Multiple New Paralytic Shellfish Toxin Vectors in Offshore North Sea Benthos, a Deep Secret Exposed
Abstract
1. Introduction
2. Results
2.1. PST Toxicity
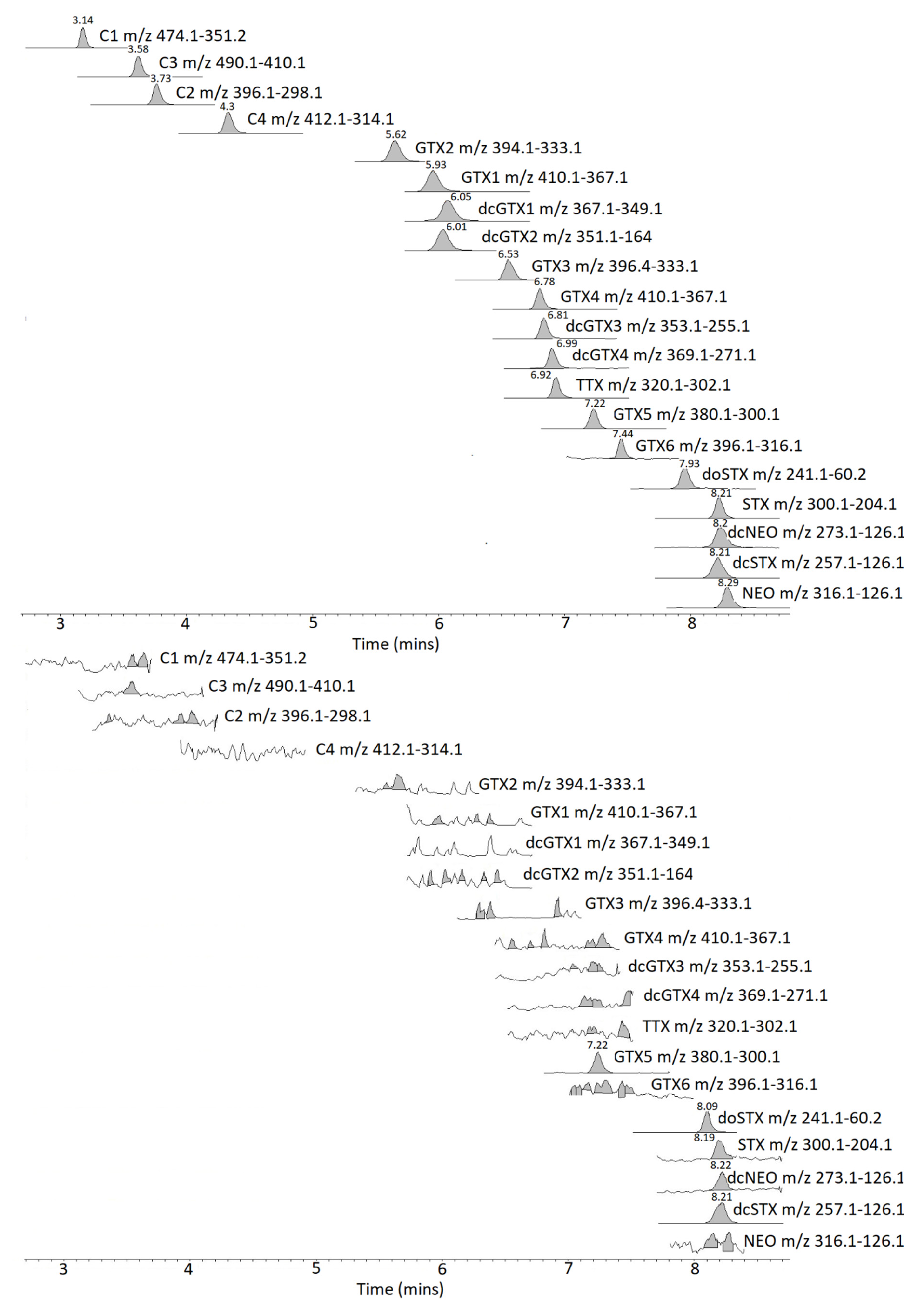
2.2. Method Comparison
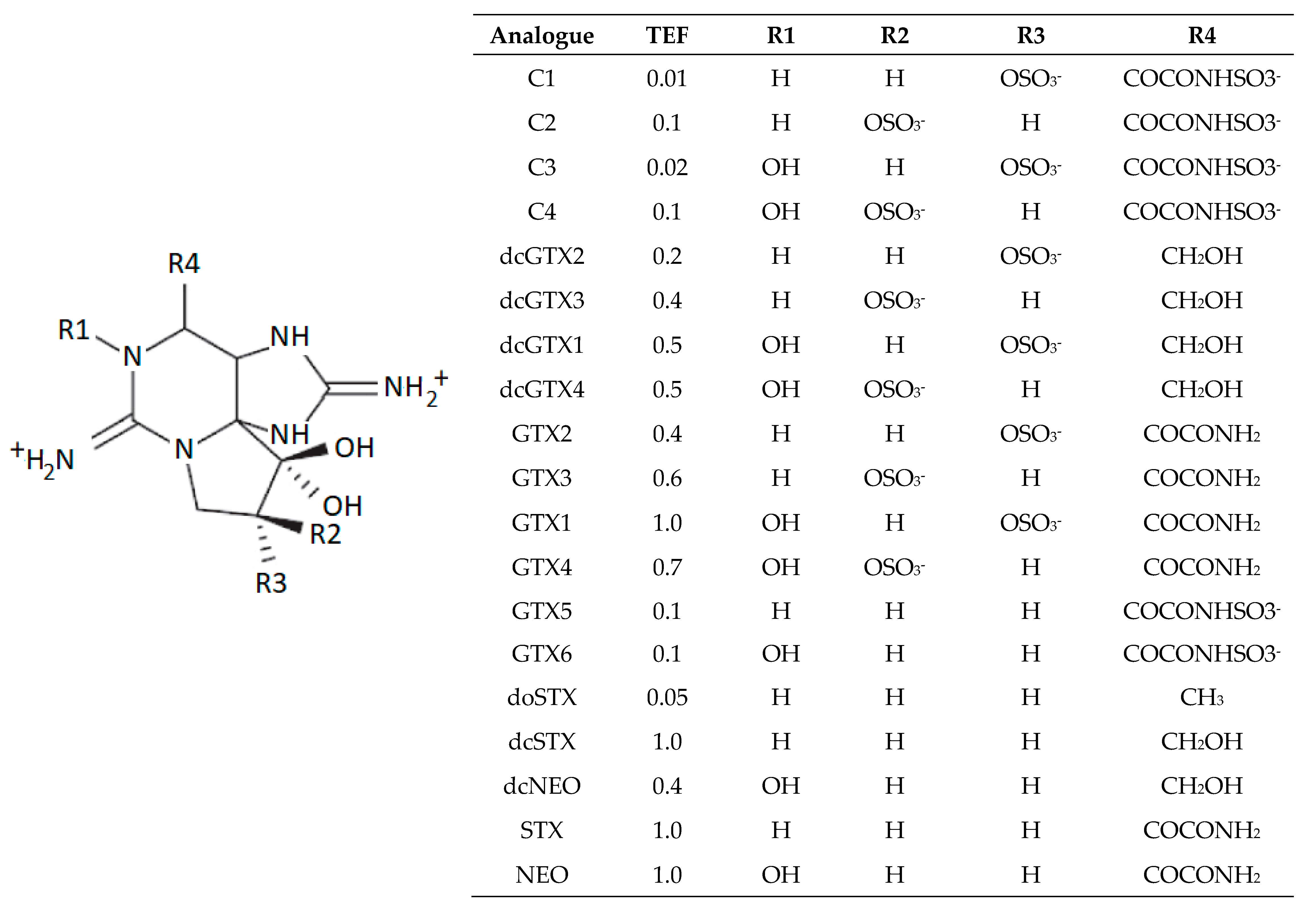
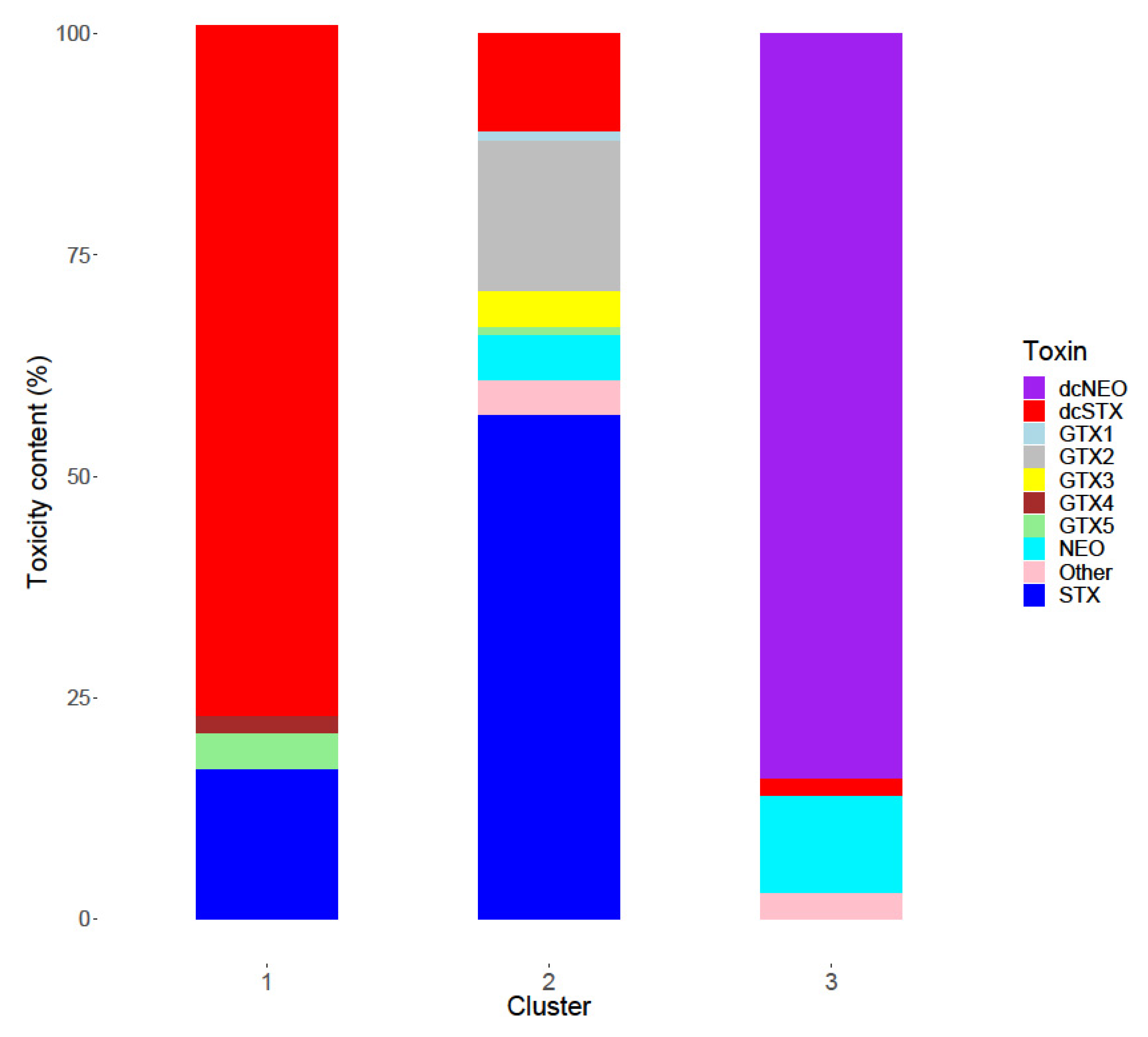
2.3. Toxin Profiles
2.4. Inter-Group Variability
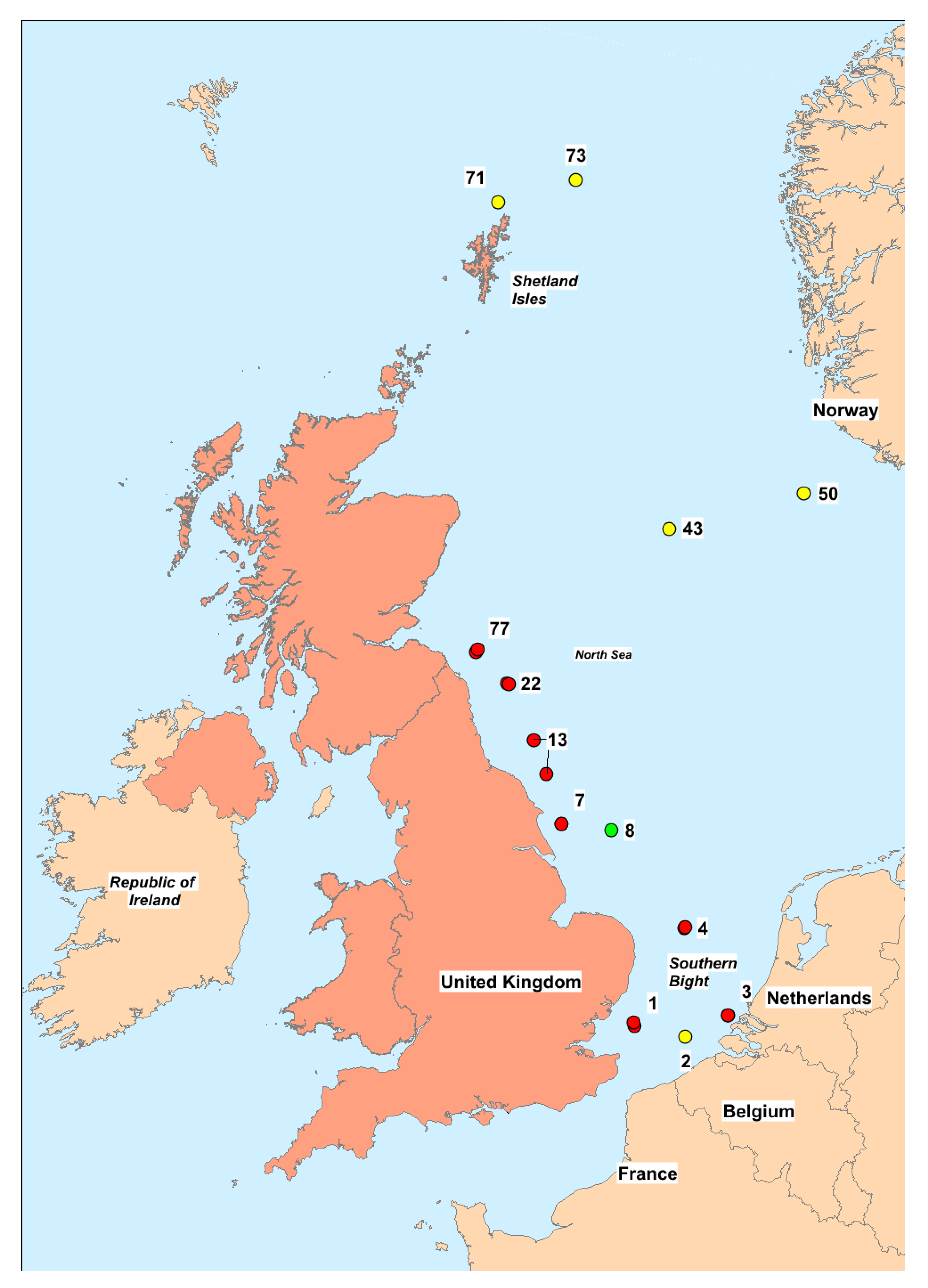
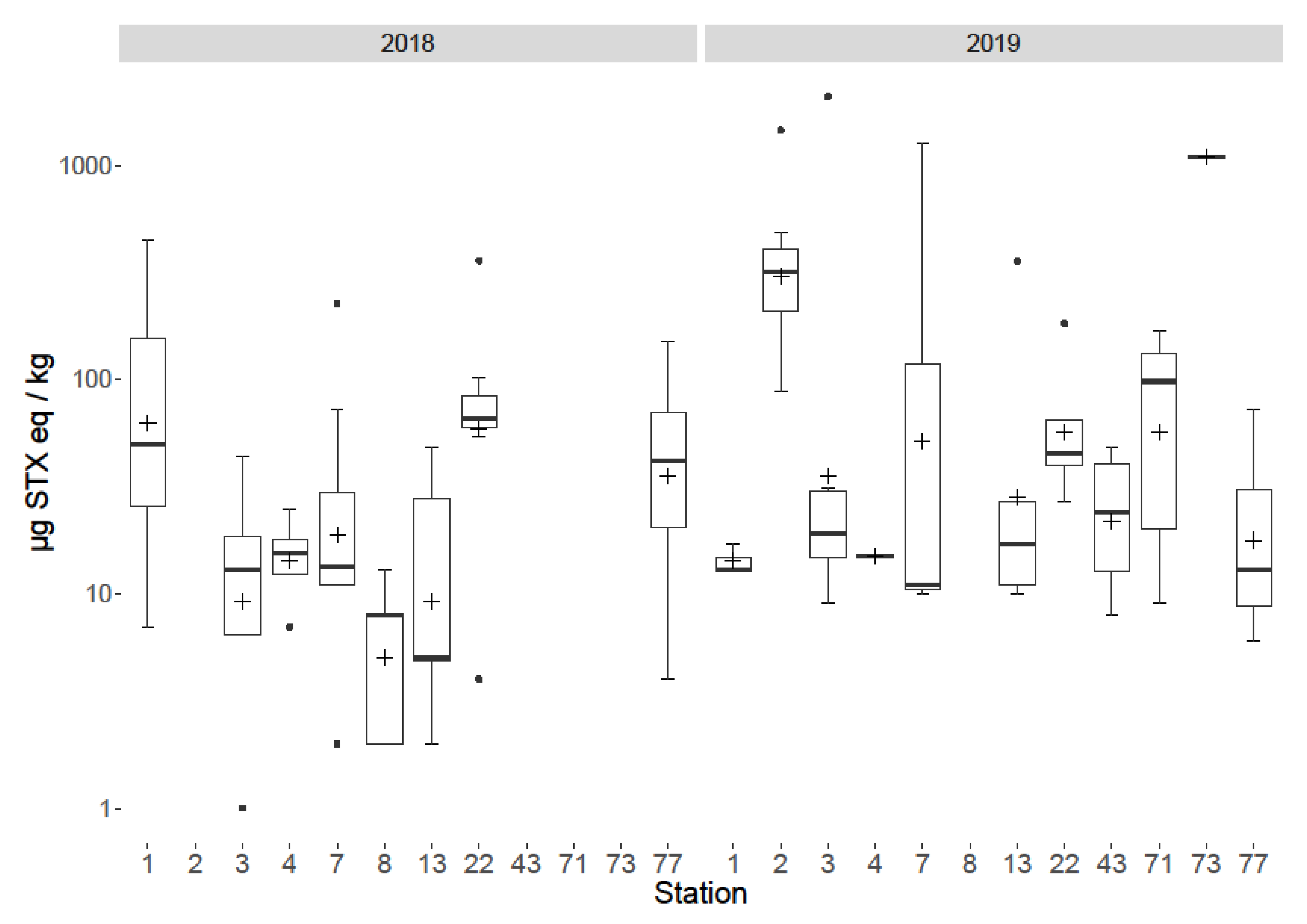
2.5. Spatial and Temporal Variability
2.6. Statistical Analysis
3. Discussion
3.1. Occurrence of Toxins
3.2. Method Comparison
3.3. Toxin Profiles
3.4. Group Variability
3.5. Spatial and Temporal Variability
3.6. One Health Considerations
4. Conclusions
5. Materials and Methods
5.1. Sample Collection Methods
5.2. Samples
5.3. Reagents and Chemicals
5.4. Sample Preparation and Extraction
5.5. Analysis of PSTs
5.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Cefas ID | Year | Station | Common Name | Species | Group | Total PST Detected by LC-FLD ( µg STX eq./kg) | Total PST Detected by LC-MS/MS ( µg STX eq./kg) |
|---|---|---|---|---|---|---|---|
| CEND 001 | 2018 | 22 | Rigid cushion starfish | Hippasteria phrygiana | Starfish & Brittlestars (excl Sunstar) | 9 | 4 |
| CEND 002 | 2018 | 22 | Whelk | Buccinidae (indet.) | Molluscs | 27 | 70 |
| CEND 003 | 2018 | 22 | Woody canoe bubble | Scaphander lignarius | Molluscs | nt | 102 |
| CEND 004 | 2018 | 22 | Sandstar | Astropecten irregularis | Starfish & Brittlestars (excl Sunstar) | 13 | 66 |
| CEND 005 | 2018 | 22 | Hermit crab | Pagurus bernhardus | Crustacean (Anomura & Brachyura) | nt | nt |
| CEND 006 | 2018 | 22 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 34 | 65 |
| CEND 007 | 2018 | 22 | Circular crab | Atelecyclus rotundatus | Crustacean (Anomura & Brachyura) | 4 | 54 |
| CEND 008 | 2018 | 22 | Sea mouse | Aphrodita aculeata | Polychaeta | 25 | 359 |
| CEND 009 | 2018 | 13 | Anemone | Actiniaria (indet.) | Actiniaria | nt | 5 |
| CEND 010 | 2018 | 13 | Dead-man’s fingers | Alcyonium digitatum | Sessile colonial fauna | 2 | 2 |
| CEND 011 | 2018 | 13 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 16 | 28 |
| CEND 012 | 2018 | 13 | Hermit crab | Pagurus bernhardus | Crustacean (Anomura & Brachyura) | nt | 48 |
| CEND 013 | 2018 | 13 | Whelk | Neptunea antiqua (eggmass) | Molluscs | nt | 5 |
| CEND 014 | 2018 | 8 | Masked crab | Corystes cassivelaunus | Crustacean (Anomura & Brachyura) | 75 | 8 |
| CEND 015 | 2018 | 8 | Square crab | Goneplax rhomboides | Crustacean (Anomura & Brachyura) | 14 | 8 |
| CEND 016 | 2018 | 8 | Swimming crabs | Liocarcinus sp. | Crustacean (Anomura & Brachyura) | 4 | 13 |
| CEND 017 | 2018 | 8 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 6 | 2 |
| CEND 018 | 2018 | 8 | Dead-man’s fingers | Alcyonium digitatum | Sessile colonial fauna | nt | 2 |
| CEND 019 | 2018 | 77 | Circular crab | Atelecyclus rotundatus | Crustacean (Anomura & Brachyura) | 15 | 13 |
| CEND 020 | 2018 | 77 | Queen scallop | Aequipecten opercularis | Molluscs | 41 | 103 |
| CEND 021 | 2018 | 77 | Swimming crabs | Liocarcinus sp. | Crustacean (Anomura & Brachyura) | 8 | 14 |
| CEND 022 | 2018 | 77 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | 32 | 39 |
| CEND 023 | 2018 | 77 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 18 | 27 |
| CEND 024 | 2018 | 77 | Rigid cushion starfish | Hippasteria phrygiana | Starfish & Brittlestars (excl Sunstar) | 21 | 23 |
| CEND 025 | 2018 | 77 | Seven-armed starfish | Luidia ciliaris | Starfish & Brittlestars (excl Sunstar) | nt | 45 |
| CEND 026 | 2018 | 77 | Squat lobster | Munida rugosa | Crustacean (Anomura & Brachyura) | 14 | 57 |
| CEND 027 | 2018 | 77 | Anemone | Actiniaria (indet.) | Actiniaria | nt | 150 |
| CEND 028 | 2018 | 77 | Sea mouse | Aphrodita aculeata | Polychaeta | nt | 98 |
| CEND 029 | 2018 | 77 | Hermit crab | Pagurus bernhardus | Crustacean (Anomura & Brachyura) | 27 | 63 |
| CEND 030 | 2018 | 77 | Sandstar | Astropecten irregularis | Starfish & Brittlestars (excl Sunstar) | 4 | 4 |
| CEND 031 | 2018 | 3 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | nt | nd |
| CEND 032 | 2018 | 3 | Shrimp | Crangon sp. | Crustacean (Natania & Stomatopoda) | nt | nd |
| CEND 033 | 2018 | 3 | Swimming crabs | Liocarcinus sp. | Crustacean (Anomura & Brachyura) | nt | 14 |
| CEND 034 | 2018 | 3 | Anemone | Actiniaria (indet.) | Actiniaria | 5 | 12 |
| CEND 035 | 2018 | 3 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | nt | 1 |
| CEND 036 | 2018 | 3 | Netted dog whelk | Hinia reticulata | Molluscs | nt | nd |
| CEND 037 | 2018 | 3 | Common shore crab | Carcinus maenas | Crustacean (Anomura & Brachyura) | 15 | 44 |
| CEND 038 | 2018 | 4 | Hermit crab | Paguris bernhardus | Crustacean (Anomura & Brachyura) | 3 | 25 |
| CEND 039 | 2018 | 4 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | nt | nd |
| CEND 040 | 2018 | 4 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 3 | nd |
| CEND 041 | 2018 | 4 | Velvet swimming crab | Necora puber | Crustacean (Anomura & Brachyura) | 13 | 7 |
| CEND 042 | 2018 | 4 | Sea urchin | Echinus sp. | Sea urchin | 13 | nd |
| CEND 043 | 2018 | 4 | Sea urchin | Echinus sp. | Sea urchin | 27 | nd |
| CEND 044 | 2018 | 4 | Swimming crabs | Liocarcinus sp. | Crustacean (Anomura & Brachyura) | 47 | 16 |
| CEND 045 | 2018 | 4 | Circular crab | Atelecyclus rotundatus | Crustacean (Anomura & Brachyura) | 188 | 15 |
| CEND 046 | 2018 | 1 | Shrimp | Crangon sp. & Pandalus sp. | Crustacean (Natania & Stomatopoda) | 181 | 446 |
| CEND 047 | 2018 | 1 | Dead-man’s fingers | Alcyonium digitatum | Sessile colonial fauna | 88 | 36 |
| CEND 048 | 2018 | 1 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | nt | 113 |
| CEND 049 | 2018 | 1 | Hermit crab | Pagurus bernhardus | Crustacean (Anomura & Brachyura) | nt | 52 |
