Flavonoid Glycosides with a Triazole Moiety for Marine Antifouling Applications: Synthesis and Biological Activity Evaluation
Abstract
1. Introduction
2. Results and Discussion
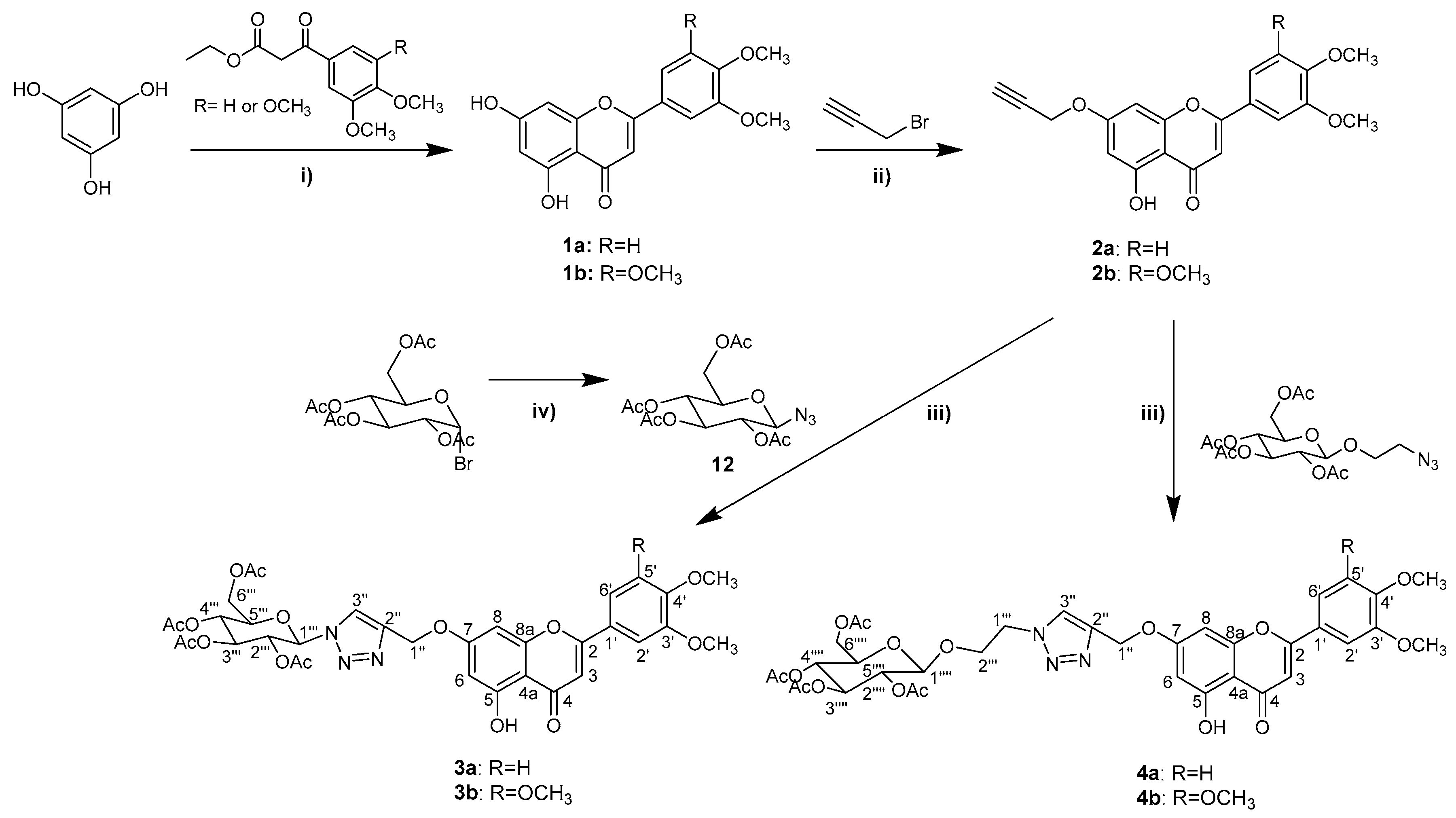
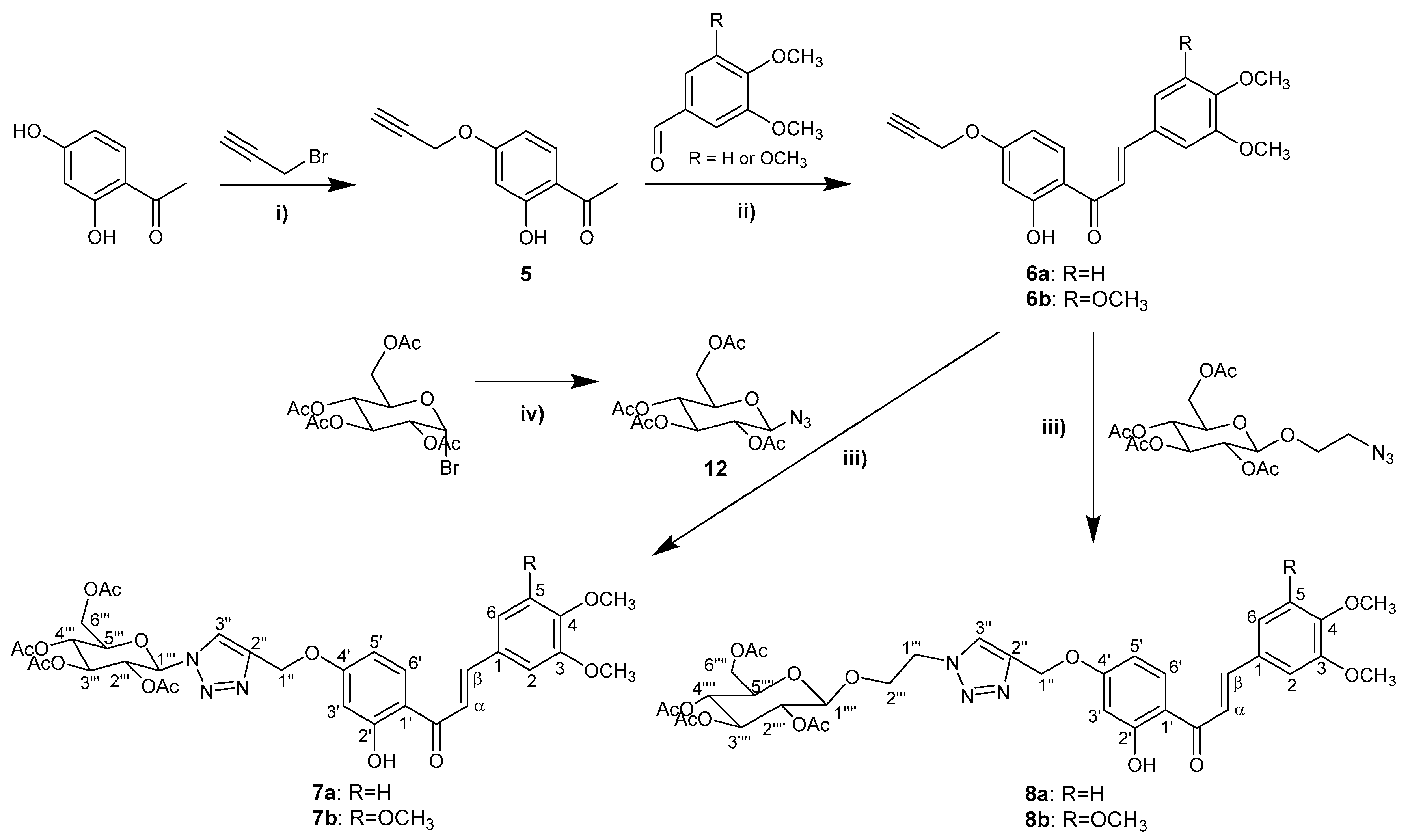
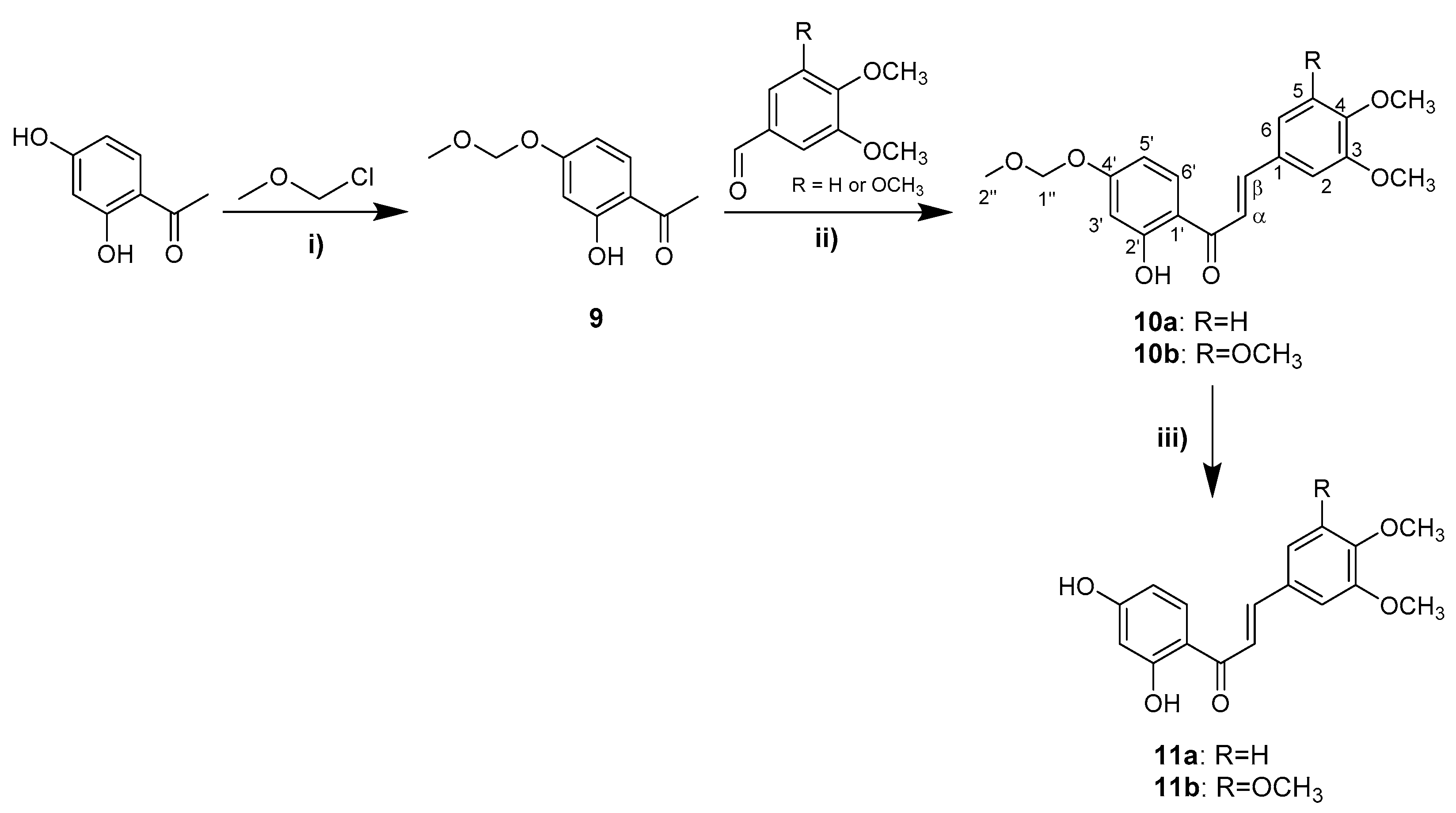
2.1. Synthesis and Structure Elucidation
2.2. Mussel (Mytilus galloprovincialis) Larvae Anti-Settlement Activity
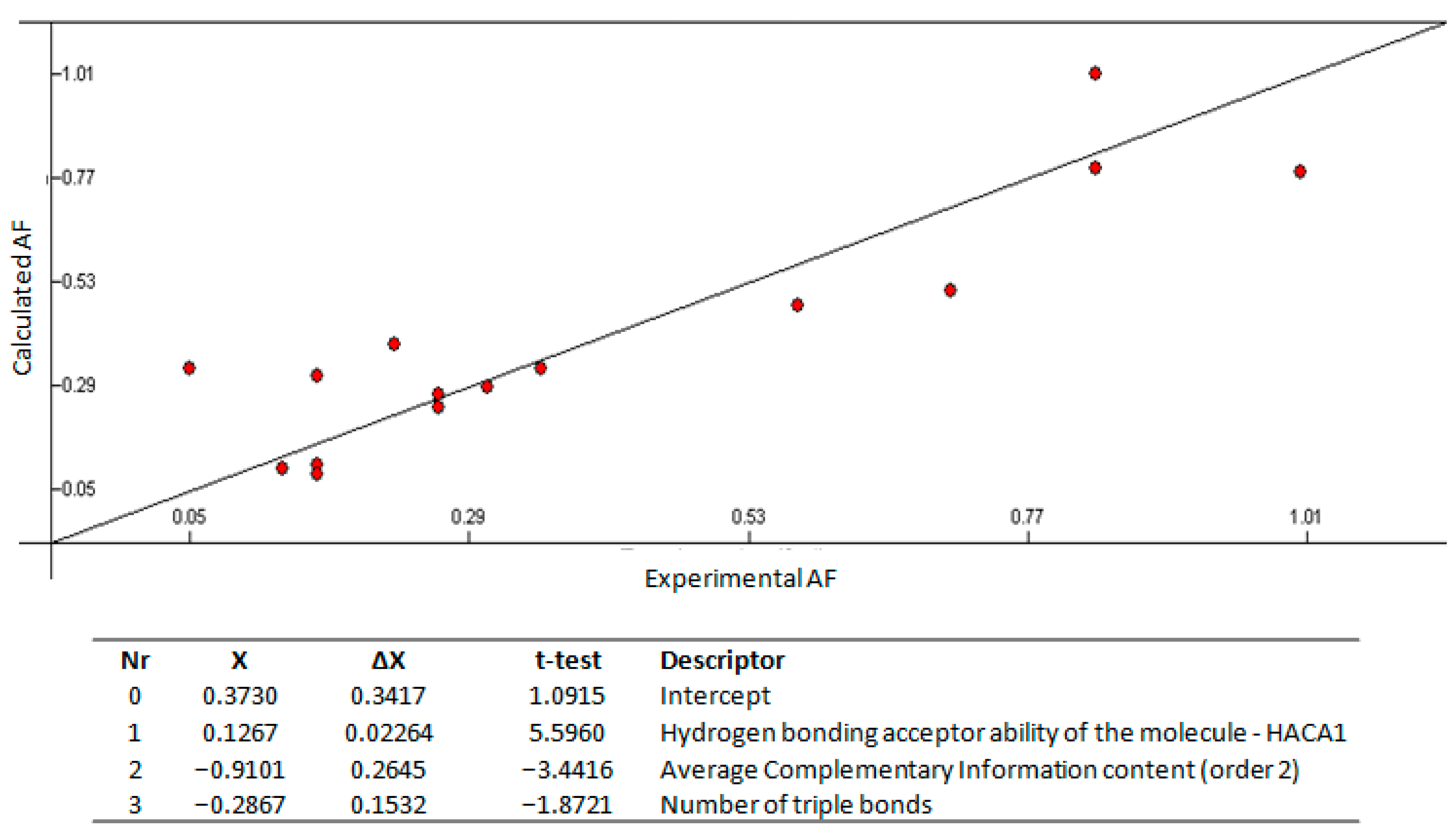
2.3. Quantitative Structure—Activity Relationship
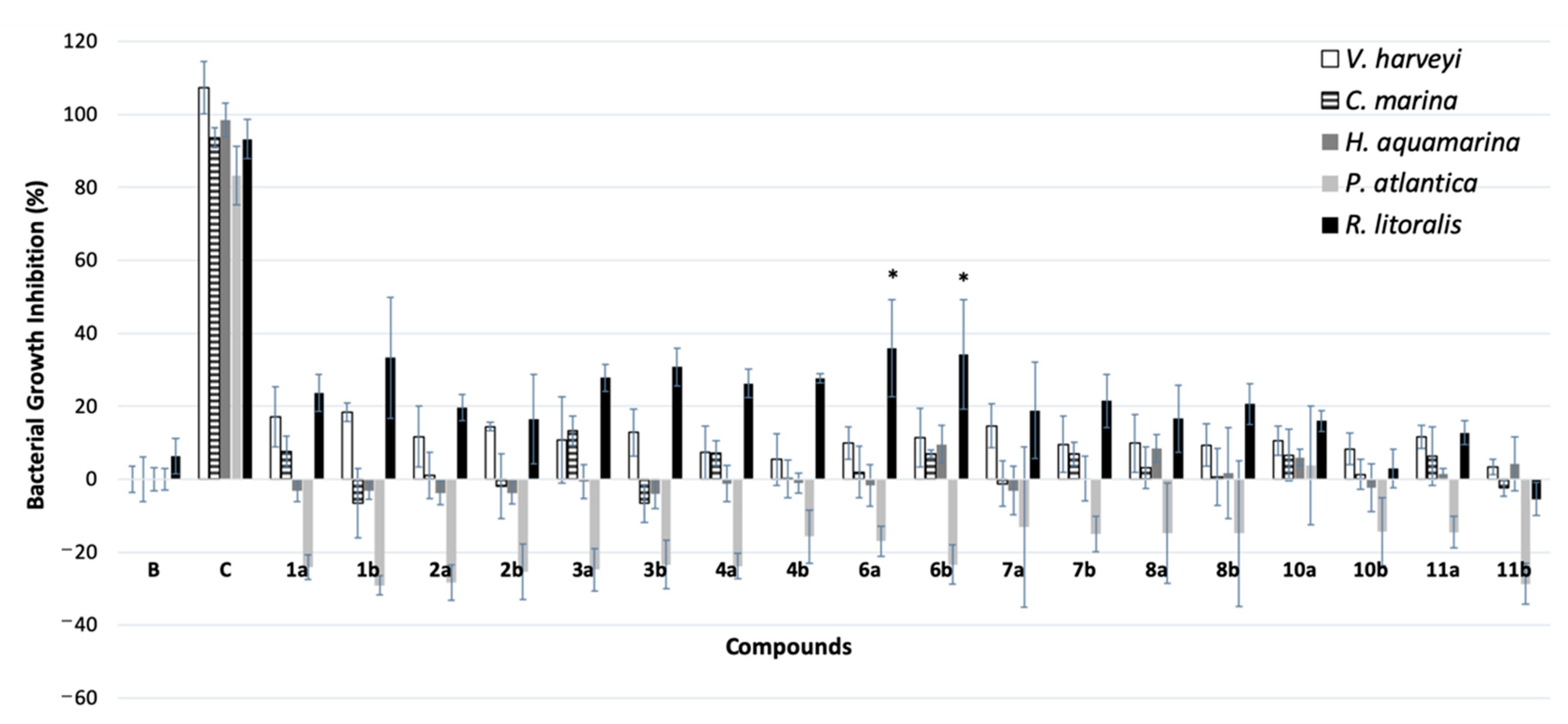
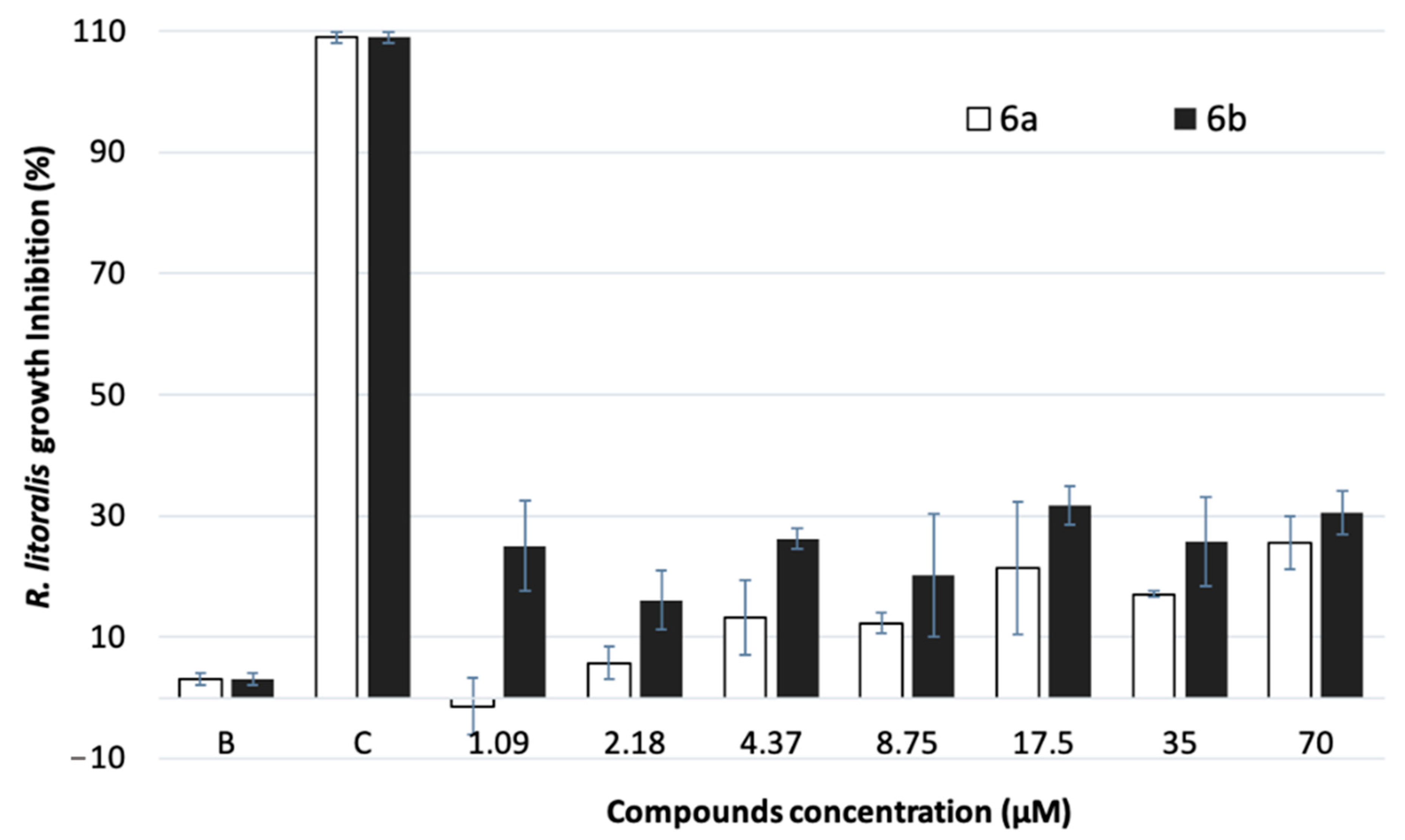
2.4. Biofilm-Forming Marine Bacteria Growth Inhibitory Activity
2.5. Biofilm—Forming Marine Diatoms Growth Inhibitory Activity
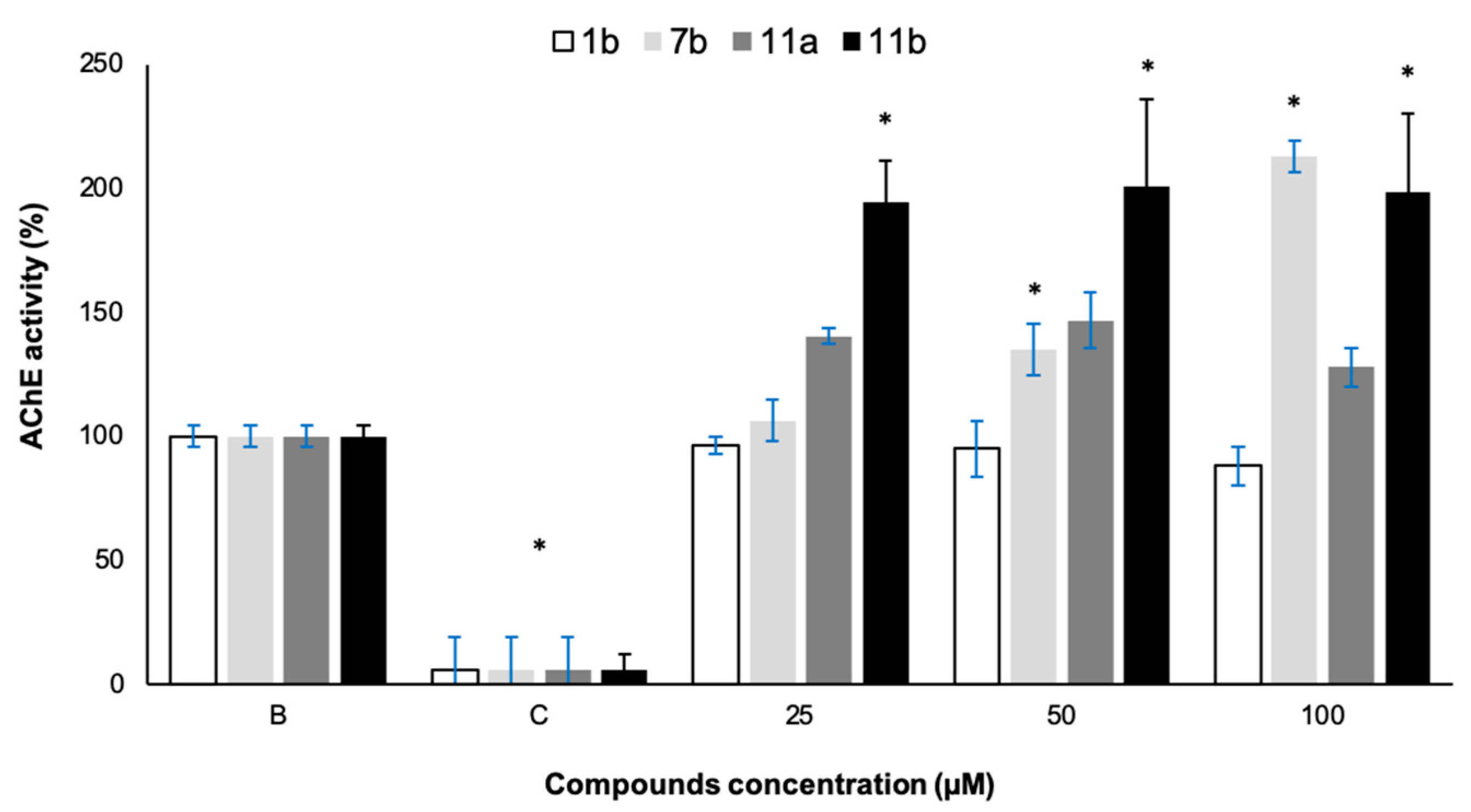
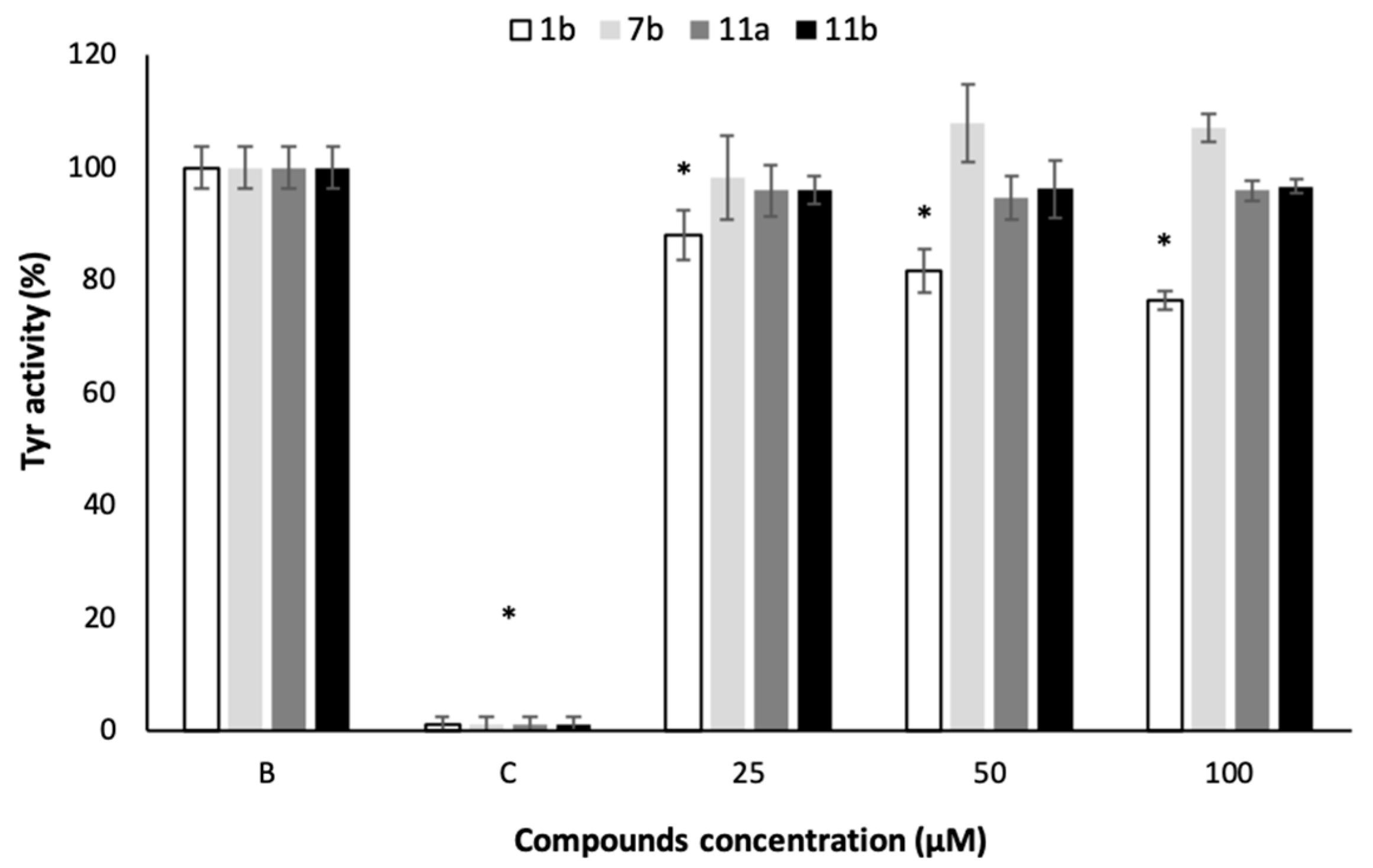
2.6. In Vitro Acetylcholinesterase (AChE) and Tyrosinase (Tyr) Activities
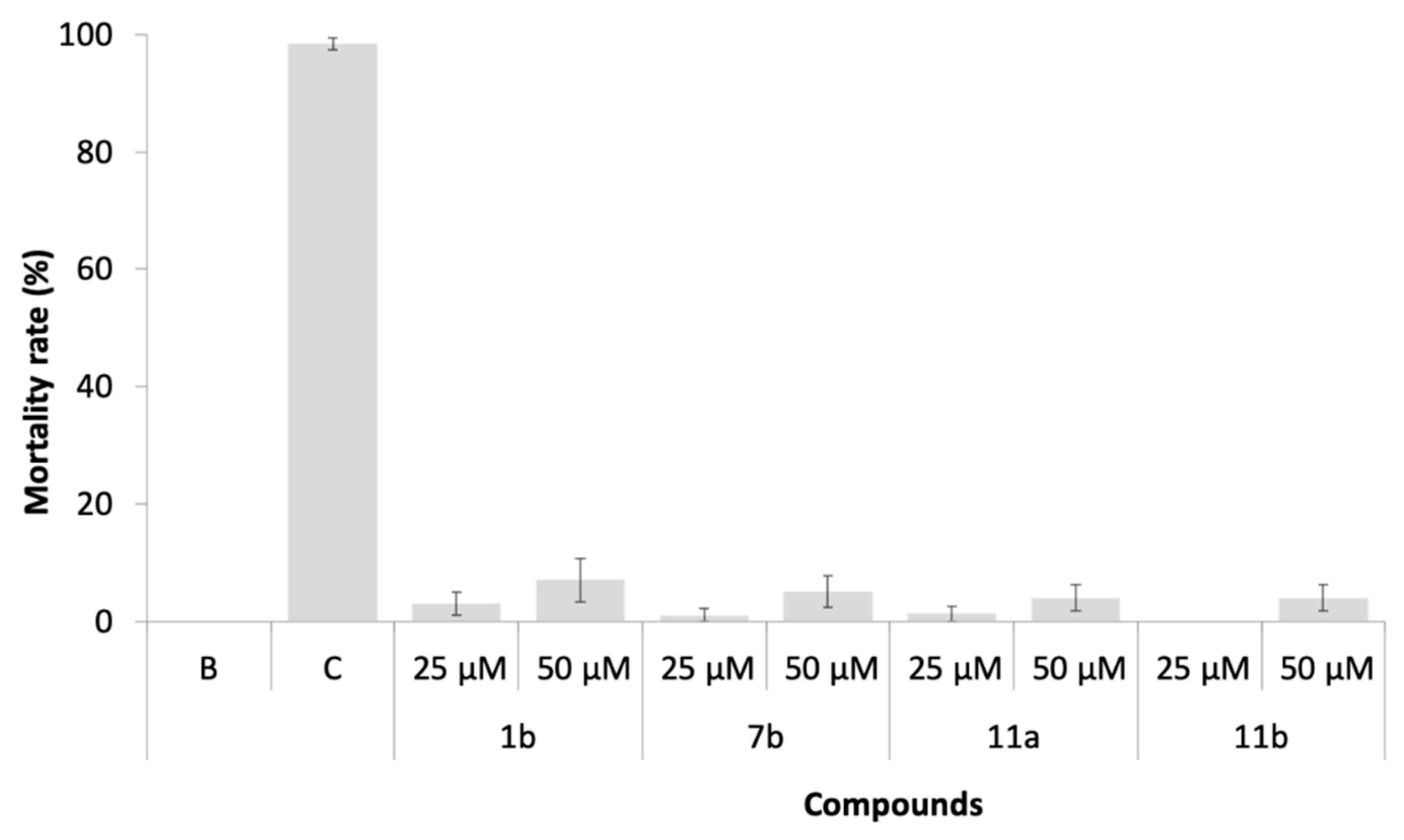
2.7. Environmental Fate Parameters: Artemia Salina Ecotoxicity Bioassay
3. Materials and Methods
3.1. Synthesis and Structure Elucidation of Chalcones and Flavones
3.1.1. Synthesis of Flavones 1a and 1b
3.1.2. Synthesis of 7-O-Propargylflavones 2a and 2b
3.1.3. Synthesis of Flavone-Triazolyl-Glycosides 3a and 3b
3.1.4. Synthesis of Flavone-Triazolyl-Glycosides 4a and 4b
3.1.5. Synthesis of Propargyloxyacetophenone 5
3.1.6. Synthesis of Propargyloxychalcones 6a and 6b
3.1.7. Synthesis of Chalcone-Triazolyl-Glycosides 7a and 7b
3.1.8. Synthesis of Chalcone-Triazolyl-Glycosides 8a and 8b
3.1.9. Synthesis of Acetophenone 9
3.1.10. Synthesis of Chalcones 10a and 10b
3.1.11. Synthesis of Chalcones 11a and 11b
3.1.12. Synthesis of 2,3,4,6-Tetra-O-Acetyl-β-D-Glucopyranosyl Azide (12)
3.2. Mussel (Mytilus galloprovincialis) Larvae Anti-Settlement Activity
3.3. Quantitative Structure–Activity Relationship
3.4. Inhibitory Activity against Biofilm-Forming Marine Bacteria Growth
3.5. Inhibitory Activity against Biofilm-Forming Marine Diatom Growth
3.6. In Vitro Acetylcholinesterase (AChE) and Tyrosinase (Tyr) Activities
