In Vitro and In Silico Characterization of G-Protein Coupled Receptor (GPCR) Targets of Phlorofucofuroeckol-A and Dieckol
Abstract
1. Introduction
2. Results
2.1. In Silico Target Prediction
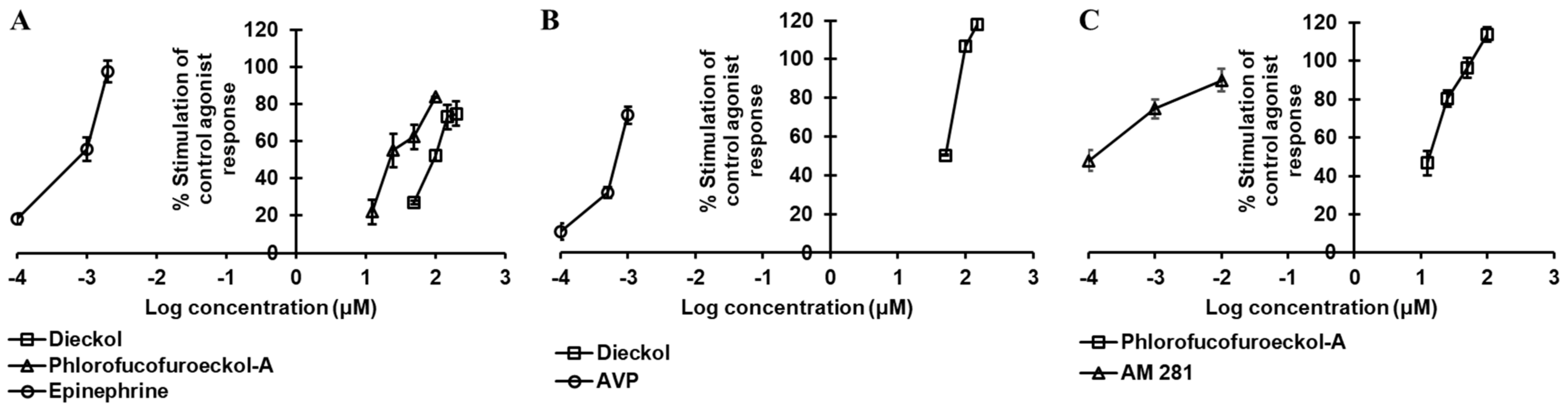
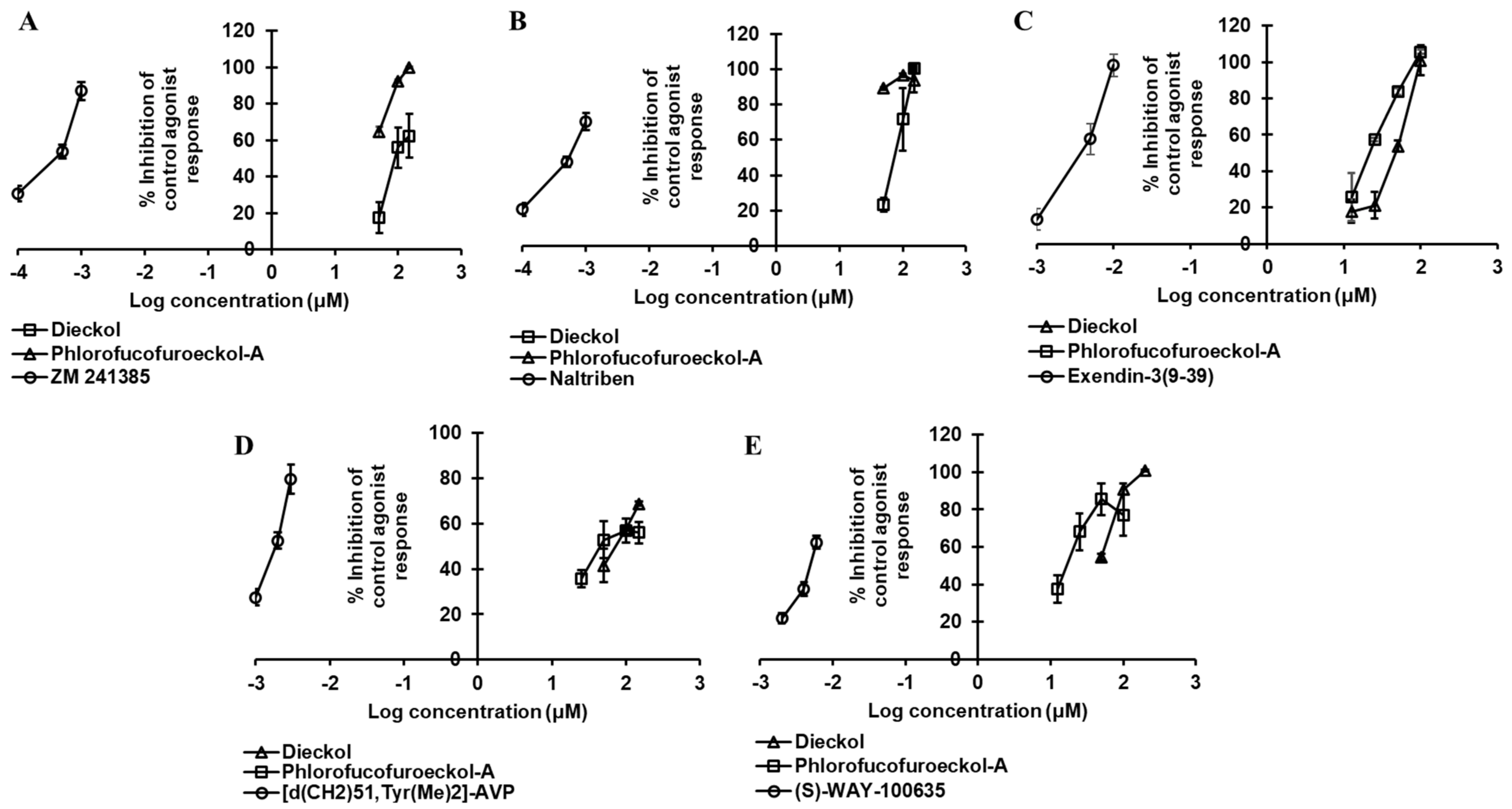
2.2. Dieckol and PFF-A as A2AR Antagonists
2.3. Dieckol and PFF-A as α2CAR Agonists
2.4. Dieckol and PFF-A as δ-OPR Antagonists
2.5. PFF-A as a CB1R Agonist
2.6. Dieckol and PFF-A as GLP-1R Antagonists
2.7. Dieckol as Agonist and PFF-A as Antagonist of hV1AR
2.8. Dieckol and PFF-A as 5-HT1AR Antagonists
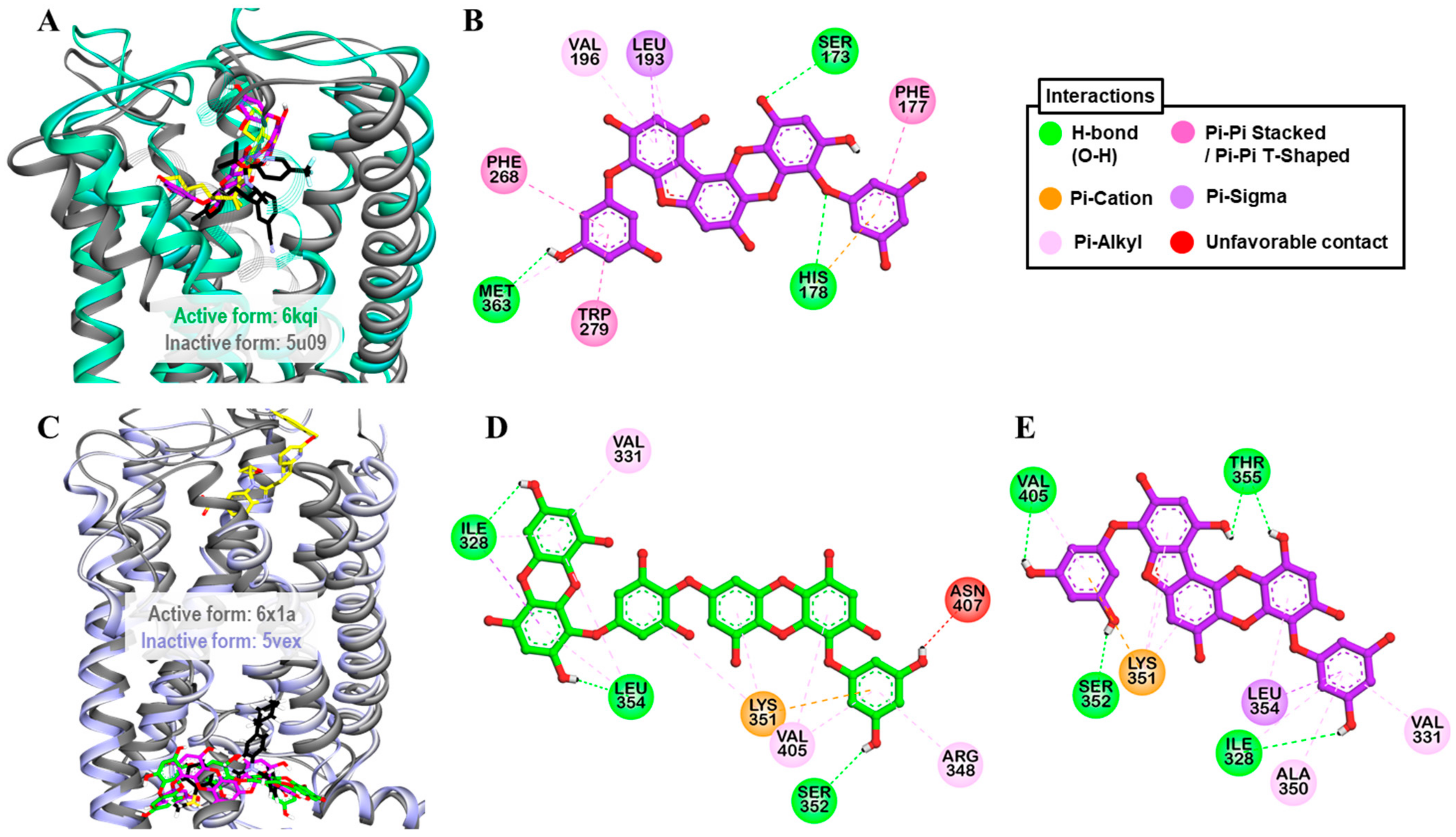
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Isolation of Phlorotannins
4.3. In Silico Prediction of Targets
4.4. Functional GPCR Assay
4.5. Measurement of cAMP Level
4.5.1. Functional Activity over hA2AR
4.5.2. Functional Activity over hα2CAR
4.5.3. Functional Activity over hCB1R
4.5.4. Functional Activity over GLP-1R
4.5.5. Functional Activity over 5-HT1BR
4.6. Measurement of Intracellular [Ca2+] Level
4.6.1. Functional Activity over hα2AAR
4.6.2. Functional Activity over hδ-OPR
4.6.3. Functional Activity over FFA1R/GPR40
4.6.4. Functional Activity over hV1AR
4.6.5. Functional Activity over h5-HT1AR
4.7. Homology Modeling and Molecular Docking
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Iliopoulos-Tsoutsouvas, C.; Kulkarni, R.N.; Makriyannis, A.; Nikas, S.P. Fluorescent probes for G-protein-coupled receptor drug discovery. Expert Opin. Drug Discov. 2018, 13, 933–947. [Google Scholar] [CrossRef]
- Schlyer, S.; Horuk, R. I want a new drug: G-protein-coupled receptors in drug development. Drug Discov. Today 2006, 11, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Xu, J.; Maeda, S.; Duc, N.M.; Ahn, D.; Du, Y.; Chung, K.Y. Structural mechanism underlying primary and secondary coupling between GPCRs and the Gi/o family. Nat. Commun. 2020, 11, 3160. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.-Y.; Zheng, J.-H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar]
- Do Valle, I.F.; Roweth, H.G.; Malloy, M.W.; Moco, S.; Barron, D.; Battinelli, E.; Loscalzo, J.; Barabási, A.-L. Network medicine framework shows that proximity of polyphenol targets and disease proteins predicts therapeutic effects of polyphenols. Nat. Food 2021, 2, 143–155. [Google Scholar] [CrossRef]
