Fatty Acid Production and Direct Acyl Transfer through Polar Lipids Control TAG Biosynthesis during Nitrogen Deprivation in the Halotolerant Alga Dunaliella tertiolecta
Abstract
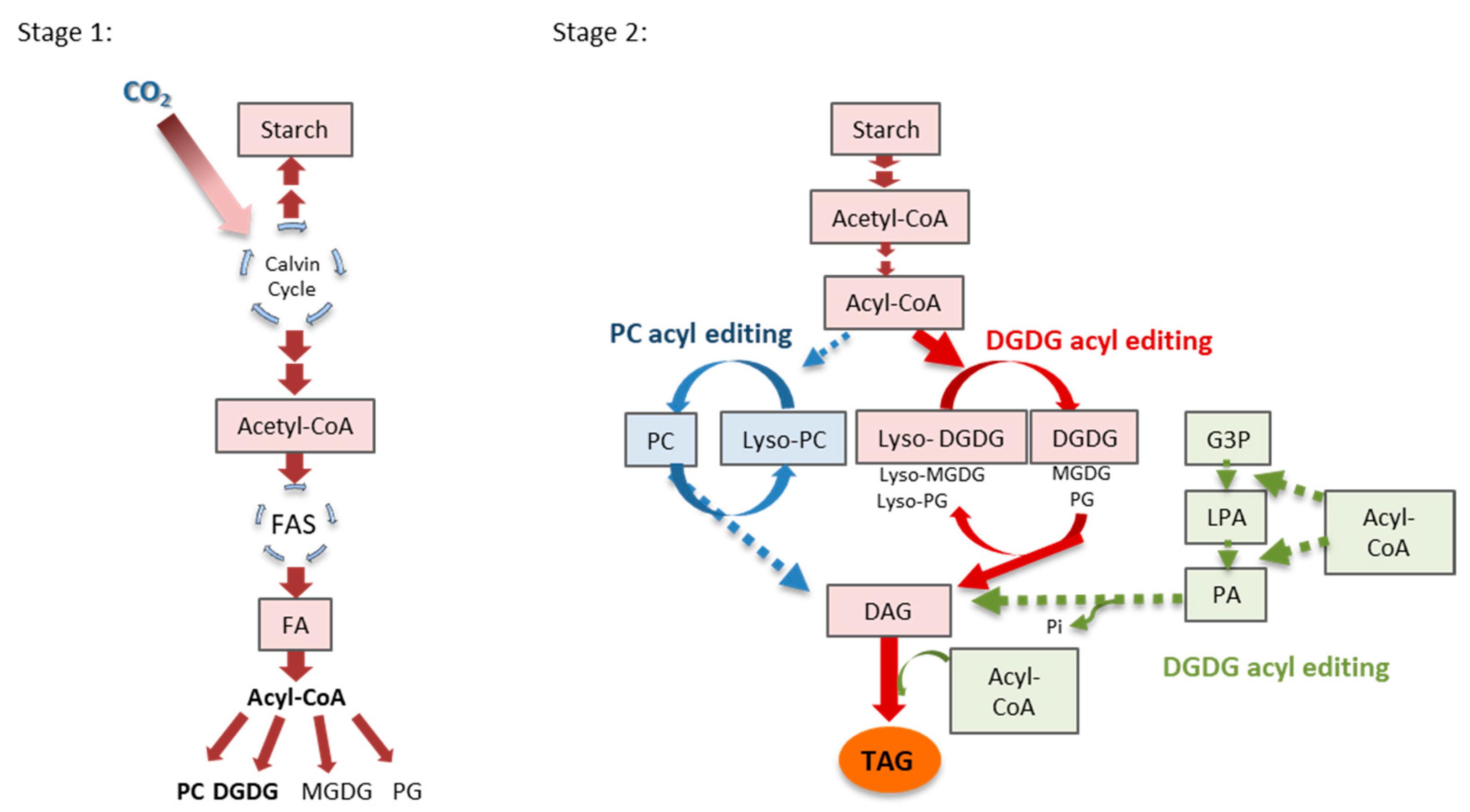
1. Introduction
2. Results
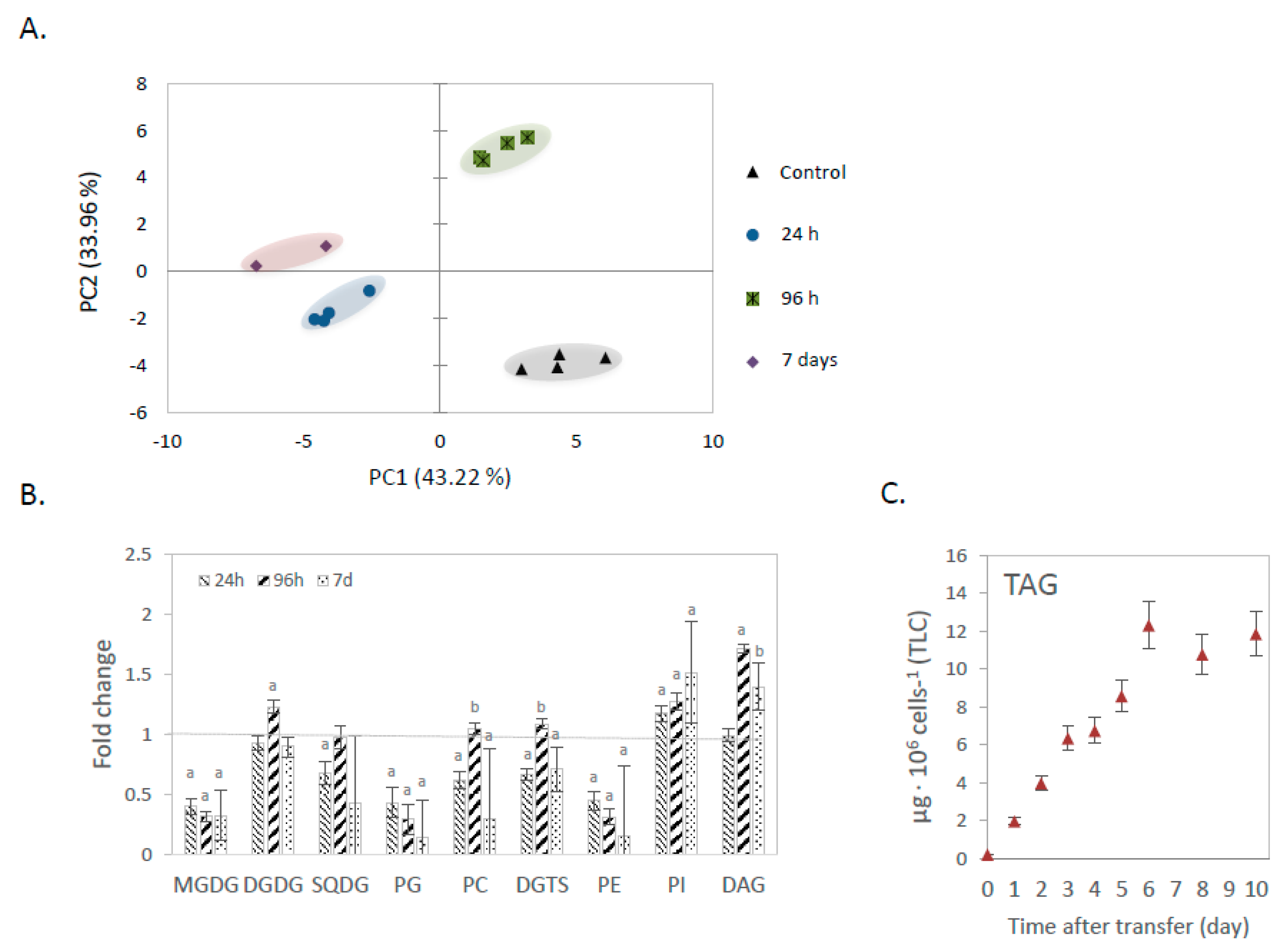
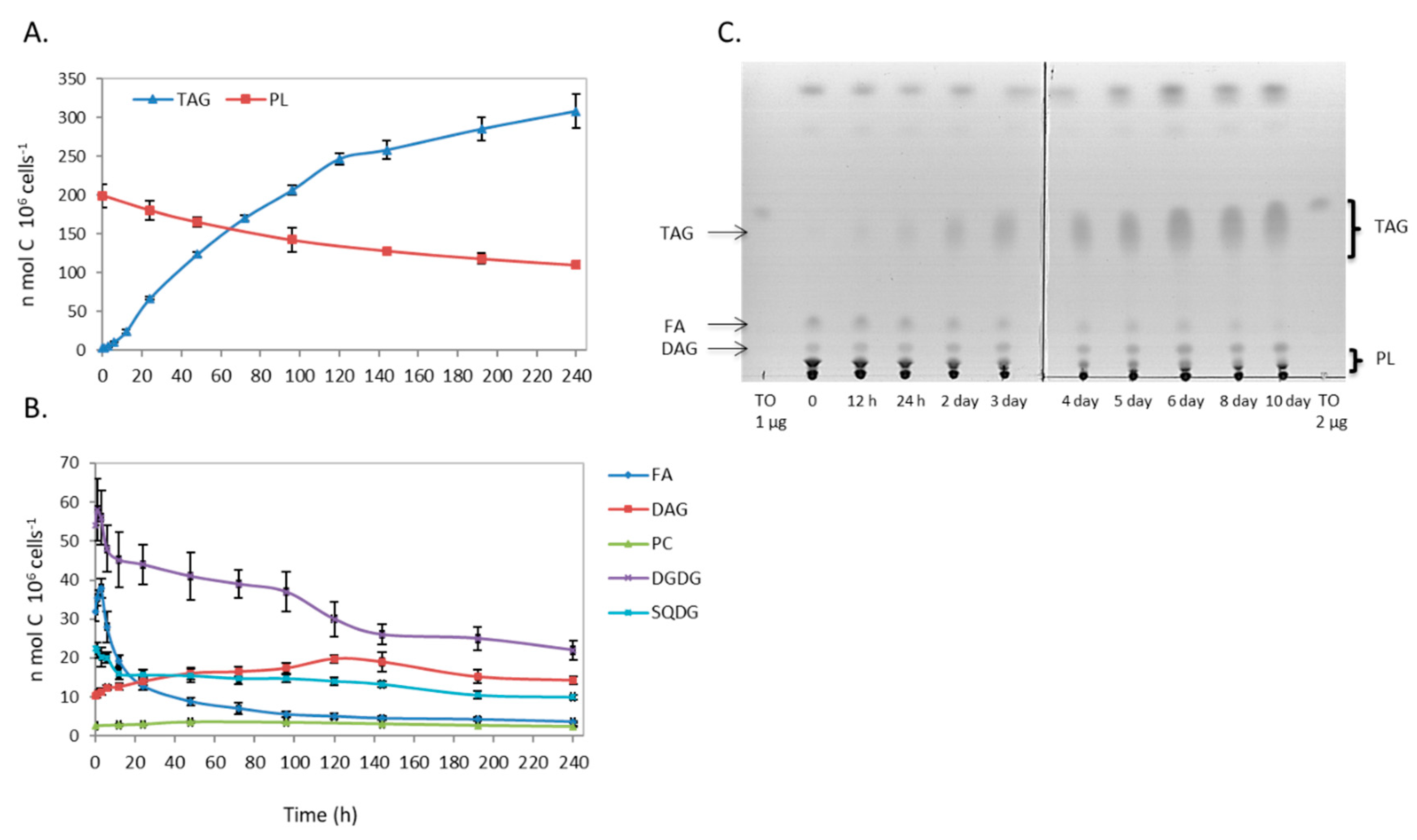
2.1. Changes in Lipidome Profile during Nitrogen Deprivation
2.1.1. Lipidome Analysis
2.1.2. Total 14C Labeling
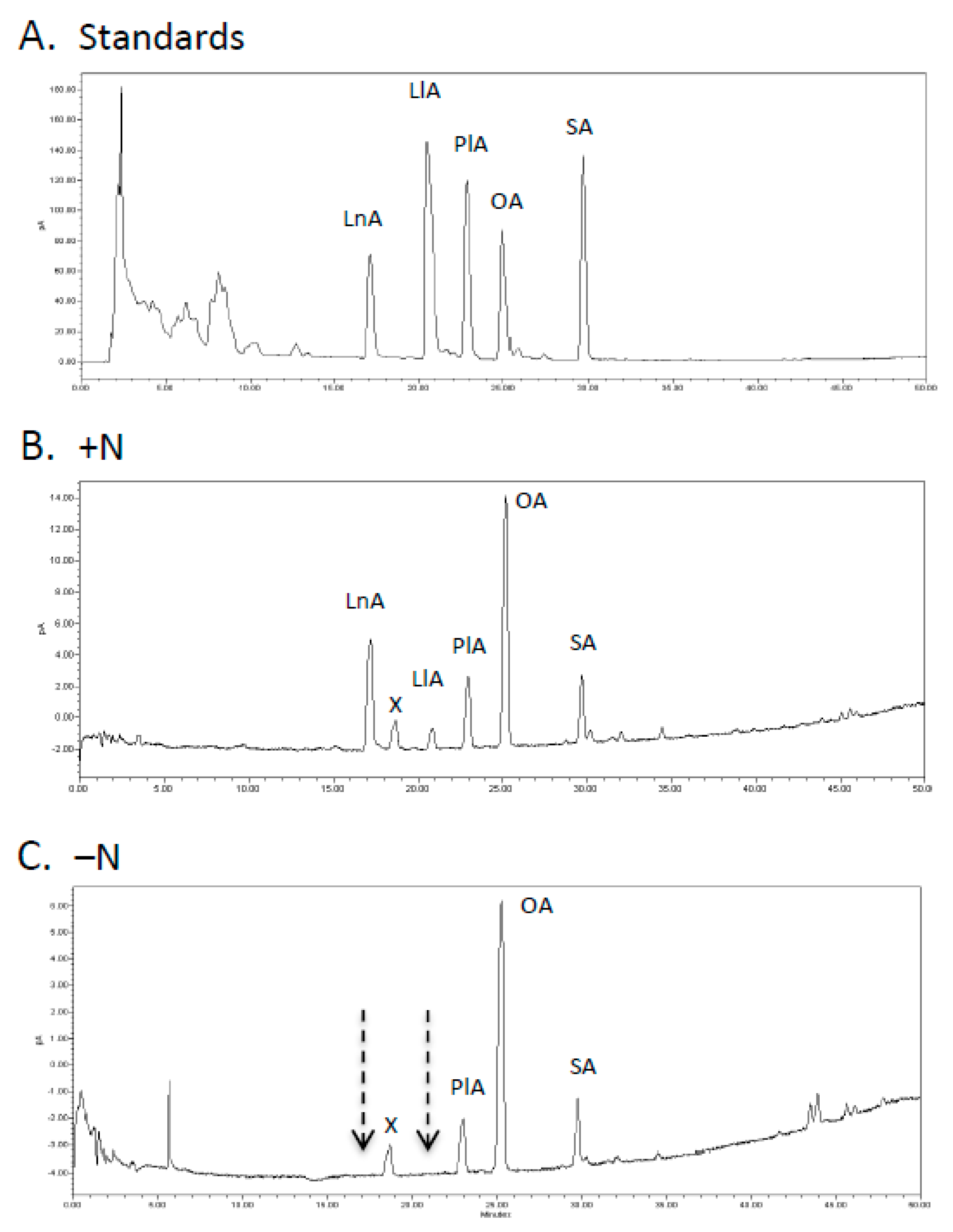
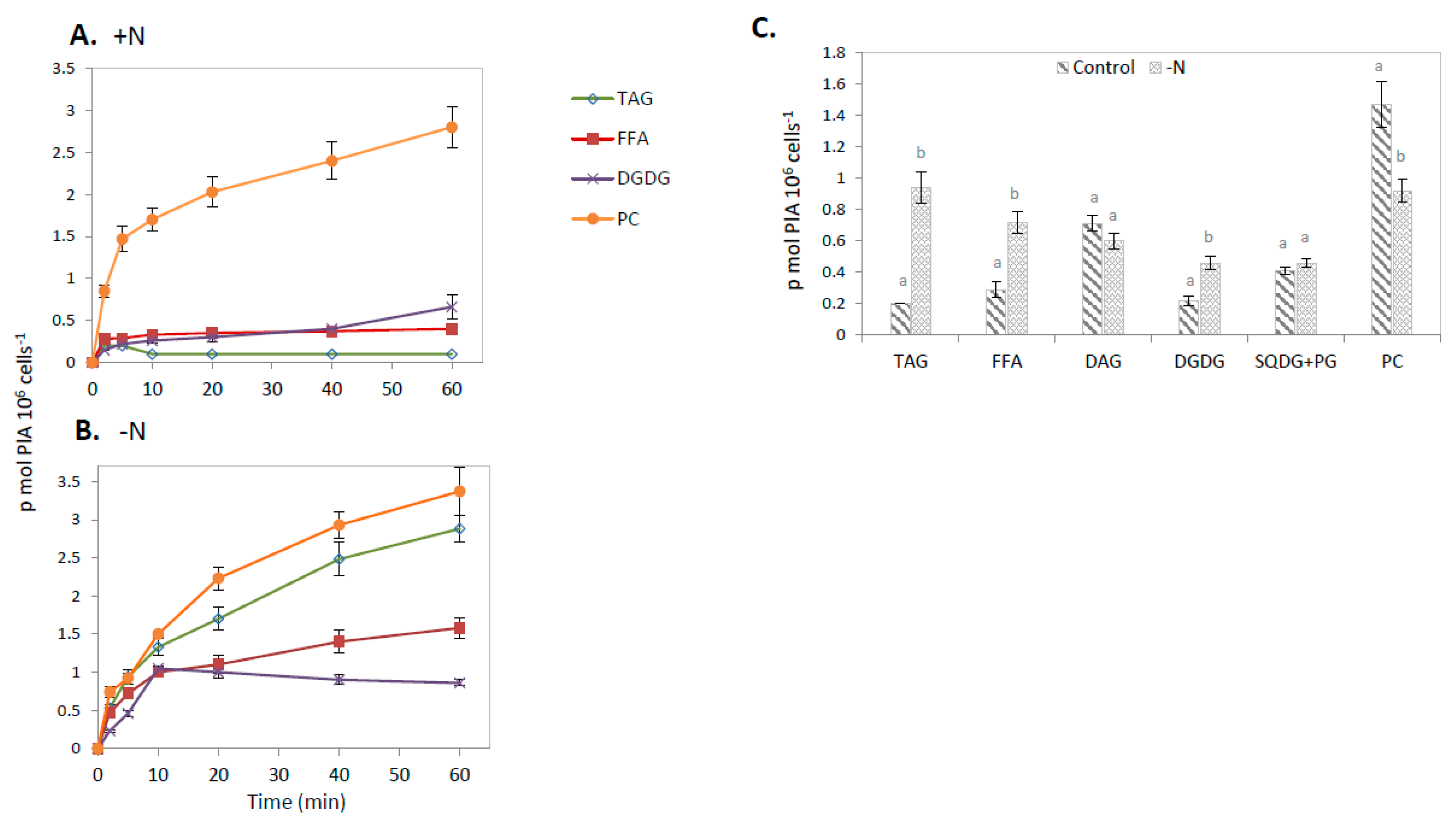
2.2. Estimation of FA Fluxes through Different Lipid Pools Using 14C-PlA
3. Discussion
3.1. The Dynamics of the FA Pool
3.2. Changes in the FA Pool Are Associated with TAG Biosynthesis
3.3. What Is the Biosynthetic Pathway Responsible for FA Integration into TAG?
3.4. Are There Alternative Acyl-Editing Pathways in D. tertiolecta?
3.5. The Origin of PUFA in TAG under N Starvation
4. Materials and Methods
4.1. Radioactive Chemicals
4.2. Algal Strains and Cultivation Conditions
4.3. Labeling with 14C-Bicabonate
4.4. Labeling with 14C-PlA
4.5. Lipid Extraction and Analysis
4.6. Lipidomic LC-MS Analysis
4.7. Determination of Starch
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cakmak, T.; Angun, P.; Demiray, Y.E.; Ozkan, A.D.; Elibol, Z.; Tekinay, T. Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2012, 109, 1947–1957. [Google Scholar] [CrossRef]
- Kropat, J.; Hong-Hermesdorf, A.; Casero, D.; Ent, P.; Castruita, M.; Pellegrini, M.; Merchant, S.S.; Malasarn, D. A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J. 2011, 66, 770–780. [Google Scholar] [CrossRef]
- Goncalves, E.C.; Koh, J.; Zhu, N.; Yoo, M.; Chen, S.; Matsuo, T.; Johnson, J. V Nitrogen starvation-induced accumulation of triacylglycerol in the green algae: Evidence for a role for ROC40, a transcription factor involved in circadian rhythm. Plant J. 2016, 743–757. [Google Scholar] [CrossRef]
- Napier, J.A. The Production of Unusual Fatty Acids in Transgenic Plants. Annu. Rev. Plant Biol. 2007, 58, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Work, V.H.; Radakovits, R.; Jinkerson, R.E.; Meuser, J.E.; Elliott, L.G.; Vinyard, D.J.; Laurens, L.M.L.; Dismukes, G.C.; Posewitz, M.C. Increased lipid accumulation in the Chlamydomonas reinhardtii sta7-10 starchless isoamylase mutant and increased carbohydrate synthesis in complemented strains. Eukaryot. Cell 2010, 9, 1251–1261. [Google Scholar] [CrossRef]