3.7. Environmental Fate Parameters: Artemia Salina Ecotoxicity Bioassay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Wu, Z.-H.; Wang, Y.; Wang, C.-Y.; Xu, Y. Mini-Review: Antifouling Natural Products from Marine Microorganisms and Their Synthetic Analogs. Mar. Drugs 2017, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Dubalska, K.; Rutkowska, M.; Bajger-Nowak, G.; Konieczka, P.; Namieśnik, J. Organotin Compounds: Environmental Fate and Analytics. Crit. Rev. Anal. Chem. 2013, 43, 35–54. [Google Scholar] [CrossRef]
- Bao, V.W.W.; Leung, K.M.Y.; Lui, G.C.S.; Lam, M.H.W. Acute and chronic toxicities of Irgarol alone and in combination with copper to the marine copepod Tigriopus japonicus. Chemosphere 2013, 90, 1140–1148. [Google Scholar] [CrossRef]
- Kyei, S.K.; Darko, G.; Akaranta, O. Chemistry and application of emerging ecofriendly antifouling paints: A review. J. Coat. Technol. Res. 2020, 17, 315–332. [Google Scholar] [CrossRef]
- Rossini, P.; Napolano, L.; Matteucci, G. Biotoxicity and life cycle assessment of two commercial antifouling coatings in marine systems. Chemosphere 2019, 237, 124475. [Google Scholar] [CrossRef]
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: Chemistry and biological activities. Nat. Prod. Res. 2019, 33, 3260–3272. [Google Scholar] [CrossRef]
- Almeida, J.R.; Correia-da-Silva, M.; Sousa, E.; Antunes, J.; Pinto, M.; Vasconcelos, V.; Cunha, I. Antifouling potential of Nature-inspired sulfated compounds. Sci. Rep. 2017, 7, 42424. [Google Scholar] [CrossRef]
- Almeida, J.R.; Moreira, J.; Pereira, D.; Pereira, S.; Antunes, J.; Palmeira, A.; Vasconcelos, V.; Pinto, M.; Correia-da-Silva, M.; Cidade, H. Potential of synthetic chalcone derivatives to prevent marine biofouling. Sci. Total Environ. 2018, 643, 98–106. [Google Scholar] [CrossRef]
- Trojer, M.A.; Movahedi, A.; Blanck, H.; Nydén, M. Imidazole and Triazole Coordination Chemistry for Antifouling Coatings. J. Chem. 2013, 2013, 23. [Google Scholar]