- Nakamura, T.; Nagayama, K.; Uchida, K.; Tanaka, R. Antioxidant activity of phlorotannins isolated from the brown alga Eisenia bicyclis. Fish. Sci. 1996, 62, 923–926. [Google Scholar] [CrossRef]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.-R.; Jung, H.A.; Choi, J.S. Phlorotannins with potential anti-tyrosinase and antioxidant activity isolated from the marine seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.-H.; Kim, Y.-M.; Kim, S.-K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Oh, S.H.; Choi, J.S. Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3211–3215. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.E. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200. [Google Scholar] [CrossRef]
- Seong, S.H.; Paudel, P.; Jung, H.A.; Choi, J.S. Identifying phlorofucofuroeckol-A as a dual inhibitor of amyloid-β25-35 self-aggregation and insulin glycation: Elucidation of the molecular mechanism of action. Mar. Drugs 2019, 17, 600. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Seong, S.H.; Wu, S.; Park, S.; Jung, H.A.; Choi, J.S. Eckol as a potential therapeutic against neurodegenerative diseases targeting dopamine D3/D4 receptors. Mar. Drugs 2019, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef]
- Lee, M.-S.; Shin, T.; Utsuki, T.; Choi, J.-S.; Byun, D.-S.; Kim, H.-R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and hepatoprotective properties in tacrine-treated HepG2 cells. J. Agri. Food Chem. 2012, 60, 5340–5349. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Roy, A.; Jung, J.H.; Choi, J.S. Evaluation of the inhibitory effects of eckol and dieckol isolated from edible brown alga Eisenia bicyclis on human monoamine oxidases A and B. Arch. Pharm. Res. 2017, 40, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish. Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Ryu, Y.B.; Kim, Y.-M.; Song, N.; Kim, C.Y.; Rho, M.-C.; Jeong, J.-H.; Cho, K.-O.; Lee, W.S.; Park, S.-J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg. Med. Chem. 2013, 21, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Chung, H.Y.; Kim, J.Y.; Son, B.W.; Jung, H.A.; Choi, J.S. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004, 27, 194–198. [Google Scholar] [CrossRef]
- Seong, S.H.; Paudel, P.; Choi, J.-W.; Ahn, D.H.; Nam, T.-J.; Jung, H.A.; Choi, J.S. Probing multi-target action of phlorotannins as new monoamine oxidase inhibitors and dopaminergic receptor modulators with the potential for treatment of neuronal disorders. Mar. Drugs 2019, 17, 377. [Google Scholar] [CrossRef] [PubMed]
