Saliniquinone Derivatives, Saliniquinones G−I and Heraclemycin E, from the Marine Animal-Derived Nocardiopsis aegyptia HDN19-252
Abstract
:1. Introduction
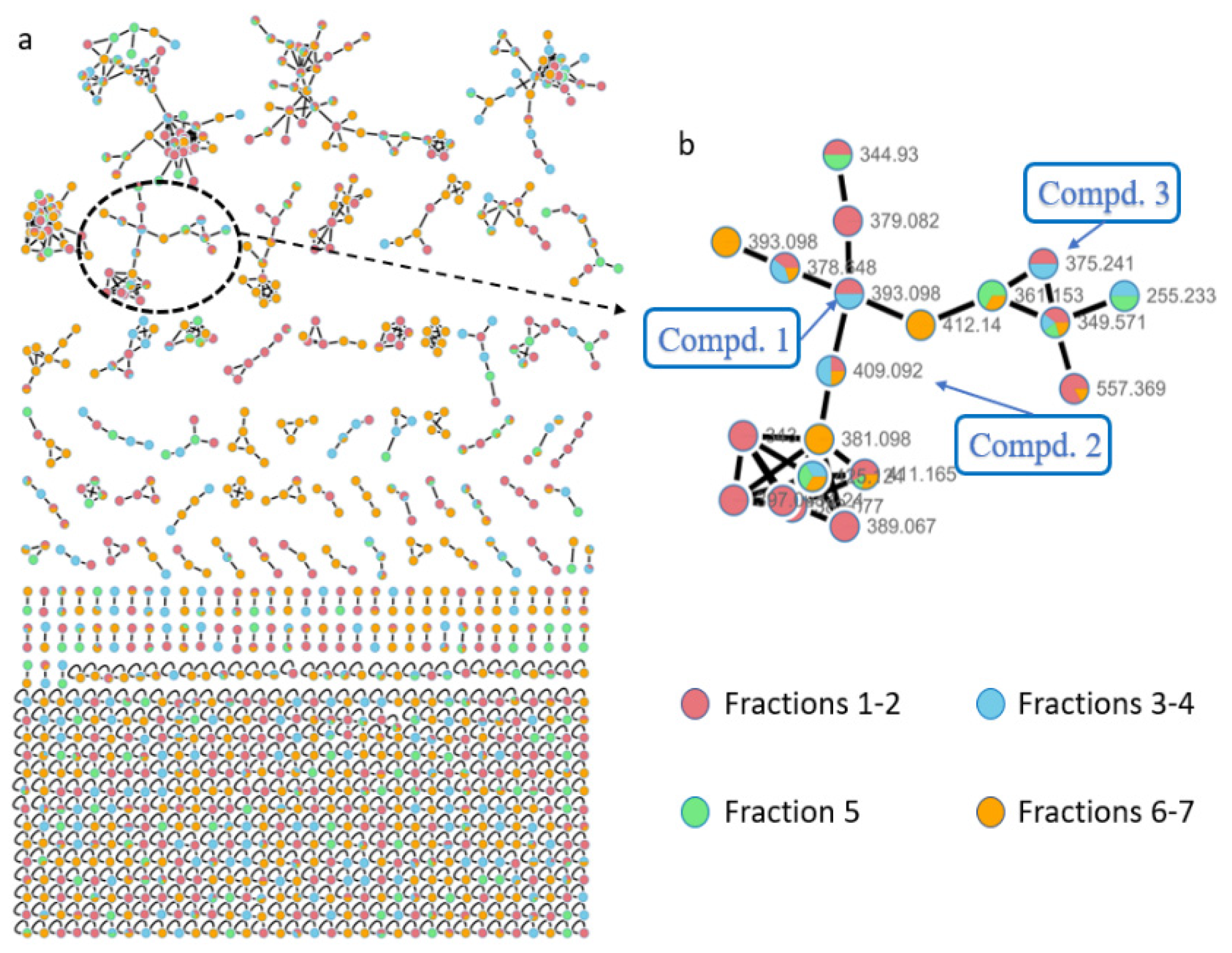
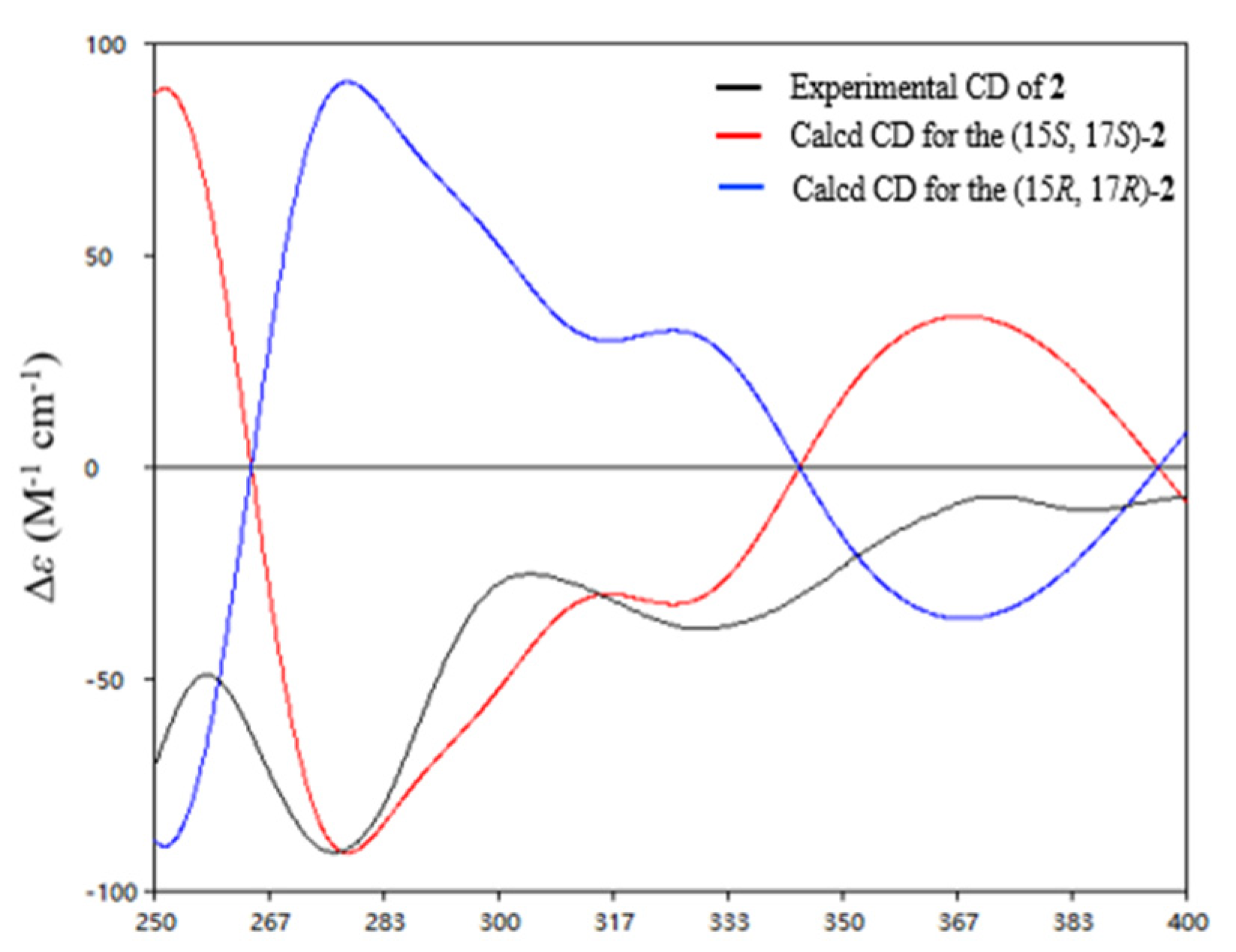
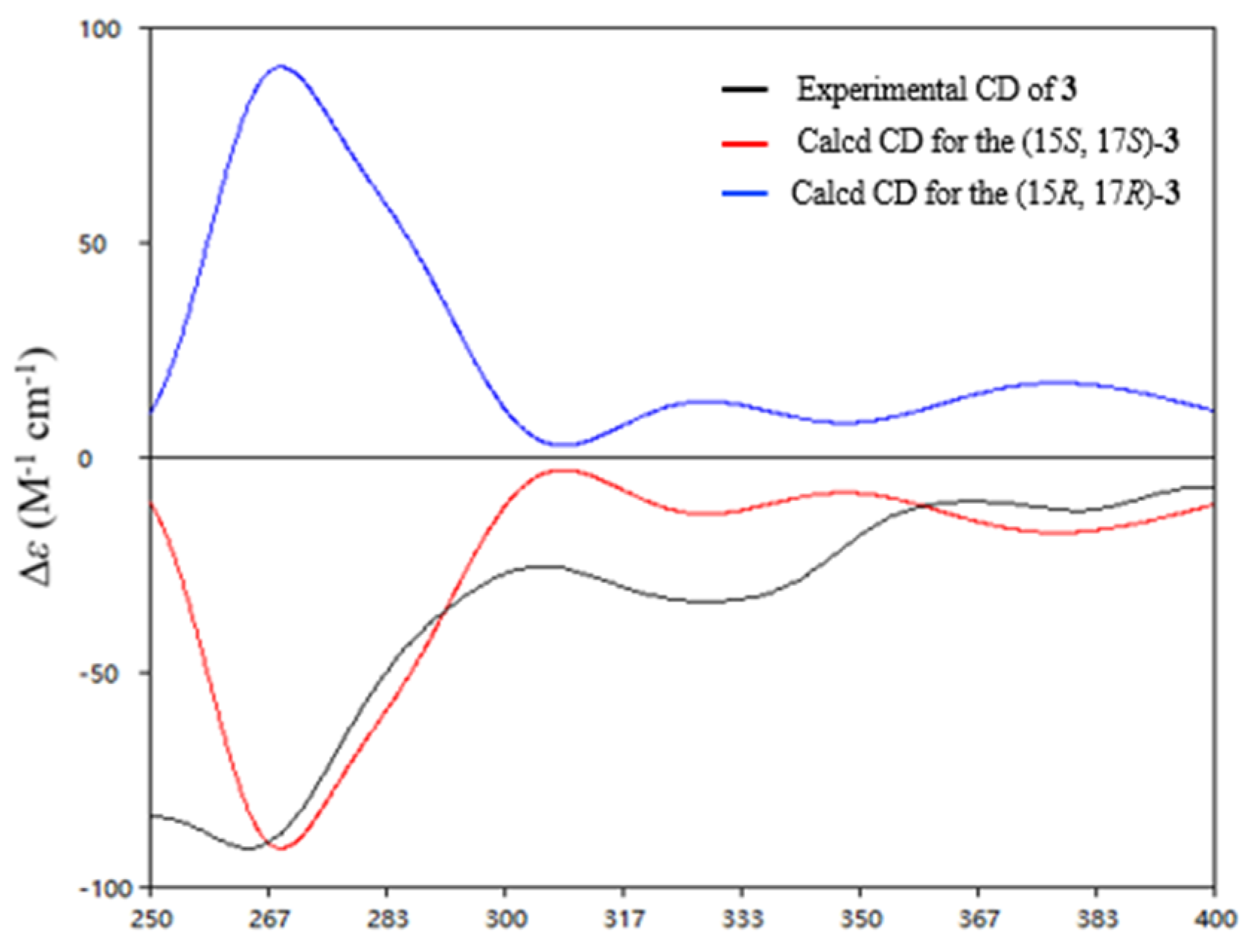
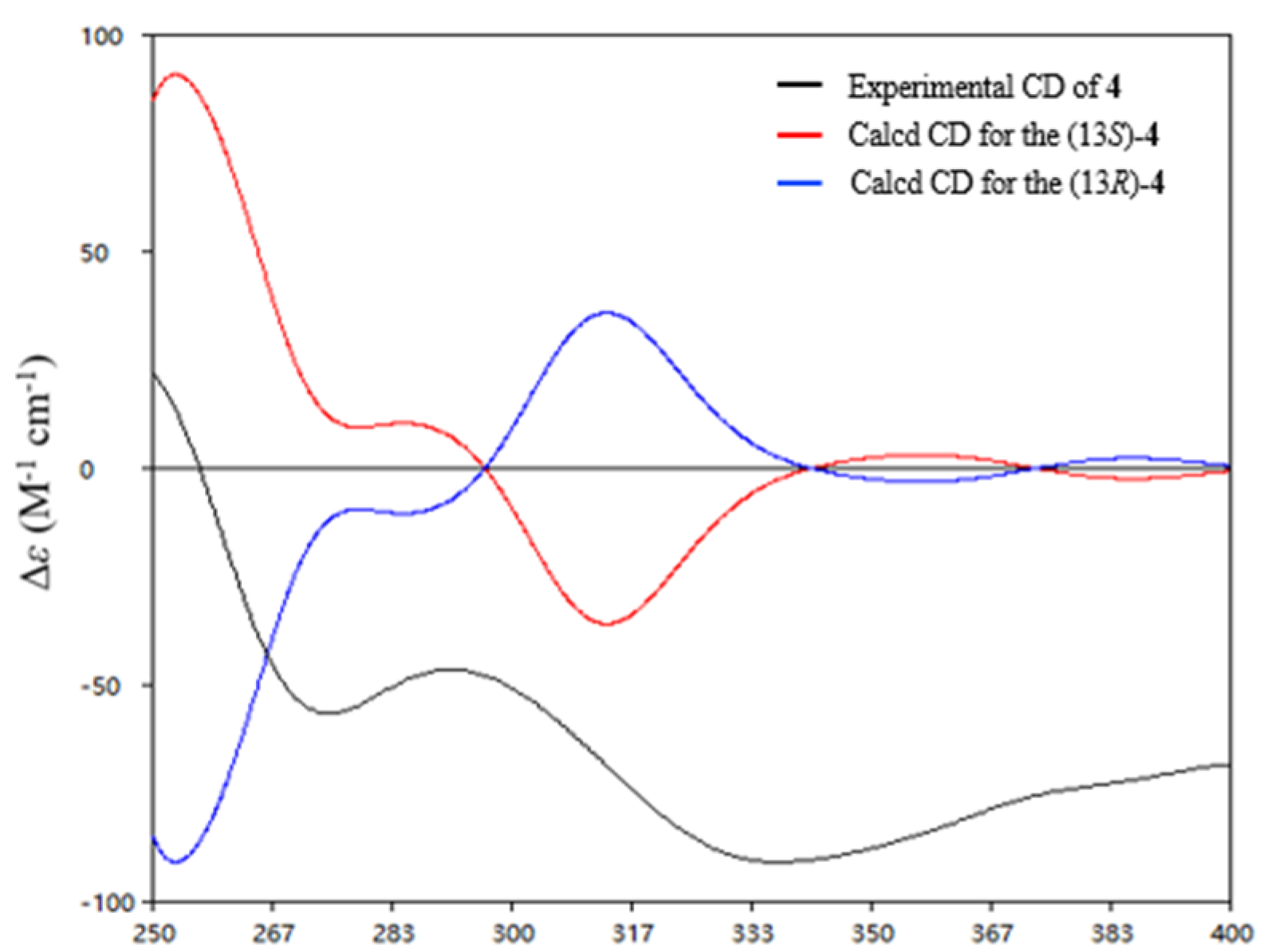
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Actinomycete Material and Fermentation
3.3. LC-MS/MS and Molecular Networking Analysis
3.4. Isolation and Purification of Compounds
3.5. Computation Section
3.6. Assay of Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, B.T.; Narender, T.; Kauffman, C.A. Saliniquinones A–F, new members of the highly cytotoxic anthraquinone-γ-pyrones from the marine actinomycete Salinispora arenicola. Cheminform 2010, 41, 929–934. [Google Scholar] [CrossRef]
