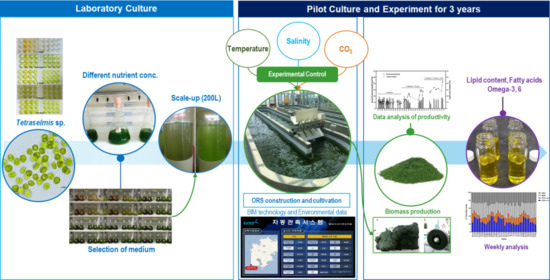
Year-Round Cultivation of Tetraselmis sp. for Essential Lipid Production in a Semi-Open Raceway System
Abstract
1. Introduction
2. Results
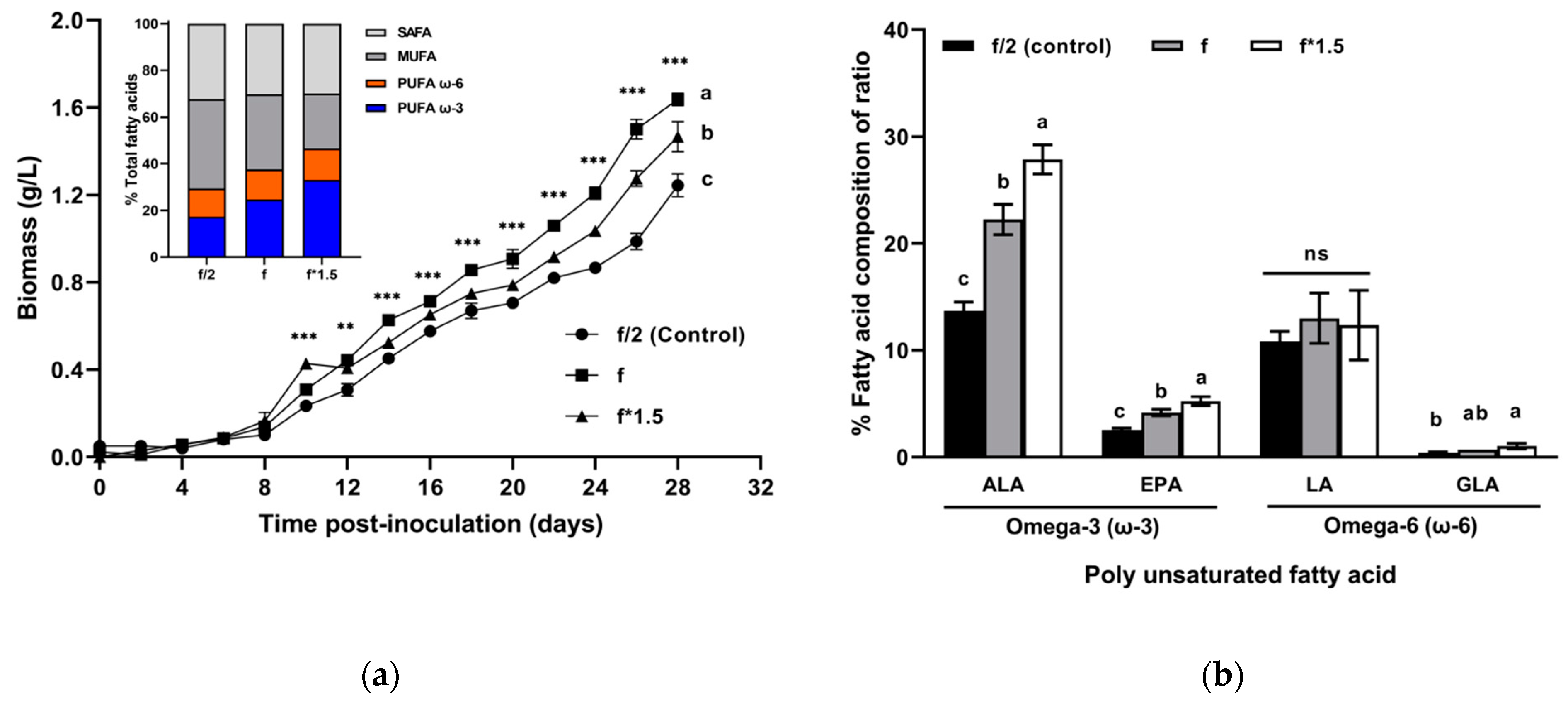
2.1. Selection of Culture Medium Concentration for High Biomass and FA Productivity
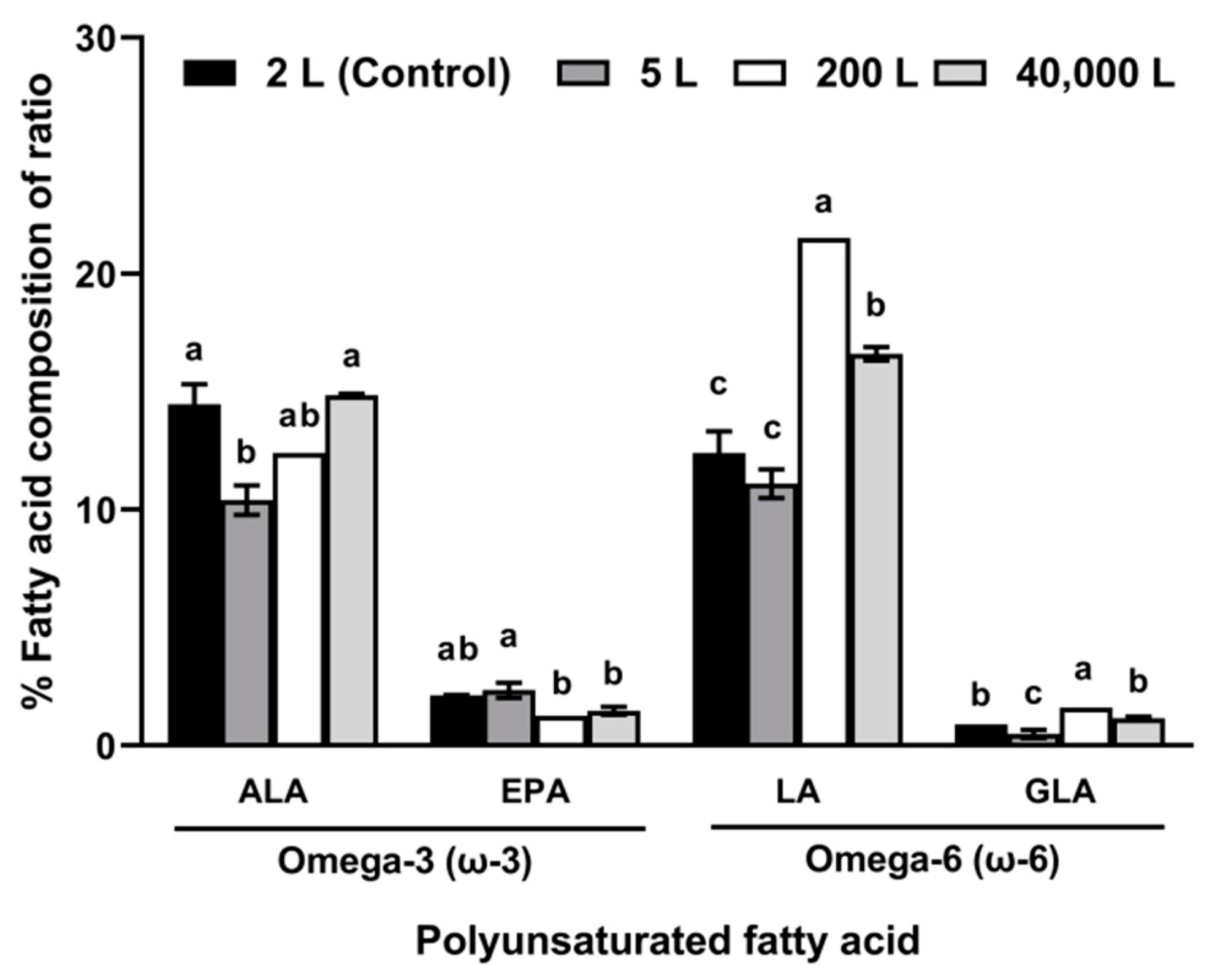
2.2. Production of Biomass, Lipids, and FAs under Four Different Culture Scales
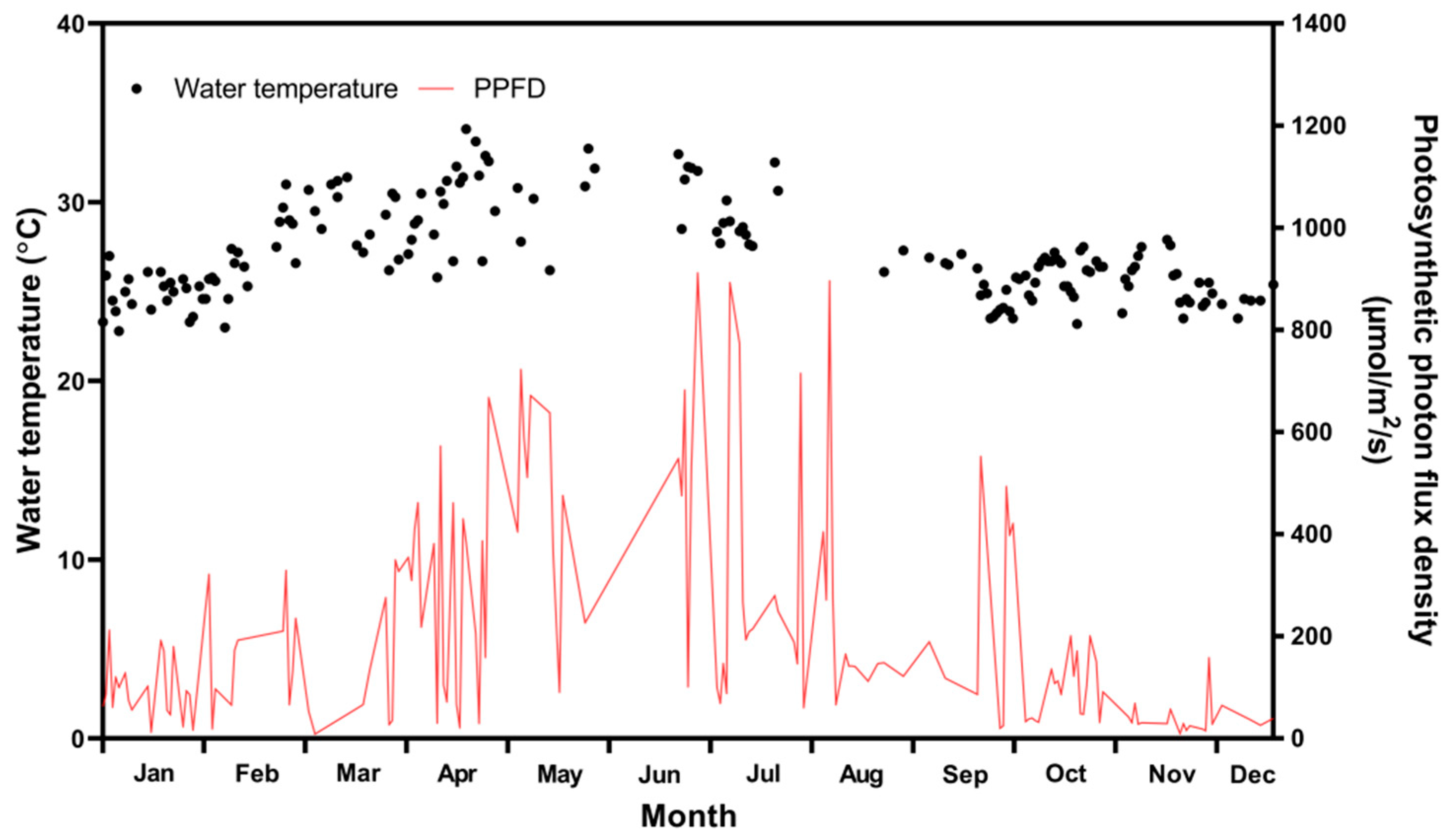
2.3. Culture Environment and Biomass and Lipid Productivities of Tetraselmis sp. in the ORS over 1 Year
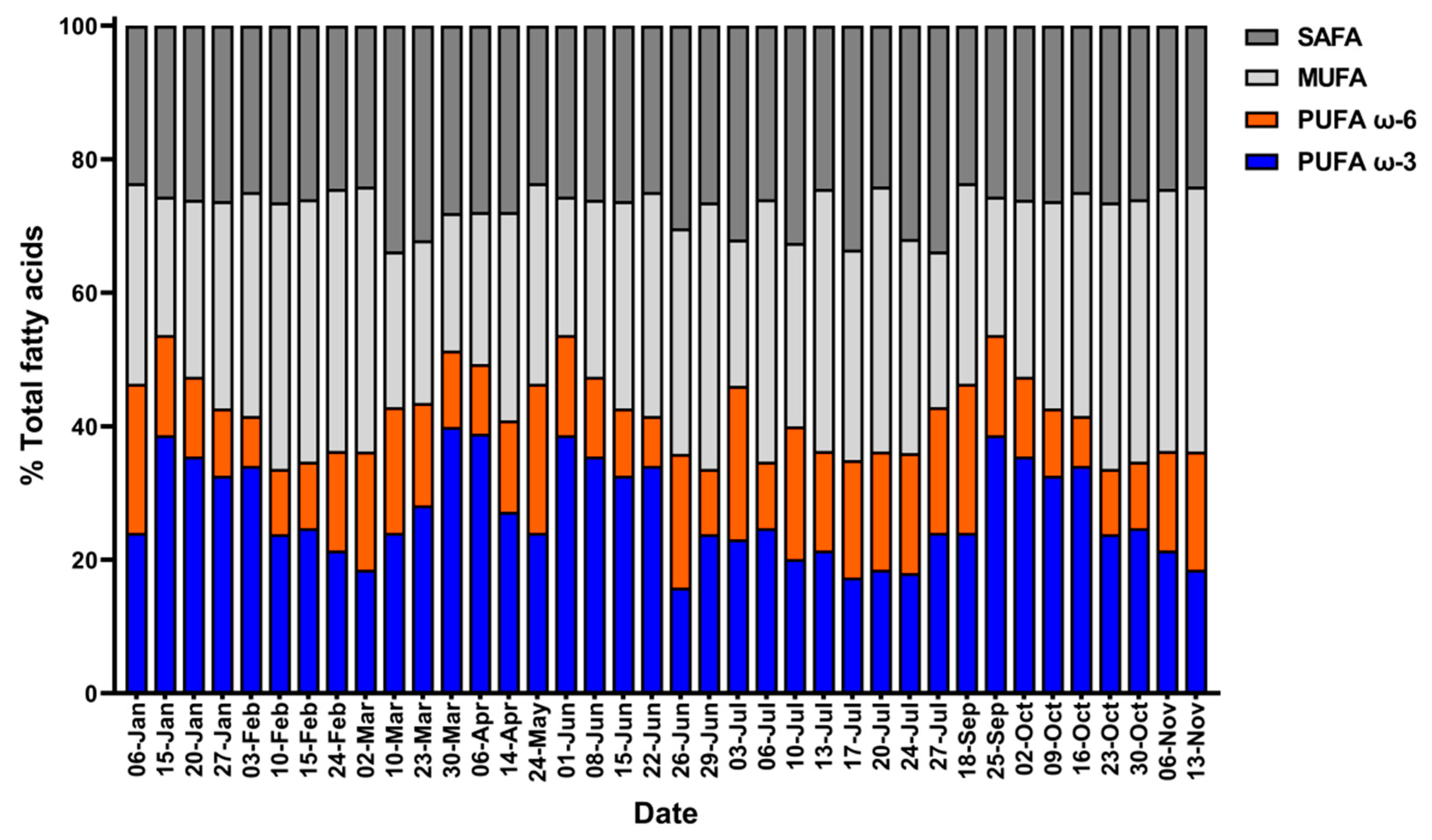
2.4. Annual Variation in AP and Lipid Content of Tetraselmis sp. in ORS
3. Discussion
3.1. Selection of Culture Medium Concentration for High Biomass Productivity
3.2. Production of Biomass, Lipids, and FAs under Four Different Culture Scales
3.3. Variations in Culture Environment and Biomass and Lipid Productivities of Tetraselmis sp. in a 1-Year ORS Study
3.4. Variations in the AP and Lipid Content of Tetraselmis sp. in a 3-Year ORS Study
4. Materials and Methods
4.1. Strain and Culture Medium
4.2. Culture Conditions
4.3. Biomass Concentration, Productivity, and Harvesting
4.4. Analysis of Lipid Content and FA Composition
4.5. System Construction and Strategies for Pilot-Scale Culture
4.5.1. Culture and Pilot Production Site
4.5.2. Construction of the Semi-ORS
4.5.3. Maintaining the Culture System
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; Lopes da Silva, T. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Nasopoulou, C.; Zabetakis, I.; Murray, P.; Saha, S.K. Bioactive lipids of marine microalga Chlorococcum sp. SABC 012504 with anti-inflammatory and anti-thrombotic activities. Mar. Drugs 2021, 19, 28. [Google Scholar] [CrossRef]
- Draaisma, R.B.; Wijffels, R.H.; (Ellen) Slegers, P.M.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef]
