The Effect of Molecular Weight on the Antimicrobial Activity of Chitosan from Loligo opalescens for Food Packaging Applications
Abstract
:1. Introduction
2. Results
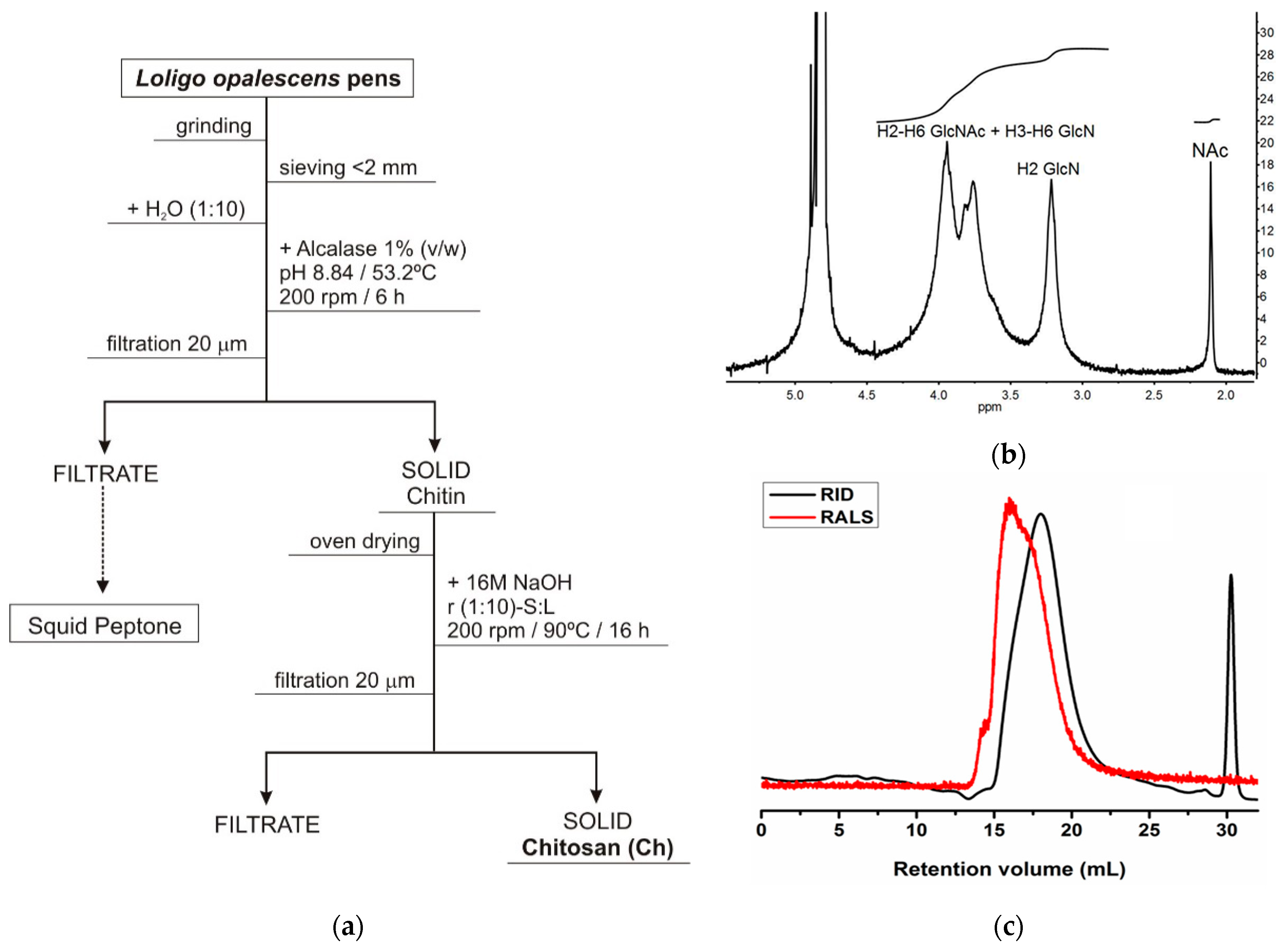
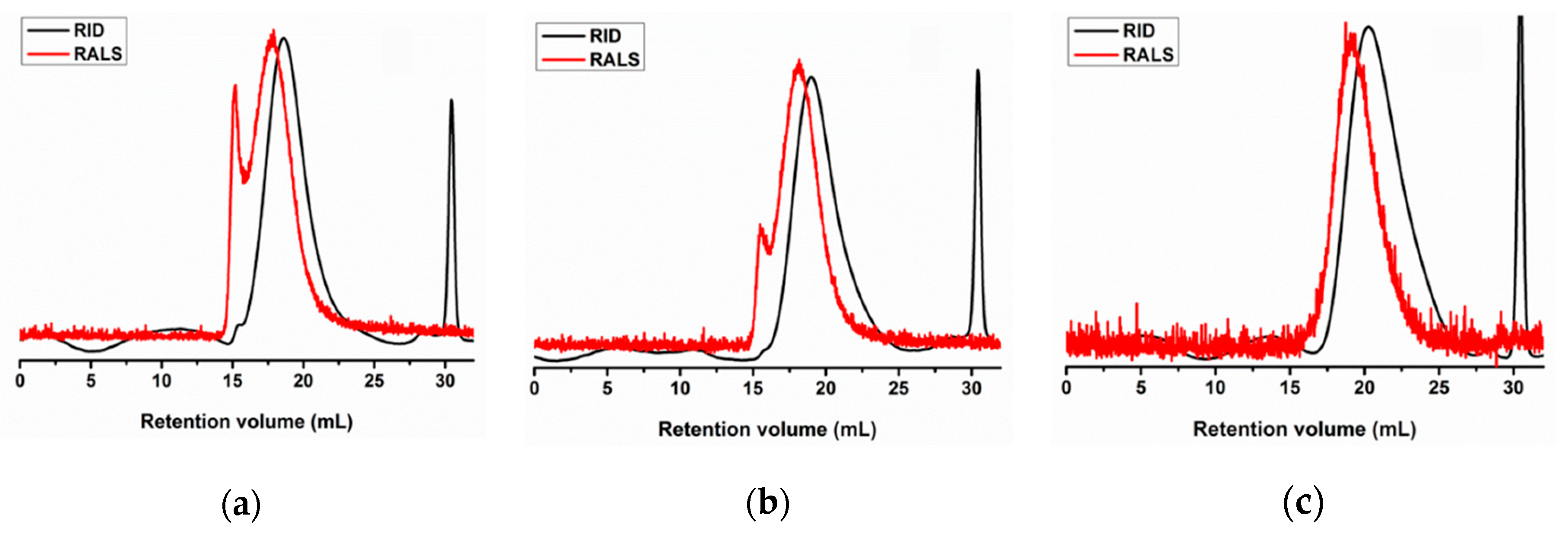
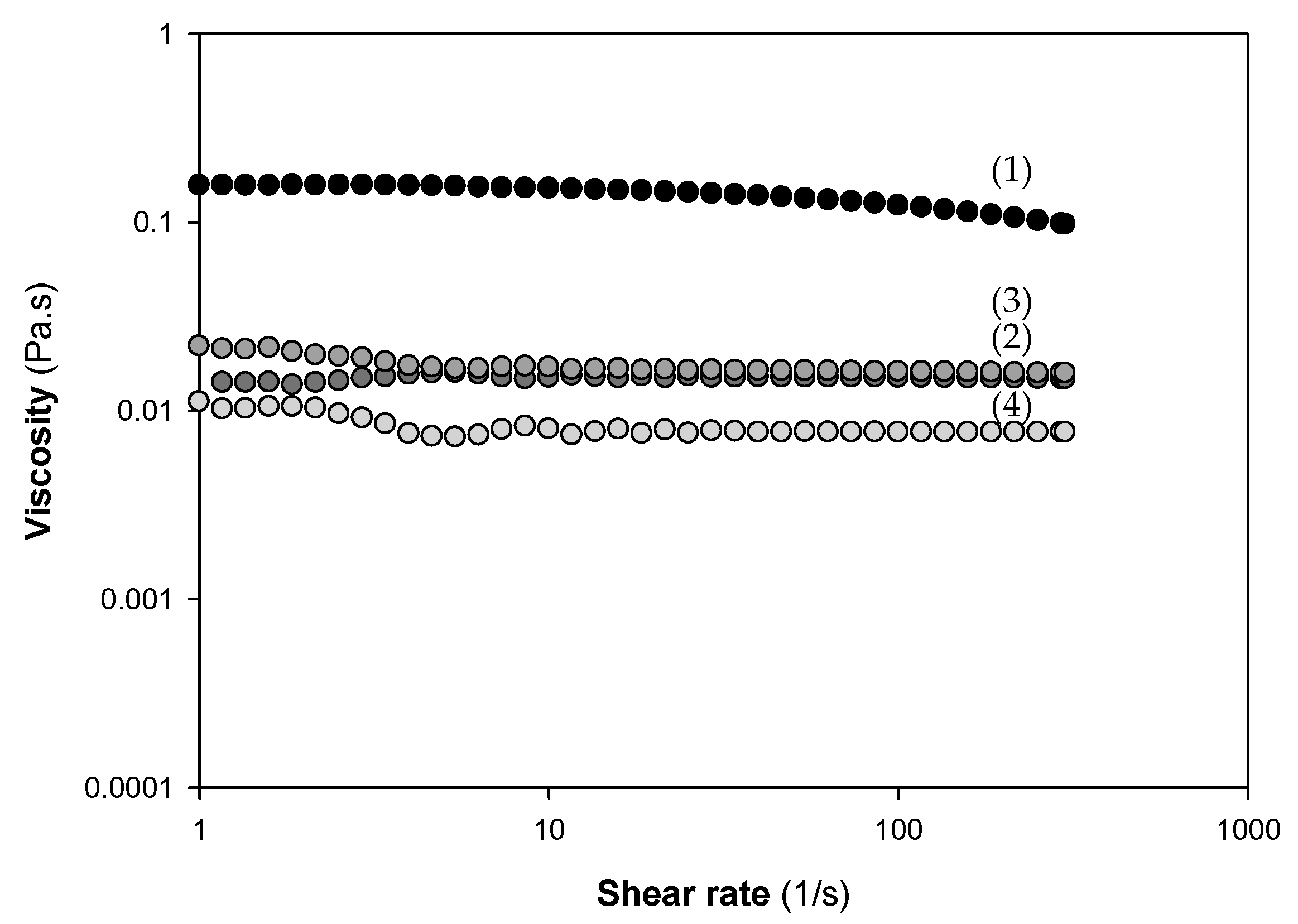
2.1. Production and Characterization of Chitosan and Derivatives
2.2. Characterization of Functionalized Poly(lactic acid) (PLA) Films
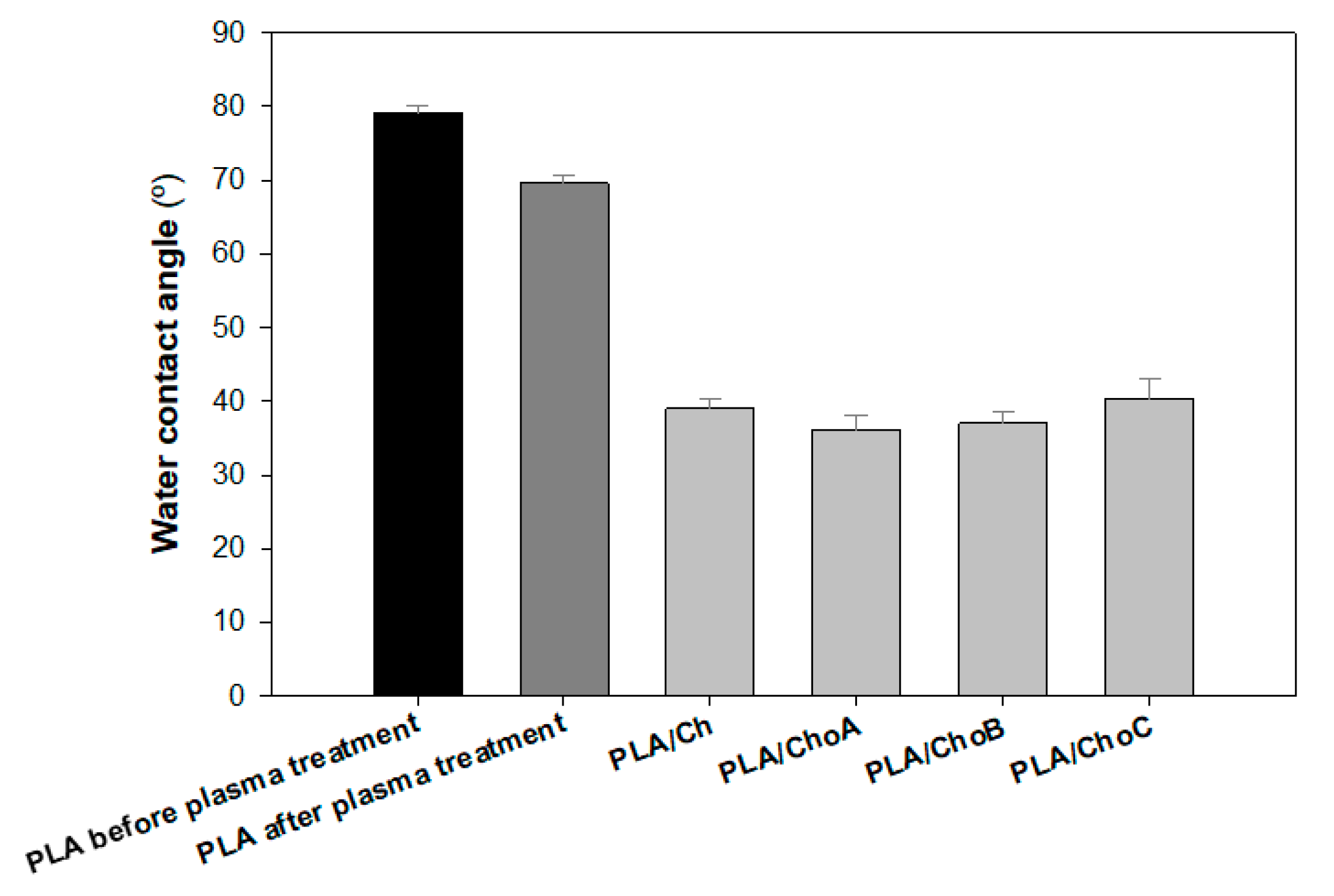
2.2.1. Water Contact Angle
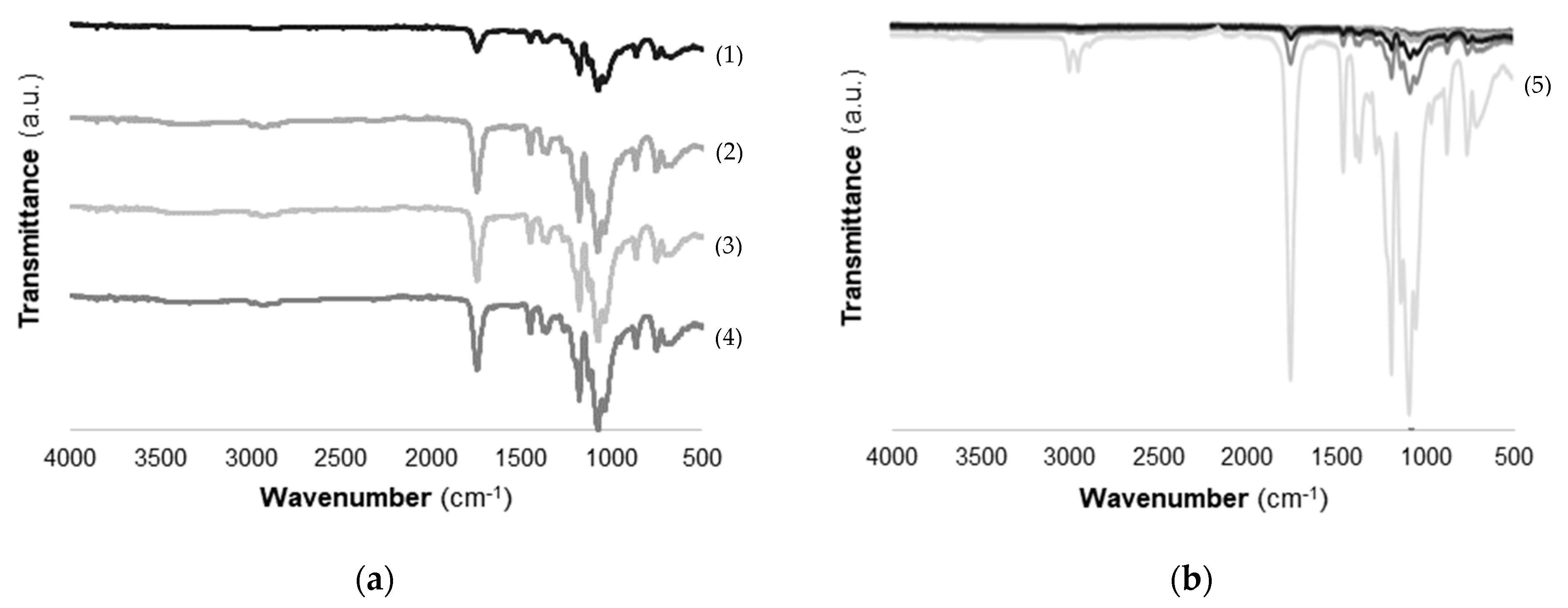
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
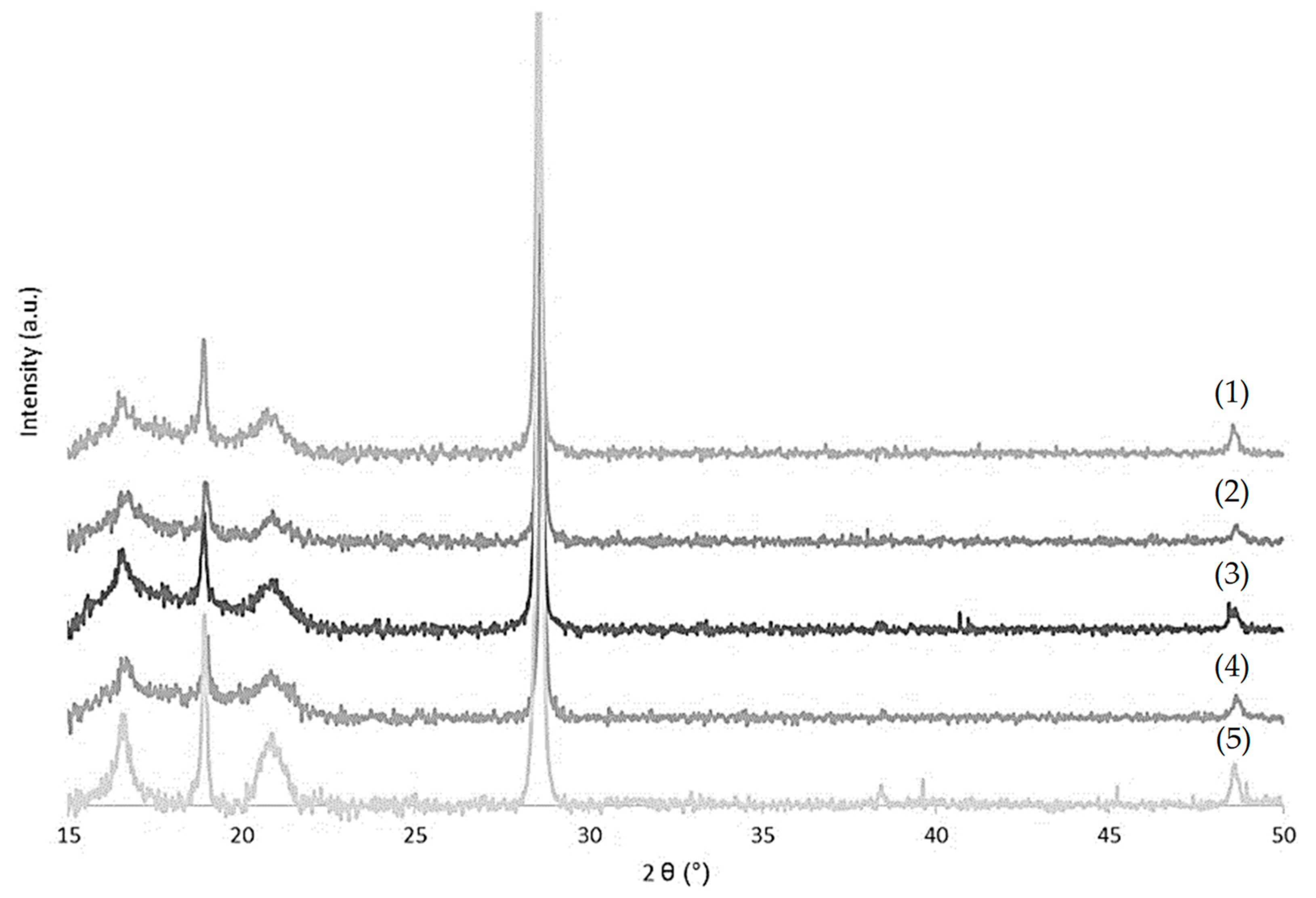
2.2.3. X-ray Diffraction (XRD)
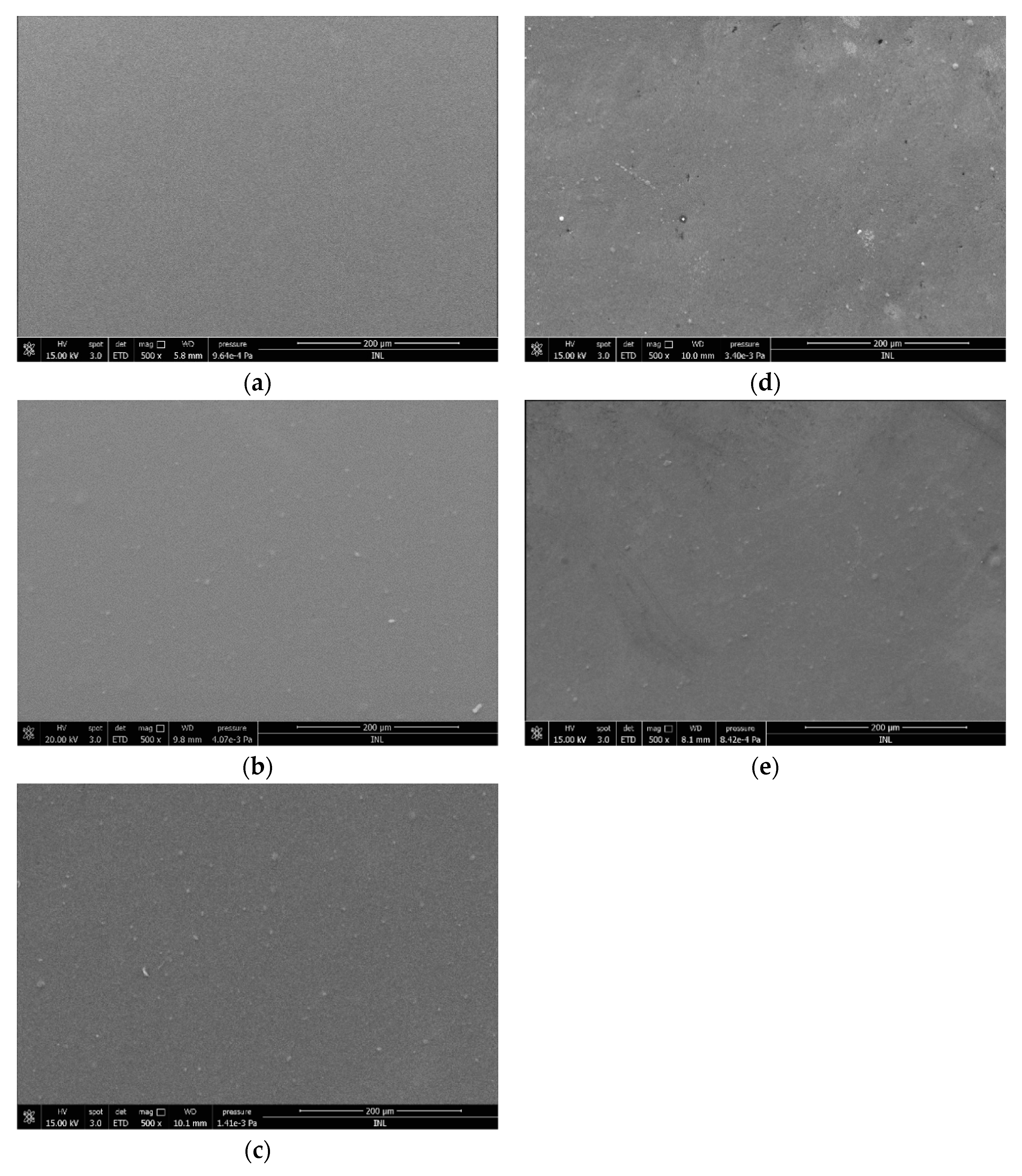
2.2.4. Morphological Studies
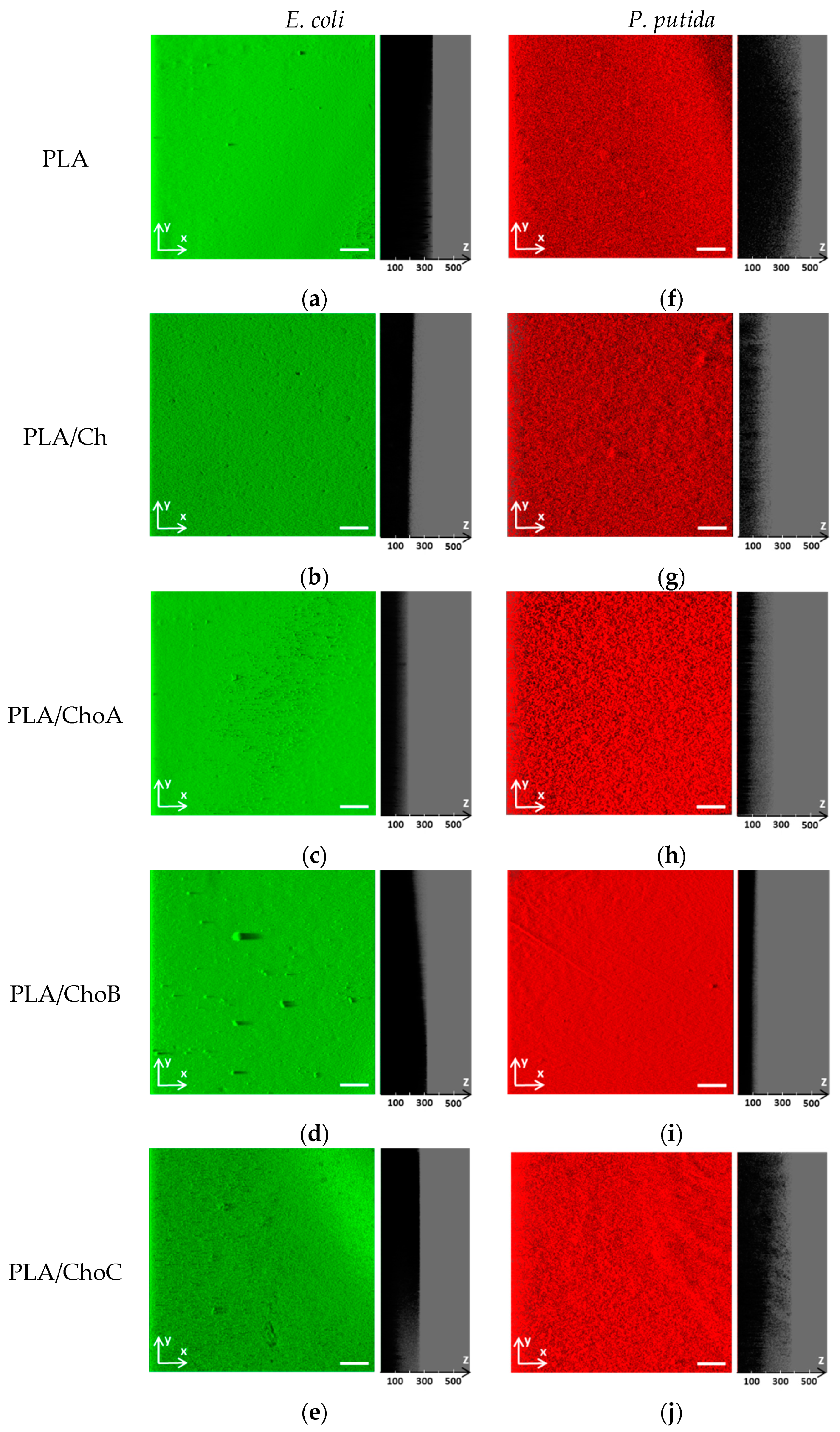
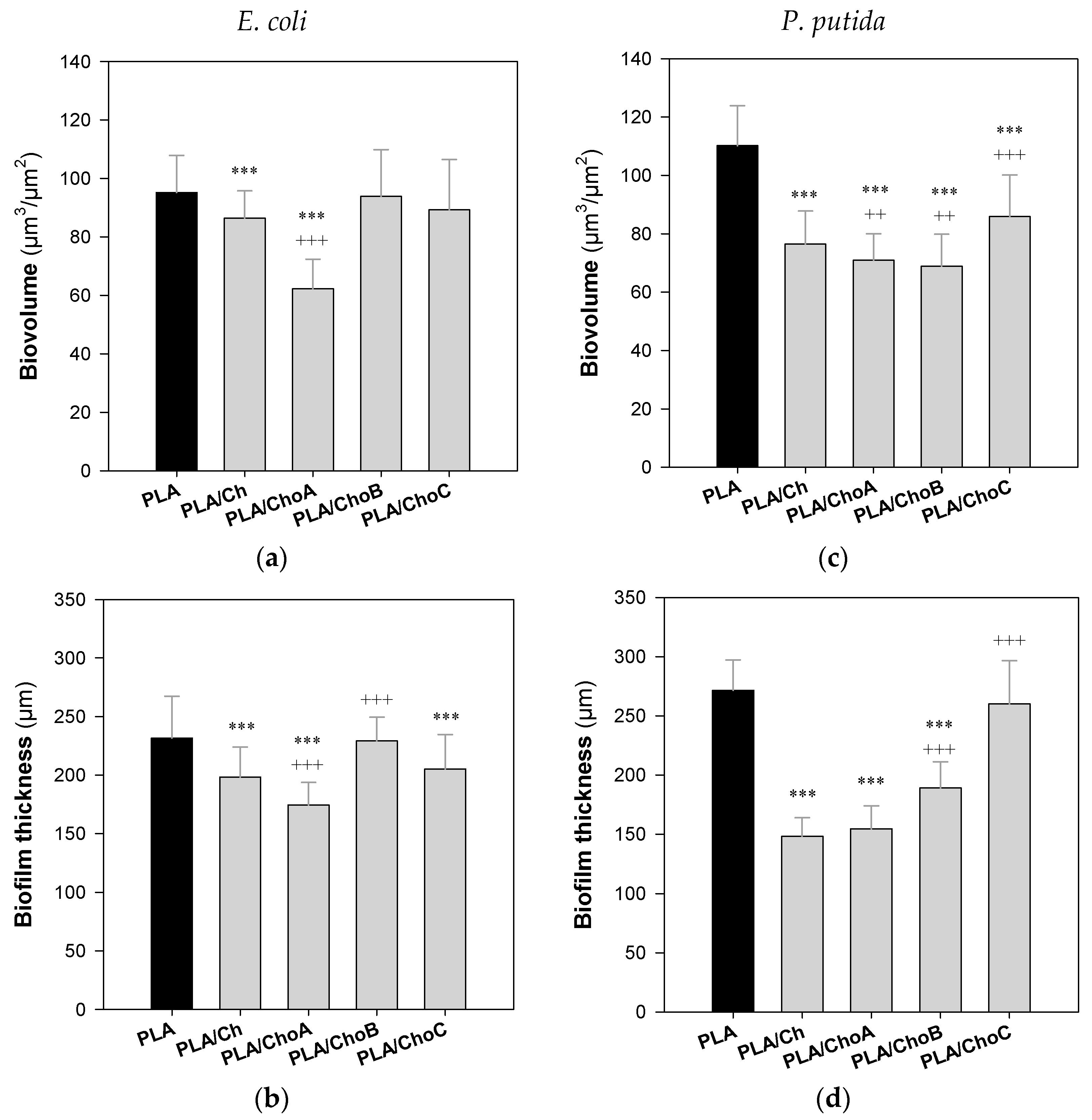
2.3. Antimicrobial Activity of Functionalized PLA Films
3. Discussion
4. Materials and Methods
4.1. Production and Chemical Characterization of Chitosan and Chitooligosaccharides
4.2. Immobilization of Chitosan and Chitooligosaccharides onto Poly(lactic acid) (PLA) Films
4.3. Rheological Measurements of Chitosan Solutions
4.4. Surface Characterization
4.4.1. Water Contact Angle
4.4.2. Fourier-Transform Infrared Spectroscopy (FTIR)
4.4.3. X-ray Diffraction (XRD)
4.4.4. Scanning Electron Microscopy (SEM)
4.5. Antimicrobial Activity of Functionalized PLA Films
4.5.1. Bacterial Strains and Culture Conditions
4.5.2. Biofilm Formation
4.5.3. Biofilm Cell Quantification
4.5.4. Confocal Scanning Electron Microscopy (CLSM)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díez-Pascual, A.M. Antimicrobial Polymer-Based Materials for Food Packaging Applications. Polymers 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, G.C.; Gombi-Vaca, M.F.; da Costa Louzada, M.L.; Azeredo, C.M.; Levy, R.B. The consumption of ultra-processed foods according to eating out occasions. Public Health Nutr. 2020, 23, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Ketelsen, M.; Janssen, M.; Hamm, U. Consumers’ response to environmentally-friendly food packaging-a systematic review. J. Clean. Prod. 2020, 254, 120123. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, A.; Lagnika, C.; Abdin, M.; Hashim, M.M.; Ahmed, W. Preparation and Characterization of Chitosan/Gelatin-Based Active Food Packaging Films Containing Apple Peel Nanoparticles. J. Polym. Environ. 2020, 28, 411–420. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Azlin-Hasim, S.; Cruz-Romero, M.; Cummins, E.; Kerry, J.P.; Morris, M.A. Antimicrobial effect of benzoic and sorbic acid salts and nano-solubilisates against Staphylococcus aureus, Pseudomonas fluorescens and chicken microbiota biofilms. Food Control 2020, 107, 106786. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Int. Food Res. J. 2020, 134, 109214. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2011, 44, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systematic study needs for food applications. Food Sci. Technol. Int. 2012, 18, 3–34. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Lago, M.A.; Sendón, R.; de Quirós, A.R.-B.; Sanches-Silva, A.; Costa, H.S.; Sánchez-Machado, D.I.; Valdez, H.S.; Angulo, I.; Aurrekoetxea, G.P.; Torrieri, E.; et al. Preparation and Characterization of Antimicrobial Films Based on Chitosan for Active Food Packaging Applications. Food Bioprocess. Tech. 2014, 7, 2932–2941. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(lactic acid)-modified films for food packaging application: Physical, mechanical, and barrier behavior. J. Appl. Polym. Sci. 2012, 125, E390–E401. [Google Scholar] [CrossRef]
- Sébastien, F.; Stéphane, G.; Copinet, A.; Coma, V. Novel biodegradable films made from chitosan and poly(lactic acid) with antifungal properties against mycotoxinogen strains. Carbohydr. Polym. 2006, 65, 185–193. [Google Scholar] [CrossRef]