- Hou, J.; Liu, X.; Shen, J.; Zhao, G.; Wang, P.G. The impact of click chemistry in medicinal chemistry. Expert Opin. Drug Discov. 2012, 7, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Totobenazara, J.; Burke, A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Ma, N.; Wang, Y.; Zhao, B.X.; Ye, W.C.; Jiang, S. The application of click chemistry in the synthesis of agents with anticancer activity. Drug Des. Devel Ther. 2015, 9, 1585–1599. [Google Scholar]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Andjouh, S.; Blache, Y. Screening of bromotyramine analogues as antifouling compounds against marine bacteria. Biofouling 2016, 32, 871–881. [Google Scholar] [CrossRef]
- Kantheti, S.; Narayan, R.; Raju, K.V.S.N. The impact of 1,2,3-triazoles in the design of functional coatings. RSC Adv. 2015, 5, 3687–3708. [Google Scholar] [CrossRef]
- Seijas, J.A.; Vazquez-Tato, M.P.; Carballido-Reboredo, R. Solvent-free synthesis of functionalized flavones under microwave irradiation. J. Org. Chem 2005, 70, 2855–2858. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord Chem Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, L.; Hong, G.; Yang, X.; Liu, T. Design, synthesis and anticancer activity of matrine-1H-1,2,3-triazole-chalcone conjugates. Bioorg. Med. Chem. Lett. 2015, 25, 2540–2544. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Khloya, P.; Seo, Y.; Kumar, S.; Lee, H.K.; Jeon, D.-K.; Jo, S.; Sharma, P.K.; Namkung, W. Potentiation of ΔF508- and G551D-CFTR-Mediated Cl- Current by Novel Hydroxypyrazolines. PLoS ONE 2016, 11, e0149131. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Fu, X.L.; Yang, N.; Wang, Q.A. Synthesis and cytotoxicity of chalcones and 5-deoxyflavonoids. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Loureiro, D.R.P.; Magalhães, Á.F.; Soares, J.X.; Pinto, J.; Azevedo, C.M.G.; Vieira, S.; Henriques, A.; Ferreira, H.; Neves, N.; Bousbaa, H.; et al. Yicathins B and C and Analogues: Total Synthesis, Lipophilicity and Biological Activities. ChemMedChem 2020, 15, 749–755. [Google Scholar] [CrossRef]