- Hartweg, J.; Perera, R.; Montori, V.M.; Dinneen, S.F.; Neil, A.H.; Farmer, A.J. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008, 1, CD003205. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Su, K.-P.; Cheng, T.-C.; Liu, H.-C.; Chang, C.-J.; Dewey, M.E.; Stewart, R.; Huang, S.-Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. ProstaglandinsLeukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Burdge, G.C. Metabolism of α-linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168. [Google Scholar] [CrossRef]
- Finco, A.M.d.O.; Mamani, L.D.G.; de Carvalho, J.C.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Soccol, C.R. Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit. Rev. Biotechnol. 2017, 37, 656–671. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Ahrens Jr, E.H.; Blankenhorn, D.H.; Tsaltas, T.T. Effect on human serum lipids of substituting plant for animal fat in diet. Proc. Soc. Exp. Biol. Med. 1954, 86, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Keys, A. Serum cholesterol response to dietary cholesterol. Am. J. Clin. Nutr. 1984, 40, 351–359. [Google Scholar] [CrossRef]
- Sijtsma, L.; De Swaaf, M.E. Biotechnological production and applications of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. Appl. Microbiol. Biotechnol. 2004, 64, 146–153. [Google Scholar] [CrossRef]
- Pulz, O. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293. [Google Scholar]
- Kang, D.-H.; Heo, S.-J.; Oh, C.; Ju, S.-J.; Jeon, S.-M.; Choi, H.-W.; Noh, J.H.; Park, S.H.; Kim, T.-Y. A review on major factors for microalgae biofuel commercialization. Ocean Polar Res. 2012, 34, 365–384. [Google Scholar] [CrossRef]
- Tredici, M.R.; Materassi, R. From open ponds to vertical alveolar panels: The Italian experience in the development of reactors for the mass cultivation of phototrophic microorganisms. J. Appl. Phycol. 1992, 4, 221–231. [Google Scholar] [CrossRef]
- Torzillo, G.; Carlozzi, P.; Pushparaj, B.; Montaini, E.; Materassi, R. A two-plane tubular photobioreactor for outdoor culture of Spirulina. Biotechnol. Bioeng. 1993, 42, 891–898. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Culturing microalgae in outdoor ponds. In Algal Culturing Techniques; Academic Press: Cambridge, MA, USA, 2005; pp. 205–218. [Google Scholar]
