Chitosan Oligosaccharide Alleviates Abnormal Glucose Metabolism without Inhibition of Hepatic Lipid Accumulation in a High-Fat Diet/Streptozotocin-Induced Diabetic Rat Model
Abstract
:1. Introduction
2. Results
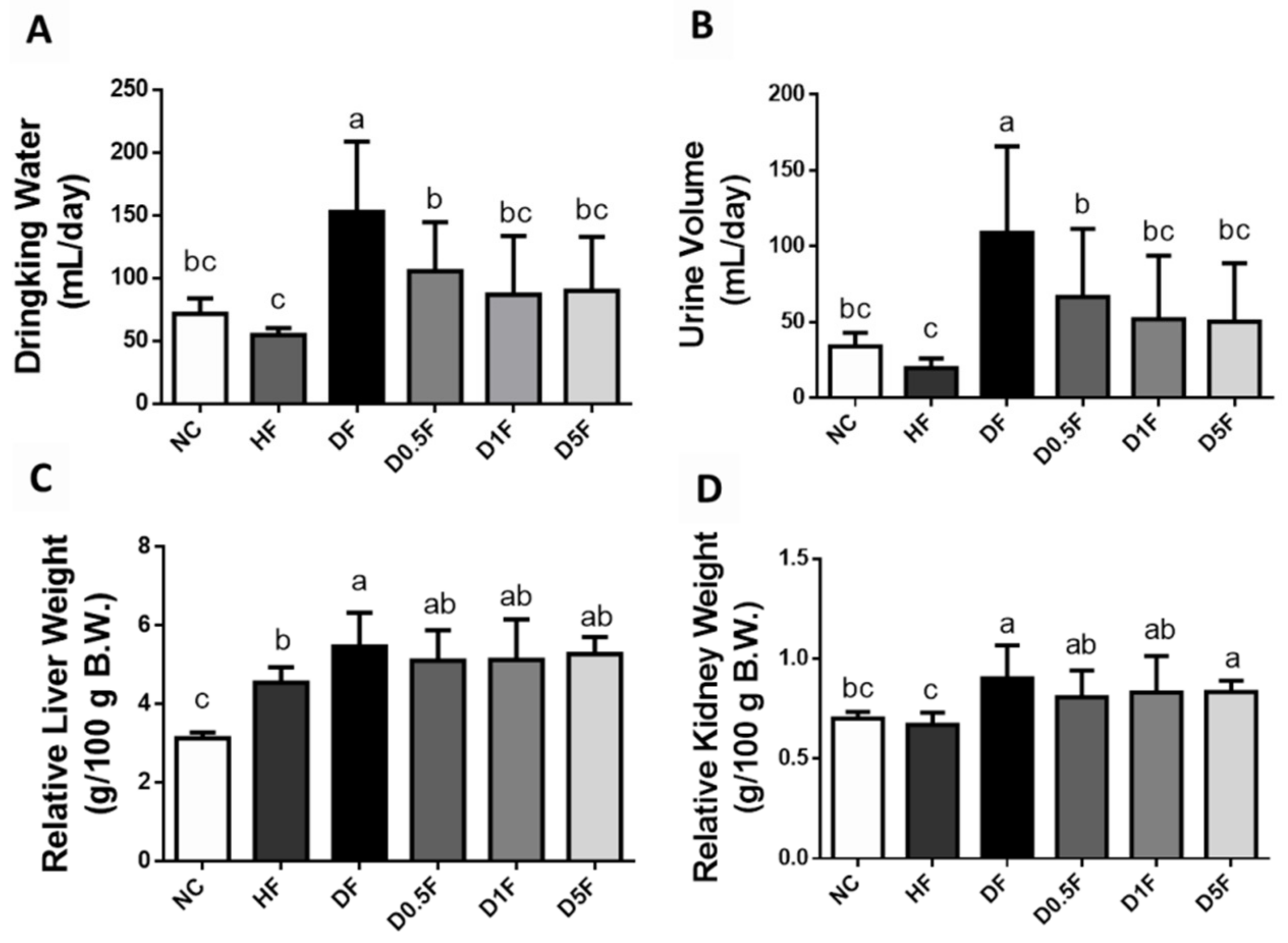
2.1. The Changes in Body Weight, Food Intake, Water Intake, Urine Volume, and Tissue Weight
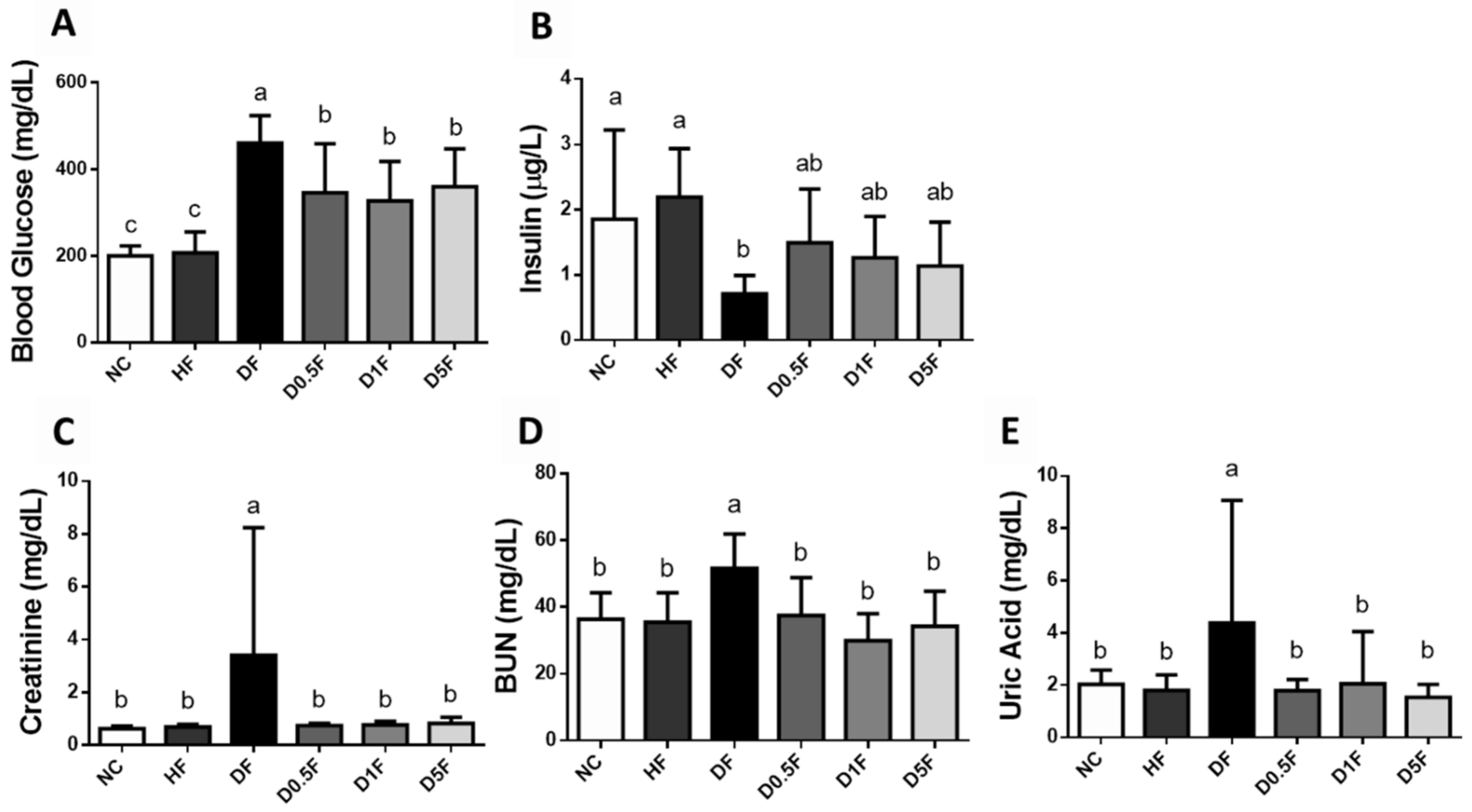
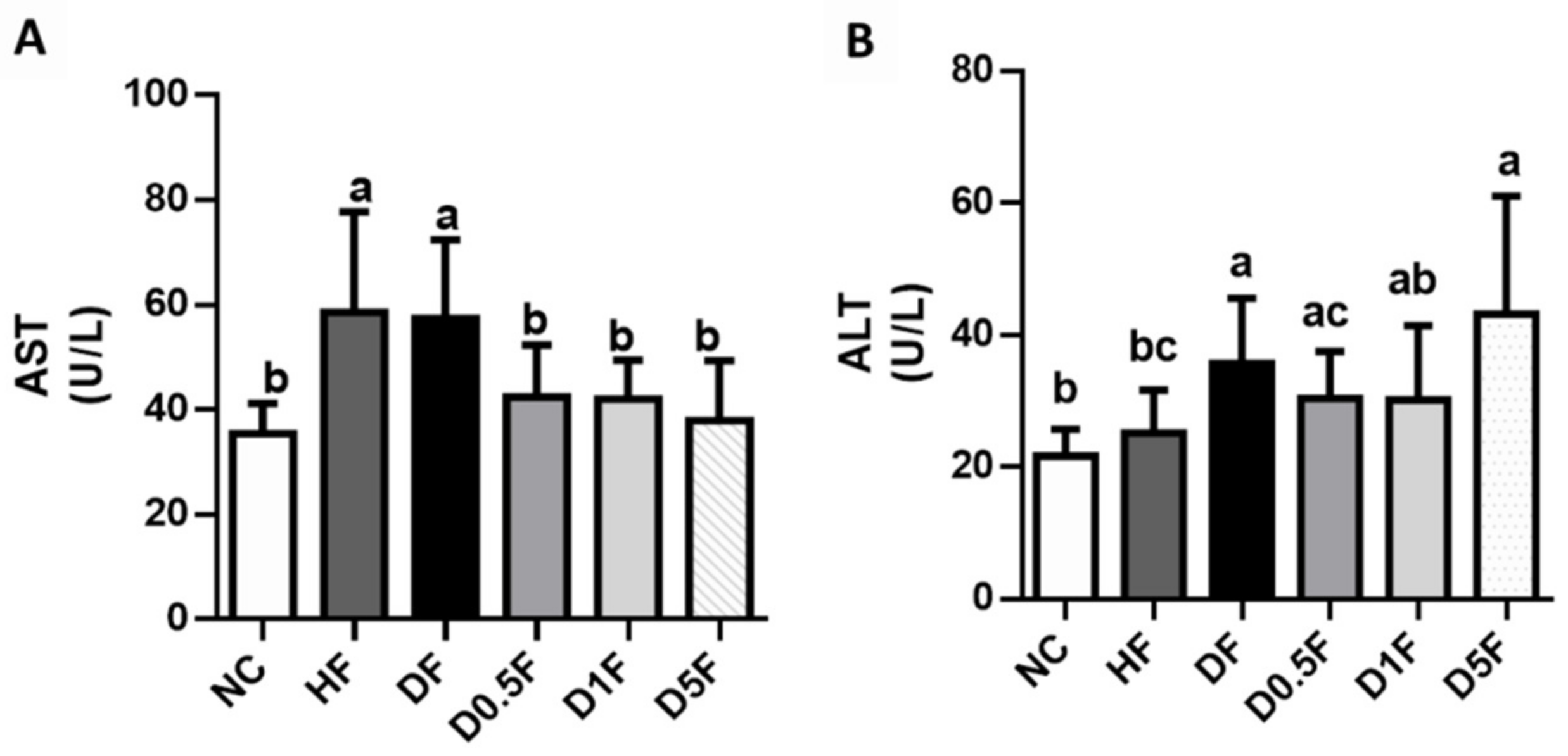
2.2. The Changes in Plasma Glucose, Insulin, Creatinine, Blood Urea Nitrogen (BUN), Uric Acid, Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), and Lipids Levels
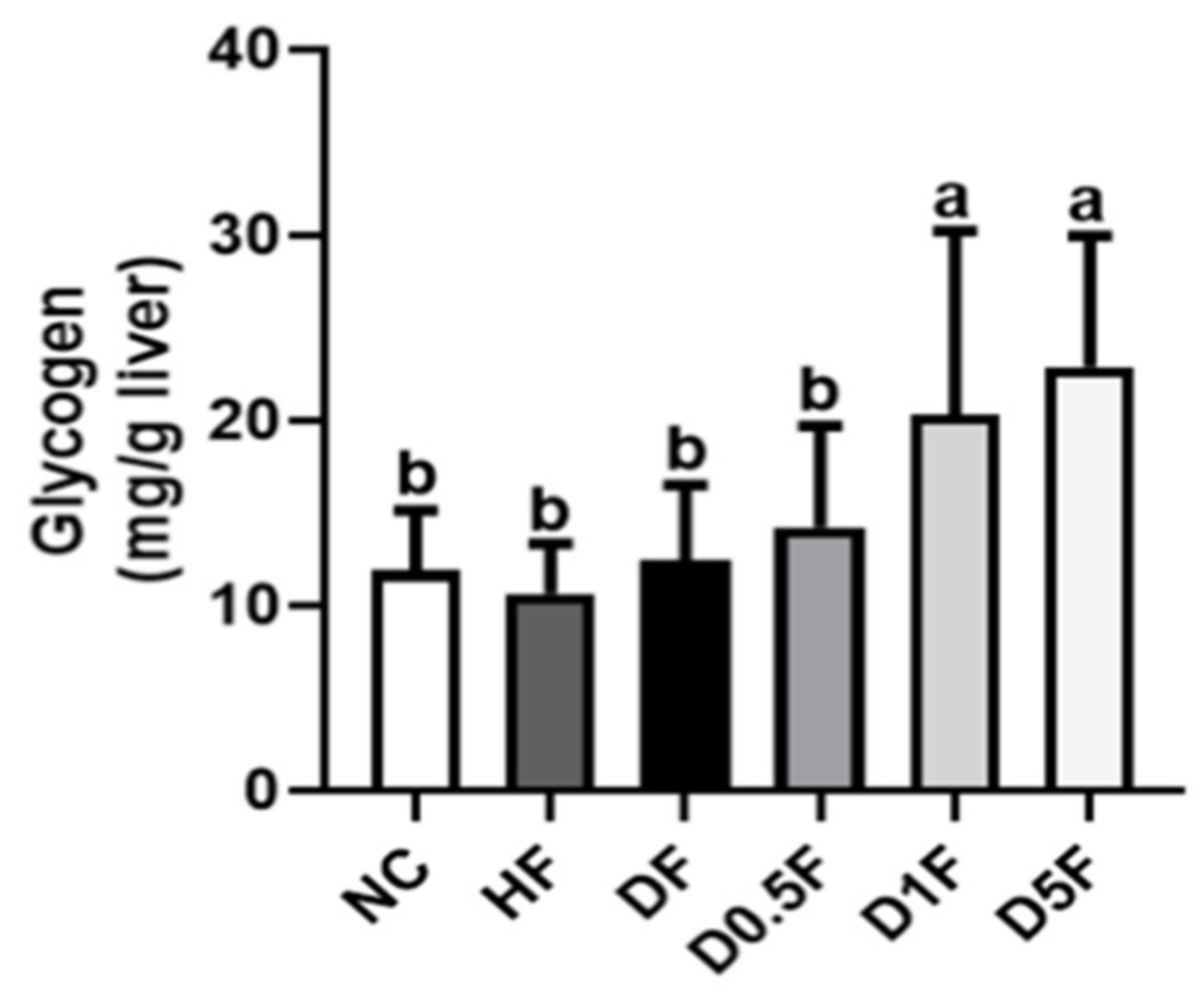
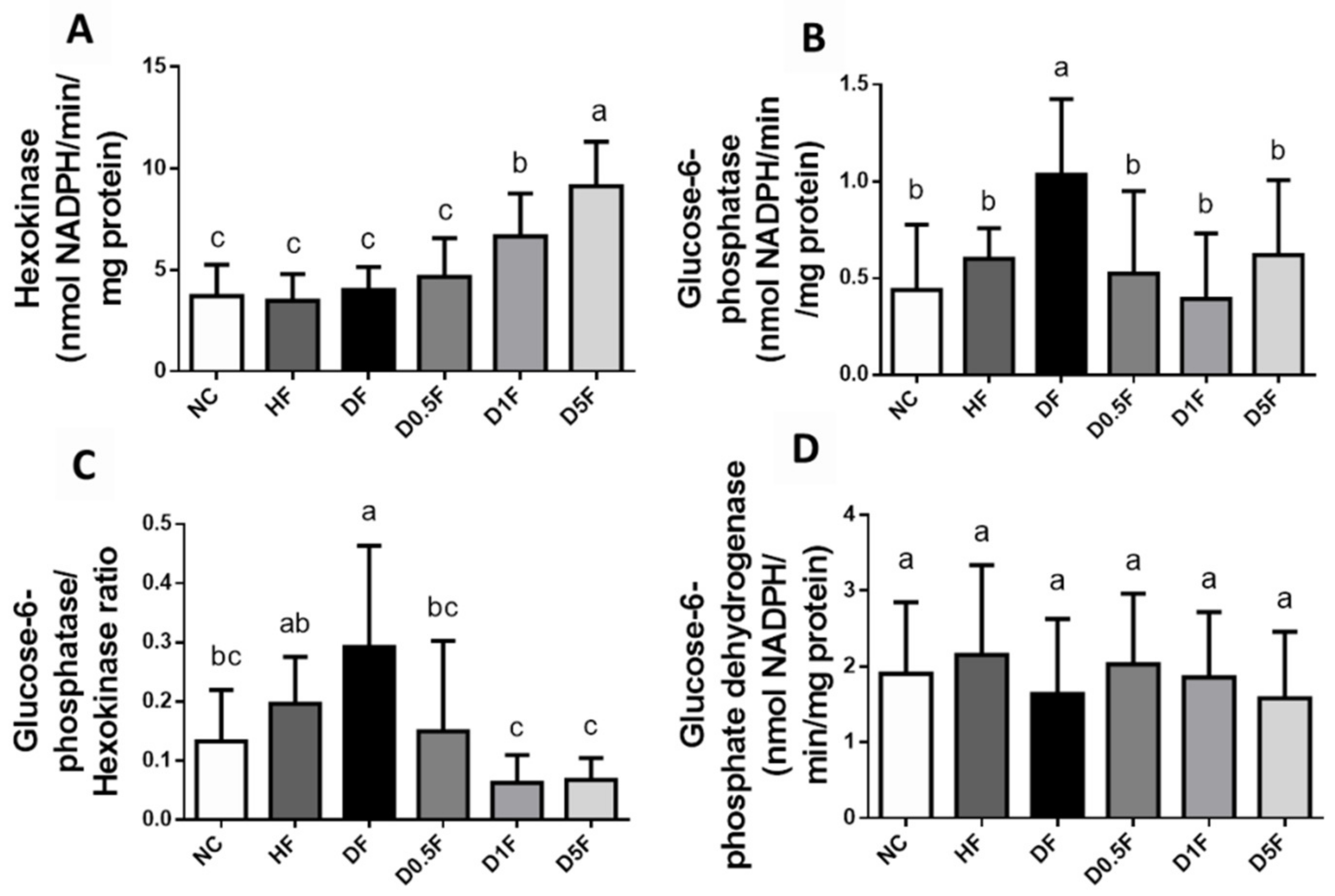
