Omega-3 Fatty Acid-Enriched Fish Oil and Selenium Combination Modulates Endoplasmic Reticulum Stress Response Elements and Reverses Acquired Gefitinib Resistance in HCC827 Lung Adenocarcinoma Cells
Abstract
1. Introduction
2. Results
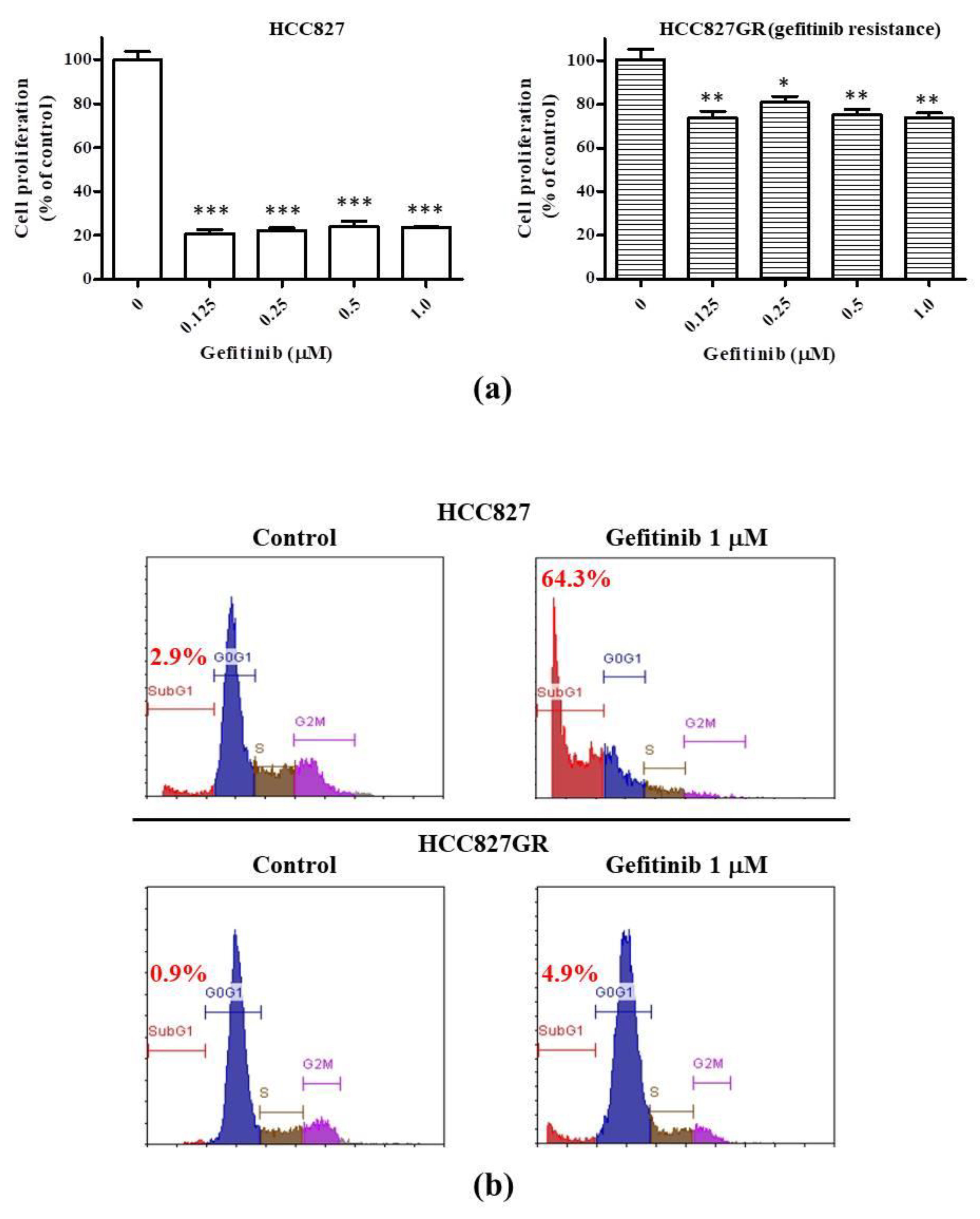
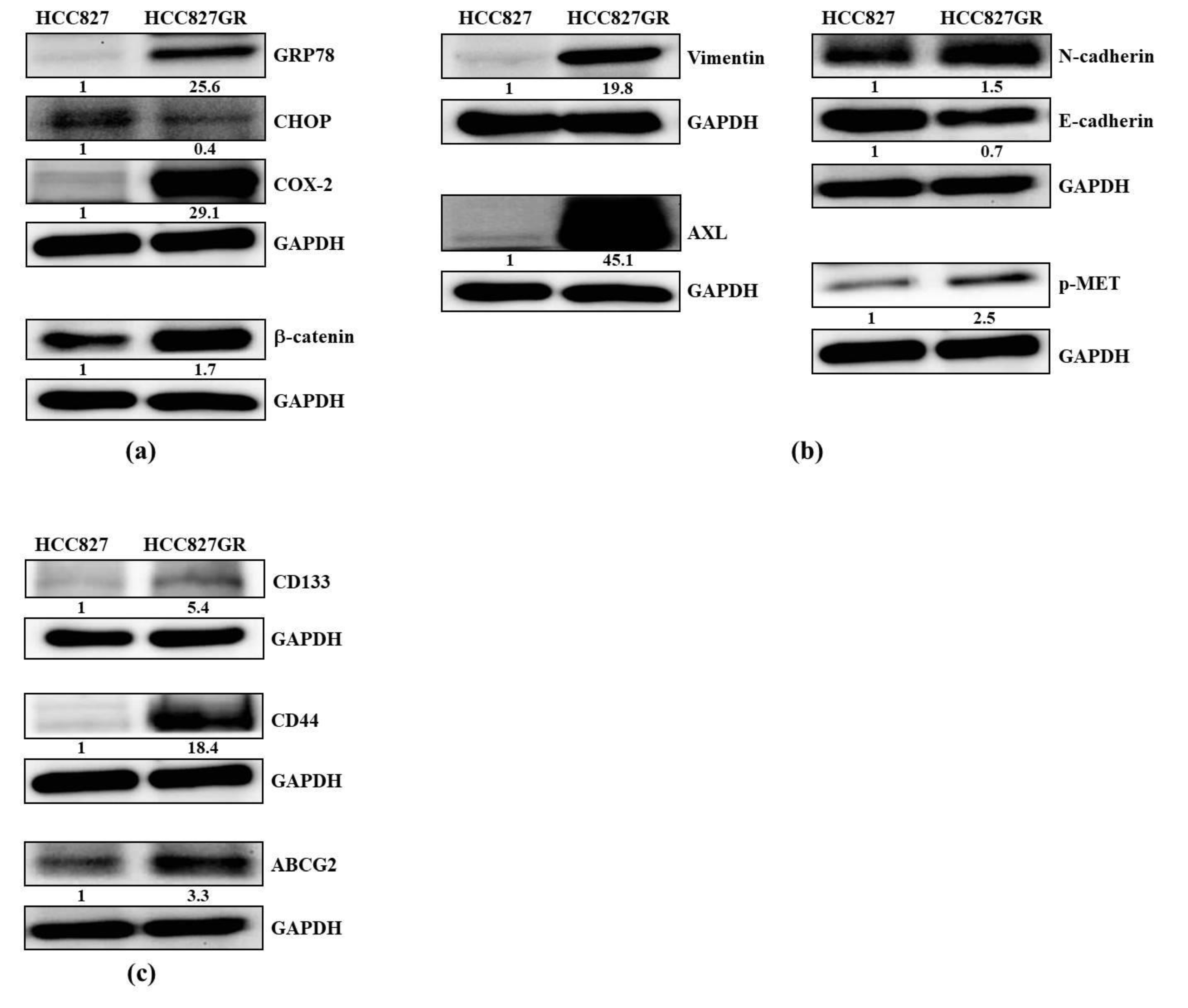
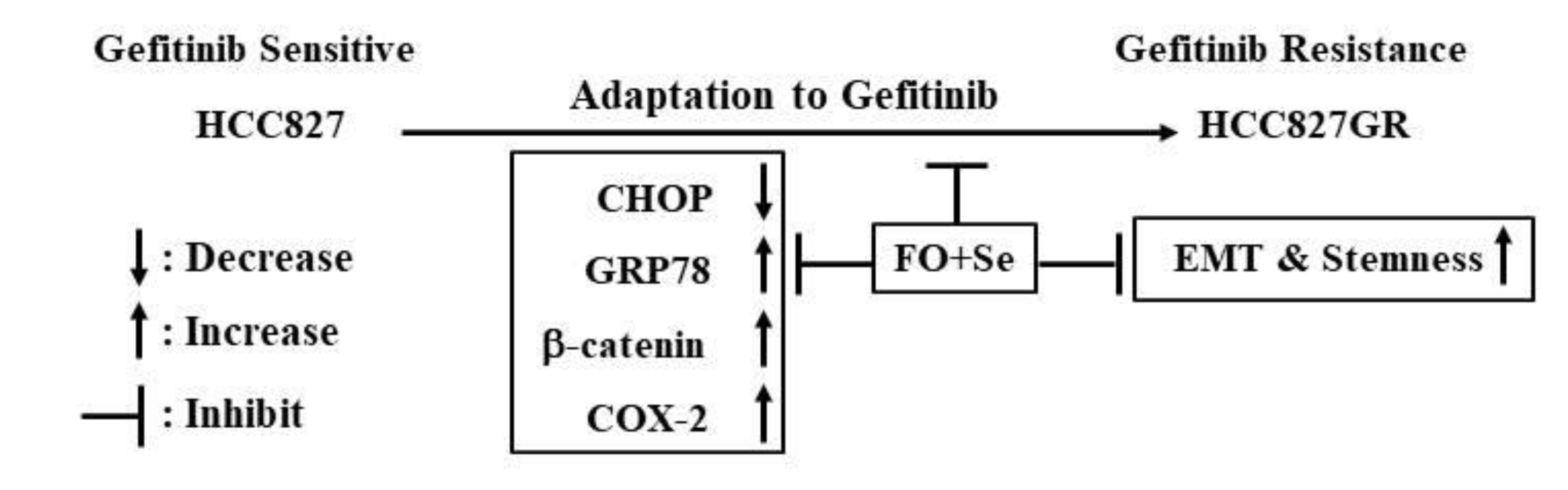
2.1. The Gefitinib-Resistant Subline HCC827GR Possesses Higher GRP78, β-Catenin, and COX-2 but Has Lower CHOP Than the Parental HCC827
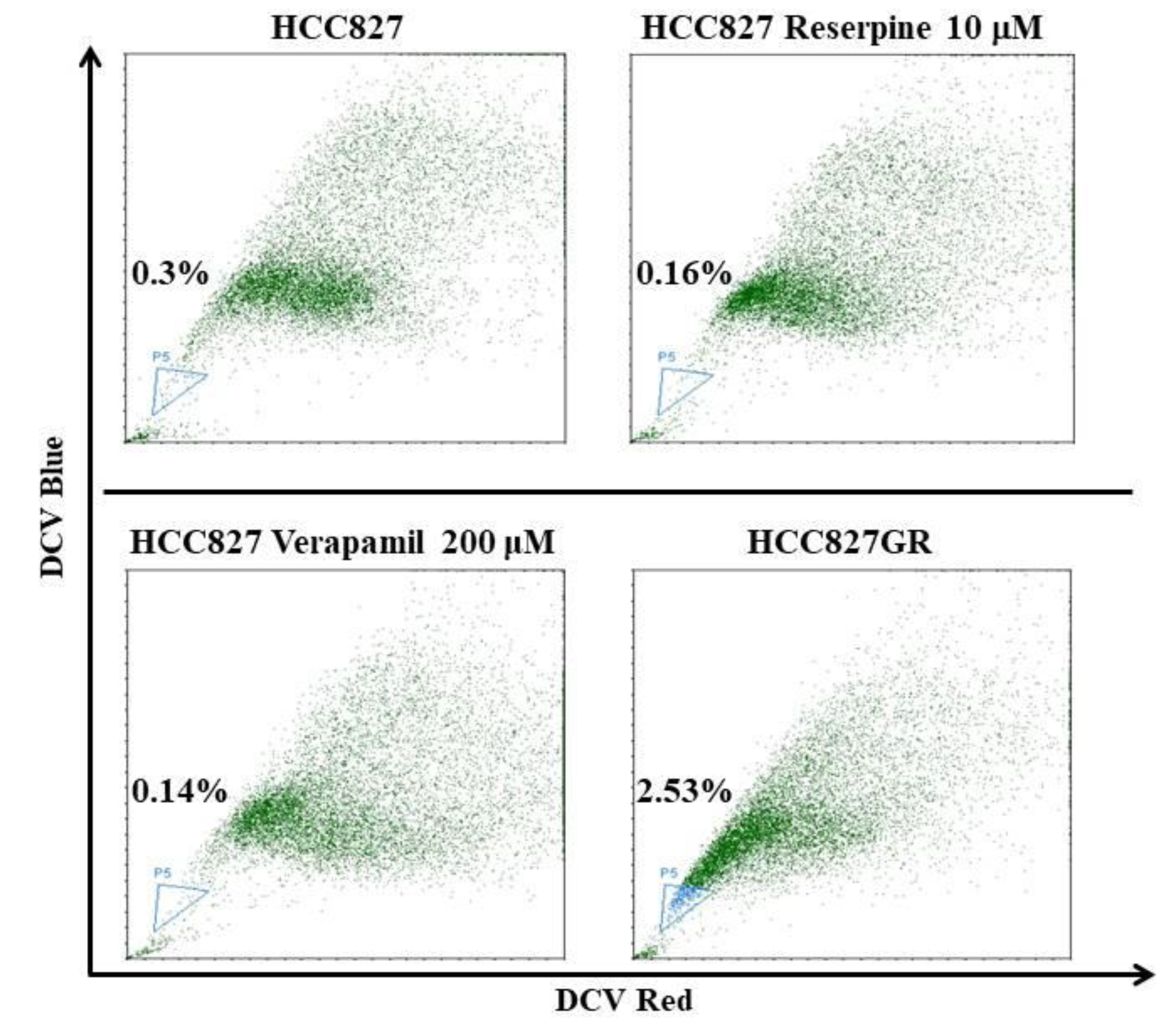
2.2. The EMT Markers and Cancer Stem Cell Traits Are Raised in Gefitinib-Resistant Subline HCC827GR
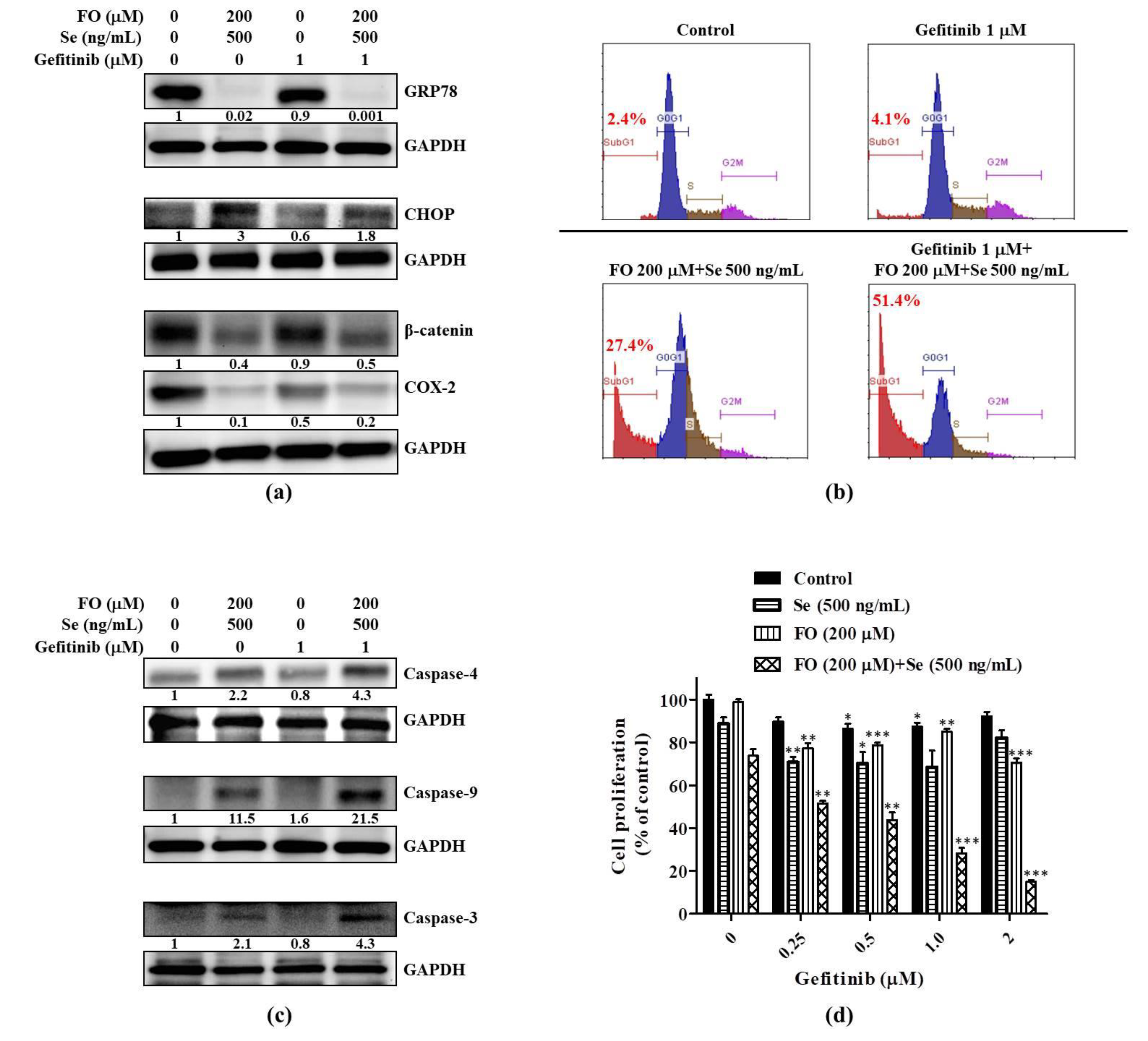
2.3. Combination of FO and Se Counteracts the Elevation of Prosurvival Effectors in HCC827GR Cells and Restores Their Sensitivity to Gefitinib
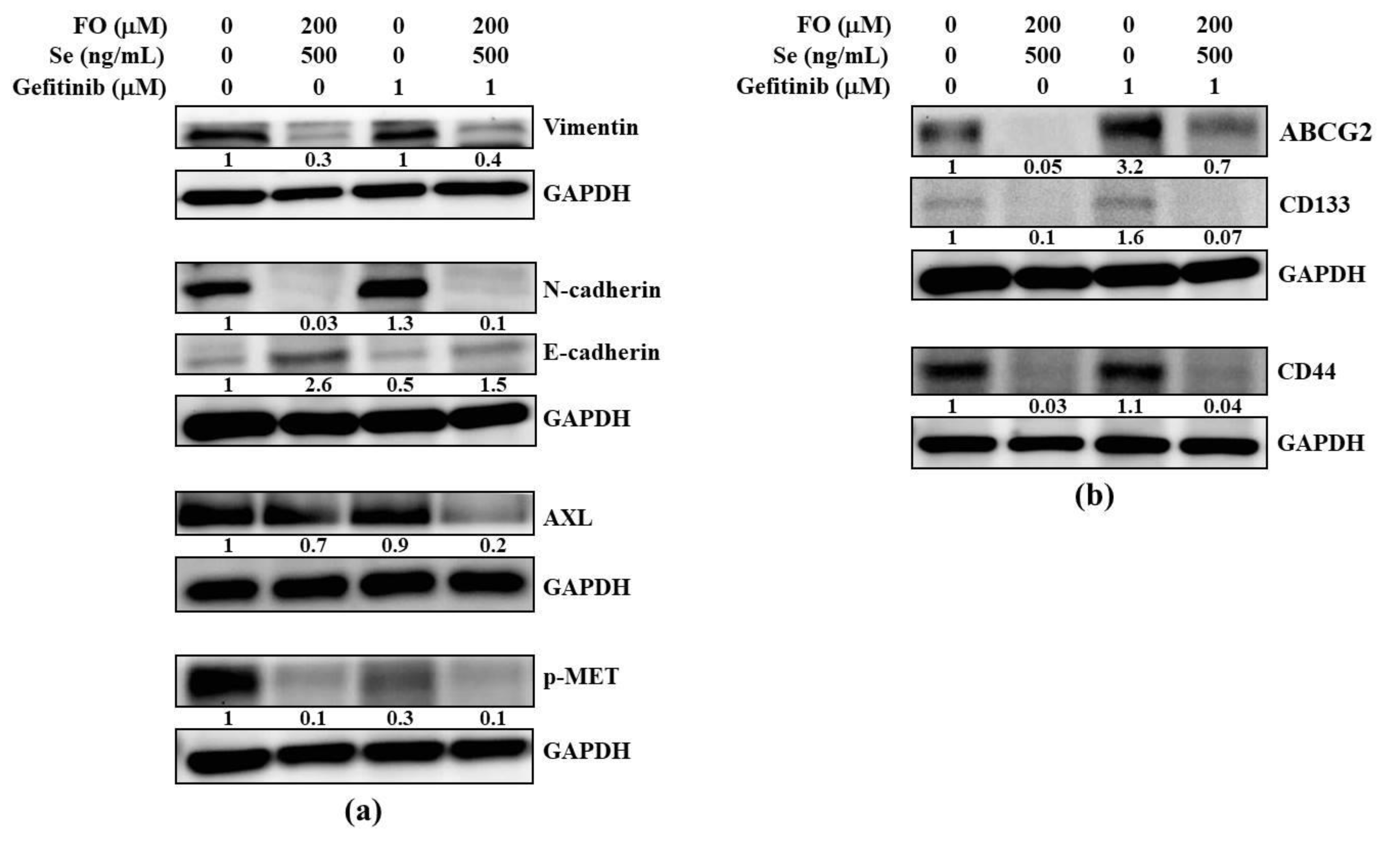
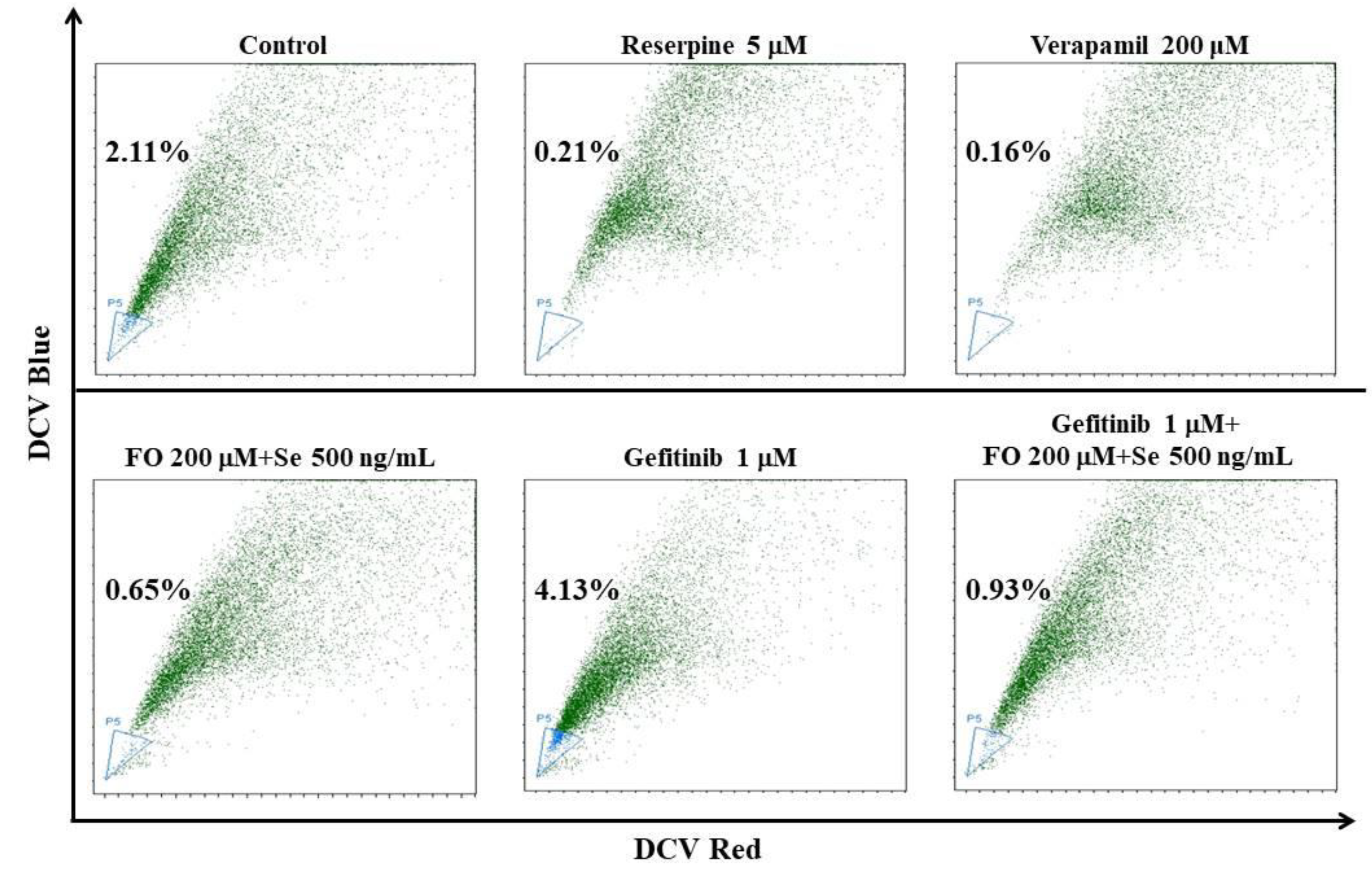
2.4. Combination of FO and Se Suppresses the EMT and Cancer Stem Cell Traits in Gefitinib-Resistant Subline HCC827GR