- Grande, R.; Carvalho, A.J. Compatible ternary blends of chitosan/poly(vinyl alcohol)/poly(lactic acid) produced by oil-in-water emulsion processing. Biomacromolecules 2011, 12, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.C.; Yamashita, F.; Müller, C.M.O.; Pires, A.T.N. Thermoplastic starch/poly(lactic acid) sheets coated with cross-linked chitosan. Polym. Test. 2013, 32, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, J.; Fortunati, E.; Vargas, M.; Chiralt, A.; Kenny, J.M. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. Food Eng. 2013, 119, 236–243. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chen, Y.-J.; Tseng, H.-J.; Hsiao, H.-I.; Chai, H.-J.; Shang, K.-C.; Pan, C.-L.; Tsai, G.-J. Antibacterial Activity of Chitosan–Polylactate Fabricated Plastic Film and Its Application on the Preservation of Fish Fillet. Polymers 2021, 13, 696. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Ramos, P.; Mirón, J.; Valcarcel, J.; Sotelo, C.G.; Pérez-Martín, R.I. Production of Chitin from Penaeus vannamei By-Products to Pilot Plant Scale Using a Combination of Enzymatic and Chemical Processes and Subsequent Optimization of the Chemical Production of Chitosan by Response Surface Methodology. Mar. Drugs 2017, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Conway, W.S.; Sams, C.E. Physicochemical Properties and Bioactivity of Fungal Chitin and Chitosan. J. Agric. Food. Chem. 2005, 53, 3888–3894. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.; Fraguas, J.; González, M.; Murado, M.Á. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747. [Google Scholar] [CrossRef] [Green Version]
- Domard, A. A perspective on 30 years research on chitin and chitosan. Carbohydr. Polym. 2011, 84, 696–703. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics 2017/FAO Annuaire; FAO: Rome, Italy, 2017. [Google Scholar]