- Maeda, K.; Takeuchi, T.; Nitta, K.; Yagishita, K.; Utahara, R.; Osato, T.; Ueda, M.; Kondo, S.; Okami, Y.; Umezawa, H. A new antitumor substance, pluramycin; studies on antitumor substances produced by Actinomycetes. J. Antibiot. 1956, 9, 75–81. [Google Scholar]
- Hsu, D.S.; Huang, J.Y. Total synthesis and determination of the absolute configuration of parimycin. Org. Lett. 2019, 21, 7665–7668. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Abdel-Mageed, W.M.; Ren, B.; He, W.; Huang, P.; Li, X.; Bolla, K.; Guo, H.; Chen, C.; Song, F.; et al. Endophytic Streptomyces sp. Y3111 from traditional Chinese medicine produced antitubercular pluramycins. Appl. Microbiol. Biotechnol. 2014, 98, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, R.W.; Davidson, B.S.; Montenegro, D.A. Gamma-indomycinone, a new pluramycin metabolite from a deep-sea derived actinomycete. J. Nat. Prod. 1995, 26, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Park, E.J.; Silva, R.R.; Kim, H.W.; Dorrestein, P.C.; Sung, S.H. Targeted isolation of neuroprotective dicoumaroyl neolignans and lignans from Sageretia theezans using in silico molecular network annotation propagation-based dereplication. J. Nat. Prod. 2018, 81, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, K.D.; Rixson, J.E.; Skelton, B.W.; Gericke, K.M.; Stewart, S.G. The total synthesis of heraclemycin B through β-ketosulfoxide and aldehyde annulation. Asian J. Org. Chem. 2015, 4, 936–942. [Google Scholar] [CrossRef]
- Ojiri, K.; Saito, K.; Nakajima, S.; Suda, H. Substance BE-26554 from Streptomyces A26554 as Neoplasm Inhibitors. JP 06228121, 16 August 1994. [Google Scholar]
- Zanardi, M.M.; Sarotti, A.M. Sensitivity analysis of DP4+ with the probability distribution terms: Development of a universal and customizable method. J. Org. Chem. 2021, 86, 8544–8548. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wu, G.; Sun, Z.; Zhang, X.; Che, Q.; Gu, Q.; Zhu, T.; Li, D.; Zhang, G. Cytotoxic tetrahydroxanthone dimers from the mangrove-associated fungus Aspergillus versicolor HDN1009. Mar. Drugs 2018, 16, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemoth. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabry, S.A.; Ghanem, N.B.; Abu-Ella, G.A. Nocardiopsis aegyptia sp. nov. isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2004, 54, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Spartan’14; Wavefunction Inc.: Irvine, CA, USA, 2013.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Hemberger, Y.; Schaumlöffel, A.; Bringmann, G. SpecDis, Version 1.53; University of Wuerzburg: Wuerzburg, Germany, 2011. [Google Scholar]
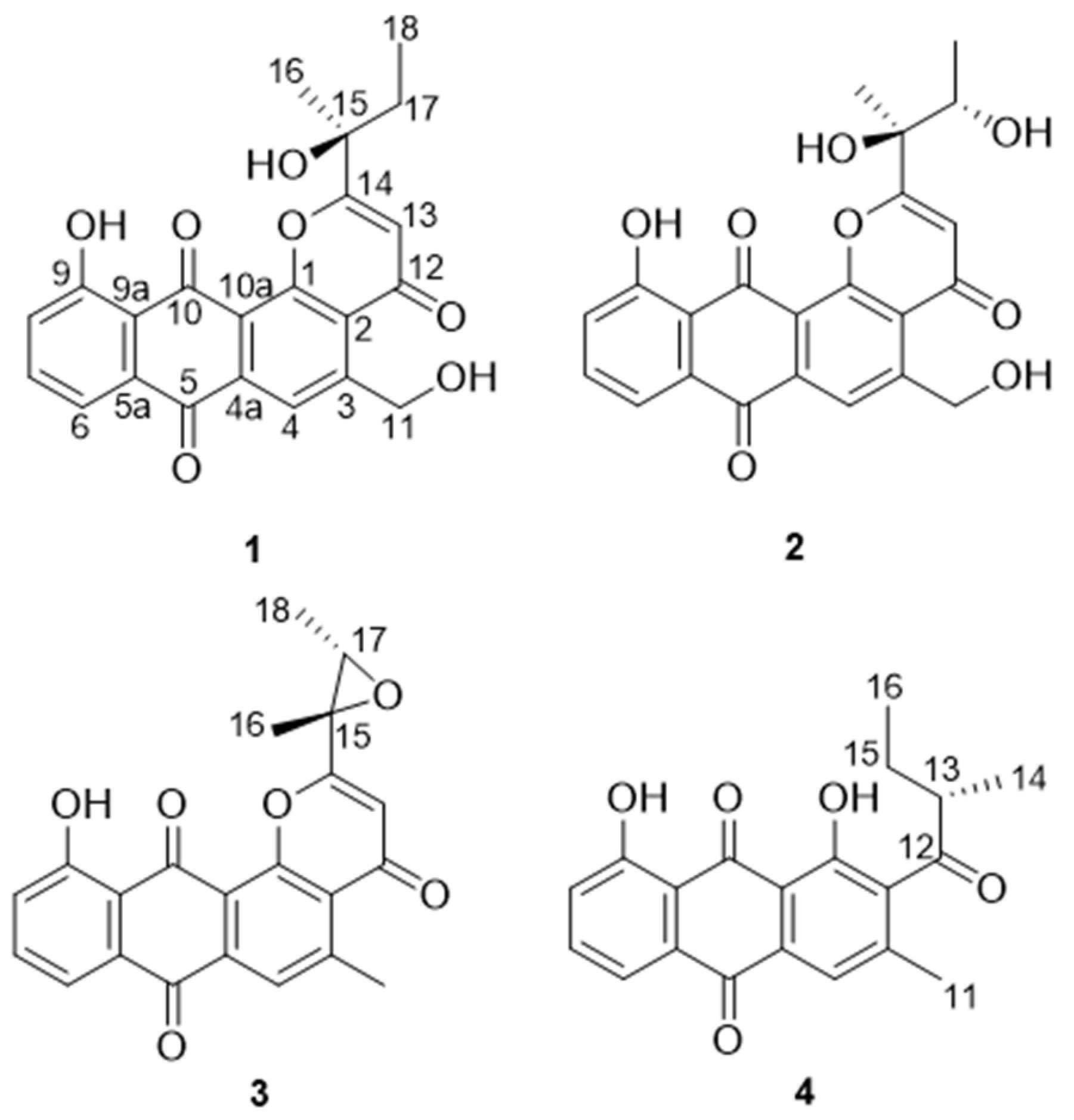