- Paricharak, S.; Cortés-Ciriano, I.; IJzerman, A.P.; Malliavin, T.E.; Bender, A. Proteochemometric modelling coupled to in silico target prediction: An integrated approach for the simultaneous prediction of polypharmacology and binding affinity/potency of small molecules. J. Cheminform. 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Dugas, A.J., Jr.; Castañeda-Acosta, J.; Bonin, G.C.; Price, K.L.; Fischer, N.H.; Winston, G.W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: Structure- activity relationships. J. Nat. Prod. 2000, 63, 327–331. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In Nineteenth International Seaweed Symposium; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Sjøtun, K., Notoya, M., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 2, pp. 255–261. [Google Scholar]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Gómez-Soler, M.; Corrales, F.; Marcellino, D.; Narvaez, M.; Frankowska, M.; Flajolet, M.; Heintz, N. Characterization of the A2AR–D2R interface: Focus on the role of the C-terminal tail and the transmembrane helices. Biochem. Biophys. Res. Commun. 2010, 402, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Agnati, L.F.; Jacobsen, K.; Hillion, J.; Canals, M.; Torvinen, M.; Tinner-Staines, B.; Staines, W.; Rosin, D.; Terasmaa, A. Receptor heteromerization in adenosine A2A receptor signaling: Relevance for striatal function and Parkinson’s disease. Neurology 2003, 61, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Ferre, S.; Von Euler, G.; Johansson, B.; Fredholm, B.B.; Fuxe, K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc. Natl. Acad. Sci. USA 1991, 88, 7238–7241. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kobayashi, M.; Kanda, T. Involvement of Adenosine A2A Receptors in Depression and Anxiety. Int. Rev. Neurobiol. 2014, 119, 373–393. [Google Scholar]
- Jaakola, V.-P.; Griffith, M.T.; Hanson, M.A.; Cherezov, V.; Chien, E.Y.; Lane, J.R.; Ijzerman, A.P.; Stevens, R.C. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 2008, 322, 1211–1217. [Google Scholar] [CrossRef]
- Kang, M.C.; Cha, S.H.; Wijesinghe, W.A.; Kang, S.M.; Lee, S.H.; Kim, E.A.; Song, C.B.; Jeon, Y.J. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef]
- Cha, S.-H.; Heo, S.-J.; Jeon, Y.-J.; Park, S.M. Dieckol, an edible seaweed polyphenol, retards rotenone-induced neurotoxicity and α-synuclein aggregation in human dopaminergic neuronal cells. RSC Adv. 2016, 6, 110040–110046. [Google Scholar] [CrossRef]
- Cui, Y.; Park, J.Y.; Wu, J.; Lee, J.H.; Yang, Y.S.; Kang, M.S.; Jung, S.C.; Park, J.M.; Yoo, E.S.; Kim, S.H.; et al. Dieckol Attenuates Microglia-mediated Neuronal Cell Death via ERK, Akt and NADPH Oxidase-mediated Pathways. Korean J. Physiol. Pharmacol. 2015, 19, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; Kang, Y.-J.; Shin, S.-A.; Bak, D.-H.; Lee, J.W.; Lee, K.B.; Yoo, Y.C.; Kim, D.-K.; Lee, B.H.; Kim, D.W. Phlorofucofuroeckol improves glutamate-induced neurotoxicity through modulation of oxidative stress-mediated mitochondrial dysfunction in PC12 cells. PLoS ONE 2016, 11, e0163433. [Google Scholar] [CrossRef] [PubMed]