- Zeidberg, L.D. Doryteuthis opalescens, Opalescent Inshore Squid. In Advances in Squid Biology, Ecology and Fisheries. Part I—Myopsid Squids; Rosa, R., O’Dor, R., Pierce, G., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 159–204. [Google Scholar]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Dai, J.; Li, F.; Fu, X. Towards Shell Biorefinery: Advances in Chemical-Catalytic Conversion of Chitin Biomass to Organonitrogen Chemicals. ChemSusChem 2020, 13, 6498–6508. [Google Scholar] [CrossRef]
- Hülsey, M.J. Shell biorefinery: A comprehensive introduction. Green Energy Environ. 2018, 3, 318–327. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Meireles, A.; Fulgêncio, R.; Machado, I.; Mergulhão, F.J.M.; Melo, L.F.; Simões, M.V. Characterization of the heterotrophic bacteria from a minimally processed vegetables plant. LWT Food Sci. Technol. 2017, 85, 293–300. [Google Scholar] [CrossRef]
- Gomes, L.C.; Piard, J.-C.; Briandet, R.; Mergulhão, F.J.M. Pseudomonas grimontii biofilm protects food contact surfaces from Escherichia coli colonization. LWT Food Sci. Technol. 2017, 85, 309–315. [Google Scholar] [CrossRef]
- Hadawey, A.; Tassou, S.A.; Chaer, I.; Sundararajan, R. Unwrapped food product display shelf life assessment. Energy Procedia 2017, 123, 62–69. [Google Scholar] [CrossRef]
- Franzetti, L.; Scarpellini, M. Characterisation of Pseudomonas spp. isolated from foods. Ann. Microbiol. 2007, 57, 39–47. [Google Scholar] [CrossRef]
- Allan, G.G.; Peyron, M. Molecular weight manipulation of chitosan II: Prediction and control of extent of depolymerization by nitrous acid. Carbohydr. Res. 1995, 277, 273–282. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of surface roughness on contact angle hysteresis and spreading work. Colloid Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria Asier, M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and antimicrobial edible chitosan films blended with stem, leaf and seed extracts of Pistacia terebinthus for active food packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef] [Green Version]
