Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Cervical Cancer Cells Hela S3
Abstract
:1. Introduction
2. Results
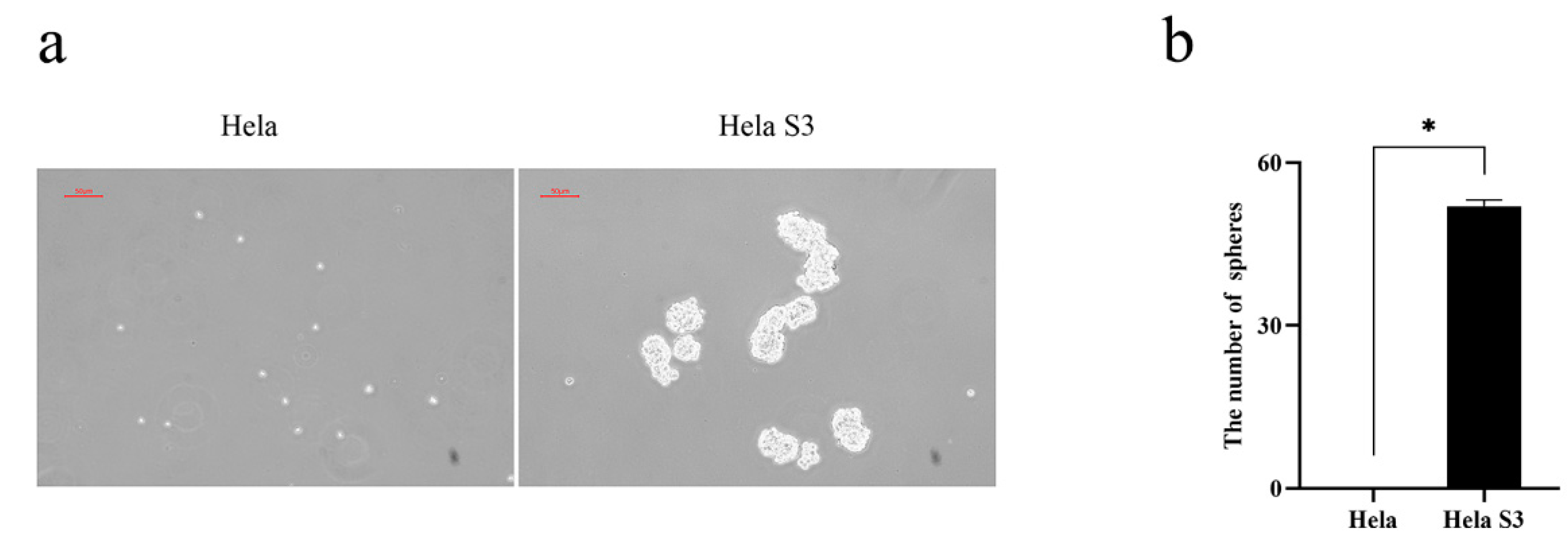
2.1. Tumorospheres Formation Assay
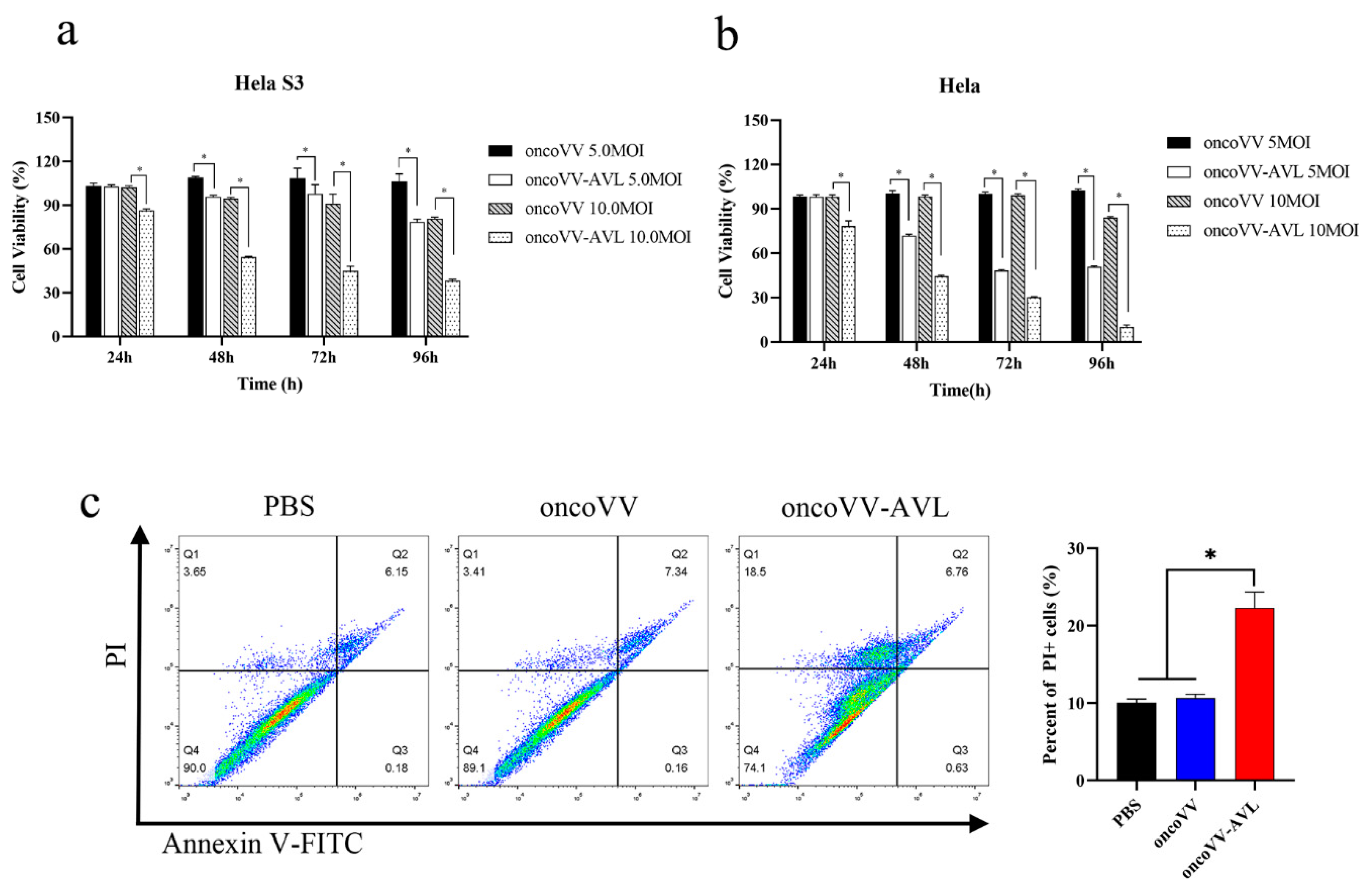
2.2. The Cytotoxic Effect of OncoVV-AVL on Cervical Cancer Cell Line Hela S3
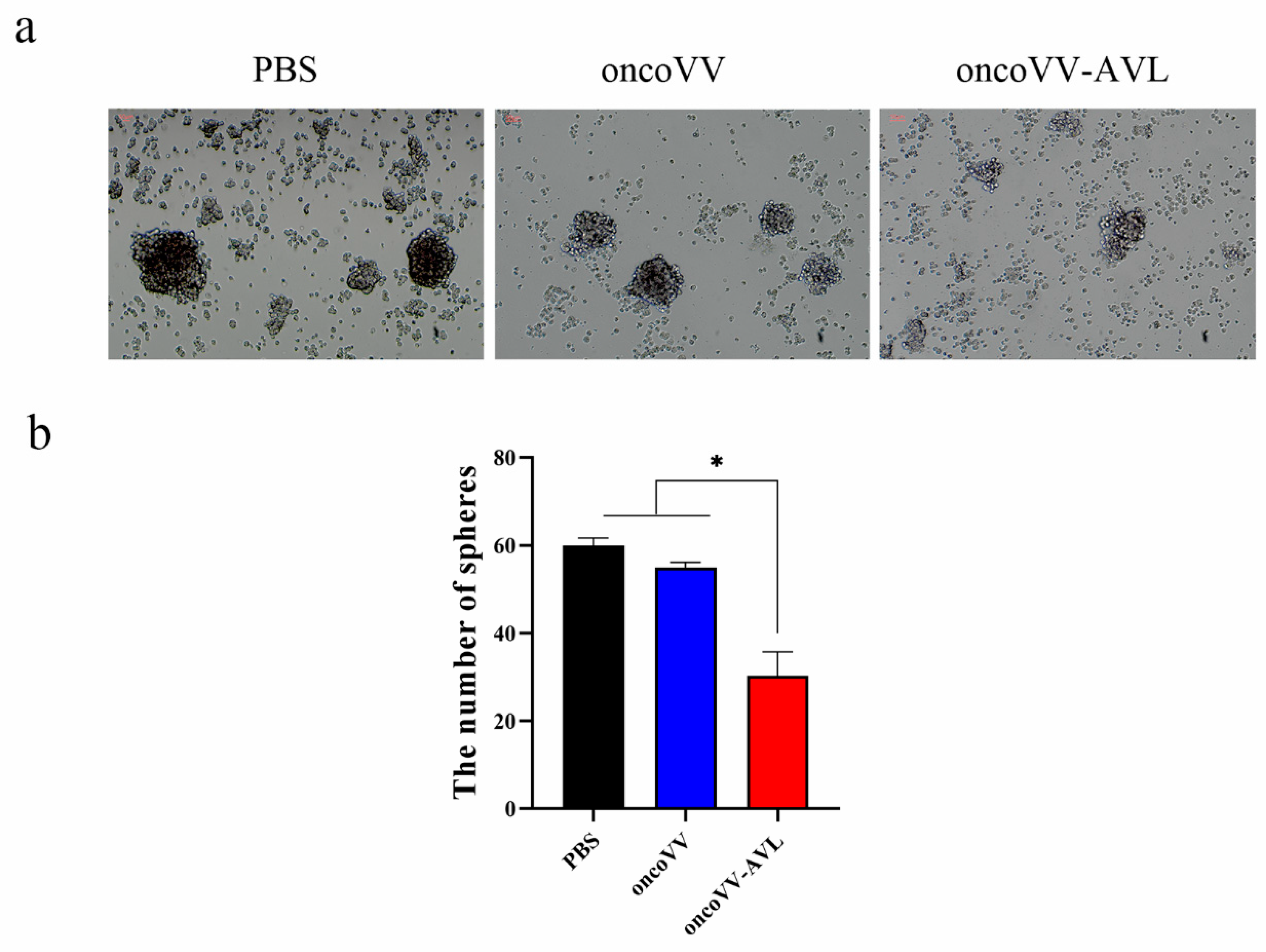
2.3. OncoVV-AVL Inhibited the Growth of Tumorospheres in Hela S3 Cells
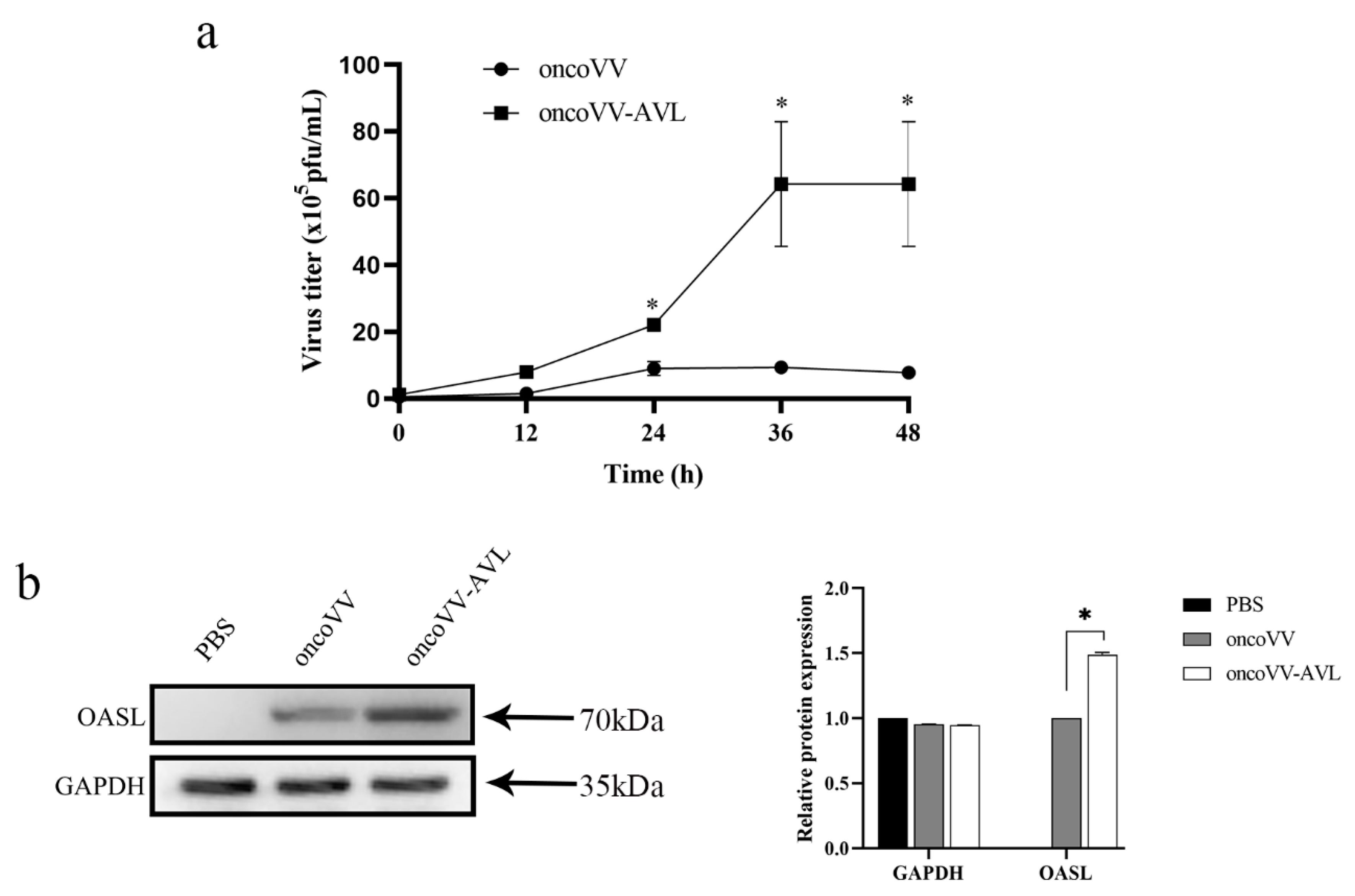
2.4. AVL Harboring Improved the Replication of Oncolytic Vaccinia Viruses in Hela S3 Cells
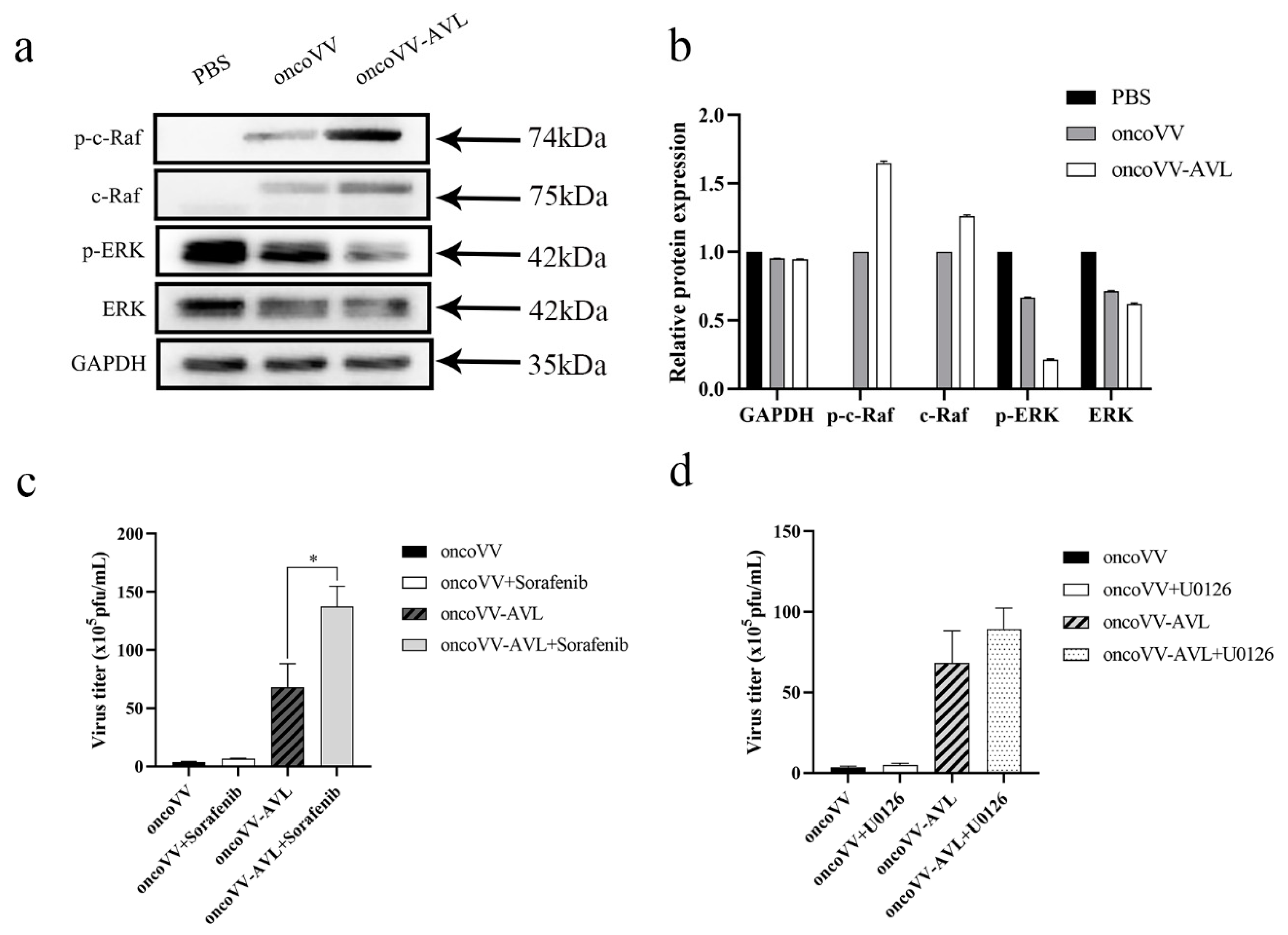
2.5. OncoVV-AVL Infection Altered the Raf/ERK Signaling Pathway in Hela S3 Cells
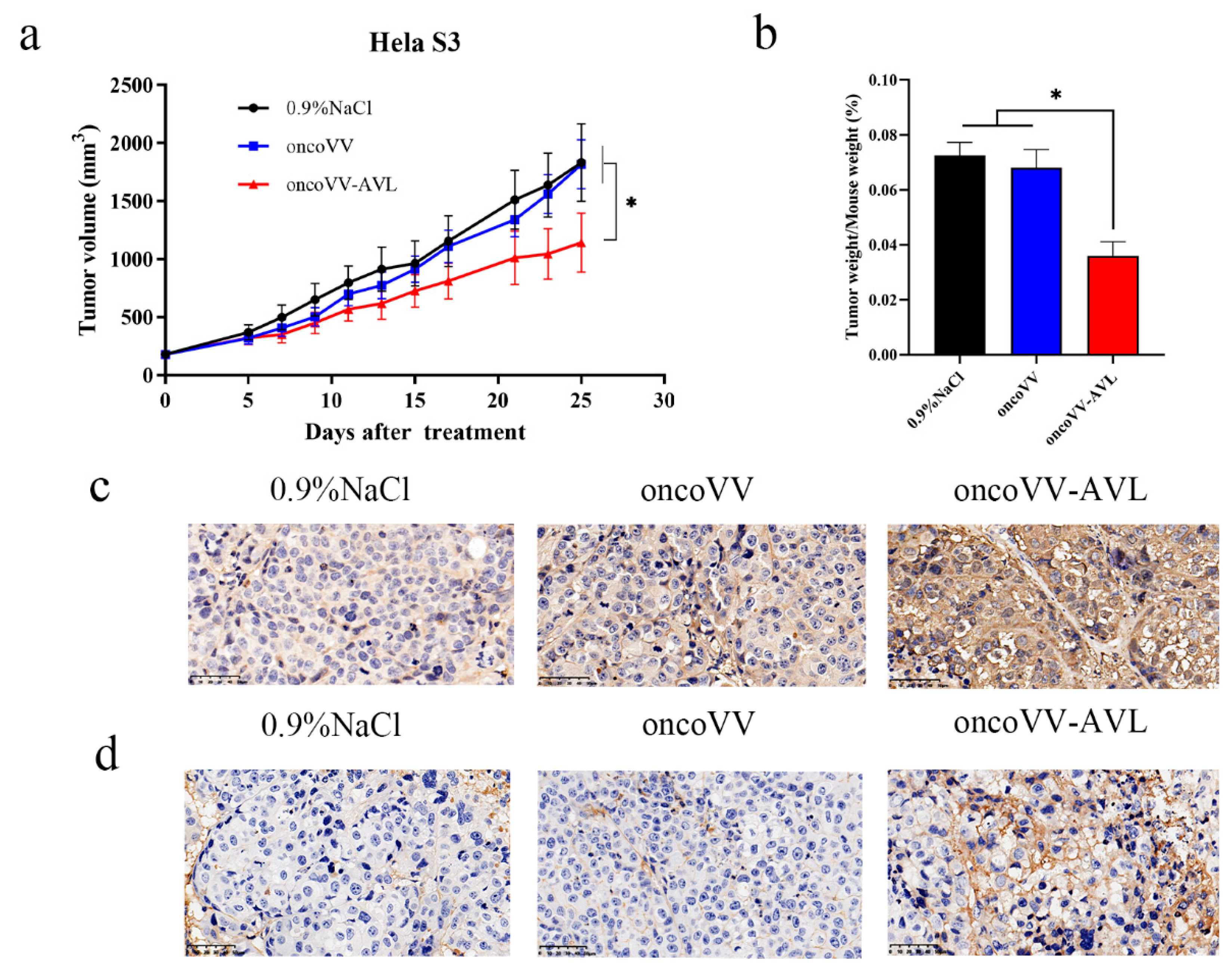
2.6. OncoVV-AVL Inhibited Tumor Growth in Mice
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Cell Proliferation and Cell Apoptosis Detection
4.3. Virus Replication Assay
4.4. Western Blot Analysis
4.5. Xenograft Tumor Model in Immunodeficient Mice