- Gaidin, S.G.; Turovskaya, M.V.; Mal’tseva, V.N.; Zinchenko, V.P.; Blinova, E.V.; Turovsky, E.A. A Complex Neuroprotective Effect of Alpha-2-Adrenergic Receptor Agonists in a Model of Cerebral Ischemia–Reoxygenation In Vitro. Biochemistry 2019, 13, 319–333. [Google Scholar] [CrossRef]
- Bücheler, M.M.; Hadamek, K.; Hein, L. Two α2-adrenergic receptor subtypes, α2A and α2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience 2002, 109, 819–826. [Google Scholar] [CrossRef]
- Scheinin, M.; Sallinen, J.; Haapalinna, A. Evaluation of the α2C-adrenoceptor as a neuropsychiatric drug target: Studies in transgenic mouse models. Life Sci. 2001, 68, 2277–2285. [Google Scholar] [CrossRef]
- Fairbanks, C.A.; Stone, L.S.; Kitto, K.F.; Nguyen, H.O.; Posthumus, I.J.; Wilcox, G.L. α2C-Adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J. Pharmacol. Exp. Ther. 2002, 300, 282–290. [Google Scholar] [CrossRef]
- Duflo, F.; Li, X.; Bantel, C.; Pancaro, C.; Vincler, M.; Eisenach, J.C. Peripheral Nerve Injury Alters the α2Adrenoceptor Subtype Activated by Clonidine for Analgesia. Anesthesiology 2002, 97, 636–641. [Google Scholar] [CrossRef]
- Quaglia, W.; Del Bello, F.; Giannella, M.; Piergentili, A.; Pigini, M. α2C-adrenoceptor modulators: A patent review. Expert. Opin. Ther. Pat. 2011, 21, 455–481. [Google Scholar] [CrossRef]
- Chilmonczyk, Z.; Bojarski, A.J.; Pilc, A.; Sylte, I. Functional selectivity and antidepressant activity of serotonin 1A receptor ligands. Int. J. Mol. Sci. 2015, 16, 18474–18506. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.G.; Guillon, C.; Fabio, K.; Heindel, N.D.; Lu, S.-f.; Miller, M.; Ferris, C.F.; Brownstein, M.J.; Garripa, C.; Koppel, G.A. Vasopressin antagonists as anxiolytics and antidepressants: Recent developments. Recent Pat. CNS Drug Discov. 2008, 3, 77–93. [Google Scholar] [CrossRef]
- Narayen, G.; Mandal, S.N. Vasopressin receptor antagonists and their role in clinical medicine. Indian J. Endocrinol. Metab. 2012, 16, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, G.; Jiang, J.; Ma, T.; Lin, X.; Jiang, L.; Cheng, J.; Tao, R. Signaling through hepatocyte vasopressin receptor 1 protects mouse liver from ischemia-reperfusion injury. Oncotarget 2016, 7, 69276. [Google Scholar] [CrossRef]
- Glass, M.; Faull, R.; Dragunow, M. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 1997, 77, 299–318. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D.; et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef] [PubMed]