- Stoleru, E.; Dumitriu, R.P.; Munteanu, B.S.; Zaharescu, T.; Tănase, E.E.; Mitelut, A.; Ailiesei, G.-L.; Vasile, C. Novel procedure to enhance PLA surface properties by chitosan irreversible immobilization. Appl. Surf. Sci. 2016, 367, 407–417. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D. Plasticizer effect on melt blending of polylactide stereocomplex. e-Polymers 2017, 17, 409–416. [Google Scholar] [CrossRef]
- Cardoso, E.; Parra, D.F.; Scagliusi, S.R.; Sales, R.M.; Caviquioli, F.; Lugao, A.B. Study of Bio-Based Foams Prepared from PBAT/PLA Reinforced with Bio-Calcium Carbonate and Compatibilized with Gamma Radiation. In Use of Gamma Radiation Techniques in Peaceful Applications; Almayah, B.A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Julkapli, N.M.; Ahmad, Z.; Akil, H.M. X-ray Diffraction Studies of Cross Linked Chitosan With Different cross Linking Agents for Waste Water Treatment Application. AIP Conf. Proc. 2010, 1202, 106–111. [Google Scholar]
- Latou, E.; Mexis, S.F.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. LWT Food Sci. Technol. 2014, 55, 263–268. [Google Scholar] [CrossRef]
- Chen, C.; Dong, L.; Cheung, M.K. Preparation and characterization of biodegradable poly(l-lactide)/chitosan blends. Eur. Polym. J. 2005, 41, 958–966. [Google Scholar] [CrossRef]
- Li, L.; Ding, S.; Zhou, C. Preparation and degradation of PLA/chitosan composite materials. J. Appl. Polym. Sci. 2004, 91, 274–277. [Google Scholar] [CrossRef]
- Calero, N.; Muñoz, J.; Ramírez, P.; Guerrero, A. Flow behaviour, linear viscoelasticity and surface properties of chitosan aqueous solutions. Food Hydrocoll. 2010, 24, 659–666. [Google Scholar] [CrossRef]
