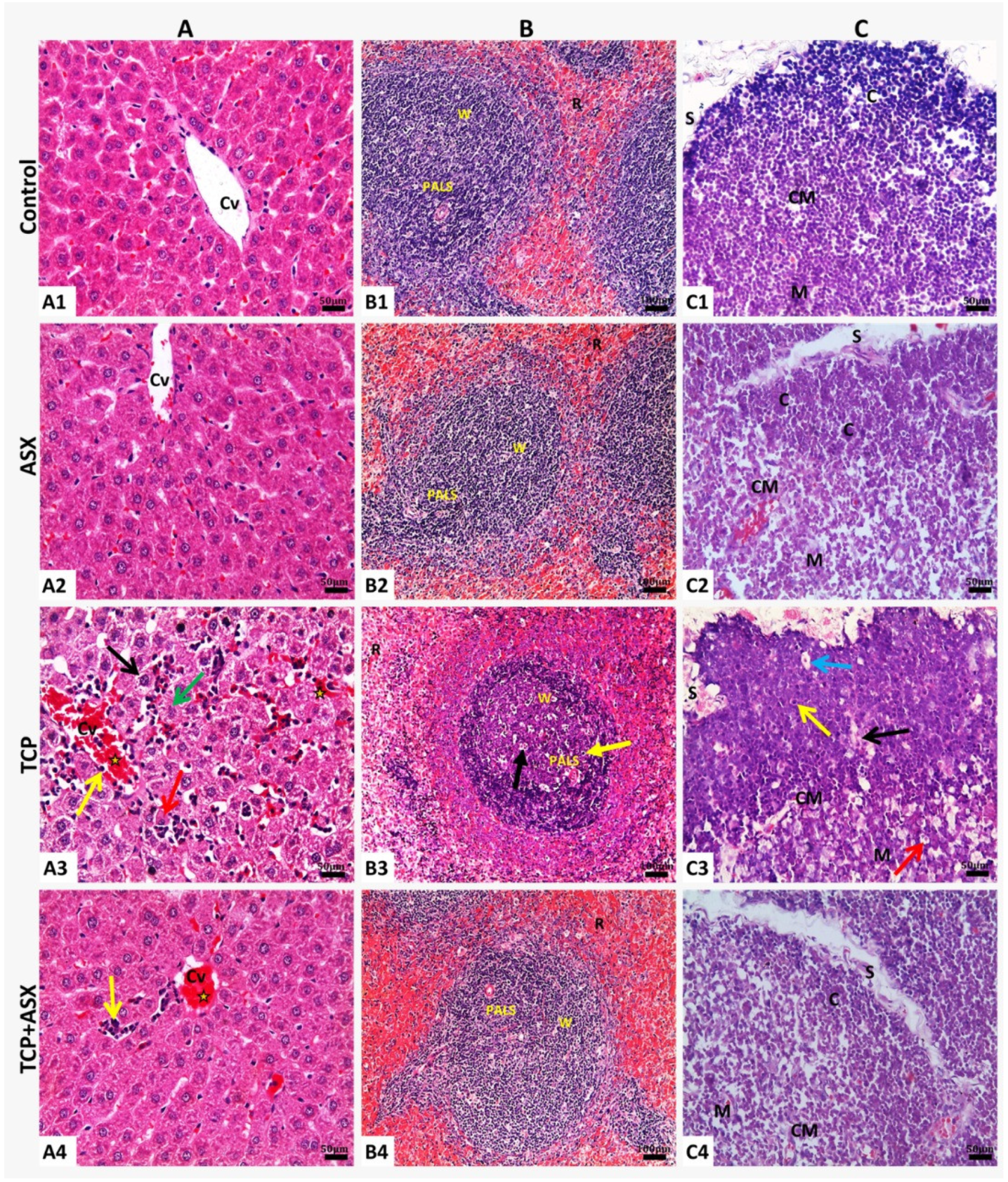
2.8. Histopathological Findings
2.8.1. Liver
2.8.2. Spleen
2.8.3. Thymus
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
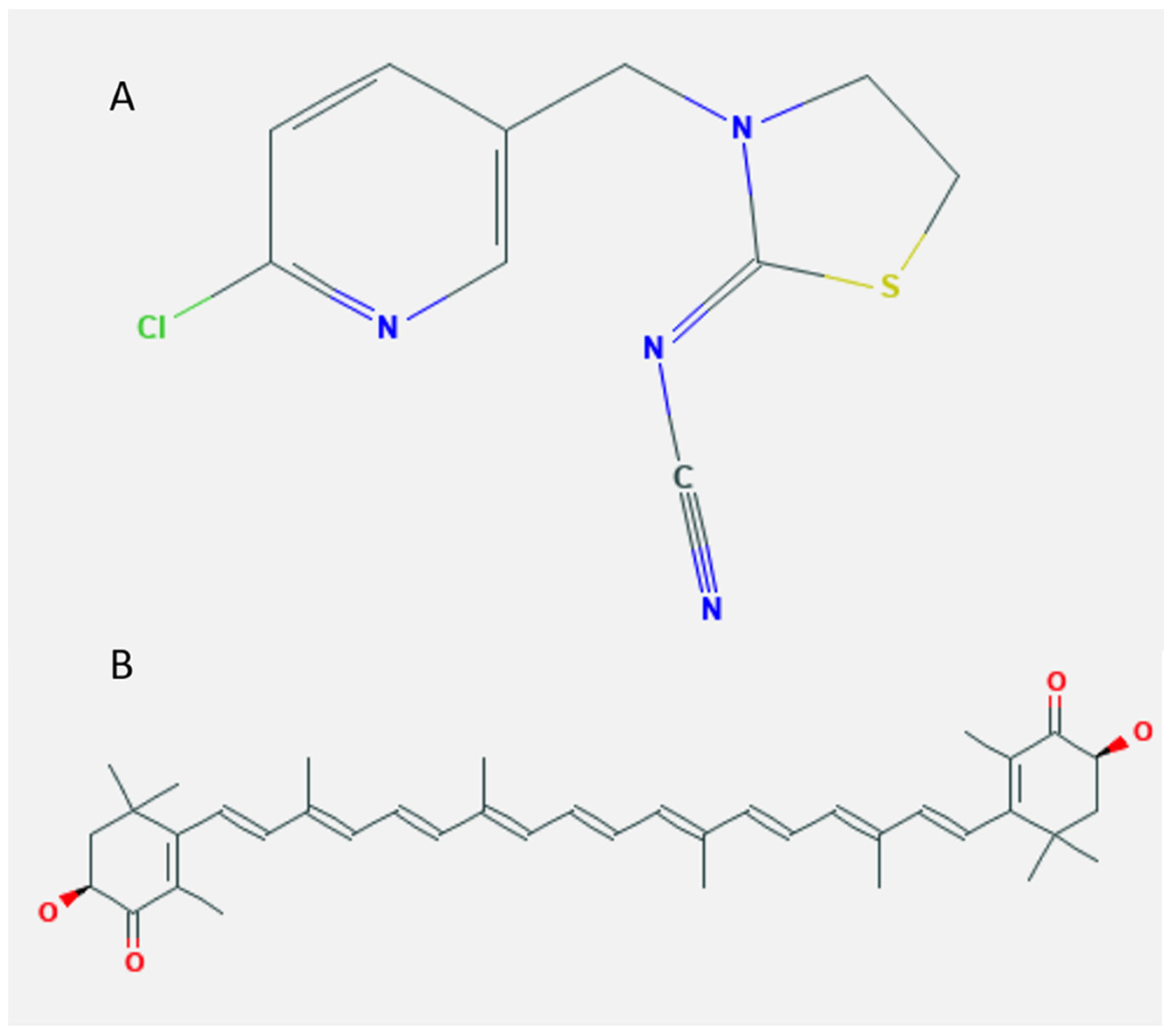
4.2. Chemicals
4.3. Antigen
4.4. Animals and Study Design
4.4.1. Assessment of Hemagglutinating Antibody Titer, iNOS and HMGB1 Gene Expression, and Histopathological Alterations
Hemagglutinating Antibody Titer
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) of iNOS and HMGB1 Genes
Histopathological Technique
4.4.2. Liver Function Biomarkers and IgM–Plaque–Forming Cell (IgM–PFC) Assay
Liver Function Biomarkers
IgM–PFC Assay
4.4.3. Delayed-Type Hypersensitivity (DTH), Serum Interleukins, and Oxidant/Antioxidant Biomarkers
Delayed-Type Hypersensitivity (DTH)
Serum IL-1β, IL-6, and IL-10
Oxidant/Antioxidant Biomarkers in Liver, Spleen, and Thymus
4.4.4. Phagocytic Activity (Carbon Clearance Test)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Houchat, J.N.; Cartereau, A.; Le Mauff, A.; Taillebois, E.; Thany, S.H. An Overview on the Effect of Neonicotinoid Insecticides on Mammalian Cholinergic Functions through the Activation of Neuronal Nicotinic Acetylcholine Receptors. Int. J. Environ. Res. Public Health 2020, 17, 3222. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef]
- Hendawi, M.Y.; Alam, R.T.; Abdellatief, S.A. Ameliorative effect of flaxseed oil against thiacloprid-induced toxicity in rats: Hematological, biochemical, and histopathological study. Environ. Sci. Pollut. Res. Int. 2016, 23, 11855–11863. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, I.; Sellem, I.; Ben Saad, H.; Boudawara, T.; Nasri, M.; Gharsallah, N.; Mallouli, L.; Amara, I.B. Potential benefits of polysaccharides derived from marine alga Ulva lactuca against hepatotoxicity and nephrotoxicity induced by thiacloprid, an insecticide pollutant. Environ. Toxicol. 2019, 34, 1165–1176. [Google Scholar] [CrossRef]
- Agency, E.P. Thiacloprid; Pesticide Tolerances. States. 2012. Available online: https://www.federalregister.gov/documents/2013/02/06/2013-02692/thiacloprid-pesticide-tolerances (accessed on 16 June 2021).
- Babelova, J.; Sefcikova, Z.; Cikos, S.; Spirkova, A.; Kovarikova, V.; Koppel, J.; Makarevich, A.V.; Chrenek, P.; Fabian, D. Exposure to neonicotinoid insecticides induces embryotoxicity in mice and rabbits. Toxicology 2017, 392, 71–80. [Google Scholar] [CrossRef]
- Schwarzbacherova, V.; Wnuk, M.; Deregowska, A.; Holeckova, B.; Lewinska, A. In vitro exposure to thiacloprid-based insecticide formulation promotes oxidative stress, apoptosis and genetic instability in bovine lymphocytes. Toxicol. In Vitro 2019, 61, 104654. [Google Scholar] [CrossRef]