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents and Chemicals
4.3. Measurement of Cell Viability
4.4. Analysis of Apoptotic Sub-G1 Fraction by Propidium Iodide Staining
4.5. Side Population Analysis by DyeCycle Violet (DCV) Exclusion
4.6. Western Blot
4.7. Antibodies
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Paez, J.G.; Janne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Shien, K.; Toyooka, S.; Yamamoto, H.; Soh, J.; Jida, M.; Thu, K.L.; Hashida, S.; Maki, Y.; Ichihara, E.; Asano, H.; et al. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 2013, 73, 3051–3061. [Google Scholar] [CrossRef]
- Morgillo, F.; Della Corte, C.M.; Fasano, M.; Ciardiello, F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open 2016, 1, e000060. [Google Scholar] [CrossRef]
- Kim, D.; Bach, D.H.; Fan, Y.H.; Luu, T.T.; Hong, J.Y.; Park, H.J.; Lee, S.K. AXL degradation in combination with EGFR-TKI can delay and overcome acquired resistance in human non-small cell lung cancer cells. Cell Death Dis. 2019, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, J.C.; Lin, L.; Olivas, V.; Au, V.; LaFramboise, T.; Abdel-Rahman, M.; Wang, X.; Levine, A.D.; Rho, J.K.; et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012, 44, 852–860. [Google Scholar] [CrossRef]
- Sugita, S.; Ito, K.; Yamashiro, Y.; Moriya, S.; Che, X.F.; Yokoyama, T.; Hiramoto, M.; Miyazawa, K. EGFR-independent autophagy induction with gefitinib and enhancement of its cytotoxic effect by targeting autophagy with clarithromycin in non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2015, 461, 28–34. [Google Scholar] [CrossRef]
- Wang, Z.; Du, T.; Dong, X.; Li, Z.; Wu, G.; Zhang, R. Autophagy inhibition facilitates erlotinib cytotoxicity in lung cancer cells through modulation of endoplasmic reticulum stress. Int. J. Oncol. 2016, 48, 2558–2566. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kulp, S.K.; Wang, D.; Yang, C.C.; Sargeant, A.M.; Hung, J.H.; Kashida, Y.; Yamaguchi, M.; Chang, G.D.; Chen, C.S. Targeting endoplasmic reticulum