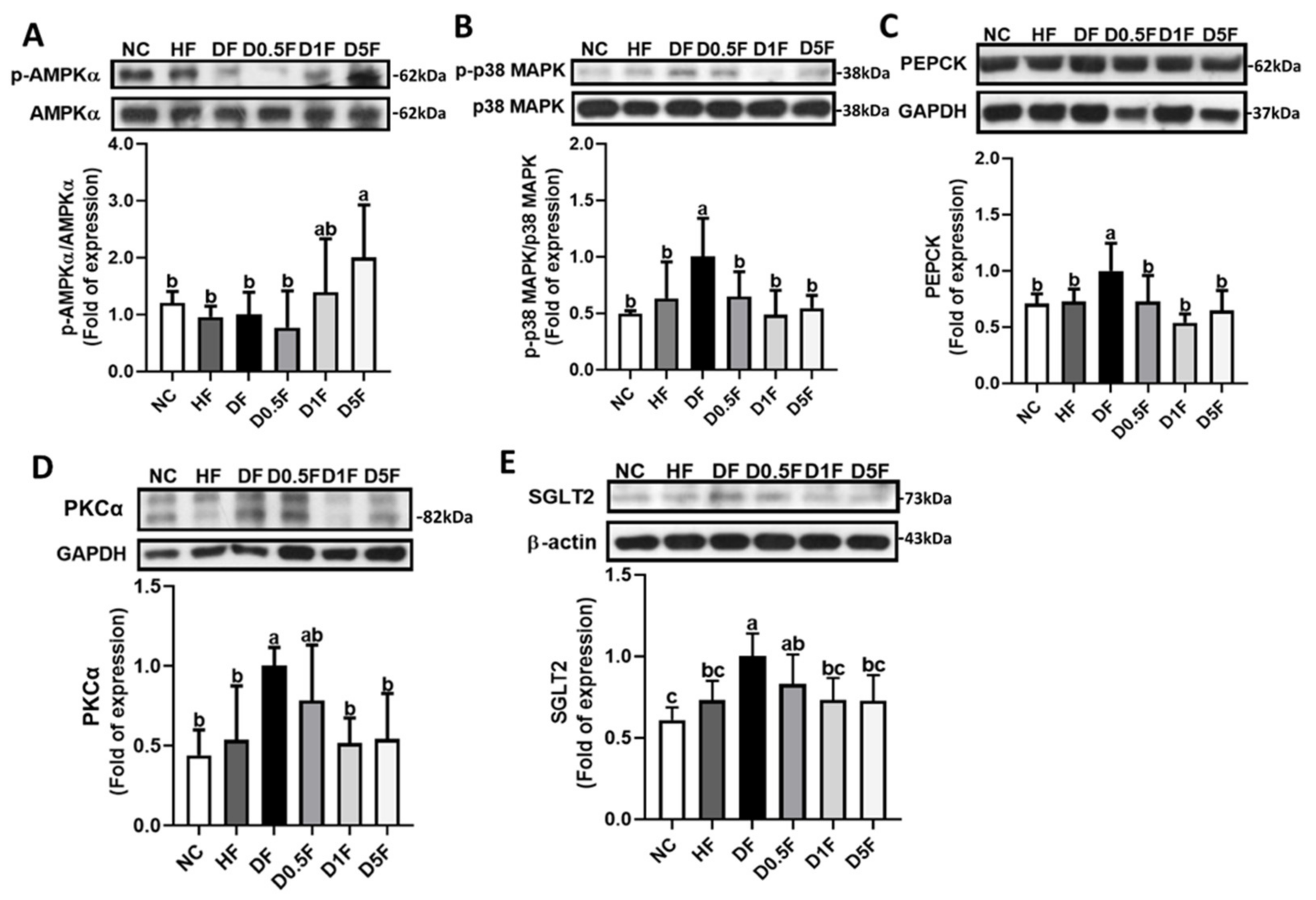
2.3. The Changes in Liver Lipids, Glycogen Content, and Glycometabolism-Related Enzymes Activities and Signaling Molecules
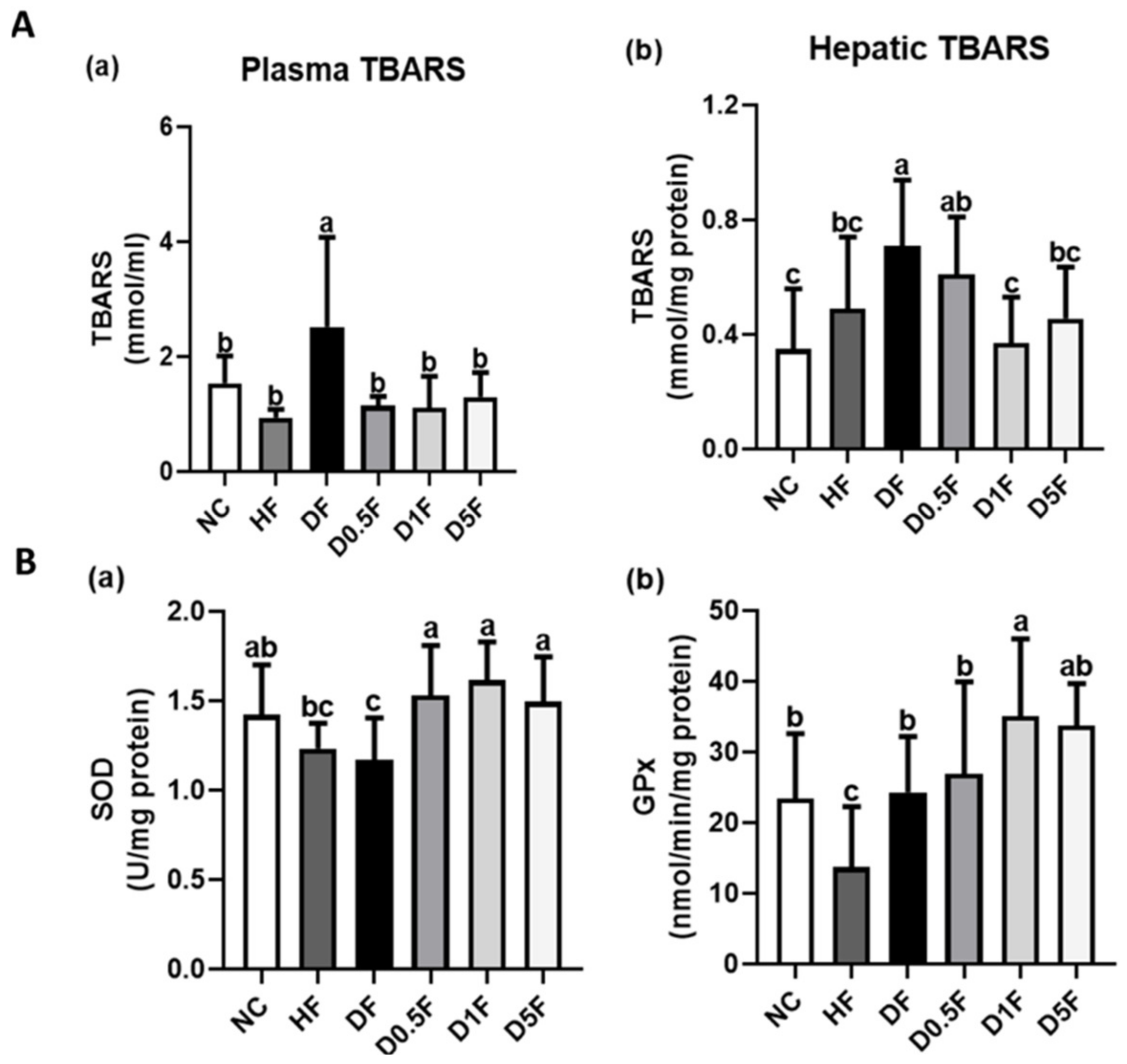
2.4. The Changes in Thiobarbituric Acid Reactive Substances (TBARS) Levels and Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx) Activities
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Sampling Blood and Tissue
4.4. Determination of Plasma Glucose, Insulin, Creatinine, BUN, Uric Acid, AST, and ALT
4.5. Measurement of Liver SOD and GPx Activities and Plasma and Liver Lipid Peroxide (Thiobarbituric Acid Reactive Substances, TBARS) Contents