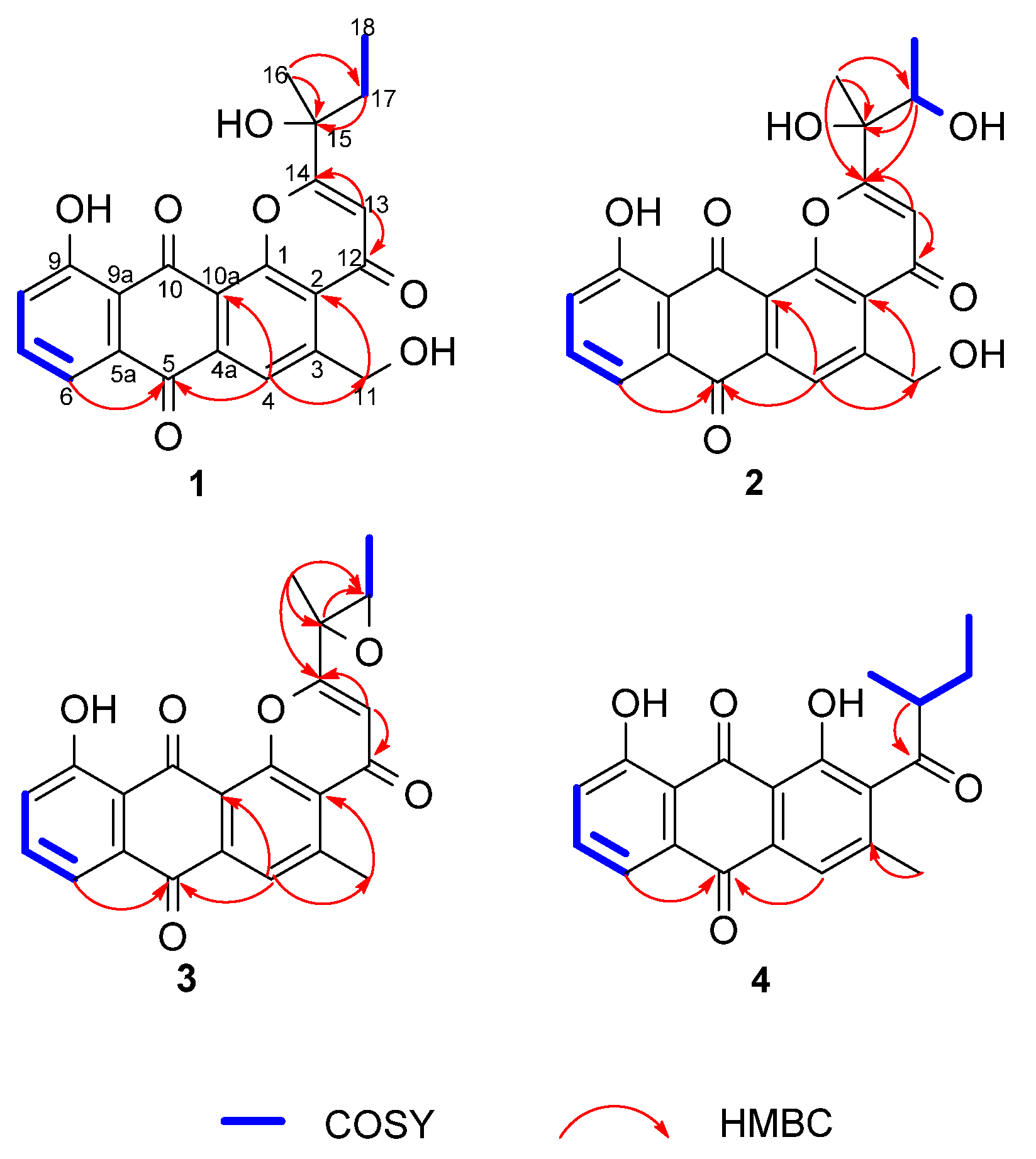
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 4 | 8.55, s | 8.55, s | 8.01, s | 7.60, s |
| 6 | 7.73, d (7.5) | 7.74, d (7.0) | 7.73, d (6.3) | 7.70, d (7.5) |
| 7 | 7.81, t (8.0) | 7.81, t (7.2) | 7.81, t (8) | 7.79, t (7.4) |
| 8 | 7.43, d (8.8) | 7.44, d (7.0) | 7.44, d (8.4) | 7.38, d (8.4) |
| 11 | 5.18, d (6.3) | 5.19, d (4.0) | 2.93, s | 2.28, s |
| 13 | 6.49, s | 6.54, s | 6.22, s | 3.00, m |
| 14 | - | - | - | 1.07, d (7.2) |
| 15 | - | - | - | 1.73, m, 1.34, m |
| 16 | 1.61, s | 1.51, s | 1.85, s | 0.88, t (7.5) |
| 17 | 1.84, m 2.07, m | 4.20, m | 3.48, q (5.0) | - |
| 18 11-OH 15-OH 17-OH | 0.83, t (7.5) 5.74, t 5.59, s - | 1.20, d (6.0) 5.73, t 5.42, s 4.67, d | 1.22, d (5.2) - - - | - - - - |
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 174.7, C | 174.9 C | 176.0, C | 158.7, C |