stress and Akt with OSU-03012 and gefitinib or erlotinib to overcome resistance to epidermal growth factor receptor inhibitors. Cancer Res. 2008, 68, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Fell, G.L.; Cho, B.S.; Pan, A.; Nose, V.; Anez-Bustillos, L.; Dao, D.T.; Baker, M.A.; Nandivada, P.; Gura, K.M.; Puder, M. A Comparison of Fish Oil Sources for Parenteral Lipid Emulsions in a Murine Model. JPEN J. Parenter. Enter. Nutr. 2017, 41, 181–187. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M. Fish oil omega-3 fatty acids and cardio-metabolic health, alone or with statins. Eur. J. Clin. Nutr. 2013, 67, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jeong, S.; Jing, K.; Shin, S.; Kim, S.; Heo, J.Y.; Kweon, G.R.; Park, S.K.; Wu, T.; Park, J.I.; et al. Docosahexaenoic Acid Induces Cell Death in Human Non-Small Cell Lung Cancer Cells by Repressing mTOR via AMPK Activation and PI3K/Akt Inhibition. BioMed Res. Int. 2015, 2015, 239764. [Google Scholar] [CrossRef]
- Yin, Y.; Sui, C.; Meng, F.; Ma, P.; Jiang, Y. The omega-3 polyunsaturated fatty acid docosahexaenoic acid inhibits proliferation and progression of non-small cell lung cancer cells through the reactive oxygen species-mediated inactivation of the PI3K /Akt pathway. Lipids Health Dis. 2017, 16, 87. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer 2011, 117, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, J.; Jiang, C.; Deng, Y.; Ozten, N.; Bosland, M.C. Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutr. Cancer 2016, 68, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising Selenium for Modulation of Cancer Treatments. Anticancer. Res. 2017, 37, 6497–6509. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, T.; Zheng, W.; Yang, Y. Selenium enhances antioxidant activity and photosynthesis in Ulva fasciata. J. Appl. Phycol. 2015, 27, 555–562. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Y.; Chen, T.; Zheng, W.; Yang, Y. Effects of selenium on antioxidant enzymes and photosynthesis in the edible seaweed Gracilaria lemaneiformis. J. Appl. Phycol. 