4.6. Measurement of Lipids in the Plasma, Liver, and Adipose Tissues and Plasma Lipoproteins
4.7. Measurement of Glycogen Content and Activities of Hexokinase, Glucose-6-Phosphatase (G6Pase), and Glucose-6-Phosphate Dehydrogenase (G6PD)
4.8. Western Blot Analysis
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Parhofer, K.G. Interaction between glucose and lipid metabolism: More than diabetic dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, W.R.; Baka, A.; Björck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of diet composition on blood glucose regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Kim, J.N.; Chang, I.Y.; Kim, H.I.; Yoon, S.P. Long-term effects of chitosan oligosaccharide in streptozotocin-induced diabetic rats. Islets 2009, 1, 111–116. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Ha, K.S.; Moon, K.S.; Kim, J.G.; Oh, C.G.; Kim, Y.C.; Apostolidis, E.; Kwon, Y.I. Molecular weight dependent glucose lowering effect of low molecular weight Chitosan Oligosaccharide (GO2KA1) on postprandial blood glucose level in SD rat model. Int. J. Mol. Sci. 2013, 14, 14214–14224. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Min Cho, J.; Kwon, Y.I.; Kim, S.C.; Yeob Shin, D.; Ho Lee, J. Chitosan oligosaccharide (GO2KA1) improves postprandial glycemic response in subjects with