- Johnson, X.; Alric, J. Interaction between starch breakdown, acetate assimilation, and photosynthetic cyclic electron flow in Chlamydomonas reinhardtii. J. Biol. Chem. 2012, 287, 26445–26452. [Google Scholar] [CrossRef]
- Matich, E.K.; Ghafari, M.; Camgoz, E.; Caliskan, E.; Pfeifer, B.A.; Haznedaroglu, B.Z.; Atilla-Gokcumen, G.E. Time-series lipidomic analysis of the oleaginous green microalga species Ettlia oleoabundans under nutrient stress. Biotechnol. Biofuels 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. B. 2010, 8, e0133. [Google Scholar] [CrossRef]
- Fan, J.; Andre, C.; Xu, C. A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. FEBS Lett. 2011, 585, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Goodson, C.; Roth, R.; Wang, Z.T.; Goodenough, U. Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryot. Cell 2011, 10, 1592–1606. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Benning, C. Lipid metabolism in microalgae distinguishes itself. Curr. Opin. Biotechnol. 2013, 24, 300–309. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Beisson, F.; Riekhof, W. Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J. 2015, 82, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Pick, U.; Avidan, O. Triacylglycerol is produced from starch and polar lipids in the green alga Dunaliella tertiolecta. J. Exp. Bot. 2017, 68, 4939–4950. [Google Scholar] [CrossRef]
- Küpper, F.C.; Gaquerel, E.; Cosse, A.; Adas, F.; Peters, A.F.; Müller, D.G.; Kloareg, B.; Salaün, J.-P.; Potin, P. Free Fatty Acids and Methyl Jasmonate Trigger Defense Reactions in Laminaria digitata. Plant Cell Physiol. 2009, 50, 789–800. [Google Scholar] [CrossRef]
- Parrish, C.C.; deFreitas, A.S.W.; Bodennec, G.; Macpherson, E.J.; Ackman, R.G. Lipid composition of the toxic marine diatom, Nitzschia pungens. Phytochemistry 1991, 30, 113–116. [Google Scholar] [CrossRef]