- Ogden, S.B.; Malamas, M.S.; Makriyannis, A.; Eckel, L.A. The novel cannabinoid CB(1) receptor agonist AM11101 increases food intake in female rats. Br. J. Pharmacol. 2019, 176, 3972–3982. [Google Scholar] [CrossRef]
- Kim, A.-R.; Lee, M.-S.; Choi, J.-W.; Utsuki, T.; Kim, J.-I.; Jang, B.-C.; Kim, H.-R. Phlorofucofuroeckol A suppresses expression of inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines via inhibition of nuclear factor-κB, c-Jun NH 2-terminal kinases, and Akt in microglial cells. Inflammation 2013, 36, 259–271. [Google Scholar] [CrossRef]
- Eo, H.J.; Kwon, T.H.; Park, G.H.; Song, H.M.; Lee, S.J.; Park, N.H.; Jeong, J.B. In Vitro Anticancer Activity of Phlorofucofuroeckol A via Upregulation of Activating Transcription Factor 3 against Human Colorectal Cancer Cells. Mar. Drugs 2016, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, A.M.; Ahn, K.H.; Kendall, D.A. Mutations of CB1 T210 produce active and inactive receptor forms: Correlations with ligand affinity, receptor stability, and cellular localization. Biochemistry 2006, 45, 5606–5617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Römpler, H.; Yu, H.-T.; Arnold, A.; Orth, A.; Schöneberg, T. Functional consequences of naturally occurring DRY motif variants in the mammalian chemoattractant receptor GPR33. Genomics 2006, 87, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Krishna Kumar, K.; Shalev-Benami, M.; Robertson, M.J.; Hu, H.; Banister, S.D.; Hollingsworth, S.A.; Latorraca, N.R.; Kato, H.E.; Hilger, D.; Maeda, S.; et al. Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex. Cell 2019, 176, 448–458.e12. [Google Scholar] [CrossRef]
- Lin, L.S.; Ha, S.; Ball, R.G.; Tsou, N.N.; Castonguay, L.A.; Doss, G.A.; Fong, T.M.; Shen, C.-P.; Xiao, J.C.; Goulet, M.T.; et al. Conformational Analysis and Receptor Docking of N-[(1S,2S)-3-(4-Chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-{[5-(trifluoromethyl)pyridin-2-yl]oxy}propanamide (Taranabant, MK-0364), a Novel, Acyclic Cannabinoid-1 Receptor Inverse Agonist. J. Med. Chem. 2008, 51, 2108–2114. [Google Scholar] [CrossRef]
- Turton, M.; O’shea, D.; Gunn, I.; Beak, S.; Edwards, C.; Meeran, K.; Choi, S.; Taylor, G.; Heath, M.; Lambert, P. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Kim, E.-A.; Lee, S.-H.; Lee, J.-H.; Kang, N.; Oh, J.-Y.; Ahn, G.; Ko, S.C.; Fernando, S.P.; Kim, S.-Y.; Park, S.-J. A marine algal polyphenol, dieckol, attenuates blood glucose levels by Akt pathway in alloxan induced hyperglycemia zebrafish model. RSC Adv. 2016, 6, 78570–78575. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Fauzi, F.M.; Bender, A.; Jung, H.A.; Choi, J.S. Establishing GPCR targets of hMAO active anthraquinones from Cassia obtusifolia Linn seeds using in silico and in vitro methods. ACS Omega 2020, 5, 7705–7715. [Google Scholar] [CrossRef]