| CEND 050 | 2018 | 1 | Whelk | Buccinum undatum | Molluscs | nt | 48 |
| CEND 051 | 2018 | 1 | Circular crab | Atelecyclus rotundatus | Crustacean (Anomura & Brachyura) | nt | 7 |
| CEND 052 | 2018 | 1 | Slipper limpet | Crepidula fornicata | Molluscs | nt | 172 |
| CEND 053 | 2018 | 1 | Green sea urchin | Psammechinus miliaris | Sea urchin | nt | 23 |
| CEND 054 | 2018 | 1 | Sea mouse | Aphrodita aculeata | Polychaeta | nt | 18 |
| CEND 055 | 2018 | 1 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | nt | 395 |
| CEND 056 | 2018 | 7 | Common sea urchin | Echinus esculentus | Sea urchin | nt | 2 |
| CEND 057 | 2018 | 7 | Bloody Henry starfish | Henricia sp. | Starfish & Brittlestars (excl Sunstar) | nt | 15 |
| CEND 058 | 2018 | 7 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 33 | 11 |
| CEND 059 | 2018 | 7 | Seven-armed starfish | Luidia ciliaris | Starfish & Brittlestars (excl Sunstar) | 26 | 72 |
| CEND 060 | 2018 | 7 | Swimming crabs | Liocarcinus holsatus & Liocarcinus depurator | Crustacean (Anomura & Brachyura) | 73 | 11 |
| CEND 061 | 2018 | 7 | Dead-man’s fingers | Alcyonium digitatum | Sessile colonial fauna | nt | 12 |
| CEND 062 | 2018 | 7 | Sunstar | Crossaster papposus | Sunstar | 302 | 227 |
| CEND 063 | 2018 | 7 | Anemone | Actiniaria (indet.) | Actiniaria | nt | 22 |
| CEND 064 | 2018 | 7 | Sea mouse | Aphrodita aculeata | Polychaeta | 14 | nd |
| CEND 065 | 2019 | 71 | Common Starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 20 | 20 |
| CEND 066 | 2019 | 71 | Sea urchin | Echinus sp. | Sea urchin | 10 | nd |
| CEND 067 | 2019 | 71 | Sea cucumber | Parastichopus tremulus | Other | 9 | nd |
| CEND 068 | 2019 | 71 | Red Cushion Starfish | Porania pulvillus | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 076 | 2019 | 71 | Sunstar | Crossaster papposus | Sunstar | 120 | 135 |
| CEND 077 | 2019 | 71 | Sunstar | Crossaster papposus | Sunstar | 132 | 131 |
| CEND 078 | 2019 | 71 | Sunstar | Crossaster papposus | Sunstar | 176 | 169 |
| CEND 079 | 2019 | 71 | Sunstar | Crossaster papposus | Sunstar | 103 | 98 |
| CEND 080 | 2019 | 71 | Sponge | Porifera sp. | Sessile colonial fauna | 7 | nd |
| CEND 081 | 2019 | 71 | Sea chervil | Alcyonidium diaphanum | Sessile colonial fauna | nd | 9 |
| CEND 082 | 2019 | 71 | Anemone | Actiniaria (indet.) | Actiniaria | 9 | nd |
| CEND 083 | 2019 | 71 | Woody canoe bubble | Scaphander lignarius | Molluscs | nd | nd |
| CEND 084 | 2019 | 71 | Seven-armed Starfish | Luidia ciliaris | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 085 | 2019 | 71 | Rosy Starfish | Stichastrella rosea | Starfish & Brittlestars (excl Sunstar) | nd | 133 |
| CEND 086 | 2019 | 71 | Goosefoot starfish | Anseropoda Placenta | Starfish & Brittlestars (excl Sunstar) | 8 | 51 |
| CEND 087 | 2019 | 71 | Portunid crabs | Portunidae (indet.) | Crustacean (Anomura & Brachyura) | 24 | nd |
| CEND 088 | 2019 | 71 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | 8 | nd |
| CEND 089 | 2019 | 71 | Shrimp | Natantia sp. | Crustacean (Natania & Stomatopoda) | 12 | nd |
| CEND 090 | 2019 | 71 | Hermit Crab | Pagurus sp. | Crustacean (Anomura & Brachyura) | 93 | 17 |
| CEND 091 | 2019 | 71 | Rigid cushion starfish | Hippasteria phrygiana | Starfish & Brittlestars (excl Sunstar) | 11 | nd |
| CEND 092 | 2019 | 43 | Common Starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 12 | nd |
| CEND 093 | 2019 | 43 | Sandstar | Astropecten irregularis | Starfish & Brittlestars (excl Sunstar) | 14 | nd |
| CEND 094 | 2019 | 43 | Rigid cushion starfish | Hippasteria phrygiana | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 095 | 2019 | 43 | Hermit Crab | Pagurus bernhardus | Crustacean (Anomura & Brachyura) | 11 | 8 |
| CEND 096 | 2019 | 43 | Flying crab | Liocarcinus holsatus | Crustacean (Anomura & Brachyura) | nd | nd |
| CEND 097 | 2019 | 43 | Anemone | Actiniaria (indet.) | Actiniaria | nd | nd |
| CEND 098 | 2019 | 43 | Sea urchin | Echinus sp. | Sea urchin | 26 | nd |
| CEND 099 | 2019 | 43 | Whelk | Buccinidae (indet.) | Molluscs | nd | nd |
| CEND 100 | 2019 | 43 | Dead-man’s fingers | Alyconium digitatum | Sessile colonial fauna | 23 | nd |
| CEND 101 | 2019 | 43 | Sea urchin | Echinus acutus | Sea urchin | 9 | nd |
| CEND 102 | 2019 | 43 | Slender colus | Colus gracilus | Molluscs | 18 | 48 |
| CEND 103 | 2019 | 43 | Whelk | Buccinidae (indet.) | Molluscs | 20 | 15 |
| CEND 104 | 2019 | 43 | Whelk | Buccinidae (indet.) | Molluscs | nd | 38 |
| CEND 105 | 2019 | 43 | Sea mouse | Aphrodita aculeata | Polychaeta | 30 | Nd |
| CEND 106 | 2019 | 13 | Rigid cushion starfish | Hippasteria phrygiana | Starfish & Brittlestars (excl Sunstar) | nd | Nd |
| CEND 107 | 2019 | 13 | Hermit Crab | Pagurus sp. | Crustacean (Anomura & Brachyura) | nd | Nd |
| CEND 108 | 2019 | 13 | Sea mouse | Aphrodita aculeata | Polychaeta | 587 | 357 |
| CEND 109 | 2019 | 13 | Dead-man’s fingers | Alyconium digitatum | Sessile colonial fauna | nd | nd |
| CEND 110 | 2019 | 13 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | 8 | nd |
| CEND 111 | 2019 | 13 | Anemone | Actiniaria (indet.) | Actiniaria | 31 | 11 |
| CEND 112 | 2019 | 13 | Common Starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 34 | 10 |
| CEND 113 | 2019 | 13 | Plumose anemone | Metridium senile | Actiniaria | 9 | 27 |
| CEND 114 | 2019 | 13 | Bivalve | Bivalvia (indet.) | Molluscs | 28 | 17 |
| CEND 115 | 2019 | 1 | Dead-man’s fingers | Alcyonium digitatum | Sessile colonial fauna | nd | nd |
| CEND 116 | 2019 | 1 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | 8 | nd |
| CEND 117 | 2019 | 1 | Sea mouse | Aphrodita aculeata | Polychaeta | nd | nd |
| CEND 118 | 2019 | 1 | Hermit crab | Pagurus bernhardus | Crustacean (Anomura & Brachyura) | nd | nd |