2019, 31, 1303–1310. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, Y.; Luo, H.; Yang, Y. Selenium-Containing Polysaccharide-Protein Complex in Se-Enriched Ulva fasciata Induces Mitochondria-Mediated Apoptosis in A549 Human Lung Cancer Cells. Mar. Drugs 2017, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Wen, Z.S.; Tang, Z.; Ma, L.; Zhu, T.L.; Wang, Y.M.; Xiang, X.W.; Zheng, B. Protective Effect of Low Molecular Weight Seleno-Aminopolysaccharide on the Intestinal Mucosal Oxidative Damage. Mar. Drugs 2019, 17, 64. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Selenium yeast: Composition, quality, analysis, and safety. Pure Appl. Chem. 2006, 78, 105–109. [Google Scholar] [CrossRef]
- Shigemi, Z.; Manabe, K.; Hara, N.; Baba, Y.; Hosokawa, K.; Kagawa, H.; Watanabe, T.; Fujimuro, M. Methylseleninic acid and sodium selenite induce severe ER stress and subsequent apoptosis through UPR activation in PEL cells. Chem. Biol. Interact. 2017, 266, 28–37. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Dong, Y.; Park, Y.M.; Ip, C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005, 65, 9073–9079. [Google Scholar] [CrossRef]
- Zu, K.; Ip, C. Synergy between selenium and vitamin E in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Res. 2003, 63, 6988–6995. [Google Scholar]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim. Biophys. Acta 2012, 1822, 1762–1772. [Google Scholar] [CrossRef]
- Jakobsen, C.H.; Storvold, G.L.; Bremseth, H.; Follestad, T.; Sand, K.; Mack, M.; Olsen, K.S.; Lundemo, A.G.; Iversen, J.G.; Krokan, H.E.; et al. DHA induces ER stress and growth arrest in human colon cancer cells: Associations with cholesterol and calcium homeostasis. J. Lipid Res. 2008, 49, 2089–2100. [Google Scholar] [CrossRef]
- Kao, R.H.; Lai, G.M.; Chow, J.M.; Liao, C.H.; Zheng, Y.M.; Tsai, W.L.; Hsia, S.; Lai, I.C.; Lee, H.L.; Chuang, S.E.; et al. Opposite Regulation of CHOP and GRP78 and Synergistic Apoptosis Induction by Selenium Yeast and Fish