- Borowitzka, M.A.; Moheimani, N.R. Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-5478-2. [Google Scholar]
- Kim, T.; Choi, W.-S.; Ye, B.-R.; Heo, S.-J.; Oh, D.; Kim, S.; Choi, K.-S.; Kang, D.-H. Cultivating Spirulina maxima: Innovative approaches. Cyanobacteria 2018, 61, 61–83. [Google Scholar]
- Williams, P.J.l.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Richmond, A. Open systems for the mass production of photoautotrophic microalgae outdoors: Physiological principles. J. Appl. Phycol. 1992, 4, 281–286. [Google Scholar] [CrossRef]
- Moheimani, N.R. Long-term outdoor growth and lipid productivity of Tetraselmis suecica, Dunaliella tertiolecta and Chlorella sp (Chlorophyta) in bag photobioreactors. J. Appl. Phycol. 2013, 25, 167–176. [Google Scholar] [CrossRef]
- Salman, A. Building information modeling (BIM): Trends, benefits, risks, and challenges for the AEC industry. Leadersh. Manag. Eng. 2011, 11, 241–252. [Google Scholar] [CrossRef]
- Lee, G.; Sacks, R.; Eastman, C.M. Specifying parametric building object behavior (BOB) for a building information modeling system. Autom. Constr. 2006, 15, 758–776. [Google Scholar] [CrossRef]
- Goedert, J.D.; Meadati, P. Integrating construction process documentation into building information modeling. J. Constr. Eng. Manag. 2008, 134, 509–516. [Google Scholar] [CrossRef]
- Mohebbi, F.; Hafezieh, M.; Seidgar, M.; Hosseinzadeh Sahhafi, H.; Mohsenpour Azari, A.; Ahmadi, R. The growth, survival rate and reproductive characteristics of Artemia urmiana fed by Dunaliella tertiolecta, Tetraselmis suecica, Nannochloropsis oculata, Chaetoceros sp., Chlorella sp. and Spirolina sp. as feeding microalgae. Iran. J. Fish. Sci. 2016, 15, 727–737. [Google Scholar]