| CEND 119 | 2019 | 1 | Common Starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 14 | nd |
| CEND 120 | 2019 | 1 | Green sea urchin | Psammechinus miliaris | Sea urchin | nd | nd |
| CEND 121 | 2019 | 1 | Echiuran worm | Echiura sp. | Other | nd | nd |
| CEND 122 | 2019 | 1 | Inachidae crab | Inachus sp. | Crustacean (Anomura & Brachyura) | 66 | 13 |
| CEND 123 | 2019 | 1 | Anemone | Actiniaria (indet.) | Actiniaria | 92 | 13 |
| CEND 124 | 2019 | 1 | Whelk | Buccinum undatum | Molluscs | 1 | nd |
| CEND 125 | 2019 | 1 | Shrimp | Crangon sp. & Rissoides desmarseti | Crustacean (Natania & Stomatopoda) | nd | 17 |
| CEND 126 | 2019 | 1 | Crabs | Portunidae sp. & Ebalia sp. | Crustacean (Anomura & Brachyura) | 25 | nd |
| CEND 127 | 2019 | 3 | Hermit Crab | Pagurus bernhardus | Crustacean (Anomura & Brachyura) | nd | 12 |
| CEND 128 | 2019 | 3 | Swimming crabs | Liocarcinus holsatus & Liocarcinus depurator | Crustacean (Anomura & Brachyura) | nd | 9 |
| CEND 129 | 2019 | 3 | Common shore crab | Carcinus maenas | Crustacean (Anomura & Brachyura) | nd | nd |
| CEND 130 | 2019 | 3 | Sea mouse | Aphrodita aculeata | Polychaeta | 44 | nd |
| CEND 131 | 2019 | 3 | Sea chervil | Alcyonidium diaphanum | Sessile colonial fauna | 1486 | 2091 |
| CEND 132 | 2019 | 3 | Common Starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 21 | nd |
| CEND 133 | 2019 | 3 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | 14 | 29 |
| CEND 134 | 2019 | 3 | Shrimp | Crangon sp. | Crustacean (Natania & Stomatopoda) | 16 | 18 |
| CEND 135 | 2019 | 3 | Spider crab | Macropodia sp. | Crustacean (Anomura & Brachyura) | 35 | 31 |
| CEND 136 | 2019 | 3 | Green sea urchin | Psammechinus miliaris | Sea urchin | 10 | 19 |
| CEND 137 | 2019 | 2 | Common Starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | 317 | 354 |
| CEND 138 | 2019 | 2 | Green sea urchin | Psammechinus miliaris | Sea urchin | 377 | 257 |
| CEND 139 | 2019 | 2 | Sea chervil | Alcyonidium diaphanum | Sessile colonial fauna | 2744 | 1461 |
| CEND 140 | 2019 | 2 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | 593 | 489 |
| CEND 141 | 2019 | 2 | Sea mouse | Aphrodita aculeata | Polychaeta | 443 | 386 |
| CEND 142 | 2019 | 2 | Swimming crabs | Liocarcinus sp. | Crustacean (Anomura & Brachyura) | 76 | 88 |
| CEND 143 | 2019 | 2 | Whelk | Baccinum undatum | Molluscs | 133 | 110 |
| CEND 144 | 2019 | 2 | Sea chervil | Alcyonidium diaphanum | Sessile colonial fauna | 562 | 288 |
| CEND 145 | 2019 | ? | Rigid cushion starfish | Hippasteria phrygiana | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 146 | 2019 | 7 | Sea mouse | Aphrodita aculeata | Polychaeta | nd | nd |
| CEND 147 | 2019 | 7 | Common Starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | nd | 10 |
| CEND 148 | 2019 | 7 | Bloody Henry starfish | Henricia oculata | Starfish & Brittlestars (excl Sunstar) | nd | 11 |
| CEND 149 | 2019 | 7 | Sunstar | Crossaster papposus | Sunstar | 654 | 1275 |
| CEND 156 | 2019 | 7 | Swimming crabs | Liocarcinus holsatus & Liocarcinus depurator | Crustacean (Anomura & Brachyura) | nd | nd |
| CEND 157 | 2019 | 7 | Masked crab | Corystes cassivelaunus | Crustacean (Anomura & Brachyura) | nd | nd |
| CEND 158 | 2019 | 7 | Dead-man’s fingers | Alcyonium digitatum | Sessile colonial fauna | nd | nd |
| CEND 159 | 2019 | 7 | Mermaids glove | Haliclona oculata | Sessile colonial fauna | 21 | nd |
| CEND 160 | 2019 | 4 | Common starfish | Asterias rubens | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 161 | 2019 | 4 | Sea mouse | Aphrodita aculeata | Polychaeta | nd | nd |
| CEND 162 | 2019 | 4 | Green sea urchin | Psammechinus miliaris | Sea urchin | nd | nd |
| CEND 163 | 2019 | 4 | Hermit Crab | Pagurus bernhardus | Crustacean (Anomura & Brachyura) | nd | nd |
| CEND 164 | 2019 | 4 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 165 | 2019 | 4 | Swimming crabs | Liocarcinus holsatus & Liocarcinus depurator | Crustacean (Anomura & Brachyura) | nd | 15 |
| CEND 166 | 2019 | 4 | Circular Crab | Atelecyclus sp. | Crustacean (Anomura & Brachyura) | nd | nd |
| CEND 167 | 2019 | 4 | Whelk | Buccinum undatum | Molluscs | nd | nd |
| CEND 168 | 2019 | 22 | Rigid cushion starfish | Hippasteria phrygiana | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 169 | 2019 | 22 | Anemone | Actiniaria (indet.) | Actiniaria | 14 | 45 |
| CEND 170 | 2019 | 22 | Sea mouse | Aphrodita aculeata | Polychaeta | 37 | 46 |
| CEND 171 | 2019 | 22 | Sandstar | Astropecten irregularis | Starfish & Brittlestars (excl Sunstar) | nd | 183 |
| CEND 172 | 2019 | 22 | Woody canoe bubble | Scaphander lignarius | Molluscs | nd | nd |
| CEND 173 | 2019 | 22 | Seven-armed Starfish | Luidia ciliaris | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 174 | 2019 | 22 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | nd | 27 |
| CEND 175 | 2019 | 22 | Shrimp | Natantia (indet.) | Crustacean (Natania & Stomatopoda) | 7 | nd |
| CEND 176 | 2019 | 77 | Sea mouse | Aphrodita aculeata | Polychaeta | 12 | 13 |
| CEND 177 | 2019 | 77 | Rigid cushion starfish | Hippasteria phrygiana | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 178 | 2019 | 77 | Brittlestar | Ophiura ophiura | Starfish & Brittlestars (excl Sunstar) | nd | nd |
| CEND 179 | 2019 | 77 | Sandstar | Astropecten irregularis | Starfish & Brittlestars (excl Sunstar) | nd | 6 |
| CEND 180 | 2019 | 77 | Anemone | Actiniaria (indet.) | Actiniaria | 22 | 72 |
| CEND 181 | 2019 | 73 | Sunstar | Crossaster papposus | Sunstar | 1600 | 1102 |
| Station | Coordinates | Bottom Salinity | Surface Salinity | Bottom Temp. (°C) | Surface Temp. (°C) | Depth (m) | Date |
|---|---|---|---|---|---|---|---|
| 1 | 51:42N 01:45E | 33.7 | 34.5 | 19.6 | 19.8 | 33 | 11 August 2018 |
| 3 | 51:50N 03:37E | 32.4 | 32.5 | 20.9 | 21 | 24 | 11 August 2018 |