- Adesoye, O.G.; Mills, I.N.; Temelkoff, D.P.; Jackson, J.A.; Norris, P. Synthesis of a d-Glucopyranosyl Azide: Spectroscopic Evidence for Stereochemical Inversion in the SN2 Reaction. J. Chem. Educ. 2012, 89, 943–945. [Google Scholar] [CrossRef]
- Briand, J.F. Marine antifouling laboratory bioassays: An overview of their diversity. Biofouling 2009, 25, 297–311. [Google Scholar] [CrossRef]
- Kojima, R.; Kobayashi, S.; Satuito, C.G.P.; Katsuyama, I.; Ando, H.; Seki, Y.; Senda, T. A Method for Evaluating the Efficacy of Antifouling Paints Using Mytilus galloprovincialis in the Laboratory in a Flow-Through System. PLoS ONE 2016, 11, e0168172. [Google Scholar] [CrossRef]
- Aldred, N.; Ista, L.K.; Callow, M.E.; Callow, J.A.; Lopez, G.P.; Clare, A.S. Mussel (Mytilus edulis) byssus deposition in response to variations in surface wettability. J. R Soc. Interface 2006, 3, 37–43. [Google Scholar] [CrossRef]
- Qian, P.Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Dudek, A.Z.; Arodz, T.; Gálvez, J. Computational methods in developing quantitative structure-activity relationships (QSAR): A review. Comb. Chem. High. Throughput Screen. 2006, 9, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.J.; Hopfinger, A.J. 3D QSAR of Flexible Molecules Using Tensor Representation; Kluwer Academic Publishers: NewYork, NY, USA, 2002; Volume 3. [Google Scholar]
- Kubinyi, H. QSAR: Hansch Analysis and Related Approaches; Wiley-VCH: Weinhem, Germany, 1993; Volume 1. [Google Scholar]
- Veerasamy, R.; Rajak, H.; Jain, A.; Sivadasan, S.; Christapher, P.V.; Agrawal, R. Validation of QSAR Models-Strategies and Importance. Int. J. Drug Design Discov. 2011, 2, 511–519. [Google Scholar]
- Alexander, D.L.J.; Tropsha, A.; Winkler, D.A. Beware of R(2): Simple, Unambiguous Assessment of the Prediction Accuracy of QSAR and QSPR Models. J. Chem. Inf. Model. 2015, 55, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Long, W. Current Mathematical Methods Used in QSAR/QSPR Studies. Int. J. Mol. Sci. 2009, 10, 1978–1998. [Google Scholar] [CrossRef] [PubMed]