4.6. Immunohistochemistry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Verardi, P.H.; Titong, A.; Hagen, C.J. A vaccinia virus renaissance: New vaccine and immunotherapeutic uses after smallpox eradication. Hum. Vaccin. Immunother. 2012, 8, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Nemunaitis, J. Fighting Cancer with Vaccinia Virus: Teaching New Tricks to an Old Dog. Mol. Ther. 2005, 11, 180–195. [Google Scholar] [CrossRef]
- Kirn, D.H.; Thorne, S.H. Targeted and armed oncolytic poxviruses: A novel multi-mechanistic therapeutic class for cancer. Nat. Rev. Cancer 2009, 9, 64–71. [Google Scholar] [CrossRef]
- Ekeke, C.N.; Russell, K.L.; Joubert, K.; Bartlett, D.L.; Luketich, J.D.; Soloff, A.C.; Guo, Z.S.; Lotze, M.T.; Dhupar, R. Fighting Fire with Fire: Oncolytic Virotherapy for Thoracic Malignancies. Ann. Surg. Oncol. 2021, 28, 2715–2727. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, J.; Park, B.; Lee, D.; Park, H.; Roh, M.; Je, J.; Yoon, J.; Thorne, S.; Kirn, D.; et al. Systemic Armed Oncolytic and Immunologic Therapy for Cancer with JX-594, a Targeted Poxvirus Expressing GM-CSF. Mol. Ther. 2006, 14, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liang, C.; Yu, Y.A.; Chen, N.; Dandekar, T.; Szalay, A.A. The highly attenuated oncolytic recombinant vaccinia virus GLV-1h68: Comparative genomic features and the contribution of F14.5L inactivation. Mol. Genet. Genom. 2009, 282, 417–435. [Google Scholar] [CrossRef] [Green Version]
- Fend, L.; Remy-Ziller, C.; Foloppe, J.; Kempf, J.; Cochin, S.; Barraud, L.; Accart, N.; Erbs, P.; Fournel, S.; Preville, X. Oncolytic virotherapy with an armed vaccinia virus in an orthotopic model of renal carcinoma is associated with modification of the tumor microenvironment. OncoImmunology 2015, 5, e1080414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.B.; Evdokimov, N.M.; Lefranc, F.; Valentão, P.; Kornienko, A.; Pereira, D.M.; Andrade, P.B.; Gomes, N.G.M. Marine-Derived Anticancer Agents: Clinical Benefits, Innovative Mechanisms, and New Targets. Mar. Drugs 2019, 17, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Xie, X.; Liu, Y.; He, J.; Benitez, R.; Buckanovich, R.J.; Lubman, D.M. Identification and Confirmation of Differentially Expressed Fucosylated Glycoproteins in the Serum of Ovarian Cancer Patients Using a Lectin Array and LC–MS/MS. J. Proteome Res. 2012, 11, 4541–4552. [Google Scholar] [CrossRef]