| 4 | 52:49N 02:45E | 34.4 | 34.4 | 17.6 | 17.6 | 38 | 12 August 2018 |
| 7 | 53:59N 00:15E | 34.5 | 34.5 | 12.6 | 12.4 | 53 | 14 August 2018 |
| 13 | 54:56N 00:16W | 34.7 | 34.5 | 7.4 | 16.3 | 77 | 14 August 2018 |
| 77 | 55:56N 01:27W | 34.8 | 34.8 | 9.5 | 13.9 | 84 | 7 September 2018 |
| 22 | 55:35N 00:49W | 34.9 | 34.9 | 7.4 | 15.3 | 96 | 7 September 2018 |
| 8 | 53:56N 01:17E | 34.8 | 34.8 | 14.2 | 16.4 | 42 | 14 August 2018 |
| 1 | 51:45N 01:44E | 35.1 | 35.0 | 18.8 | 19 | 30 | 7 August 2019 |
| 2 | 51:35N 02:46E | 34.8 | 34.8 | 19.6 | 19.6 | 32 | 7 August 2019 |
| 3 | 51:50N 03:38E | 33.5 | 33.4 | 20.1 | 20.2 | 27 | 8 August 2019 |
| 4 | 52:50N 02:46E | 34.7 | 34.7 | 17.8 | 18.1 | 41 | 8 August 2019 |
| 7 | 53:60N 00:16E | 34.4 | 34.4 | 13.1 | 14.5 | 55 | 11 August 2019 |
| 13 | 54:33N 00:02W | 34.6 | 34.6 | 9.6 | 15.9 | 65 | 11 August 2019 |
| 22 | 55:35N 00:48W | 34.9 | 34.5 | 8.4 | 16.2 | 99 | 18 August 2019 |
| 71 | 61:01N 00:60W | 35.4 | 35.3 | 9.6 | 13.3 | 132 | 24 August 2019 |
| 43 | 57:19N 02:27E | 35.1 | 34.8 | 7.7 | 16.9 | 84 | 28 August 2019 |
| 73 | 61:16N 00:34E | 35.4 | 35.4 | 9.3 | 13.4 | 170 | 24 August 2019 |
| 50 | 57:43N 05:10E | 35.3 | 34.0 | 8 | 16 | 110 | 27 August 2019 |
| 77 | 55:58N 01:25W | 34.7 | 34.5 | 9.3 | 15 | 90 | 18 August 2019 |
References
- Botana, L.M. Seafood and freshwater toxins: Pharmacology, physiology and detection, chapter 2: Diversity of marine and freshwateralgal toxins. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 20–24. [Google Scholar]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef]
- Cadoret, J.-P.; Garnier, M.; Saint-Jean, B. Microalgae, functional genomics and biotechnology. Adv. Bot. Res. 2012, 64, 285–341. [Google Scholar] [CrossRef]
- Pearson, L.A.; Mihali, T.; Moffitt, M.C.; Kellmann, R.; Neilan, B.A. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- Thottumkara, A.P.; Parsons, W.H.; DuBois, J. Saxitoxin. Angew. Chemie Int. Ed. 2014, 53, 5760–5784. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Anon. Regulation (EC) No 854/2004 of the european parliament and of the council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Off. J. Eur. Union 2004, 139, 206–320. [Google Scholar]
- Anon. Regulation (EC) No 853/2004 of the european parliament and of the council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. 2004, 7, 69–74. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Domenico, D.D.; Fernández-Cruz, M.L.; Fink-Gremmels, J.; Fürst, P.; Galli, C.; et al. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on marine biotoxins in shellfish—Saxitoxin group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- Quiblier, C.; Susanna, W.; Isidora, E.-S.; Mark, H.; Aurélie, V.; Jean-François, H. A review of current knowledge on toxic benthic freshwater cyanobacteria–Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Aráoz, R.; Molgó, J.; De Marsac, N.T. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.M.J.; Wood, S.A.; Van Ginkel, R.; Broady, P.A.; Gaw, S. First report of saxitoxin production by a species of the freshwater benthic cyanobacterium, Scytonema Agardh. Toxicon 2011, 57, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Borges, H.; Branco, L.H.; Martins, M.; Lima, C.; Barbosa, P.; Lira, G.; Bittencourt-Oliveira, M.D.C.; Molica, R. Cyanotoxin production and phylogeny of benthic cyanobacterial strains isolated from the northeast of Brazil. Harmful Algae 2015, 43, 46–57. [Google Scholar] [CrossRef]
- Ahern, K.S.; Ahern, C.R.; Savige, G.M.; Udy, J.W. Mapping the distribution, biomass and tissue nutrient levels of a marine benthic cyanobacteria bloom (Lyngbya majuscula). Mar. Freshw. Res. 2007, 58, 883. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Screening for competition effects and allelochemicals in benthic marine diatoms and cyanobacteria isolated from an intertidal flat (southern North Sea). Phycology 2012, 51, 432–450. [Google Scholar] [CrossRef]
- Stal, L.J.; van Gemerden, H.; Krumbein, W.E. Structure and development of a benthic marine microbial mat. FEMS Microbiol. Lett. 1985, 31, 111–125. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar]
- Brown, L. Distribution, diversity and toxin composition of the genus alexandrium (Dinophyceae) in Scottish waters distribution, diversity and toxin composition of the genus alexandrium (Dinophyceae) in Scottish waters. Eur. J. Phycol. 2010, 45, 375–393. [Google Scholar] [CrossRef]
- Lewis, A.M.; Coates, L.N.; Turner, A.D.; Percy, L.; Lewis, J. A review of the global distribution of Alexandrium minutum (Dinophyceae) and comments on ecology and associated paralytic shellfish toxin profiles, with a focus on Northern Europe. J. Phycol. 2018, 54, 581–598. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef]
- Nascimento, S.M.; Purdie, D.A.; Lilly, E.L.; Larsen, J.; Morris, S. Toxin profile, pigment composition, and large subunit rDNA phylogenetic analysis of an Alexandrium minutum (Dinophyceae) strain isolated from the fleet lagoon, United Kingdom. J. Phycol. 2005, 41, 343–353. [Google Scholar] [CrossRef]
- Martin, J.L.; LeGresley, M.M.; Hanke, A.R. Thirty years-Alexandrium fundyense cyst, bloom dynamics and shellfish toxicity in the Bay of Fundy, eastern Canada. Deep. Res. Part II Top. Stud. Oceanogr. 2014, 103, 27–39. [Google Scholar] [CrossRef]
- Anderson, D.M.; Stock, C.A.; Keafer, B.A.; Nelson, A.B.; Thompson, B.; McGillicuddy, J.D.; Keller, M.; Matrai, P.A.; Martin, J. Alexandrium fundyense cyst dynamics in the gulf of maine. Deep. Sea Res. Part II: Top. Stud. Oceanogr. 2005, 52, 2522–2542. [Google Scholar] [CrossRef]
- Natsuike, M.; Nagai, S.; Matsuno, K.; Saito, R.; Tsukazaki, C.; Yamaguchi, A.; Imai, I. Abundance and distribution of toxic alexandrium tamarense resting cysts in the sediments of the chukchi sea and the eastern bering sea. Harmful Algae 2013, 27, 52–59. [Google Scholar] [CrossRef]