- Gramatica, P. On the Development and Validation of QSAR Models. In Computational Toxicology: Volume II; Reisfeld, B., Mayeno, A.N., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 499–526. [Google Scholar]
- Golbraikh, A.; Shen, M.; Xiao, Z.; Xiao, Y.-D.; Lee, K.-H.; Tropsha, A. Rational selection of training and test sets for the development of validated QSAR models. J. Comput.-Aided Mol. Des. 2003, 17, 241–253. [Google Scholar] [CrossRef]
- OECD. Guidance Document on the Validation of (Quantitative) Structure-Activity Relationships [(Q)SAR] Models; OECD: Paris, France, 2007. [Google Scholar]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics; Wiley-VCH: Weinheim, Germany, 2009; Volume I. [Google Scholar]
- Dunn, W.J. Handbook of Molecular Descriptors. Methods and Principles in Medicinal Chemistry Series. Volume 11 By Roberto Todeschini and Viviana Consonni (Universita degli Studi di Milano-Bicocca). Edited by R. Mannold, H. Kubinyi, and H. Timmerman. Wiley-VCH: Weinheim and New York. 2000. xxi + 668 pp. 498 DM. ISBN 3-527-29913-O. J. Am. Chem. Soc. 2001, 123, 7198. [Google Scholar]
- Roy, K.; Kar, S.; Das, R.N. Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment; Academic Press: Boston, MA, USA, 2015. [Google Scholar]
- Almeida, J.R.; Vasconcelos, V. Natural antifouling compounds: Effectiveness in preventing invertebrate settlement and adhesion. Biotechnol. Adv. 2015, 33, 343–357. [Google Scholar] [CrossRef]
- Qian, P.Y.; Chen, L.; Xu, Y. Mini-review: Molecular mechanisms of antifouling compounds. Biofouling 2013, 29, 381–400. [Google Scholar] [CrossRef]
- Chen, L.; Qian, P.Y. Review on Molecular Mechanisms of Antifouling Compounds: An Update since 2012. Mar. Drugs 2017, 15, 264. [Google Scholar] [CrossRef]