- Fry, S.A.; Afrough, B.; Lomax-Browne, H.J.; Timms, J.; Velentzis, L.S.; Leathem, A.J.C. Lectin microarray profiling of metastatic breast cancers. Glycobiology 2011, 21, 1060–1070. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.P.; Yang, M.C.; Liu, H.S.; Lin, Y.S.; Lei, H.Y. Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma model. Hepatology 2007, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Terada, D.; Kawai, F.; Noguchi, H.; Unzai, S.; Hasan, I.; Fujii, Y.; Park, S.-Y.; Ozeki, Y.; Tame, J.R.H. Crystal structure of MytiLec, a galactose-binding lectin from the mussel Mytilus galloprovincialis with cytotoxicity against certain cancer cell types. Sci. Rep. 2016, 6, 28344. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Sugawara, S.; Fujii, Y.; Koide, Y.; Terada, D.; Iimura, N.; Fujiwara, T.; Takahashi, K.G.; Kojima, N.; Rajia, S.; et al. MytiLec, a Mussel R-Type Lectin, Interacts with Surface Glycan Gb3 on Burkitt’s Lymphoma Cells to Trigger Apoptosis through Multiple Pathways. Mar. Drugs 2015, 13, 7377–7389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gundacker, D.; Leys, S.; Schröder, H.C.; Müller, I.M.; Müller, W.E.G. Isolation and cloning of a C-type lectin from the hexactinellid sponge Aphrocallistes vastus: A putative aggregation factor. Glycobiology 2001, 11, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Xiang, Y.; Liu, T.; Wang, X.; Ren, X.; Ye, T.; Li, G. Oncolytic Vaccinia Virus Expressing Aphrocallistes vastus Lectin as a Cancer Therapeutic Agent. Mar. Drugs 2019, 17, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Qi, C.; He, X. Stem cell protein Piwil1 endowed endometrial cancer cells with stem-like properties via inducing epithelial-mesenchymal transition. BMC Cancer 2015, 15, 811. [Google Scholar]
- Shen, C.; Hu, G.; Zhang, S.; Ao, X.; Zhou, Q.; Xiao, P.; Zhong, Y. Immunophenotypic characterization of sphere-forming cells derived from the human renal cell carcinoma cell line 786-O. Am. J. Transl. Res. 2018, 10, 3978–3990. [Google Scholar]