- Persson, A.; Smith, B.C.; Wikfors, G.H.; Quilliam, M. Grazing on toxic alexandrium fundyense resting cysts and vegetative cells by the eastern oyster (Crassostrea virginica). Harmful Algae 2006, 5, 678–684. [Google Scholar] [CrossRef]
- Suarez-isla, B.A. Saxitoxin and other paralytic toxins: Toxicological profile. Mar. Freshw. Toxins 2015, 1–16. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- White, A.W. Sensitivity of marine fishes to toxins from the red-tide dinoflagellate Gonyaulax excavata and implications for fish kills. Mar. Boil. 1981, 65, 255–260. [Google Scholar] [CrossRef]
- Costa, P.R. Impact and effects of paralytic shellfish poisoning toxins derived from harmful algal blooms to marine fish. Fish Fish. 2014, 17, 226–248. [Google Scholar] [CrossRef]
- Montoya, N.; Akselman, R.; Franco, J.; Carreto, J.I. Paralytic shellfish toxins and mackerel (Scomber japonicus) mortality in the argentine sea. Harmful Toxic Algal. Bloom. 1996, 1, 417–420. [Google Scholar]
- Jensen, S.; Lacazeb, J.P.; Hermannb, G.; Kershawa, J.; Brownlowc, A.; Turnerd, A.; Halla, A. Toxicon Detection and effects of harmful algal toxins in Scottish harbour seals and potential links to population decline. Toxicon 2015, 97, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Robinson, I.; Aguilar, A.; González, L.M.; López-Jurado, L.F.; Reyero, M.I.; Cacho, E.; Franco, J.; López-Rodas, V.; Costas, E. Did algal toxins cause monk seal mortality? Nature 1998, 393, 28–29. [Google Scholar] [CrossRef]
- Kvitek, R.G.; DeGunge, A.R.; Beitler, M.K. Paralytic shellfish poisoning toxins mediate feeding behavior of sea otters. Limnol. Oceanogr. 1991, 36, 393–404. [Google Scholar] [CrossRef]
- Fraser, S.M. Mortality of shags and other sea birds caused by paralytic shellfish poison. Nature 1968, 220, 23–24. [Google Scholar]
- Mann, R.; Allen, S.M.; Boersma, P.D. Marine birds and harmful algal blooms: Sporadic victims or under-reported events? Harmful Algae 2003, 2, 1–17. [Google Scholar]
- Bif, M.B.; Yunes, J.S.; Resgalla, C. Evaluation of mysids and sea urchins exposed to saxitoxins. Environ. Toxicol. Pharmacol. 2013, 36, 819–825. [Google Scholar]
- Ferrer, R.P.; Lunsford, E.T.; Candido, C.M.; Strawn, M.L.; Pierce, K.M. Saxitoxin and the ochre sea star: Molecule of keystone significance and a classic keystone species. Integr. Comp. Boil. 2015, 55, 533–542. [Google Scholar] [CrossRef][Green Version]
- Andrade-Villagrán, P.V.; Navarro, J.M.; Aliste, S.; Chaparro, O.R.; Ortíz, A. Trophic transfer of paralytic shellfish toxin (PST): Physiological and reproductive effects in the carnivorous gastropod Acanthina monodon (Pallas, 1774). Aquat. Toxicol. 2019, 212, 37–46. [Google Scholar] [CrossRef]
- Li, S.-C.; Wang, W.-X.; Hsieh, D.P. Effects of toxic dinoflagellate alexandrium tamarense on the energy budgets and growth of two marine bivalves. Mar. Environ. Res. 2002, 53, 145–160. [Google Scholar] [CrossRef]
- Navarro, J.; González, K.; Cisternas, B.; López, J.A.; Chaparro, O.R.; Segura, C.J.; Córdova, M.; Suárez-Isla, B.; Fernández-Reiriz, M.J.; Labarta, U. Contrasting physiological responses of two populations of the razor clam tagelus dombeii with different histories of exposure to paralytic shellfish poisoning (PSP). PLoS ONE 2014, 9, e105794. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Dean, K.; Milligan, S.; Hamilton, M.; Thomas, J.; Poole, C.; Haycock, J.; Spelman-Marriott, J.; Watson, A.; et al. Fatal canine intoxications linked to the presence of saxitoxins in stranded marine organisms following winter storm activity. Toxins 2018, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Stubbs, B.; Coates, L.; Dhanji-Rapkova, M.; Hatfield, R.G.; Lewis, A.M.; Rowland-Pilgrim, S.; O’Neil, A.; Stubbs, P.; Ross, S.; et al. Variability of paralytic shellfish toxin occurrence and profiles in bivalve molluscs from Great Britain from official control monitoring as determined by pre-column oxidation liquid chromatography and implications for applying immunochemical tests. Harmful Algae 2014, 31, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Lewis, A.M.; O’Neil, A.; Hatfield, R.G. Transformation of paralytic shellfish poisoning toxins in UK surf clams (Spisula solida) for targeted production of reference materials. Toxicon 2013, 65, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Artigas, M.L.; Vale, P.; Gomes, S.S.; Botelho, M.J.; Rodrigues, S.M.; Amorim, A.; Henriques, M.J.B. Profiles of paralytic shellfish poisoning toxins in shellfish from portugal explained by carbamoylase activity. J. Chromatogr. 2007, 1160, 99–105. [Google Scholar] [CrossRef]
- Lin, H.-P.; Cho, Y.; Yashiro, H.; Yamada, T.; Oshima, Y. Purification and characterization of paralytic shellfish toxin transforming enzyme from Mactra chinensis. Toxicon 2004, 44, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Ogawa, N.; Takahashi, M.; Lin, H.-P.; Oshima, Y. Purification and characterization of paralytic shellfish toxin-transforming enzyme, sulfocarbamoylase I, from the Japanese bivalve peronidia venulosa. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2008, 1784, 1277–1285. [Google Scholar] [CrossRef]
- Smith, A.E.; Grant, F.; Ferguson, C.M.J.; Gallacher, S. Biotransformations of paralytic shellfish toxins by bacteria isolated from bivalve molluscs. Appl. Environ. Microbiol. 2001, 67, 2345–2353. [Google Scholar] [CrossRef]
- Shumway, S.E. Phycotoxin related shellfish poisoning: Bivalve molluscs are not the only vectors. Rev. Fish. Sci. 1995, 3, 1–31. [Google Scholar] [CrossRef]
- Jester, R.; Rhodes, L.; Beuzenberg, V. Uptake of paralytic shellfish poisoning and spirolide toxins by paddle crabs (Ovalipes catharus) via a bivalve vector. Harmful Algae 2009, 8, 369–376. [Google Scholar] [CrossRef]