- Chen, L.; Au, D.W.; Hu, C.; Peterson, D.R.; Zhou, B.; Qian, P.Y. Identification of Molecular Targets for 4,5-Dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) in Teleosts: New Insight into Mechanism of Toxicity. Environ. Sci. Technol. 2017, 51, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, H.; Sun, J.; Wong, Y.H.; Han, Z.; Au, D.W.; Bajic, V.B.; Qian, P.Y. Proteomic changes in brain tissues of marine medaka (Oryzias melastigma) after chronic exposure to two antifouling compounds: Butenolide and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT). Aquat. Toxicol. (Amst. Neth.) 2014, 157, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.H.; Wang, Y.F.; Zhang, X.Y.; Zhou, M.P.; Xu, X.Y.; Qi, S.H. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus. Mar. Drugs 2014, 12, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.; Svenson, J.; Sepcic, K.; Trembleau, L.; Engqvist, M.; Andersen, J.H.; Jaspars, M.; Stensvag, K.; Haug, T. Isolation and synthesis of pulmonarins A and B, acetylcholinesterase inhibitors from the colonial ascidian Synoicum pulmonaria. J. Nat. Prod. 2014, 77, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Duysen, E.G.; Lockridge, O. Induction of plasma acetylcholinesterase activity and apoptosis in mice treated with the organophosphorus toxicant, tri-o-cresyl phosphate. Toxicol. Res. 2012, 1, 55–61. [Google Scholar] [CrossRef]
- Martin-Rodriguez, A.J.; Babarro, J.M.; Lahoz, F.; Sanson, M.; Martin, V.S.; Norte, M.; Fernandez, J.J. From broad-spectrum biocides to quorum sensing disruptors and mussel repellents: Antifouling profile of alkyl triphenylphosphonium salts. PLoS ONE 2015, 10, e0123652. [Google Scholar] [CrossRef]
- Katranitsas, A.; Castritsi-Catharios, J.; Persoone, G. The effects of a copper-based antifouling paint on mortality and enzymatic activity of a non-target marine organism. Mar. Pollut. Bull. 2003, 46, 1491–1494. [Google Scholar] [CrossRef]
- Koutsaftis, A.; Aoyama, I. Toxicity of four antifouling biocides and their mixtures on the brine shrimp Artemia salina. Sci. Total Environ. 2007, 387, 166–174. [Google Scholar] [CrossRef]