- Mohd Fauzi, F.; John, C.M.; Karunanidhi, A.; Mussa, H.Y.; Ramasamy, R.; Adam, A.; Bender, A. Understanding the mode-of-action of Cassia auriculata via in silico and in vivo studies towards validating it as a long term therapy for type II diabetes. J. Ethnopharmacol. 2017, 197, 61–72. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing fucoxanthin as a selective dopamine D3/D4 receptor agonist: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2019, 310, 108757. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Morris, G.M.; Olson, A.J. Automated docking of flexible ligands: Applications of AutoDock. J. Mol. Recognit. 1996, 9, 1–5. [Google Scholar] [CrossRef]
- Granier, S.; Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Weis, W.I.; Kobilka, B.K. Structure of the δ-opioid receptor bound to naltrindole. Nature 2012, 485, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Yan, W.; Chapman, K.; Ramesh, K.; Ferrell, A.J.; Yin, J.; Wang, X.; Xu, Q.; Rosenbaum, D.M. Structure of an allosteric modulator bound to the CB1 cannabinoid receptor. Nat. Chem. Biol. 2019, 15, 1199–1205. [Google Scholar] [CrossRef]
- Song, G.; Yang, D.; Wang, Y.; de Graaf, C.; Zhou, Q.; Jiang, S.; Liu, K.; Cai, X.; Dai, A.; Lin, G.; et al. Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators. Nature 2017, 546, 312–315. [Google Scholar] [CrossRef]



| Rank | Dieckol | Phlorofucofuroeckol-A | ||
|---|---|---|---|---|
| Protein Name | Average Score | Protein Name | Average Score | |
| 1 | Vasopressin 1A receptor | 0.513 | Vasopressin 1A receptor | 0.797 |
| 2 | Vasopressin 1B receptor | 0.742 | ||
| 3 | Oxytocin receptor | 0.737 | ||
| 4 | B2 bradykinin receptor | 0.735 | ||
| 5 | B1 bradykinin receptor | 0.727 | ||
| 6 | Histamine H1 receptor | 0.721 | ||
| 7 | Serotonin 1D receptor | 0.717 | ||
| 8 | Type-1 angiotensin II receptor | 0.716 | ||
| 9 | Dopamine D2 receptor | 0.713 | ||
| 10 | Cannabinoid receptor 1 | 0.711 | ||
| 11 | Prostanoid EP3 receptor | 0.710 | ||
| 12 | Rho-associated protein kinase 1 | 0.710 | ||
| 13 | Muscarinic acetylcholine receptor M3 | 0.709 | ||
| 14 | Cholecystokinin A receptor | 0.709 | ||
| 15 | Serotonin 1A receptor | 0.706 | ||
| 16 | Neurokinin 1 receptor | 0.706 | ||
| 17 | Cysteinyl leukotriene receptor 1 | 0.706 | ||
| 18 | Alpha-1D adrenergic receptor | 0.705 | ||
| 19 | Cholecystokinin B receptor | 0.704 | ||