- Chen, S.; Zhang, W.; Wu, Z.; Zhang, J.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Sun, K.; Cheng, A.; et al. Goose Mx and OASL Play Vital Roles in the Antiviral Effects of Type I, II, and III Interferon against Newly Emerging Avian Flavivirus. Front. Immunol. 2017, 8, 1006. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.E.; Lee, M.S.; Kim, Y.-J.; Lee, H.K. OASL1 deficiency promotes antiviral protection against genital herpes simplex virus type 2 infection by enhancing type I interferon production. Sci. Rep. 2016, 6, 19089. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zhang, Y.; Ghosh, A.; Cuevas, R.; Forero, A.; Dhar, J.; Ibsen, M.; Schmid-Burgk, J.; Schmidt, T.; Ganapathiraju, M.; et al. Antiviral Activity of Human OASL Protein Is Mediated by Enhancing Signaling of the RIG-I RNA Sensor. Immunity 2014, 40, 936–948. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Shao, L.; Sampath, P.; Zhao, B.; Patel, N.V.; Zhu, J.; Behl, B.; Parise, R.A.; Beumer, J.H.; O’Sullivan, R.J.; et al. Oligoadenylate-Synthetase-Family Protein OASL Inhibits Activity of the DNA Sensor cGAS during DNA Virus Infection to Limit Interferon Production. Immunity 2019, 50, 51–63.e5. [Google Scholar] [CrossRef] [Green Version]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Lee, C. Extracellular signal-regulated kinase (ERK) activation is required for porcine epidemic diarrhea virus replication. Virology 2015, 484, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Abbot, A. Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef]
- Ivascu, A.; Kubbies, M. Rapid Generation of Single-Tumor Spheroids for High-Throughput Cell Function and Toxicity Analysis. J. Biomol. Screen. 2006, 11, 922–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and In vitro Propagation of Tumorigenic Breast Cancer Cells with Stem/Progenitor Cell Properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar] [CrossRef] [Green Version]
- Vitiani, L.R.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2006, 445, 111–115. [Google Scholar] [CrossRef]
- Zhang, S.; Balch, C.; Chan, M.; Lai, H.-C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.-M.; Nephew, K.P. Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef] [Green Version]
- Molina, J.R.; Adjei, A.A. The Ras/Raf/MAPK pathway. J. Thorac. Oncol. 2006, 1, 7–9. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, J.; Feng, H.; Xu, X.; Liu, T.; Ye, T.; Chen, K.; Li, G. Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Cervical Cancer Cells Hela S3. Mar. Drugs 2021, 19, 532. https://doi.org/10.3390/md19100532
Ni J, Feng H, Xu X, Liu T, Ye T, Chen K, Li G. Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Cervical Cancer Cells Hela S3. Marine Drugs. 2021; 19(10):532. https://doi.org/10.3390/md19100532
Chicago/Turabian StyleNi, Jiajun, Hualin Feng, Xiang Xu, Tingting Liu, Ting Ye, Kan Chen, and Gongchu Li. 2021. "Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Cervical Cancer Cells Hela S3" Marine Drugs 19, no. 10: 532. https://doi.org/10.3390/md19100532
APA StyleNi, J., Feng, H., Xu, X., Liu, T., Ye, T., Chen, K., & Li, G. (2021). Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Cervical Cancer Cells Hela S3. Marine Drugs, 19(10), 532. https://doi.org/10.3390/md19100532