- Lananan, F.; Jusoh, A.; Ali, N.; Lam, S.S.; Endut, A. Effect of conway medium and f/2 medium on the growth of six genera of South China Sea marine microalgae. Bioresour. Technol. 2013, 141, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Erkelens, M.; Ward, A.J.; Ball, A.S.; Lewis, D.M. Microalgae digestate effluent as a growth medium for Tetraselmis sp. in the production of biofuels. Bioresour. Technol. 2014, 167, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, Z.; Wen, W.; Yan, J. Effects of nitrogen supplementation of the culture medium on the growth, total lipid content and fatty acid profiles of three microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis). J. Appl. Phycol. 2013, 25, 129–137. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, X.; Fan, X.; Han, L.; Zeng, C. Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp.(Eustigmatophyta). J. Appl. Phycol. 2001, 13, 463–469. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Lee, Y.-K. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, B.; Latrille, E.; Steyer, J.-P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Hadiyanto, H.; Elmore, S.; Van Gerven, T.; Stankiewicz, A. Hydrodynamic evaluations in high rate algae pond (HRAP) design. Chem. Eng. J. 2013, 217, 231–239. [Google Scholar] [CrossRef]
- Prussi, M.; Buffi, M.; Casini, D.; Chiaramonti, D.; Martelli, F.; Carnevale, M.; Tredici, M.R.; Rodolfi, L. Experimental and numerical investigations of mixing in raceway ponds for algae cultivation. Biomass Bioenergy 2014, 67, 390–400. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Wang, S.-K.; Hu, Y.-R.; Wang, F.; Stiles, A.R.; Liu, C.-Z. Scale-up cultivation of Chlorella ellipsoidea from indoor to outdoor in bubble column bioreactors. Bioresour. Technol. 2014, 156, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Zepka, L.Q.; Queiroz, M.I. Energy from Microalgae; Springer: Berlin, Germany, 2018; ISBN 3319690930. [Google Scholar]
- Lee, S.-H.; Oh, H.-M.; Jo, B.-H.; Lee, S.-A.; Shin, S.-Y.; Kim, H.-S.; Lee, S.-H.; Ahn, C.-Y. Higher biomass productivity of microalgae in an attached growth system, using wastewater. J. Microbiol. Biotechnol. 2014, 24, 1566–1573. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Turnbull, M.H.; Craggs, R.J. Increased pond depth improves algal productivity and nutrient removal in wastewater treatment high rate algal ponds. Water Res. 2014, 53, 271–281. [Google Scholar] [CrossRef]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.P.; Del Pino, V.; Uronen, P.; Legrand, C. Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies 2012, 5, 1577–1592. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Li, J.; Zhu, Q.; Liu, Z. Variation of lipid and fatty acid compositions of the marine microalga Pavlova viridis (Prymnesiophyceae) under laboratory and outdoor culture conditions. World J. Microbiol. Biotechnol. 2008, 24, 1209–1214. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Meseck, S.L.; Alix, J.H.; Wikfors, G.H. Photoperiod and light intensity effects on growth and utilization of nutrients by the aquaculture feed microalga, Tetraselmis chui (PLY429). Aquaculture 2005, 246, 393–404. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Kim, Z.-H.; Park, H.; Lee, C.-G. Seasonal assessment of biomass and fatty acid productivity by Tetraselmis sp. in the ocean using semi-permeable membrane photobioreactors. J. Microbiol. Biotechnol. 2016, 26, 1098–1102. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 2011, 102, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1–16. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, H.; Li, B.; Yang, J. Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour. Technol. 2014, 152, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.; Idris, A.; Bukhari, A.; Wahidin, S. Intensity of blue LED light: A potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour. Technol. 2013, 148, 373–378. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Xie, G.-J.; Ren, N.-Q. Enhanced lipid accumulation of green microalga Scenedesmus sp. by metal ions and EDTA addition. Bioresour. Technol. 2014, 169, 763–767. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.; Liu, T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G. Effects of competition between two introduced Caulerpa. Mar. Ecol. Prog. Ser. 2002, 225, 189–195. [Google Scholar] [CrossRef]
- Belay, A. Mass culture of Spirulina outdoors—the Earthrise Farms experience. Spirulina Platensis 1997, 1, 131–158. [Google Scholar]