| 20 | Serotonin 1B receptor | 0.704 | ||
| GPCRs | Functional Effect at 100 µM Concentration | |||
|---|---|---|---|---|
| Dieckol | Phlorofucofuroeckol-A | |||
| Agonist Effect | Antagonist Effect | Agonist Effect | Antagonist Effect | |
| Adenosine A2A receptor (A2AR) | −0.1 ± 1.41 | 55.55 ± 4.03 | −0.7 ± 0.57 | 66.6 ± 2.26 |
| Alpha-2A adrenergic receptor (α2AAR) | 13.4 ± 19.87 | 46.15 ± 20.15 | −0.5 ± 0.85 | 20.95 ± 1.77 |
| Alpha-2C adrenergic receptor (α2CAR) | 52.4 ± 4.24 | −1.2 ± 6.08 | 83.8 ± 0.07 | 19.2 ± 9.76 |
| δ-opioid receptor (δ-OPR) | −5.7 ± 0.14 | 66.95 ± 0.92 | 14.7 ± 7.35 | 73.55 ± 5.44 |
| Cannabinoid receptor 1(CB1R) | −23.3 ± 12.09 | 158.75 ± 17.18 | 113.8 ± 3.68 | 21.35 ± 0.49 |
| Free fatty acid receptor 1 (FFA1R) (GPR40) | 0.2 ± 1.56 | 22.55 ± 5.44 | −1.0 ± 0.07 | 30.15 ± 0.78 |
| Glucagon-like peptide-1 receptor (GLP-1) | −16.3 ± 1.13 | 101 ± 8.20 | −15.5 ± 2.55 | 105.7 ± 1.27 |
| Vasopressin 1A receptor (V1AR) | 106.73 ± 2.97 | 57.77 ± 0.32 b | 38.45 ± 7.14 a | 56.90 ± 5.37 b |
| 5-hydroxytryptophan 1A (5-HT1AR) | 1.75 ± 0.64 a | 91.0 ± 3.11 | 1.65 ± 0.49 a | 77.00 ± 11.03 |
| 5-hydroxytryptophan 1B (5-HT1BR) | −7.3 ± 3.96 | −18.5 ± 2.69 | ||
| Compounds (µM) | Target GPCRs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| hA2AR | hα2AAR | hα2CAR | hδ-OPR | CB1R | GPR40 | GLP-1 | hV1AR | h5-HT1AR | ||
| Dieckol | 12.5 | – | – | – | – | – | – | – | – | – |
| 25 | – | – | – | – | – | – | – | – | – | |
| 50 | – | – | – | – | – | – | – | 50.40 ± 0.42 | – | |
| 100 | −0.1 ± 1.41 | 13.4 ± 19.87 | 52.4 ± 4.24 | −5.7 ± 0.14 | −23.3 ± 12.09 | 0.2 ± 1.56 | −16.3 ± 1.13 | 106.73 ± 2.97 | 1.75 ± 0.64 c | |
| 150 | – | – | 73.0 ± 6.42 | – | – | – | – | 118.1 ± 2.83 | – | |
| 200 | – | – | 74.77 ± 6.60 | – | – | – | – | – | – | |
| EC50 (µM) a | NA | NA | 98.80 ± 7.71 | NA | NA | NA | NA | 39.12 ± 2.12 | NA | |
| PFF-A | 12.5 | – | – | 22.03 ± 6.61 | – | 46.7 ± 6.22 | – | – | – | – |
| 25 | – | – | 55.0 ± 9.13 | – | 80.3 ± 4.10 | – | – | – | – | |
| 50 | – | – | 62.27 ± 6.53 | – | 96.45 ± 5.02 | – | – | – | – | |
| 100 | −0.7 ± 0.57 | −0.5 ± 0.85 | 83.8 ± 0.07 | 14.7 ± 7.35 | 113.8 ± 3.68 | −1.0 ± 0.07 | −15.5 ± 2.55 | 38.45 ± 7.14 c | 1.65 ± 0.49 c | |
| 150 | – | – | – | – | – | – | – | – | – | |
| 200 | – | – | – | – | – | – | – | – | – | |
| EC50 (µM) a | NA | NA | 23.67 ± 3.32 | NA | 13.42 ± 2.03 | NA | NA | NA | NA | |
| Reference Drugs, EC50 (nM) b | 9.1 | 0.74 | 0.86 | 4.4 | 0.21 | 10000 | 0.049 | 0.72 | 2.5 c | |
| Compounds (µM) | Target GPCRs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| hA2AR | hα2AAR | hα2CAR | hδ-OPR | CB1R | GPR40 | GLP-1 | hV1AR | h5-HT1AR | h5-HT1BR | ||