impaired glucose tolerance and impaired fasting glucose and in healthy subjects: A crossover, randomized controlled trial. Nutr. Diabetes 2019, 9, 31. [Google Scholar] [CrossRef]
- Hsu, H.C.; Dozen, M.; Matsuno, N.; Obara, H.; Tanaka, R.; Enosawa, S. Experimental nonalcoholic steatohepatitis induced by neonatal streptozotocin injection and a high-fat diet in rats. Cell Med. 2013, 6, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.T.; Huang, S.Y.; Chiang, M.T. A comparative study on hypoglycemic and hypocholesterolemic effects of high and low molecular weight chitosan in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2008, 46, 1525–1534. [Google Scholar] [CrossRef]
- Mostafavinia, A.; Amini, A.; Ghorishi, S.K.; Pouriran, R.; Bayat, M. The effects of dosage and the routes of administrations of streptozotocin and alloxan on induction rate of type1 diabetes mellitus and mortality rate in rats. Lab. Anim. Res. 2016, 32, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, E.A.; Dimitriadis, G. Integration of biochemical and physiologic effects of insulin on glucose metabolism. Exp. Clin. Endocrinol. Diabetes 2001, 109, S122–S134. [Google Scholar] [CrossRef]
- Melkonian, E.A.; Asuka, E.; Schury, M.P. Physiology, Gluconeogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541119/ (accessed on 26 March 2021).