- Kwong, R.W.; Wang, W.-X.; Lam, P.K.; Yu, P.K.N.; Lam, P.K. The uptake, distribution and elimination of paralytic shellfish toxins in mussels and fish exposed to toxic dinoflagellates. Aquat. Toxicol. 2006, 80, 82–91. [Google Scholar] [CrossRef]
- Turner, A.D.; Tarnovius, S.; Goya, A.B. Paralytic shellfish toxins in the marine gastropods zidona dufresnei and adelomelon beckii from argentina: Toxicity and toxin profiles. J. Shellfish. Res. 2014, 33, 519–530. [Google Scholar] [CrossRef]
- Terrazas, J.O.; Contreras, H.R.; García, C. Prevalence, variability and bioconcentration of saxitoxin-group in different marine species present in the food chain. Toxins 2017, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Felpeto, A.B.; Rodriguez, P.; Otero, P.; Azevedo, J.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New invertebrate vectors for PST, spirolides and okadaic acid in the north atlantic. Mar. Drugs 2013, 11, 1936–1960. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, M.; Nishimura, F.; Miyazawa, K.; Noguchi, T. Occurance of paralytic shellfish poison in the starfish Asteria amurensis in Kure Bay, Hiroshima prefecture, Japan. Toxins 1997, 35, 1081–1087. [Google Scholar]
- Ito, K.; Asakawa, M.; Sida, Y.; Miyazawa, K. Occurrence of paralytic shellfish poison (PSP) in the starfish Asterina pectinifera collected from the Kure Bay, Hiroshima Prefecture, Japan. Toxicon 2003, 41, 291–295. [Google Scholar] [CrossRef]
- Lin, S.-J.; Tsai, Y.-H.; Lin, H.; Hwang, D. Paralytic toxins in Taiwanese starfish Astrpecten scoparius. Toxicon 1998, 36, 799–803. [Google Scholar] [CrossRef]
- Silva, M.; Rey, V.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Botana, A.; Botana, L.M.; Vasconcelos, V. Paralytic shellfish toxins occurrence in non-traditional invertebrate vectors from north atlantic waters (Azores, Madeira, and Morocco). Toxins 2018, 10, 362. [Google Scholar] [CrossRef]
- Cusson, M.; Bourget, E. Global patterns of macroinvertebrate production in marine benthic habitats. Mar. Ecol. Prog. Ser. 2005, 297, 1–14. [Google Scholar] [CrossRef]
- Bremner, J.; Rogers, S.; Frid, C. Assessing functional diversity in marine benthic ecosystems: A comparison of approaches. Mar. Ecol. Prog. Ser. 2003, 254, 11–25. [Google Scholar] [CrossRef]
- Aravind, H.; Rajgopal, C.; Soman, K.P. A simple approach to clustering in excel. Int. J. Comput. Appl. 2010, 11, 19–25. [Google Scholar]
- EFSA. Marine biotoxins in shellfish-Saxitoxin group. EFSA J. 2009, 7, 1019. [Google Scholar]
- Jen, H.-C.; Nguyen, T.A.-T.; Wu, Y.-J.; Hoang, T.; Arakawa, O.; Lin, W.-F.; Hwang, D.-F. Tetrodotoxin and paralytic shellfish poisons in gastropod species from Vietnam analyzed by high-performance liquid chromatography and liquid chromatography–tandem mass spectrometry. J. Food Drug Anal. 2014, 22, 178–188. [Google Scholar] [CrossRef]
- Alderman, D.J. Monitoring and surveillance of biological contaminants and disease in the aquatic environment. Minist. Agric. For. Fish. 1993, 19, 1–35. [Google Scholar]
- Oikawa, H.; Fujita, T.; Satomi, M.; Suzuki, T.; Kotani, Y.; Yano, Y. Accumulation of paralytic shellfish poisoning toxins in the edible shore crab telmessus acutidens. Toxicon 2002, 40, 1593–1599. [Google Scholar] [CrossRef]
- Jester, R.J.; Baugh, K.A.; Lefebvre, K.A. Presence of Alexandrium catenella and paralytic shellfish toxins in finfish, shellfish and rock crabs in Monterey Bay, California, USA. Mar. Boil. 2009, 156, 493–504. [Google Scholar] [CrossRef]
- McLeod, C.; Kiermeier, A.; Stewart, I.; Tan, J.; Turnbull, A.; Madigan, T. Paralytic shellfish toxins in Australian southern rock lobster (Jasus edwardsii): Acute human exposure from consumption of hepatopancreas. Hum. Ecol. Risk Assessment: Int. J. 2018, 24, 1872–1886. [Google Scholar] [CrossRef]
- Costa, P.R.; Costa, S.T.; Braga, A.; Rodrigues, S.M.; Vale, P. Relevance and challenges in monitoring marine biotoxins in non-bivalve vectors. Food Control. 2017, 76, 24–33. [Google Scholar] [CrossRef]
- DeGrasse, S.; Vanegas, C.; Conrad, S. Paralytic shellfish toxins in the sea scallop placopecten magellanicus on georges bank: Implications for an offshore roe-on and whole scallop fishery. Deep. Sea Res. Part II: Top. Stud. Oceanogr. 2014, 103, 301–307. [Google Scholar] [CrossRef]
- Horner, R.; Greengrove, C.; Davies-Vollum, K.; Gawel, J.; Postel, J.; Cox, A. Spatial distribution of benthic cysts of Alexandrium catenella in surface sediments of Puget Sound, Washington, USA. Harmful Algae 2011, 11, 96–105. [Google Scholar] [CrossRef]
- Oshima, Y.; Bolch, C.J.; Hallegraeff, G.M. Toxin composition of resting cysts of Alexandrium tamerense (Dinophycae). Toxicon 1992, 30, 1539–1544. [Google Scholar] [CrossRef]
- Anderson, D.M.; Taylor, C.D.; Armbrust, E.V. The effects of darkness and anaerobiosis dinoflagellate cyst germination. Limnol. Ocean. 1987, 32, 340–351. [Google Scholar] [CrossRef]
- Ayres, P.; Cullum, M. Paralytic shellfish poisoning: An account of investigations into mussel toxicity in England. Fis.Res.Tec. MAFF 1978, 40, 1–23. [Google Scholar]
- Joint, I.; Lewis, J.; Aiken, J.; Proctor, R.; Moore, G.; Higman, W.; Donald, M. Interannual variability of PSP outbreaks on the north east UK coast. J. Plankton Res. 1997, 19, 937–956. [Google Scholar] [CrossRef]
- Lewis, J.; Higman, W.; Kuenstner, S. Occurrence of alexandrium sp. Cysts in sediments from the north east coast of britain. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard-Le Denn, E., Gentien, P., Macrcaillou-Le Baut, C., Eds.; Lavoisier: Paris, France, 1995; pp. 175–180. [Google Scholar]
- Cefas. Harmful Algal Blooms (HABS) Surveillance Programmes and Monitoring. Available online: https://www.cefas.co.uk/data-and-publications/habs/ (accessed on 13 July 2020).