- Senyildiz, M.; Kilinc, A.; Ozden, S. Investigation of the genotoxic and cytotoxic effects of widely used neonicotinoid insecticides in HepG2 and SH-SY5Y cells. Toxicol. Ind. Health 2018, 34, 375–383. [Google Scholar] [CrossRef]
- Khalil, S.R.; Awad, A.; Mohammed, H.H.; Nassan, M.A. Imidacloprid insecticide exposure induces stress and disrupts glucose homeostasis in male rats. Environ. Toxicol. Pharmacol. 2017, 55, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.; Tennekes, H.; Sd nchez-Bayo, F.; Jepsen, P.U. Immune Suppression by Neonicotinoid Insecticides at the Root of Global Wildlife Declines. J. Environ. Immunol. Toxicol. 2013, 1, 3–12. [Google Scholar] [CrossRef]
- Badgujar, P.C.; Jain, S.K.; Singh, A.; Punia, J.S.; Gupta, R.P.; Chandratre, G.A. Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice. Environ. Toxicol. Pharmacol. 2013, 35, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Shakthi Devan, R.K.; Prabu, P.C.; Panchapakesan, S. Immunotoxicity assessment of sub-chronic oral administration of acetamiprid in Wistar rats. Drug Chem. Toxicol. 2015, 38, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Thaker, A.M. Study on the impact of lead acetate pollutant on immunotoxicity produced by thiamethoxam pesticide. Indian J. Pharmacol. 2014, 46, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Choi, K.C. Adverse effects of pesticides on the functions of immune system. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 235, 108789. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Iannaccone, M.; Ianniello, F.; Ferrara, R.; Caprio, E.; Pennacchio, F.; Capparelli, R. The neonicotinoid insecticide Clothianidin adversely affects immune signaling in a human cell line. Sci. Rep. 2017, 7, 13446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, R.G. Nitric oxide synthases. Biochem. Soc. Trans. 1996, 24, 875–878. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.; Siddiqui, M.H.; Sharma, P.K.; Shukla, Y. Deltamethrin induced RIPK3-mediated caspase-independent non-apoptotic cell death in rat primary hepatocytes. Biochem. Biophys. Res. Commun. 2016, 479, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M.; AbuBakr, H.O.; Mohamed, M.A.; El-Bahrawy, A. Ameliorative effect of pumpkin seed oil against emamectin induced toxicity in mice. Biomed. Pharmacother. 2018, 98, 242–251. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Destefanis, S.; Giretto, D.; Muscolo, M.C.; Di Cerbo, A.; Guidetti, G.; Canello, S.; Giovazzino, A.; Centenaro, S.; Terrazzano, G. Clinical evaluation of a nutraceutical diet as an adjuvant to pharmacological treatment in dogs affected by Keratoconjunctivitis sicca. BMC Vet. Res. 2016, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Di Cerbo, A.; Morales-Medina, J.C.; Palmieri, B.; Pezzuto, F.; Cocco, R.; Flores, G.; Iannitti, T. Functional foods in pet nutrition: Focus on dogs and cats. Res. Vet. Sci. 2017, 112, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; Di Cerbo, A.; Giovazzino, A.; Rubino, V.; Palatucci, A.T.; Centenaro, S.; Fraccaroli, E.; Cortese, L.; Bonomo, M.G.; Ruggiero, G.; et al. In Vitro Effects of Some Botanicals with Anti-Inflammatory and Antitoxic Activity. J. Immunol. Res. 2016, 2016, 5457010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B. Basic liver immunology. Cell Mol. Immunol. 2016, 13, 265–266. [Google Scholar] [CrossRef] [Green Version]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Aliyu, M.; Zohora, F.; Saboor-Yaraghi, A.A. Spleen in innate and adaptive immunity regulation. AIMS Allergy Immunol. 2021, 5, 1–17. [Google Scholar] [CrossRef]
- Whalan, J.E. (Ed.) Clinical Chemistry. In A Toxicologist’s Guide to Clinical Pathology in Animals; Springer International Publishing: Basel, Switzerland, 2015; pp. 67–94. [Google Scholar]
- Karaca, B.U.; Arican, Y.E.; Boran, T.; Binay, S.; Okyar, A.; Kaptan, E.; Ozhan, G. Toxic effects of subchronic oral acetamiprid exposure in rats. Toxicol. Ind. Health 2019, 35, 679–687. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chang, C.C.; Lin, S.T.; Chyau, C.C.; Peng, R.Y. Improved Hepatoprotective Effect of Liposome-Encapsulated Astaxanthin in Lipopolysaccharide-Induced Acute Hepatotoxicity. Int. J. Mol. Sci. 2016, 17, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.A.; Al Mamun, M.A.; Faruk, M.; Ul Islam, M.T.; Rahman, M.M.; Alam, M.N.; Rahman, A.; Reza, H.M.; Alam, M.A. Astaxanthin Ameliorates Hepatic Damage and Oxidative Stress in Carbon Tetrachloride-administered Rats. Pharmacogn. Res. 2017, 9, S84–S91. [Google Scholar] [CrossRef]
- Gawade, L.; Dadarkar, S.S.; Husain, R.; Gatne, M. A detailed study of developmental immunotoxicity of imidacloprid in Wistar rats. Food Chem. Toxicol. 2013, 51, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Marzouki, S.; Bini Dhouib, I.; Benabdessalem, C.; Rekik, R.; Doghri, R.; Maroueni, A.; Bellasfar, Z.; Fazaa, S.; Bettaieb, J.; Barbouche, M.R.; et al. Specific immune responses in mice following subchronic exposure to acetamiprid. Life Sci. 2017, 188, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ladics, G. Assays for Antibody Production. In Encyclopedic Reference of Immunotoxicology; Vohr, H.W., Ed.; Springer: New York, NY, USA, 2005; pp. 50–53. [Google Scholar]