- Li, N.; Gügel, I.L.; Giavalisco, P.; Zeisler, V.; Schreiber, L.; Soll, J.; Philippar, K. FAX1, a Novel Membrane Protein Mediating Plastid Fatty Acid Export. PLoS Biol. 2015, 13, e1002053. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, J.; Taylor, D.C.; Ohlrogge, J.B. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 2009, 21, 3885–3901. [Google Scholar] [CrossRef]
- Bates, P.D.; Stymne, S.; Ohlrogge, J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013, 16, 358–364. [Google Scholar] [CrossRef]
- Miller, R.; Wu, G.; Deshpande, R.R.; Vieler, A.; Gartner, K.; Li, X.; Moellering, E.R.; Zauner, S.; Cornish, A.J.; Liu, B.; et al. Changes in transcript abundance in chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol. 2010, 154, 1737–1752. [Google Scholar] [CrossRef] [PubMed]
- Boyle, N.R.; Page, M.D.; Liu, B.; Blaby, I.K.; Casero, D.; Kropat, J.; Cokus, S.J.; Hong-Hermesdorf, A.; Shaw, J.; Karpowicz, S.J.; et al. Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J. Biol. Chem. 2012, 287, 15811–15825. [Google Scholar] [CrossRef]
- Msanne, J.; Xu, D.; Konda, A.R.; Casas-Mollano, J.A.; Awada, T.; Cahoon, E.B.; Cerutti, H. Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 2012, 75, 50–59. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Ko, S.R.; Oh, H.M.; Kim, H.S. Lipid droplet synthesis is limited by acetate availability in starchless mutant of Chlamydomonas reinhardtii. FEBS Lett. 2013, 587, 370–377. [Google Scholar] [CrossRef]
- Chen, J.E.; Smith, A.G. A look at diacylglycerol acyltransferases (DGATs) in algae. J. Biotechnol. 2012, 162, 28–39. [Google Scholar] [CrossRef]
- Goncalves, E.C.; Wilkie, A.C.; Kirst, M.; Rathinasabapathi, B. Metabolic regulation of triacylglycerol accumulation in the green algae: Identification of potential targets for engineering to improve oil yield. Plant Biotechnol. J. 2016, 14, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Anderson, M.; Kumar, A.; Zhang, Y.; Michael, E.M.; Abrams, S.R.; Zaharia, L.I.; Taylor, D.C.; Fobert, P.R. Transgenic increases in seed oil content are associated with the differential expression of novel Brassica-specific transcripts. BMC Genom. 2008, 9, 619. [Google Scholar] [CrossRef]