- Gallacher, S.; Smith, E.A. Bacteria and paralytic shellfish toxins. Protist 1999, 150, 245–255. [Google Scholar] [CrossRef]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, Toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Narita, H. Vibrio alginolyticus, a TTX-producing bacterium isolated from the starfish astropecten polyacanthus. Nippon. Suisan Gakkaishi 1987, 53, 617–621. [Google Scholar] [CrossRef]
- Turner, A.D.; Hatfield, R.G.; Rapkova, M.; Higman, W.; Algoet, M.; Suarez-Isla, B.A.; Córdova, M.; Caceres, C.; Riet, J.; Gibbs, R.S.; et al. Comparison of AOAC 2005.06 LC official method with other methodologies for the quantitation of paralytic shellfish poisoning toxins in UK shellfish species. Anal. Bioanal. Chem. 2010, 399, 1257–1270. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Rodríguez-Velasco, M.; Otero, A.; Vieites, J.; Cabado, A.G. A comparative study for PSP toxins quantification by using MBA and HPLC official methods in shellfish. Toxicon 2012, 60, 864–873. [Google Scholar] [CrossRef]
- Dean, K.J.; Hatfield, R.G.; Turner, A.D. Performance characteristics of a refined AOAC 2005.06 pre-column oxidation liquid chromatography with fluorescence detection and hydrophilic interaction liquid chromatography with tandem mass spectrometry methods for the determination of paralytic shellfi. J. AOAC Int. 2020, 98, 628–635. [Google Scholar]
- Medina-Elizalde, J.; García-Mendoza, E.; Turner, A.D.; Sánchez-Bravo, Y.A.; Murillo-Martínez, R. Transformation and depuration of paralytic shellfish toxins in the geoduck clam panopea globosa from the northern gulf of california. Front. Mar. Sci. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Taleb, H.; Vale, P.; Jaime, E.; Blaghen, M. Study of paralytic shellfish poisoning toxin profile in shellfish from the Mediterranean shore of Morocco. Toxicon 2001, 39, 1855–1861. [Google Scholar] [CrossRef]
- Botelho, M.J.; Vale, C.; Grilo, R.V.; Ferreira, J.; Henriques, M.J.B. Uptake and release of paralytic shellfish toxins by the clam Ruditapes decussatus exposed to Gymnodinium catenatum and subsequent depuration. Mar. Environ. Res. 2012, 77, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kotaki, Y.; Oshima, Y.; Yasumoto, T. Bacterial transformation of paralytic shellfish toxins in coral reef crabs and a marine snail. Nippon. Suisan Gakkaishi 1985, 51, 1009–1013. [Google Scholar] [CrossRef]
- Smith, E.; Mackintosh, F.; Grant, F.; Gallacher, S. Sodium channel blocking (SCB) activity and transformation of paralytic shellfish toxins (PST) by dinoflagellate-associated bacteria. Aquat. Microb. Ecol. 2002, 29, 1–9. [Google Scholar] [CrossRef]
- Bricelj, V.; Lee, J.; Cembella, A.; Anderson, D. Uptake kinetics of paralytic shellfish toxins from the dinoflagellate Alexandrium fundyense in the mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 1990, 63, 177–188. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Shumway, S.E. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Sakamoto, S.; Sato, S.; Ogata, T.; Kodama, M. Formation of intermediate conjugates in the reductive transformation of gonyautoxins to saxitoxins by thiol compounds. Fish. Sci. 2000, 66, 136–141. [Google Scholar] [CrossRef]
- Jaime, E.; Gerdts, G.; Luckas, B. In vitro transformation of PSP toxins by different shellfish tissues. Harmful Algae 2007, 6, 308–316. [Google Scholar] [CrossRef]
- Sharp, J.H.; Winson, M.K.; Porter, J.S. Bryozoan metabolites: An ecological perspective. Nat. Prod. Rep. 2007, 24, 659–673. [Google Scholar] [CrossRef]
- Carle, J.; Christophersen, C. Correction. dogger bank itch. the allergen is (2-Hydroxyethyl)dimethylsulfonium Ion. J. Am. Chem. Soc. 1981, 103, 2143. [Google Scholar] [CrossRef]
- Porter, J.; Ellis, J.; Hayward, P.; Rogers, S.; Callaway, R. Geographic variation in the abundance and morphology of the bryozoan alcyonidium diaphanum (Ctenostomata: Alcyonidiidae) in UK coastal waters. J. Mar. Boil. Assoc. UK 2002, 82, 529–535. [Google Scholar] [CrossRef]
- Carlson, H.R.; Pfister, C.A. A seventeen-year study of the rose star crossaster papposus population in a coastal bay in southeast Alaska. Mar. Boil. 1999, 133, 223–230. [Google Scholar] [CrossRef]
- Harwood, T.; Selwood, A.I.; Van Ginkel, R.; Waugh, C.; McNabb, P.S.; Munday, R.; Hay, B.; Thomas, K.; Quilliam, M.A.; Malhi, N.; et al. Paralytic shellfish toxins, including deoxydecarbamoyl-STX, in wild-caught Tasmanian abalone (Haliotis rubra). Toxicon 2014, 90, 213–225. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.; Dowsett, N.; Hallegraeff, G.; Harwood, D.T.; Hay, B.; Ibbott, S.; Malhi, N.; Murray, S.A.; Smith, K.; Tan, J.; et al. Accumulation and depuration of paralytic shellfish toxins by Australian abalone Haliotis rubra: Conclusive association with Gymnodinium catenatum dinoflagellate blooms. Food Control. 2017, 73, 971–980. [Google Scholar] [CrossRef]
- Etheridge, S.M.; Pitcher, G.C.; Roesler, C.S. Depuration and transformation of PSP toxins in the South African abalone haliotis midae. Harmful Algae 2002, 10, 98–101. [Google Scholar]
- Ellis, J.; Rogers, S. The distribution, relative abundance and diversity of echinoderms in the eastern English Channel, Bristol Channel, and Irish Sea. J. Mar. Boil. Assoc. U. K. 2000, 80, 127–138. [Google Scholar] [CrossRef]
- Paramor, O.A. MEFEPO North Sea Atlas; University of Liverpool: Liverpool, UK, 2009. [Google Scholar]
- Anderson, D.M.; Keafer, B.A.; Kleindinst, J.L.; McGillicuddy, J.D.; Martin, J.L.; Norton, K.; Pilskaln, C.H.; Smith, J.L.; Sherwood, C.R.; Butman, B. Alexandrium fundyense cysts in the Gulf of Maine: Long-term time series of abundance and distribution, and linkages to past and future blooms. Deep. Sea Res. Part II: Top. Stud. Oceanogr. 2013, 103, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; White, A.W.; Baden, D.G. The occurrence of PSP toxins in intertidal organisms. In Toxic Dinoflagellates; Elsevier: Amsterdam, The Netherlands, 1985; pp. 467–472. [Google Scholar]
- Cefas, P.-H.M.; Masefield, R.; Bell, D.E. Edible Crab (Cancer Pagurus); Technical Report for Cefas Stock Status: Lowestoft, UK, 2014.
- Lawton, P. Predatory interaction between the brachyuran crab Cancer pagurus and decapod crustacean prey. Mar. Ecol. Prog. Ser. 1989, 52, 169–179. [Google Scholar] [CrossRef]
- Lawton, P.; Hughes, R. Foraging behaviour of the crab Cancer pagurus feeding on the gastropods Nucella lapillus and Littorina littorea: Comparisons with optimal foraging theory. Mar. Ecol. Prog. Ser. 1985, 27, 143–154. [Google Scholar] [CrossRef]
- Hall, S.; Basford, D.; Robertson; Raffaelli, D.; Tuck, I. Patterns of recolonisation and the importance of pit-digging by the crab Cancer pagurus in a subtidal sand habitat. Mar. Ecol. Prog. Ser. 1991, 72, 93–102. [Google Scholar] [CrossRef]
- Mascaro, M.; Seed, R. Foraging behavior of juvenile Carcinus maenas (L.) and Cancer pagurus L. Mar. Boil. 2001, 139, 1135–1145. [Google Scholar]
- Oikawa, H.; Fujita, T.; Saito, K.; Watabe, S.; Satomi, M.; Yano, Y. Comparison of paralytic shellfish poisoning toxin between carnivorous crabs (Telmessus acutidens and Charybdis japonica) and their prey mussel (Mytilus galloprovincialis) in an inshore food chain. Toxicon 2004, 43, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Niu, T.; Xu, Y.-X. Transfer and metabolism of paralytic shellfish poisoning from scallop (Chlamys nobilis) to spiny lobster (Panulirus stimpsoni). Toxicon 2006, 48, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, L.E. Haemolymph protein in xanthid crabs: Its selective binding of saxitoxin and possible role in toxin bioaccumulation. Mar. Boil. 1997, 128, 599–606. [Google Scholar] [CrossRef]
- Shumway, S.E.; Sherman, S.A.; Cembella, A.D.; Selvin, R. Accumulation of Paralytic Shellfish Toxins by Surfclams, Spisula solidissima (Dilwyn, 1897) in the Gulf of maine: Seasonal changes, Distribution between Tissues, and Notes on Feeding Habits. Nat. Toxins 1994, 2, 236–251. [Google Scholar] [CrossRef]
- Graneli, E.; Sundstrom, B.; Edler, L.; Anderson, D.M. Uptake and distribution of PSP toxins in butter clams. In Toxic Marine Phytoplankton; Elsevier: Amsterdam, The Netherlands, 1990; pp. 257–262. [Google Scholar]
- Ellis, J.R. Personal communication, 13 July 2020.
- Bbc. Beach Safe for Dogs after Alert. Available online: http://news.bbc.co.uk/1/hi/england/northamptonshire/4662752.stm (accessed on 13 July 2006).