- Benemann, J.R. Open ponds and closed photobioreactors–comparative economics. In Proceedings of the 5th Annual World Congress on Industrial Biotechnology and Bioprocessing, Chicago, IL, USA, 27–30 April 2008; Volume 30. [Google Scholar]
- Fon-Sing, S.; Borowitzka, M.A. Isolation and screening of euryhaline Tetraselmis spp. suitable for large-scale outdoor culture in hypersaline media for biofuels. J. Appl. Phycol. 2016, 28, 1–14. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Qiang, H.; Richmond, A.; Zarmi, Y. Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (cyanobacteria). Eur. J. Phycol. 1998, 33, 165–171. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Sakamoto, A.; Nishiyama, Y.; Inaba, M.; Murata, N. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000, 123, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.F.; Tabatabaei, M.; MohtashamiI, S.K.; Tohidfar, M.; Moradi, F. Comparative salt stress study on intracellular ion concentration in marine and salt-adapted freshwater strains of microalgae. Not. Sci. Biol. 2013, 5, 309–315. [Google Scholar] [CrossRef]
- Asulabh, K.S.; Supriya, G.; Ramachandra, T.V. Effect of salinity concentrations on growth rate and lipid concentration in Microcystis sp., Chlorococcum sp. and Chaetoceros sp. In Proceedings of the National Conference on Conservation and Management of Wetland Ecosystems, School of Environmental Sciences, Mahatma Gandhi University, Kottayam, Kerala, 6–9 November 2012. [Google Scholar]
- Kassim, M.A.; Meng, T.K. Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production. Sci. Total Environ. 2017, 584, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Santos, T.; Mendoza-Martín, J.L.; Fernández, F.G.A.; Molina, E.; Vieira-Costa, J.A.; Heaven, S. Optimization of carbon dioxide supply in raceway reactors: Influence of carbon dioxide molar fraction and gas flow rate. Bioresour. Technol. 