| 2 | 124.5, C | 124.6, C | 126.3, C | 145.5, C |
| 3 | 153.9, C | 153.9, C | 156.4, C | 136.0, C |
| 4 4a | 119.3, CH 120.5, C | 119.4, CH 120.5, C | 125.6, CH 120.5, C | 121.8, CH 114.9, C |
| 5 5a | 182.5, C 132.9, C | 182.3, C 132.9, C | 182.1, C 132.8, C | 181.7, C 133.9, C |
| 6 | 119.3, CH | 119.3, CH | 119.3, CH | 120.0, CH |
| 7 | 137.3, CH | 137.3, CH | 137.5, CH | 138.1, CH |
| 8 | 125.3, CH | 125.3, CH | 125.4, CH | 125.1, CH |
| 9 9a | 161.9, C 117.5, C | 163.3, C 117.4, C | 161.8, C 117.4, C | 161.9, C 116.6, C |
| 10 10a | 187.8, C 136.8, C | 187.7, C 136.7, C | 187.8, C 137.3, C | 192.3, C 153.2, C |
| 11 | 62.8, CH2 | 62.9, CH2 | 24.1, CH3 | 20.3, CH3 |
| 12 | 179.0, C | 179.1, C | 178.5, C | 208.9, C |
| 13 | 109.4, CH | 110.2, CH | 111.2, CH | 48.2, CH |
| 14 | 174.7, C | 174.9, C | 165.7, C | 15.0, CH3 |
| 15 | 73.3, C | 76.5, C | 59.9, C | 24.9, CH2 |