- Hwang, J.; Shin, H.-H. Rheological properties of chitosan solutions. Korea Aust. Rheol. J. 2000, 12, 175–179. [Google Scholar]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; Nunes de Oliveira, P.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, D. Viscosity and flow properties of concentrated solutions of chitosan with different degrees of deacetylation. Int. J. Biol. Macromol. 1994, 16, 149–152. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P.; Inamdar, M.S. Aqueous Behaviour of Chitosan. Int. J. Polym. Sci. 2010, 2010, 939536. [Google Scholar] [CrossRef]
- Elhefian, E.A.; Yahaya, A. Rheological Study of Chitosan and Its Blends: An Overview. Maejo Int. J. Sci. Technol. 2010, 4, 210–220. [Google Scholar]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Rivera Aguayo, P.; Bruna Larenas, T.; Alarcón Godoy, C.; Cayupe Rivas, B.; González-Casanova, J.; Rojas-Gómez, D.; Caro Fuentes, N. Antimicrobial and Antibiofilm Capacity of Chitosan Nanoparticles against Wild Type Strain of Pseudomonas sp. Isolated from Milk of Cows Diagnosed with Bovine Mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- Tokura, S.; Ueno, K.; Miyazaki, S.; Nishi, N. Molecular weight dependent antimicrobial activity by Chitosan. Macromol. Symp 1997, 120, 199–207. [Google Scholar] [CrossRef]
- Hirano, S.; Tsuchida, H.; Nagao, N. N-acetylation in chitosan and the rate of its enzymic hydrolysis. Biomaterials 1989, 10, 574–576. [Google Scholar] [CrossRef]
- Li, X.-F.; Feng, X.-Q.; Yang, S.; Fu, G.-Q.; Wang, T.-P.; Su, Z.-X. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B 2020, 185, 110627. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Kim, K.Y.; Yoo, Y.J.; Oh, S.J.; Choi, J.H.; Kim, C.Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Malcata, X.F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Oliver, J.D. The viable but non-culturable state and its relevance in food safety. Curr. Opin. Food Sci. 2016, 8, 127–133. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.-W.; Ding, T. Current Perspectives on Viable but Non-culturable State in Foodborne Pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Ramos, P.; Valcarcel, J.; Antelo, L.T.; Novoa-Carballal, R.; Reis, R.L.; Pérez-Martín, R.I. An integral and sustainable valorisation strategy of squid pen by-products. J. Clean. Prod. 2018, 201, 207–218. [Google Scholar] [CrossRef]