| Dieckol | 12.5 | – | – | – | – | – | – | 17.87 ± 6.32 | – | – | – |
| 25 | – | – | – | – | – | – | 21.23 ± 7.31 | – | – | – | |
| 50 | 17.5 ± 8.50 | – | – | 23.23 ± 4.04 | – | – | 53.80 ± 3.12 | 41.53 ± 7.39 c | 54.7 ± 1.7 | – | |
| 100 | 56.0 ± 11.11 | 46.15 ± 20.15 | −1.20 ± 6.08 | 71.6 ± 17.85 | −1.20 ± 6.08 | 22.55 ± 5.44 | 101.0 ± 8.20 | 57.77 ± 0.32 c | 91.0 ± 3.11 | −7.3 ± 3.96 | |
| 150 | 62.43 ± 12.19 | – | – | 100.1 ± 1.53 | – | – | – | 68.80 ± 0.85 c | – | – | |
| 200 | – | – | – | – | – | – | – | – | 100.9 ± 0.57 | −8.1 ± 0.85 | |
| IC50 (µM) a | 87.18 ± 2.63 | NA | NA | 80.46 ± 13.74 | NA | NA | 47.19 ± 2.46 | 82.71 ± 8.73 | 43.31 ± 3.22 | NA | |
| PFF-A | 12.5 | – | – | – | – | – | – | 25.63 ± 13.14 | – | 37.40 ± 2.19 | – |
| 25 | – | – | – | – | – | – | 57.37 ± 1.15 | 35.67 ± 3.88 | 68.20 ± 9.89 | – | |
| 50 | 64.7 ± 2.72 | – | – | 89.03 ± 0.70 | – | – | 83.87 ± 2.03 | 52.80 ± 8.09 | 85.55 ± 8.41 | – | |
| 100 | 92.2 ± 0.95 | 20.95 ± 1.77 | 19.2 ± 9.76 | 96.47 ± 0.84 | 21.35 ± 0.49 | 30.15 ± 0.78 | 105.7 ± 1.27 | 56.90 ± 5.37 c | 77.00 ± 11.03 | −18.5 ± 2.69 | |
| 150 | 99.93 ± 0.31 | – | – | 93.67 ± 6.67 | – | – | – | 56.07 ± 4.72 | – | – | |
| 200 | – | – | – | – | – | – | – | – | – | −35.5 ± 4.38 | |
| IC50 (µM) a | < 50 | NA | NA | < 50 | NA | NA | 21.56 ± 2.16 | 42.25 ± 0.41 | 17.75 ± 3.42 | NA | |
| Reference Drugs, IC50 (nM) b | 0.41 | 17 | 22 | 9 | 77 | ND | 4.6 | 1.9 | 4.4 | 23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, P.; Seong, S.H.; Park, S.E.; Ryu, J.H.; Jung, H.A.; Choi, J.S. In Vitro and In Silico Characterization of G-Protein Coupled Receptor (GPCR) Targets of Phlorofucofuroeckol-A and Dieckol. Mar. Drugs 2021, 19, 326. https://doi.org/10.3390/md19060326
Paudel P, Seong SH, Park SE, Ryu JH, Jung HA, Choi JS. In Vitro and In Silico Characterization of G-Protein Coupled Receptor (GPCR) Targets of Phlorofucofuroeckol-A and Dieckol. Marine Drugs. 2021; 19(6):326. https://doi.org/10.3390/md19060326
Chicago/Turabian StylePaudel, Pradeep, Su Hui Seong, Se Eun Park, Jong Hoon Ryu, Hyun Ah Jung, and Jae Sue Choi. 2021. "In Vitro and In Silico Characterization of G-Protein Coupled Receptor (GPCR) Targets of Phlorofucofuroeckol-A and Dieckol" Marine Drugs 19, no. 6: 326. https://doi.org/10.3390/md19060326
APA StylePaudel, P., Seong, S. H., Park, S. E., Ryu, J. H., Jung, H. A., & Choi, J. S. (2021). In Vitro and In Silico Characterization of G-Protein Coupled Receptor (GPCR) Targets of Phlorofucofuroeckol-A and Dieckol. Marine Drugs, 19(6), 326. https://doi.org/10.3390/md19060326