- Tarazona, R.; Solana, R.; Ouyang, Q.; Pawelec, G. Basic biology and clinical impact of immunosenescence. Exp. Gerontol. 2002, 37, 183–189. [Google Scholar] [CrossRef]
- Hirano, T.; Minagawa, S.; Furusawa, Y.; Yunoki, T.; Ikenaka, Y.; Yokoyama, T.; Hoshi, N.; Tabuchi, Y. Growth and neurite stimulating effects of the neonicotinoid pesticide clothianidin on human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 2019, 383, 114777. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yanai, S.; Omotehara, T.; Hashimoto, R.; Umemura, Y.; Kubota, N.; Minami, K.; Nagahara, D.; Matsuo, E.; Aihara, Y.; et al. The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice. J. Vet. Med. Sci. 2015, 77, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Dietert, R.R.; Bunn, T.L.; Lee, J.E. The delayed type hypersensitivity assay using protein and xenogeneic cell antigens. In Immunotoxicity Testing Methods and Protocols; Humana Press: New York, NY, USA, 2010; pp. 185–194. [Google Scholar]
- Fratelli, M.; Demol, H.; Puype, M.; Casagrande, S.; Eberini, I.; Salmona, M.; Bonetto, V.; Mengozzi, M.; Duffieux, F.; Miclet, E.; et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 3505–3510. [Google Scholar] [CrossRef] [Green Version]
- Vos, J.G. Immune suppression as related to toxicology. J. Immunotoxicol. 2007, 4, 175–200. [Google Scholar] [CrossRef]
- Julius, A.; Abernathy, L.; Yung, R. Defective Dendritic Cell Phagocytic Function in Aging (134.36). J. Immunol. 2009, 182, 134–136. [Google Scholar]
- Salema, L.H.; Alwan, M.J.; Yousif, A.A. Immunotoxic effect of thiamethoxam in immunized mice with Brucella abortus cultural filtrate antigen. Vet. World 2016, 9, 1407–1412. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Xing, L.; Lin, H.; Leng, K.; Zhai, Y.; Liu, X. Assessment and comparison of in vitro immunoregulatory activity of three astaxanthin stereoisomers. J. Ocean Univ. China 2016, 15, 283. [Google Scholar] [CrossRef]
- De la Fuente, M.; Hernanz, A.; Vallejo, M.C. The immune system in the oxidative stress conditions of aging and hypertension: Favorable effects of antioxidants and physical exercise. Antioxid. Redox Signal. 2005, 7, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B. Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rats. Pestic. Biochem. Physiol. 