- Allan, G.G.; Peyron, M. Molecular weight manipulation of chitosan. I: Kinetics of depolymerization by nitrous acid. Carbohydr. Res. 1995, 277, 257–272. [Google Scholar] [CrossRef]
- Fernandez-Megia, E.; Novoa-Carballal, R.; Quiñoá, E.; Riguera, R. Optimal routine conditions for the determination of the degree of acetylation of chitosan by 1H-NMR. Carbohydr. Polym. 2005, 61, 155–161. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Fernandez-Megia, E.; Riguera, R. Dynamics of Chitosan by 1H NMR Relaxation. Biomacromolecules 2010, 11, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Rochas, C.; Morfin, I.; Viton, C.; Domard, A. Light scattering studies of the solution properties of chitosans of varying degrees of acetylation. Biomacromolecules 2003, 4, 1034–1040. [Google Scholar] [CrossRef]
- Martins, V.D.F.; Cerqueira, M.A.; Fuciños, P.; Garrido-Maestu, A.; Curto, J.M.R.; Pastrana, L.M. Active bi-layer cellulose-based films: Development and characterization. Cellulose 2018, 25, 6361–6375. [Google Scholar] [CrossRef]
- Costa, M.J.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Characterization of PHBV films loaded with FO1 bacteriophage using polyvinyl alcohol-based nanofibers and coatings: A comparative study. Innov. Food Sci Emerg. Technol. 2021, 69, 102646. [Google Scholar] [CrossRef]
- Martins, A.J.; Silva, P.; Maciel, F.; Pastrana, L.M.; Cunha, R.L.; Cerqueira, M.A.; Vicente, A.A. Hybrid gels: Influence of oleogel/hydrogel ratio on rheological and textural properties. Food Res. Int. 2019, 116, 1298–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, L.C.; Deschamps, J.; Briandet, R.; Mergulhão, F.J. Impact of modified diamond-like carbon coatings on the spatial organization and disinfection of mixed-biofilms composed of Escherichia coli and Pantoea agglomerans industrial isolates. Int. J. Food Microbiol. 