- La Russa, M.; Bogen, C.; Uhmeyer, A.; Doebbe, A.; Filippone, E.; Kruse, O.; Mussgnug, J.H. Functional analysis of three type-2 DGAT homologue genes for triacylglycerol production in the green microalga Chlamydomonas reinhardtii. J. Biotechnol. 2012, 162, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.F.; Zhang, M.H.; Li, D.W.; Yang, W.D.; Liu, J.S.; Bai, W.B.; Li, H.Y. Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum. Mar. Drugs 2013, 11, 4558–4569. [Google Scholar] [CrossRef]
- Bates, P.D.; Browse, J. The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Front. Plant Sci. 2012, 3, 147. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Everard, J.D.; Kinney, A.J.; Quant, P.A.; Harwood, J.L. Studies on the regulation of lipid biosynthesis in plants: Application of control analysis to soybean. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1488–1500. [Google Scholar] [CrossRef]
- Hurlock, A.K.; Roston, R.L.; Wang, K.; Benning, C. Lipid trafficking in plant cells. Traffic 2014, 15, 915–932. [Google Scholar] [CrossRef]
- Park, J.J.; Wang, H.; Gargouri, M.; Deshpande, R.R.; Skepper, J.N.; Holguin, F.O.; Juergens, M.T.; Shachar-Hill, Y.; Hicks, L.M.; Gang, D.R. The response of Chlamydomonas reinhardtii to nitrogen deprivation: A systems biology analysis. Plant J. 2015, 81, 611–624. [Google Scholar] [CrossRef]
- Blaby, I.K.; Glaesener, A.G.; Mettler, T.; Fitz-Gibbon, S.T.; Gallaher, S.D.; Liu, B.; Boyle, N.R.; Kropat, J.; Stitt, M.; Johnson, S.; et al. Systems-level analysis of nitrogen starvation-induced modifications of carbon metabolism in a Chlamydomonas reinhardtii starchless mutant. Plant Cell 2013, 25, 4305–4323. [Google Scholar] [CrossRef]
- Goodenough, U.; Blaby, I.; Casero, D.; Gallaher, S.D.; Goodson, C.; Johnson, S.; Lee, J.H.; Merchant, S.S.; Pellegrini, M.; Roth, R.; et al. The path to triacylglyceride obesity in the sta6 strain of Chlamydomonas reinhardtii. Eukaryot. Cell 2014, 13, 591–613. [Google Scholar] [CrossRef]
- Siaut, M.; Cuiné, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F.; Triantaphylidès, C.; Li-Beisson, Y.; et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yan, C.; Andre, C.; Shanklin, J.; Schwender, J.; Xu, C. Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant Cell Physiol. 2012, 53, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Avidan, O.; Brandis, A.; Rogachev, I.; Pick, U. Enhanced acetyl-CoA production is associated with increased triglyceride accumulation in the green alga Chlorella desiccata. J. Exp. Bot. 2015, 66, 3725–3735. [Google Scholar] [CrossRef]