2016, 212, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Dayananda, C.; Sarada, R.; Rani, M.U.; Shamala, T.R.; Ravishankar, G.A. Autotrophic cultivation of Botryococcus braunii for the production of hydrocarbons and exopolysaccharides in various media. Biomass Bioenergy 2007, 31, 87–93. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-H.; Ramanan, R.; Heo, J.; Kang, Z.; Kim, B.-H.; Ahn, C.-Y.; Oh, H.-M.; Kim, H.-S. Organic carbon, influent microbial diversity and temperature strongly influence algal diversity and biomass in raceway ponds treating raw municipal wastewater. Bioresour. Technol. 2015, 191, 481–487. [Google Scholar] [CrossRef]
- Hanwool, P.; Donghee, H.; Dong-Woo, S.; Z-Hun, K.; Seong-Joo, H.; Sang-Min, L.; Choul-Gyun, L. Isolation and characterization of five isolates of Tetraselmis sp. with rapid growth rates in low temperatures. Korean Soc. Mar. Biotechnol. 2019, 11, 23–28. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Springer: Berlin, Germany, 1975; pp. 29–60. [Google Scholar]
- De Castro, M.D.L.; Garcıa-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]









| Month | ALA (C18:3n3) | EPA (C20:5n3) | LA (C18:2n6) | GLA (C18:3n6) | AA (C20:4n6) | Ratio (ω-6/ω-3) |
|---|---|---|---|---|---|---|
| Jan | 27.55 ± 5.00 | 0.12 ± 0.04 ab | 11.86 ± 4.69 ab | 0.88 ± 0.36 | 1.91 ± 0.40 | 0.49/1 |
| Feb | 20.21 ± 4.90 | 0.16 ± 0.02 a | 8.02 ± 2.89 ab | 0.85 ± 0.27 | 1.50 ± 0.11 | 0.43/1 |
| Mar | 21.56 ± 8.64 | 0.07 ± 0.02 b | 13.38 ± 2.53 ab | 0.72 ± 0.42 | 1.43 ± 0.37 | 0.70/1 |
| Apr | 27.33 ± 7.75 | 0.11 ± 0.01 ab | 9.01 ± 1.08 ab | 1.61 ± 1.18 | 1.26 ± 0.05 | 0.39/1 |
| May | 21.13 | 0.08 | 18.21 | 1.41 | 2.5 | 0.93/1 |
| Jun | 24.61 ± 7.48 | 0.15 ± 0.03 a | 10.00 ± 4.71 ab | 0.83 ± 0.36 | 1.36 ± 0.68 | 0.49/1 |
| Jul | 16.88 ± 2.55 | 0.09 ± 0.04 b | 15.44 ± 4.27 a | 1.04 ± 0.29 | 0.83 ± 0.90 | 0.85/1 |
| Sep | 26.94 ± 8.22 | 0.09 ± 0.02 ab | 15.34 ± 4.05 ab | 1.05 ± 0.51 | 2.07 ± 0.61 | 0.66/1 |
| Oct | 24.19 ± 5.19 | 0.16 ± 0.01 a | 7.37 ± 1.46 b | 0.72 ± 0.12 | 1.59 ± 0.17 | 0.34/1 |
| Nov | 15.09 ± 1.60 | 0.12 ± 0.02 ab | 13.16 ± 1.61 ab | 1.32 ± 0.16 | 1.68 ± 0.21 | 0.83/1 |
| Semi-ORS (a) | Scenario 1 (b) | Scenario 2 (c) | |