2018, 277, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Silva, L.N.; Simões, M.; Melo, L.F.; Mergulhão, F.J. Escherichia coli adhesion, biofilm development and antibiotic susceptibility on biomedical materials. J. Biomed. Mater. Res. 2015, 103, 1414–1423. [Google Scholar] [CrossRef] [Green Version]
- Alves, P.; Gomes, L.C.; Vorobii, M.; Rodriguez-Emmenegger, C.; Mergulhão, F.J. The potential advantages of using a poly(HPMA) brush in urinary catheters: Effects on biofilm cells and architecture. Colloids Surf. B 2020, 191, 110976. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.C.; Faria, S.I.; Valcarcel, J.; Vázquez, J.A.; Cerqueira, M.A.; Pastrana, L.; Bourbon, A.I.; Mergulhão, F.J. The Effect of Molecular Weight on the Antimicrobial Activity of Chitosan from Loligo opalescens for Food Packaging Applications. Mar. Drugs 2021, 19, 384. https://doi.org/10.3390/md19070384
Gomes LC, Faria SI, Valcarcel J, Vázquez JA, Cerqueira MA, Pastrana L, Bourbon AI, Mergulhão FJ. The Effect of Molecular Weight on the Antimicrobial Activity of Chitosan from Loligo opalescens for Food Packaging Applications. Marine Drugs. 2021; 19(7):384. https://doi.org/10.3390/md19070384
Chicago/Turabian StyleGomes, Luciana C., Sara I. Faria, Jesus Valcarcel, José A. Vázquez, Miguel A. Cerqueira, Lorenzo Pastrana, Ana I. Bourbon, and Filipe J. Mergulhão. 2021. "The Effect of Molecular Weight on the Antimicrobial Activity of Chitosan from Loligo opalescens for Food Packaging Applications" Marine Drugs 19, no. 7: 384. https://doi.org/10.3390/md19070384
APA StyleGomes, L. C., Faria, S. I., Valcarcel, J., Vázquez, J. A., Cerqueira, M. A., Pastrana, L., Bourbon, A. I., & Mergulhão, F. J. (2021). The Effect of Molecular Weight on the Antimicrobial Activity of Chitosan from Loligo opalescens for Food Packaging Applications. Marine Drugs, 19(7), 384. https://doi.org/10.3390/md19070384