2011, 100, 165–171. [Google Scholar] [CrossRef]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Yonar, M.E.; Sakin, F. Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pestic. Biochem. Physiol. 2011, 99, 226–231. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Mohany, M.; El-Feki, M.; Refaat, I.; Garraud, O.; Badr, G. Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. J. Toxicol. Sci. 2012, 37, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdikova, M.; Holeckova, B.; Sivikova, K.; Schwarzbacherova, V.; Kolenicova, S. Evaluating the genotoxic damage in bovine whole blood cells in vitro after exposure to thiacloprid. Toxicol. In Vitro 2019, 61, 104616. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.L.; Han, X.D.; Li, Y.; Chu, X.F.; Miao, W.M.; Zhang, J.L.; Fan, S.J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In Vitro Antioxidant, Antityrosinase, and Cytotoxic Activities of Astaxanthin from Shrimp Waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Qu, Y.; Li, Q.; An, T.; Chen, Z.; Chen, Y.; Deng, X.; Bai, D. Protective effect of astaxanthin against La2O3 nanoparticles induced neurotoxicity by activating PI3K/AKT/Nrf-2 signaling in mice. Food Chem. Toxicol. 2020, 144, 111582. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Li, A.; Song, M. Tetrachlorobisphenol A induced immunosuppression and uterine injury in mice. Ecotoxicol. Environ. Saf. 2021, 207, 111527. [Google Scholar] [CrossRef]
- Duzguner, V.; Erdogan, S. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats. Pestic. Biochem. Physiol. 2012, 104, 58–64. [Google Scholar] [CrossRef]
- Tewari, P.; Roy, R.; Mishra, S.; Mandal, P.; Yadav, A.; Chaudhari, B.P.; Chaturvedi, R.K.; Dwivedi, P.D.; Tripathi, A.; Das, M. Benzanthrone induced immunotoxicity via oxidative stress and inflammatory mediators in Balb/c mice. Immunobiology 2015, 220, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Jarvelainen, H.A.; Fang, C.; Ingelman-Sundberg, M.; Lindros, K.O. Effect of chronic coadministration of endotoxin and ethanol on rat liver pathology and proinflammatory and anti-inflammatory cytokines. Hepatology 1999, 29, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- Robertson, S.A.; Care, A.S.; Skinner, R.J. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol. Reprod. 2007, 76, 738–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthwal, M.K.; Srivastava, N.; Shukla, R.; Nag, D.; Seth, P.K.; Srimal, R.C.; Dikshit, M. Polymorphonuclear leukocyte nitrite content and antioxidant enzymes in Parkinson’s disease patients. Acta. Neurol. Scand. 