- Malitsky, S.; Ziv, C.; Rosenwasser, S.; Zheng, S.; Schatz, D.; Porat, Z.; Ben-Dor, S.; Aharoni, A.; Vardi, A. Viral infection of the marine alga Emiliania huxleyi triggers lipidome remodeling and induces the production of highly saturated triacylglycerol. New Phytol. 2016, 210, 88–96. [Google Scholar] [CrossRef]
- Martin, G.J.O.; Hill, D.R.A.; Olmstead, I.L.D.; Bergamin, A.; Shears, M.J.; Dias, D.A.; Kentish, S.E.; Scales, P.J.; Botté, C.Y.; Callahan, D.L. Lipid profile remodeling in response to nitrogen deprivation in the microalgae Chlorella sp. (Trebouxiophyceae) and Nannochloropsis sp. (Eustigmatophyceae). PLoS ONE 2014, 9, e103389. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Jia, J.; Li, J.; Sommerfeld, M.; Xu, J.; Hu, Q. Metabolic remodeling of membrane glycerolipids in the microalga Nannochloropsis oceanica under nitrogen deprivation. Front. Mar. Sci. 2017, 4, 242. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Moellering, E.R.; Liu, B.; Johnny, C.; Fedewa, M.; Sears, B.B.; Kuo, M.H.; Benning, C. A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in chlamydomonas reinhardtiic w. Plant Cell 2012, 24, 4670–4686. [Google Scholar] [CrossRef]
- Davidi, L.; Shimoni, E.; Khozin-Goldberg, I.; Zamir, A.; Pick, U. Origin of β-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol. 2014, 164, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E. Molecular Properties of Short Chain Acyl Thioesters of Acyl Carrier Protein*. J. Biol. Chem. 1982, 257, 5013–5017. [Google Scholar] [CrossRef]
- Davidi, L.; Katz, A.; Pick, U. Characterization of major lipid droplet proteins from Dunaliella. Planta 2012, 236, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Ho Cho, S.; Thompson, G.A. On the Metabolic Relationships between Monogalactosyldiacylglycerol and Digalactosyldiacylglycerol Molecular Species in Dunaliella salina. J. Biol. Chem. 1987, 262, 75867593. [Google Scholar]