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T. Ultrahigh-Performance hydrophilic interaction liquid chromatography with tandem mass spectrometry method for the determination of paralytic shellfish toxins and tetrodotoxin in mussels, oysters, clams, cockles, and scallops: Collaborative study. J. AOAC Int. 2019, 103, 1–30. [Google Scholar] [CrossRef]
- Jinrong, X.; Zhongming, C. AOAC Official Method 2005.06 Paralytic shellfish poisoning toxins in shellfish prechromatographic oxidation and liquid chromatography with fluorescence detection. J. AOAC Int. 2005, 23, 366–369. [Google Scholar]
- Turner, A.D.; McNabb, P.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single-Laboratory Validation of a Multitoxin ultra-Performance LC-Hydrophilic interaction LC-MS/MS method for quantitation of paralytic shellfish toxins in bivalve shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [CrossRef]
- Hatfield, R.G.; Punn, R.; Algoet, M.; Turner, A.D. A rapid method for the analysis of paralytic shellfish toxins utilizing standard pressure HPLC: Refinement of AOAC 2005.06. J. AOAC Int. 2016, 99, 475–480. [Google Scholar] [CrossRef]
- Chambers, T.M.; Hastie, T. Statistical Models in S.; Wadsworth & Brooks: Cole, OH, USA, 1992. [Google Scholar]
- Lloyd, D. An introduction to. Ind. Commer. Train. 1978, 10, 11–18. [Google Scholar] [CrossRef]
- Chavent, M.; Kuentz-Simonet, V.; Labenne, A.; Saracco, J. Multivariate Analysis of Mixed Data: The R Package PCAmixdata; Cornel University: Ithaca, NY, USA, 2015. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core team. nlme: Linear and Nonlinear Mixed Effects Models; R Package Nlme Version; R Core team: Vienna, Austria, 2018; Volume 3, pp. 1–83. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
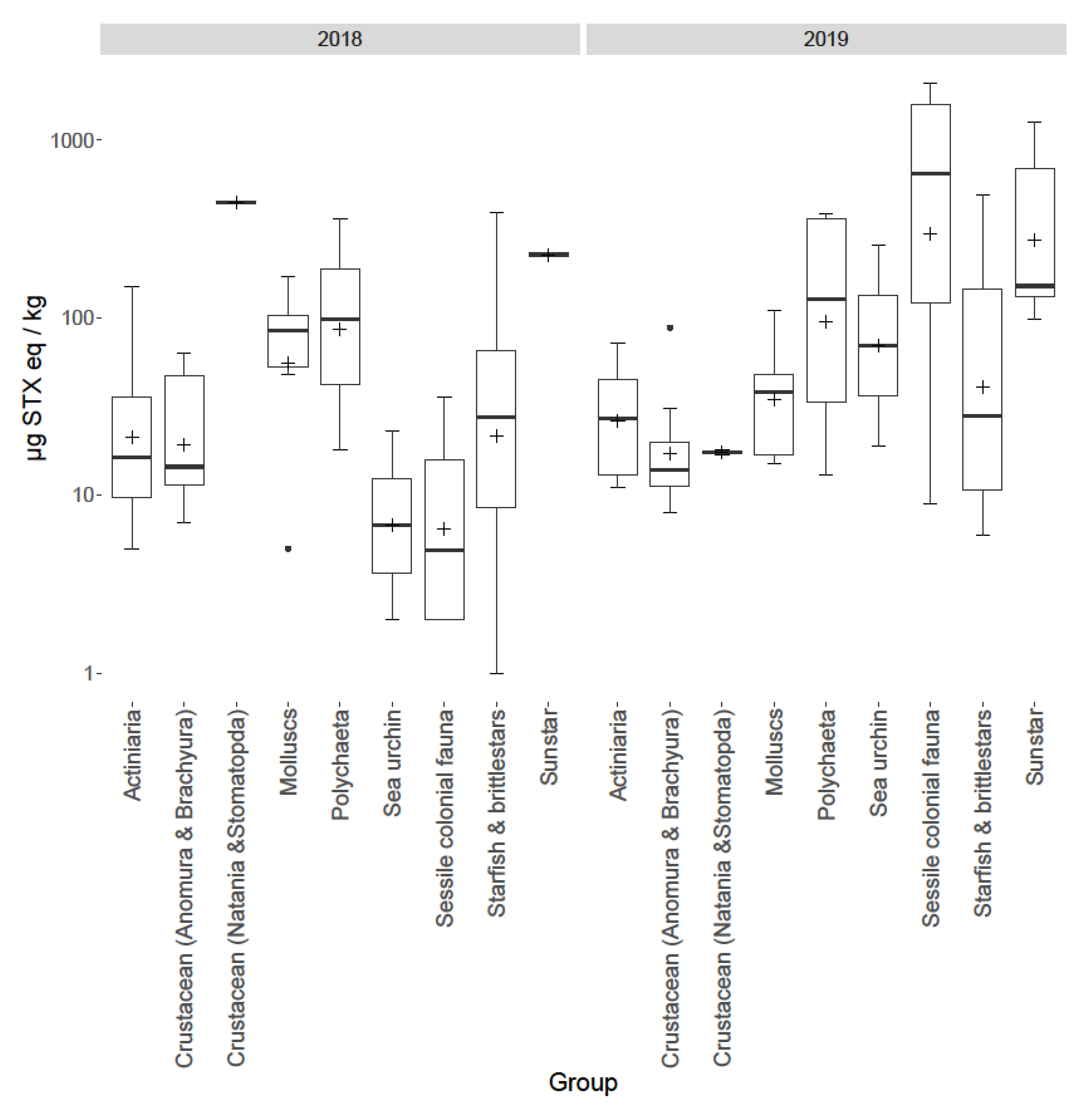



| Faunal Group | Species | Feeding Guild | Mean Toxicity | s.d | Toxicity Range | Number of Samples | % Samples > LOD |
|---|---|---|---|---|---|---|---|
| Sunstar | Crossaster papposus | SP | 448 | 472 | 98–1275 | 7 | 100% |
| Starfish & Brittlestar (excluding C. papposus) | Anseropoda placenta, Asterias rubens, Astropecten irregularis, Henricia oculata, Henricia sp., Hippasteria phrygiana, Luidia ciliaris, Porania pulvillus, Stichastrella rosea, Ophiura ophiura | SP | 80 | 126 | nd–488 | 50 | 56% |
| Sea urchins | Echinus esculentus, Echinus sp., Psammechinus miliaris | OG | 75 | 122 | nd–257 | 11 | 36% |
| Crustaceans (Natantia and Stomatopoda) | Rissoides desmarseti | SP | 161 | 247 | nd–446 | 6 | 50% |
| Crangon sp., Pandalus sp., unidentified Natantia | DS/SP | ||||||
| Crustaceans (Anomura and Brachyura) | Atelecyclus rotundatus, Atelecyclus sp., Carcinus maenas, Corystes cassivelaunus, Inachus sp., Liocarcinus depurator, Liocarcinus holsatus, Liocarcinus sp., Munida rugosa, Necora puber, Pagurus bernhardus, Pagurus sp., Portunidae (indet.) | SP | 25 | 22 | nd–88 | 37 | 70% |
| Goneplax rhomboides | DS | ||||||
| Polychaetes | Aphrodita aculeata | SP | 182 | 175 | nd–386 | 13 | 54% |
| Molluscs | Aequipecten opercularis, Bivalvia (indet.) | FF | 66 | 51 | nd–172 | 17 | 65% |
| Crepidula fornicata | FF/OG | ||||||
| Hinia reticulata, Scaphander lignarius | DS | ||||||
| Buccinidae (indet.), Buccinum undatum, Colus gracilus | DS/SP | ||||||
| Neptunea antiqua eggmass | N/A | ||||||
| Actiniaria | Metridium senile | FF | 40 | 44 | nd–150 | 11 | 82% |
| Anemone (indet.) | P | ||||||
| Sessile colonial fauna | Alcyonidium diaphanum, Alcyonium digitatum, Haliclona oculata, Porifera (indet.) | FF | 488 | 818 | nd–2090 | 14 | 57% |
| Other | Parastichopus tremulus, Echiura (indet.) | DF | nd | nd | 2 | 0% | |
| Total | 127 | 301 | nd–2090 | 168 | 61% |
| Criteria | LC-FLD | LC-MS/MS |
|---|---|---|
| Mean toxicity (µg STX eq./kg) | 130 | 145 |
| Sd | 366 | 334 |
| Maximum toxicity (µg STX eq./kg) | 2744 | 2091 |
| Correlation | 0.87 | |
| t score | −0.47 | |
| t crit | 2 | |
| Means tested | 15% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dean, K.J.; Hatfield, R.G.; Lee, V.; Alexander, R.P.; Lewis, A.M.; Maskrey, B.H.; Teixeira Alves, M.; Hatton, B.; Coates, L.N.; Capuzzo, E.; et al. Multiple New Paralytic Shellfish Toxin Vectors in Offshore North Sea Benthos, a Deep Secret Exposed. Mar. Drugs 2020, 18, 400. https://doi.org/10.3390/md18080400
Dean KJ, Hatfield RG, Lee V, Alexander RP, Lewis AM, Maskrey BH, Teixeira Alves M, Hatton B, Coates LN, Capuzzo E, et al. Multiple New Paralytic Shellfish Toxin Vectors in Offshore North Sea Benthos, a Deep Secret Exposed. Marine Drugs. 2020; 18(8):400. https://doi.org/10.3390/md18080400
Chicago/Turabian StyleDean, Karl J., Robert G. Hatfield, Vanessa Lee, Ryan P. Alexander, Adam M. Lewis, Benjamin H. Maskrey, Mickael Teixeira Alves, Benjamin Hatton, Lewis N. Coates, Elisa Capuzzo, and et al. 2020. "Multiple New Paralytic Shellfish Toxin Vectors in Offshore North Sea Benthos, a Deep Secret Exposed" Marine Drugs 18, no. 8: 400. https://doi.org/10.3390/md18080400
APA StyleDean, K. J., Hatfield, R. G., Lee, V., Alexander, R. P., Lewis, A. M., Maskrey, B. H., Teixeira Alves, M., Hatton, B., Coates, L. N., Capuzzo, E., Ellis, J. R., & Turner, A. D. (2020). Multiple New Paralytic Shellfish Toxin Vectors in Offshore North Sea Benthos, a Deep Secret Exposed. Marine Drugs, 18(8), 400. https://doi.org/10.3390/md18080400