1999, 100, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Wang, M.; Chen, A.; Zhang, C.; Lin, L.; Zhang, Z.; Chen, A. Protective effect of caffeic acid phenethyl ester against imidacloprid-induced hepatotoxicity by attenuating oxidative stress, endoplasmic reticulum stress, inflammation and apoptosis. Pestic. Biochem. Physiol. 2020, 164, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Asp. Med. 2014, 40, 1–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.Y.; Yao, Y.M.; Yan, Y.H.; Dong, N.; Sheng, Z.Y. High mobility group box 1 protein suppresses T cell-mediated immunity via CD11c(low)CD45RB(high) dendritic cell differentiation. Cytokine 2011, 54, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Hou, C.; Li, J.; Peng, H.; Wang, Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp. Eye Res. 2020, 197, 108113. [Google Scholar] [CrossRef]
- Otton, R.; Marin, D.P.; Bolin, A.P.; Santos Rde, C.; Polotow, T.G.; Sampaio, S.C.; de Barros, M.P. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, X.H.; Wu, Y.; Dong, N.; Tong, Y.L.; Yao, Y.M. Inhibition of Cerebral High-Mobility Group Box 1 Protein Attenuates Multiple Organ Damage and Improves T Cell-Mediated Immunity in Septic Rats. Mediat. Inflamm. 2019, 2019, 6197084. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, M. Sex Differences in Human and Animal Toxicology. Toxicol. Pathol. 2017, 45, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, H.; Shao, Y.; Wang, P.; Wei, Y.; Zhang, W.; Jiang, J.; Chen, Y.; Zhang, Z. Nephroprotective effect of astaxanthin against trivalent inorganic arsenic-induced renal injury in wistar rats. Nutr. Res. Pract. 2014, 8, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nfambi, J.; Bbosa, G.S.; Sembajwe, L.F.; Gakunga, J.; Kasolo, J.N. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 603–611. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Aroonvilairat, S.; Tangjarukij, C.; Sornprachum, T.; Chaisuriya, P.; Siwadune, T.; Ratanabanangkoon, K. Effects of topical exposure to a mixture of chlorpyrifos, cypermethrin and captan on the hematological and immunological systems in male Wistar rats. Environ. Toxicol. Pharmacol. 2018, 59, 53–60. [Google Scholar] [CrossRef]
- Allen, I.C. Delayed-type hypersensitivity models in mice. Methods Mol. Biol. 2013, 1031, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hussein, U.K.; Hassan, N.E.Y.; Elhalwagy, M.E.A.; Zaki, A.R.; Abubakr, H.O.; Nagulapalli Venkata, K.C.; Jang, K.Y.; Bishayee, A. Ginger and Propolis Exert Neuroprotective Effects against Monosodium Glutamate-Induced Neurotoxicity in Rats. Molecules 2017, 22, 1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganeshpurkar, A.; Saluja, A.K. Protective effect of catechin on humoral and cell mediated immunity in rat model. Int. Immunopharmacol. 2018, 54, 261–266. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Control | ASX | TCP | TCP + ASX |