|---|---|---|---|
| Scale (ha) | 0.04 | 0.04 | 0.04 |
| Biomass yield | |||
| g/m2/d | 32.14 | 32.14 | <32.14 |
| tons/ha/year | 117.31 | 58.66 | <58.66 |
| Capital cost ($) | |||
| Major purchased equipment | 25,000 | 25,000 | 25,000 |
| Installation | 70,000 | 70,000 | 70,000 |
| Construction | 390,000 | - | - |
| Infrastructure | 141,000 | 141,000 | 141,000 |
| Other | 4500 | 4500 | 4500 |
| Total capital costs | 630,500 | 240,500 | 240,500 |
| Depreciation (30 years, $/year) | 24,017 | 11,017 | 11,017 |
| Operating cost ($/year) | |||
| Fertilizers | 3000 | 1500 | 1500 |
| Labor | 5000 | 5000 | 5000 |
| Electricity | 4500 | 2250 | 2250 |
| Water | - | - | - |
| Analytical cost | 2200 | 2200 | 2200 |
| Total operating costs | 14,700 | 10,950 | 10,950 |
| Total production cost ($/year) | 38,717 | 21,967 | 21,967 |
| Biomass production cost ($/kg) | 8.15 | 9.25 | >9.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-K.; Ryu, Y.-K.; Choi, W.-Y.; Kim, T.; Park, A.; Lee, Y.-J.; Jeong, Y.; Lee, C.-G.; Kang, D.-H. Year-Round Cultivation of Tetraselmis sp. for Essential Lipid Production in a Semi-Open Raceway System. Mar. Drugs 2021, 19, 314. https://doi.org/10.3390/md19060314
Lee W-K, Ryu Y-K, Choi W-Y, Kim T, Park A, Lee Y-J, Jeong Y, Lee C-G, Kang D-H. Year-Round Cultivation of Tetraselmis sp. for Essential Lipid Production in a Semi-Open Raceway System. Marine Drugs. 2021; 19(6):314. https://doi.org/10.3390/md19060314
Chicago/Turabian StyleLee, Won-Kyu, Yong-Kyun Ryu, Woon-Yong Choi, Taeho Kim, Areumi Park, Yeon-Ji Lee, Younsik Jeong, Choul-Gyun Lee, and Do-Hyung Kang. 2021. "Year-Round Cultivation of Tetraselmis sp. for Essential Lipid Production in a Semi-Open Raceway System" Marine Drugs 19, no. 6: 314. https://doi.org/10.3390/md19060314
APA StyleLee, W.-K., Ryu, Y.-K., Choi, W.-Y., Kim, T., Park, A., Lee, Y.-J., Jeong, Y., Lee, C.-G., & Kang, D.-H. (2021). Year-Round Cultivation of Tetraselmis sp. for Essential Lipid Production in a Semi-Open Raceway System. Marine Drugs, 19(6), 314. https://doi.org/10.3390/md19060314