| 16 | 27.4, CH3 | 23.7, CH3 | 20.8, CH3 | 12.0, CH3 |
| 17 | 33.6, CH2 | 70.9, CH | 62.2, CH | - |
| 18 | 8.5, CH3 | 17.4, CH3 | 13.7, CH3 | - |
| Compd. | MIC (μM) | |||||
|---|---|---|---|---|---|---|
| MRCNS | B. subtilis | P. species | B. cereus | E. coli | M. Phlei | |
| 1 | 6.2 | 6.2 | 12.5 | 6.2 | 6.2 | 6.2 |
| 2 | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 | 3.1 |
| 3 | >50 | >50 | >50 | >50 | >50 | >50 |
| 4 | >50 | >50 | >50 | >50 | >50 | >50 |
| CPFX | 50 | 0.01 | 0.2 | 3.1 | 3.1 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Chen, X.; Sun, C.; Chang, Y.; Huang, X.; Zhu, T.; Zhang, G.; Che, Q.; Li, D. Saliniquinone Derivatives, Saliniquinones G−I and Heraclemycin E, from the Marine Animal-Derived Nocardiopsis aegyptia HDN19-252. Mar. Drugs 2021, 19, 575. https://doi.org/10.3390/md19100575
Zhou L, Chen X, Sun C, Chang Y, Huang X, Zhu T, Zhang G, Che Q, Li D. Saliniquinone Derivatives, Saliniquinones G−I and Heraclemycin E, from the Marine Animal-Derived Nocardiopsis aegyptia HDN19-252. Marine Drugs. 2021; 19(10):575. https://doi.org/10.3390/md19100575
Chicago/Turabian StyleZhou, Luning, Xuedong Chen, Chunxiao Sun, Yimin Chang, Xiaofei Huang, Tianjiao Zhu, Guojian Zhang, Qian Che, and Dehai Li. 2021. "Saliniquinone Derivatives, Saliniquinones G−I and Heraclemycin E, from the Marine Animal-Derived Nocardiopsis aegyptia HDN19-252" Marine Drugs 19, no. 10: 575. https://doi.org/10.3390/md19100575
APA StyleZhou, L., Chen, X., Sun, C., Chang, Y., Huang, X., Zhu, T., Zhang, G., Che, Q., & Li, D. (2021). Saliniquinone Derivatives, Saliniquinones G−I and Heraclemycin E, from the Marine Animal-Derived Nocardiopsis aegyptia HDN19-252. Marine Drugs, 19(10), 575. https://doi.org/10.3390/md19100575