|---|---|---|---|---|
| ALT (U/L) | 31.46 ± 0.93 | 28.8 ± 0.76 | 81.36 ± 1.39 ** | 38.2 ±1.33 **,## |
| AST (U/L) | 50.56 ±1.18 | 47.04 ± 1.58 | 121.38 ± 2.35 ** | 58.18 ± 1.47 **,## |
| Total protein (g/dL) | 7.45 ± 0.11 | 7.49 ± 0.10 | 4.46 ± 0.11 ** | 6.53 ± 0.14 **,## |
| Albumin (g/dL) | 3.94 ± 0.06 | 3.92 ± 0.08 | 2.63 ± 0.04 ** | 3.46 ± 0.05 **,## |
| Globulin(g/dL) | 3.43 ± 0.07 | 3.57 ± 0.15 | 1.83 ± 0.13 ** | 3.07 ± 0.10 *,## |
| Control | ASX | TCP | TCP + ASX | ||
|---|---|---|---|---|---|
| Log2 HA Titer | 5.6 ± 0.24 | 6.2 ± 0.37 | 4 ± 0.32 ** | 5.2 ± 0.37 *,## | |
| IgM-PFC/106 spleen cells | 1148 ± 35.23 | 1212 ± 33.77 | 835.4 ± 25.71 ** | 1022.4 ± 37.57 | |
| DTH (mm) | 24 h | 0.93 ± 0.01 | 0.95 ± 0.02 | 0.68 ± 0.04 ** | 0.81 ± 0.03 **,## |
| 48 h | 0.53 ± 0.02 | 0.61 ± 0.01 ** | 0.41 ± 0.01 ** | 0.49 ± 0.01 ## | |
| Phagocytic index | 0.032 ± 0.002 | 0.034 ± 0.002 | 0.015 ± 0.001 ** | 0.024 ± 0.001 **,## | |
| IL-1β (pg/mL) | 201.68 ± 3.01 | 195.74 ± 3.18 | 282.41 ± 3.26 ** | 242.02 ± 3.49 **,## | |
| IL-6 (pg/mL) | 331.33 ± 5.7 | 324.74 ± 5.13 | 390.65 ± 6.85 ** | 343.50 ± 5.28 | |
| IL-10 (pg/mL) | 64.75 ± 1.63 | 65.07± 1.77 | 101.22 ± 2.61 ** | 81.51 ± 2.19 **,## | |
| Organ | Lesion | Lesion Scoring | |||
|---|---|---|---|---|---|
| Control | ASX | TCP | TCP + ASX | ||
| Liver | Congestion of central and portal vein | 0.30 ± 0.01 | 0.12 ± 0.01 | 68.70 ± 3.24 ** | 13.10 ± 0.07 **,## |
| Congestion in hepatic sinusoids | 0.96 ± 0.14 | 0.61 ± 0.30 | 32.40 ± 0.21 ** | 9.97 ± 0.03 **,## | |
| Hydropic degeneration | 0.00 ± 0.00 | 0.00 ± 0.00 | 26.22 ± 0.17 ** | 2.12 ± 0.11 | |
| Granular degeneration of hepatocytes | 0.00 ± 0.00 | 0.00 ± 0.00 | 83.00 ± 0.24 ** | 2.90 ± 0.29 | |
| Coagulative necrosis in hepatocytes | 0.00 ± 0.00 | 0.00 ± 0.00 | 41.70 ± 0.24 ** | 2.07 ± 0.30 | |
| Inflammatory cells infiltration | 0.00 ± 0.00 | 0.00 ± 0.00 | 93.06 ± 5.24 ** | 23.70 ± 1.24 **,## | |
| Area of coagulative necrosis infiltrated by inflammatory cells | 0.00 ± 0.00 | 0.00 ± 0.00 | 34.00 ± 2.27 ** | 0.78 ± 0.37 | |
| Spleen | Decreased population of lymphocytes | 0.00 ± 0.00 | 0.00 ± 0.00 | 59.41 ± 5.06 ** | 0.00 ± 0.00 |
| Weakly stained lymphocytes | 0.00 ± 0.00 | 0.00 ± 0.00 | 57.33 ± 2.72 ** | 1.70 ± 0.13 | |