- Sugamoto, K.; Matsusita, Y.-i.; Matsui, K.; Kurogi, C.; Matsui, T. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 2011, 67, 5346–5359. [Google Scholar] [CrossRef]
- Lim, S.S.; Jung, S.H.; Ji, J.; Shin, K.H.; Keum, S.R. Synthesis of flavonoids and their effects on aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. J. Pharm. Pharmacol. 2001, 53, 653–668. [Google Scholar] [CrossRef]
- Neves, M.P.; Cravo, S.; Lima, R.T.; Vasconcelos, M.H.; Nascimento, M.S.J.; Silva, A.M.S.; Pinto, M.; Cidade, H.; Corrêa, A.G. Solid-phase synthesis of 2′-hydroxychalcones. Effects on cell growth inhibition, cell cycle and apoptosis of human tumor cell lines. Bioorg. Med. Chem. 2012, 20, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Freitas, M.; Cruz, S.; Leão, P.N.; Vasconcelos, V.; Cunha, I. Acetylcholinesterase in Biofouling Species: Characterization and Mode of Action of Cyanobacteria-Derived Antifouling Agents. Toxins 2015, 7, 2739–2756. [Google Scholar] [CrossRef] [PubMed]
- Katritsky, A.; Karelson, M.; Lobanov, V.S.; Dennington, R.; Keith, T. CODESSA 2.7.10; Semichem, Inc.: Shawnee, KS, USA, 2004. [Google Scholar]
- Ćwik, J.; Koronacki, J. A Heuristic Method of Model Choice for Nonlinear Regression. In Proceedings of the Rough Sets and Current Trends in Computing, Berlin, Heidelberg, 22–26 June 1998; pp. 68–74. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cunha, I.; Garcia, L.M.; Guilhermino, L. Sea-urchin (Paracentrotus lividus) glutathione S-transferases and cholinesterase activities as biomarkers of environmental contamination. J. Environ. Monit 2005, 7, 288–294. [Google Scholar] [CrossRef]
- Adhikari, A.; Devkota, H.P.; Takano, A.; Masuda, K.; Nakane, T.; Basnet, P.; Skalko-Basnet, N. Screening of Nepalese crude drugs traditionally used to treat hyperpigmentation: In vitro tyrosinase inhibition. Int. J. Cosmet. Sci. 2008, 30, 353–360. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
| Compound | EC50 (μM) | EC50 (μg·mL−1) | LC50 (µM) | LC50/EC50 |
|---|---|---|---|---|