- Bates, P.D.; Durrett, T.P.; Ohlrogge, J.B.; Pollard, M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 2009, 150, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Liping, W.; Shen, W.; Kazachkov, M.; Chen, G.; Chen, Q.; Carlsson, A.S.; Stymne, S.; Weselake, R.J.; Zou, J. Metabolic interactions between the lands cycle and the kennedy pathway of glycerolipid synthesis in arabidopsis developing seedsw. Plant Cell 2012, 24, 4652–4669. [Google Scholar] [CrossRef]
- Allen, D.K.; Bates, P.D.; Tjellström, H. Tracking the metabolic pulse of plant lipid production with isotopic labeling and flux analyses: Past, present and future. Prog. Lipid Res. 2015, 58, 97–120. [Google Scholar] [CrossRef]
- Bates, P.D.; Ohlrogge, J.B.; Pollard, M. Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J. Biol. Chem. 2007, 282, 31206–31216. [Google Scholar] [CrossRef]
- Scrimgeour, C.M.; Harwood, J.L. The Lipidomic Handbook; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849396885. [Google Scholar]
- Zienkiewicz, K.; Du, Z.Y.; Ma, W.; Vollheyde, K.; Benning, C. Stress-induced neutral lipid biosynthesis in microalgae—Molecular, cellular and physiological insights. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1269–1281. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Lippold, F.; vom Dorp, K.; Abraham, M.; Hölzl, G.; Wewer, V.; Yilmaz, J.L.; Lager, I.; Montandon, C.; Besagni, C.; Kessler, F.; et al. Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis. Plant Cell 2012, 24, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Froehlich, J.E.; Zienkiewicz, A.; Hersh, H.L.; Benning, C. A plastid phosphatidylglycerol lipase contributes to the export of acyl groups from plastids for seed oil biosynthesis. Plant Cell 2017, 29, 1678–1696. [Google Scholar] [CrossRef]
- Davidi, L.; Levin, Y.; Ben-Dor, S.; Pick, U. Proteome analysis of cytoplasmatic and plastidic β-carotene lipid droplets in Dunaliella bardawil1[OPEN]. Plant Physiol. 2015, 167, 60–79. [Google Scholar] [CrossRef]
- Dahlqvist, A.; Ståhl, U.; Lenman, M.; Banas, A.; Lee, M.; Sandager, L.; Ronne, H.; Stymne, S. Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 2000, 97, 6487–6492. [Google Scholar] [CrossRef]