| Degenerative changes of lymphocytes (necrosis, apoptosis) | 0.00 ± 0.00 | 0.00 ± 0.00 | 66.96 ± 4.04 ** | 17.44 ± 0.89 **,## | |
| Tingible body macrophages | 0.00 ± 0.00 | 0.00 ± 0.00 | 63.76 ± 3.70 ** | 12.90 ± 1.71 **,## | |
| Intercellular space dilatation | 0.00 ± 0.00 | 0.00 ± 0.00 | 28.27 ± 0.67 ** | 0.00 ± 0.00 | |
| Edema and hyperemia of trabeculae | 0.00 ± 0.00 | 0.00 ± 0.00 | 19.21 ± 1.97 ** | 0.00 ± 0.00 | |
| Thymus | Thickened interlobular septum between the thymus lobules | 0.00 ± 0.00 | 0.00 ± 0.00 | 29.07 ± 4.14 ** | 0.00 ± 0.00 |
| Lymphocyte depletion | 0.00 ± 0.00 | 0.00 ± 0.00 | 63.88 ± 5.07 ** | 20.14 ± 2.27 **,## | |
| Necrosis | 0.00 ± 0.00 | 0.00 ± 0.00 | 51.36 ± 3.04 ** | 16.18 ±1.35 **,## | |
| Tangible body macrophages @ | 0.00 ± 0.00 | 0.00 ± 0.00 | 58.04 ± 3.1 ** | 24.33 ± 0.31 **,## | |
| Degenerated epithelial reticular cells in the medulla | 0.00 ± 0.00 | 0.00 ± 0.00 | 68.38 ± 6.00 ** | 28.09 ± 2.71 **,## | |
| Calcified Hassall’s corpuscles in the medulla | 0.00 ± 0.00 | 0.00 ± 0.00 | 27.20 ± 3.04 ** | 0.00 ± 0.00 | |
| Target Genes | Accession No. | Sequence (5′ to 3′) | Tm | Product Size |
|---|---|---|---|---|
| GAPDH (reference gene) | NM_017008.4 | F: 5′-GAGACAGCCGCATCTTCTTG-3′ R: 5′-TGACTGTGCCGTTGAACTTG-3′ | 58.99 58.99 | 224 bp |
| iNOS | XM_006246949.3 | F: 5′-GTTTGACCAGAGGACCCAGA-3′ R: 5′-GTGAGCTGGTAGGTTCCTGT-3′ | 59 59 | 175 bp |
| HMGB1 | NM_012963.2 | F: 5′-TCCTTCGGCCTTCTTCTTGT-3′ R: 5′-CGGCCTTCTTTTCATAGGGC-3′ | 58.94 58.97 | 152 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou-Zeid, S.M.; Aljuaydi, S.H.; AbuBakr, H.O.; Tahoun, E.A.; Di Cerbo, A.; Alagawany, M.; Khalil, S.R.; Farag, M.R. Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats. Mar. Drugs 2021, 19, 525. https://doi.org/10.3390/md19090525
Abou-Zeid SM, Aljuaydi SH, AbuBakr HO, Tahoun EA, Di Cerbo A, Alagawany M, Khalil SR, Farag MR. Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats. Marine Drugs. 2021; 19(9):525. https://doi.org/10.3390/md19090525
Chicago/Turabian StyleAbou-Zeid, Shimaa M., Samira H. Aljuaydi, Huda O. AbuBakr, Enas A. Tahoun, Alessandro Di Cerbo, Mahmoud Alagawany, Samah R. Khalil, and Mayada R. Farag. 2021. "Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats" Marine Drugs 19, no. 9: 525. https://doi.org/10.3390/md19090525
APA StyleAbou-Zeid, S. M., Aljuaydi, S. H., AbuBakr, H. O., Tahoun, E. A., Di Cerbo, A., Alagawany, M., Khalil, S. R., & Farag, M. R. (2021). Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats. Marine Drugs, 19(9), 525. https://doi.org/10.3390/md19090525