| 1b | 8.34 (95% CI: 4.2–13.36) | 2.87 | > 200 | > 23.98 |
| 4a | 42.55 (95% CI: 34.90–52.80) | 32.75 | > 200 | > 4.70 |
| 4b | 48.22 (95% CI: 30.57–85.40) | 38.56 | > 200 | > 4.15 |
| 6a | 84.52 (95% CI: 45.07–267.02) | 28.60 | > 200 | > 2.37 |
| 6b | 85.56 (95% CI: 44.84–291.41) | 31.52 | > 200 | > 2.34 |
| 7b | 3.28 (95% CI: 1.97–4.74) | 2.43 | > 200 | > 60.98 |
| 8a | 35.83 (95% CI: 19.22–74.74) | 27.07 | > 200 | > 5.58 |
| 8b | 53.90 (95% CI: 29.98–126.88) | 42.35 | > 200 | > 3.71 |
| 11a | 18.10 (95% CI: 13.95–23.44) | 5.44 | > 200 | > 11.05 |
| 11b | 9.64 (95% CI: 3.85–17.22) | 3.18 | > 200 | > 20.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.; Gonçalves, C.; Martins, B.T.; Palmeira, A.; Vasconcelos, V.; Pinto, M.; Almeida, J.R.; Correia-da-Silva, M.; Cidade, H. Flavonoid Glycosides with a Triazole Moiety for Marine Antifouling Applications: Synthesis and Biological Activity Evaluation. Mar. Drugs 2021, 19, 5. https://doi.org/10.3390/md19010005
Pereira D, Gonçalves C, Martins BT, Palmeira A, Vasconcelos V, Pinto M, Almeida JR, Correia-da-Silva M, Cidade H. Flavonoid Glycosides with a Triazole Moiety for Marine Antifouling Applications: Synthesis and Biological Activity Evaluation. Marine Drugs. 2021; 19(1):5. https://doi.org/10.3390/md19010005
Chicago/Turabian StylePereira, Daniela, Catarina Gonçalves, Beatriz T. Martins, Andreia Palmeira, Vitor Vasconcelos, Madalena Pinto, Joana R. Almeida, Marta Correia-da-Silva, and Honorina Cidade. 2021. "Flavonoid Glycosides with a Triazole Moiety for Marine Antifouling Applications: Synthesis and Biological Activity Evaluation" Marine Drugs 19, no. 1: 5. https://doi.org/10.3390/md19010005
APA StylePereira, D., Gonçalves, C., Martins, B. T., Palmeira, A., Vasconcelos, V., Pinto, M., Almeida, J. R., Correia-da-Silva, M., & Cidade, H. (2021). Flavonoid Glycosides with a Triazole Moiety for Marine Antifouling Applications: Synthesis and Biological Activity Evaluation. Marine Drugs, 19(1), 5. https://doi.org/10.3390/md19010005