- Yoon, K.; Han, D.; Li, Y.; Sommerfeld, M.; Hu, Q. Phospholipid:Diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga chlamydomonas reinhardtii. Plant Cell 2012, 24, 3708–3724. [Google Scholar] [CrossRef]
- Allen, J.W.; DiRusso, C.C.; Black, P.N. Triacylglycerol synthesis during nitrogen stress involves the prokaryotic lipid synthesis pathway and acyl chain remodeling in the microalgae Coccomyxa subellipsoidea. Algal Res. 2015, 10, 110–120. [Google Scholar] [CrossRef]
- Sakurai, K.; Mori, N.; Sato, N. Detection and characterization of phosphatidylcholine in various strains of the genus Chlamydomonas (Volvocales, Chlorophyceae). J. Plant Res. 2014, 127, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Liu, K.H.; Lee, S.Y.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effects of Light Intensity and Nitrogen Starvation on Glycerolipid, Glycerophospholipid, and Carotenoid Composition in Dunaliella tertiolecta Culture. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Du, Z.Y.; Lucker, B.F.; Zienkiewicz, K.; Miller, T.E.; Zienkiewicz, A.; Sears, B.B.; Kramer, D.M.; Benning, C. Galactoglycerolipid lipase PGD1 is involved in thylakoid membrane remodeling in response to adverse environmental conditions in chlamydomonas. Plant Cell 2018, 30, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Moellering, E.R.; Benning, C. Galactoglycerolipid metabolism under stress: A time for remodeling. Trends Plant Sci. 2011, 16, 98–107. [Google Scholar] [CrossRef]
- Avidan, O.; Pick, U. Acetyl-CoA synthetase is activated as part of the PDHbypass in the oleaginous green alga Chlorella desiccata. J. Exp. Bot. 2015, 66, 7287–7298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bates, P.D.; Fatihi, A.; Snapp, A.R.; Carlsson, A.S.; Browse, J.; Lu, C. Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol. 2012, 160, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of Action of the Massively Accumulated β-Carotene of Dunaliella bardawil in Protecting the Alga against Damage by Excess Irradiation. Plant Physiol. 1989, 91, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Zalogin, T.R.; Pick, U. Azide improves triglyceride yield in microalgae. Algal Res. 2014, 3, 8–16. [Google Scholar] [CrossRef]