- Ashokkumar, N.; Pari, L. Effect of N-benzoyl-D-phenylalanine and metformin on carbohydrate metabolic enzymes in neonatal streptozotocin diabetic rats. Clin. Chim. Acta 2005, 351, 105–113. [Google Scholar] [CrossRef]
- Qiao, L.; MacDougald, O.A.; Shao, J. CCAAT/enhancer-binding protein α mediates induction of hepatic phosphoenolpyruvate carboxykinase by p38 mitogen-activated protein kinase. J. Biol. Chem. 2006, 281, 24390–24397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berasi, S.; Huard, C.; Li, D.; Shih, H.H.; Sun, Y.; Zhong, W.; Paulsen, J.E.; Brown, E.L.; Gimeno, R.E.; Martinez, R.V. Inhibition of gluconeogenesis through transcriptional activation of EGR1 and DUSP4 by AMP-activated kinase. J. Biol. Chem. 2006, 281, 27167–27177. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Kanemoto, N.; Ban, T.; Sudo, T.; Nagano, K.; Niki, I. Establishment and characterization of a novel method for evaluating gluconeogenesis using hepatic cell lines, H4IIE and HepG2. Arch. Biochem. Biophys. 2009, 491, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Kishimoto, A.; Inoue, M.; Nishizuka, Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J. Biol. Chem. 1977, 252, 7603–7609. [Google Scholar] [CrossRef]
- Paolisso, G.; Giugliano, D. Oxidative stress and insulin action: Is there a relationship? Diabetologia 1996, 39, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; King, G.L. The role of protein kinase C activation in diabetic nephropathy. Kidney Int. 2007, 72, S49–S53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiyar, D.; Singh, B.; Lall, A.M.; Haldar, C. Efficacy of chitooligosaccharides for the management of diabetes in alloxan induced mice: A correlative study with antihyperlipidemic and antioxidative activity. Eur. J. Pharm. Sci. 2011, 44, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Chao, E.C.; Henry, R.R. SGLT2 inhibition—A novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan oligosaccharide attenuates nonalcoholic fatty liver disease induced by high fat diet through reducing lipid accumulation, inflammation and oxidative stress in C57BL/6 mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Chen, R.Y.; Chiang, M.T. Effects and mechanisms of chitosan and chitosan oligosaccharide on hepatic lipogenesis and lipid peroxidation, adipose lipolysis, and intestinal lipid absorption in rats with high-fat diet-induced obesity. Int. J. Mol. Sci. 2021, 22, 1139. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Sun, T.; Zhong, R.; Ma, L.; You, C.; Tian, M.; Li, H.; Wang, C. Effects of chitosan oligosaccharides on human blood components. Front. Pharmacol. 2018, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef]
- Hwang, J.S.; Park, J.W.; Nam, M.S.; Cho, H.; Han, I.O. Glucosamine enhances body weight gain and reduces insulin response in mice fed chow diet but mitigates obesity, insulin resistance and impaired glucose tolerance in mice high-fat diet. Metabolism 2015, 64, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chen, R.Y.; Chiang, M.T. Effects of chitosan oligosaccharide on plasma and hepatic lipid metabolism and liver histomorphology in normal Sprague-Dawley rats. Mar. Drugs 2020, 18, 408. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Takahisa, F.; Suzuki, Y. Effect of guar gum and cholestyramine on plasma lipoprotein cholesterol in rats. J. Jpn. Soc. Nutr. Food Sci. 1990, 43, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Murat, J.C.; Serfaty, A. Simple enzymatic determination of polysaccharide (glycogen) content of animal tissues. Clin. Chem. 1974, 20, 1576–1577. [Google Scholar] [CrossRef]
- DeWaal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Taussky, H.H.; Shorr, E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chang, T.C.; Liu, S.H.; Chiang, M.T. The regulatory effects of fish oil and chitosan on hepatic lipogenic signals in high-fat diet-induced obese rats. J. Food Drug Anal. 2017, 25, 919–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Diet | NC | HF | DF | D0.5F | D1F | D5F |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 374.3 ± 23.7 a | 374.2 ± 19.6 a | 336.5 ± 31.8 b | 342.0 ± 16.4 b | 337.5 ± 30.7 b | 341.2 ± 22.8 b |