- Dakhma, W.S.; Zarrouk, M.; Cherif, A. Effects of drought-stress on lipids in rape leaves. Phytochemistry 1995, 40, 1383–1386. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
| FA | 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | X |
|---|---|---|---|---|---|---|
| +N | 10.2 ± 0.2 | 9.0 ± 0.9 | 48.2 ± 5.0 | 2.3 ± 0.1 | 25.5 ± 1.9 | 4.8 ± 0.5 |
| −N | 10 ± 1.3 | 12.2 ± 2.1 | 70.4 ± 3.6 | 0 | 0 | 7.2 ± 0.5 |
| Lipid |
Incorporation Rate [f mol PlA × min−1 × 106 cells−1] |
Steady-State Concentration [n mol C 106 cells−1] | ||||
|---|---|---|---|---|---|---|
| +N | −N | −N/+N | +N | −N | −N/+N | |
| PC | 294 ± 30 | 184 ± 14 | 0.62 ± 0.13 | 2.6 ± 0.3 | 3.6 ± 0.2 | 1.38 ± 0.13 |
| FA | 58 ± 10 | 144 ± 14 | 2.48 ± 0.2 | 32 ± 4 | 8.8 ± 1 | 0.28 ± 0.17 |
| DAG | 142 ± 10 | 120 ± 10 | 0.85 ± 0.1 | 10.4 ± 1 | 16.1 ± 2 | 1.54 ± 0.16 |
| DGDG | 44 ± 6 | 92 ± 8 | 2.09 ± 0.16 | 54 ± 4 | 45 ± 6 | 0.83 ± 0.17 |
| SQDG + PG | 82 ± 4 | 92 ± 6 | 1.12 ± 0.08 | 22.4 ± 1.7 | 15.5 ± 1.1 | 0.69 ± 0.1 |
| TAG | 40 ± 2 | 188 ± 20 | 4.7 ± 0.09 | 2 ± 0.2 | 123 ± 3 | 6.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avidan, O.; Malitsky, S.; Pick, U. Fatty Acid Production and Direct Acyl Transfer through Polar Lipids Control TAG Biosynthesis during Nitrogen Deprivation in the Halotolerant Alga Dunaliella tertiolecta. Mar. Drugs 2021, 19, 368. https://doi.org/10.3390/md19070368
Avidan O, Malitsky S, Pick U. Fatty Acid Production and Direct Acyl Transfer through Polar Lipids Control TAG Biosynthesis during Nitrogen Deprivation in the Halotolerant Alga Dunaliella tertiolecta. Marine Drugs. 2021; 19(7):368. https://doi.org/10.3390/md19070368
Chicago/Turabian StyleAvidan, Omri, Sergey Malitsky, and Uri Pick. 2021. "Fatty Acid Production and Direct Acyl Transfer through Polar Lipids Control TAG Biosynthesis during Nitrogen Deprivation in the Halotolerant Alga Dunaliella tertiolecta" Marine Drugs 19, no. 7: 368. https://doi.org/10.3390/md19070368
APA StyleAvidan, O., Malitsky, S., & Pick, U. (2021). Fatty Acid Production and Direct Acyl Transfer through Polar Lipids Control TAG Biosynthesis during Nitrogen Deprivation in the Halotolerant Alga Dunaliella tertiolecta. Marine Drugs, 19(7), 368. https://doi.org/10.3390/md19070368