| Final body weight (g) | 484.3 ± 29.0 ab | 512.2 ± 22.2 a | 444.8 ± 51.9 b | 450.9 ± 26.0 b | 449.7 ± 38.1 b | 445.6 ± 44.3 b |
| Body weight gain (g) | 110.1 ± 14.9 b | 138.0 ± 17.7 a | 108.3 ± 29.2 b | 108.9 ± 21.0 b | 112.2 ± 25.7 b | 104.4 ± 25.4 b |
| Food intake (g/day) | 30.7 ± 2.12 ab | 28.4 ± 1.85 b | 35.1 ± 7.40 a | 33.4 ± 5.74 ab | 31.9 ± 5.38 ab | 32.1 ± 4.80 ab |
| Feed efficiency (%) 1 | 3.58 ± 0.34 b | 4.84 ± 0.39 a | 3.15 ± 0.88 b | 3.35 ± 0.87 b | 3.67 ± 1.21 b | 3.38 ± 1.14 b |
| Diet | NC | HF | DF | D0.5F | D1F | D5F |
|---|---|---|---|---|---|---|
| Total adipose tissue (g) | 14.5 ± 3.52 a | 15.5 ± 2.46 a | 8.89 ± 4.39 b | 13.0 ± 4.64 a | 10.5 ± 2.64 ab | 10.1 ± 2.74 ab |
| Relative adipose tissue weight (g/100g B.W.) | 3.11 ± 0.66 a | 3.14 ± 0.54 a | 2.04 ± 0.83 b | 2.91 ± 0.96 a | 2.35 ± 0.41 ab | 2.33 ± 0.46 ab |
| Perirenal adipose (g) | 6.84 ± 1.82 a | 8.21 ± 1.30 a | 4.20 ± 2.88 b | 7.15 ± 2.77 a | 5.84 ± 1.72 ab | 5.05 ± 1.71 ab |
| Relative perirenal adipose (g/100g B.W.) | 1.48 ± 0.38 a | 1.67 ± 0.30 a | 0.98 ± 0.58 b | 1.59 ± 0.59 a | 1.30 ± 0.28 ab | 1.16 ± 0.30 ab |
| Epididymal adipose (g) | 6.60 ± 1.34 a | 7.83 ± 1.08 a | 4.81 ± 1.72 b | 5.95 ± 1.50 ab | 5.14 ± 0.70 ab | 5.23 ± 1.06 ab |
| Relative epididymal adipose weight (g/100g B.W.) | 1.45 ± 0.29 a | 1.59 ± 0.22 a | 1.08 ± 0.32 b | 1.34 ± 0.29 ab | 1.16 ± 0.08 b | 1.20 ± 0.19 ab |
| Diet | NC | HF | DF | D0.5F | D1F | D5F |
|---|---|---|---|---|---|---|
| Triglyceride | ||||||
| (mg/g liver) | 13.8 ± 4.46 b | 43.6 ± 11.0 a | 47.9 ± 10.0 a | 48.3 ± 22.9 a | 41.9 ± 18.0 a | 34.0 ± 10.8 a |
| (g/liver) | 0.20 ± 0.06 b | 0.99 ± 0.31 a | 1.11 ± 0.30 a | 1.07 ± 0.50 a | 0.91 ± 0.34 a | 0.75 ± 0.22 a |
| Total cholesterol | ||||||
| (mg/g liver) | 3.22 ± 0.49 c | 62.6 ± 8.50 b | 83.8 ± 12.5 a | 86.0 ± 17.4 a | 74.8 ± 10.9 a | 74.9 ± 10.7 a |
| (g/liver) | 0.05 ± 0.01 c | 1.41 ± 0.24 b | 1.94 ± 0.46 a | 1.97 ± 0.57 a | 1.67 ± 0.38 ab | 1.67 ± 0.26 ab |
| Ingredient (%) | NC | HF | DF | D0.5F | D1F | D5F |
|---|---|---|---|---|---|---|
| Chow diet | 100 | 89.4 | 89.4 | 88.9 | 88.4 | 84.4 |
| Lard | 10 | 10 | 10 | 10 | 10 | |
| Cholesterol | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Cholic acid | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Chitosan oligosaccharide 1 | 0.5 | 1 | 5 | |||
| Total calories (kcal/100g) | 336.2 | 390.6 | 390.6 | 389.9 | 389.2 | 383.8 |
| Carbohydrates (% kcal) | 57.9 | 44.6 | 44.6 | 44.6 | 44.7 | 45.42 |
| Protein (% kcal) | 28.7 | 22.1 | 22.1 | 22.0 | 21.9 | 21.22 |
| Fat (% kcal) | 13.4 | 33.3 | 33.3 | 33.4 | 33.4 | 33.36 |
| 100 | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Chen, F.-W.; Chiang, M.-T. Chitosan Oligosaccharide Alleviates Abnormal Glucose Metabolism without Inhibition of Hepatic Lipid Accumulation in a High-Fat Diet/Streptozotocin-Induced Diabetic Rat Model. Mar. Drugs 2021, 19, 360. https://doi.org/10.3390/md19070360
Liu S-H, Chen F-W, Chiang M-T. Chitosan Oligosaccharide Alleviates Abnormal Glucose Metabolism without Inhibition of Hepatic Lipid Accumulation in a High-Fat Diet/Streptozotocin-Induced Diabetic Rat Model. Marine Drugs. 2021; 19(7):360. https://doi.org/10.3390/md19070360
Chicago/Turabian StyleLiu, Shing-Hwa, Fan-Wen Chen, and Meng-Tsan Chiang. 2021. "Chitosan Oligosaccharide Alleviates Abnormal Glucose Metabolism without Inhibition of Hepatic Lipid Accumulation in a High-Fat Diet/Streptozotocin-Induced Diabetic Rat Model" Marine Drugs 19, no. 7: 360. https://doi.org/10.3390/md19070360
APA StyleLiu, S.-H., Chen, F.-W., & Chiang, M.-T. (2021). Chitosan Oligosaccharide Alleviates Abnormal Glucose Metabolism without Inhibition of Hepatic Lipid Accumulation in a High-Fat Diet/Streptozotocin-Induced Diabetic Rat Model. Marine Drugs, 19(7), 360. https://doi.org/10.3390/md19070360