Oil via AMPK Activation in Lung Adenocarcinoma Cells. Nutrients 2018, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Gu, P.; Zhou, C.; Liang, A.; Ren, S.; Liu, F.; Zeng, Y.; Wu, Y.; Zhao, Y.; Huang, B.; et al. beta-Catenin overexpression is associated with gefitinib resistance in non-small cell lung cancer cells. Pulm. Pharmacol. Ther. 2014, 28, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, F.; Lu, H.; Xu, S.; Zou, L.; Tian, Q.; Fu, Y.; Lin, X.; Liu, L.; Yuan, P.; et al. Targeting the COX2/MET/TOPK signaling axis induces apoptosis in gefitinib-resistant NSCLC cells. Cell Death Dis. 2019, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Starrett, J.H.; Stewart, T.; Ashtekar, K.; Walther, Z.; Wurtz, A.; Lu, D.; Park, J.H.; DeVeaux, M.; Song, X.; et al. The EGFR Exon 19 Mutant L747-A750>P Exhibits Distinct Sensitivity to Tyrosine Kinase Inhibitors in Lung Adenocarcinoma. Clin. Cancer Res. 2019, 25, 6382–6391. [Google Scholar] [CrossRef]
- Reid, M.E.; Stratton, M.S.; Lillico, A.J.; Fakih, M.; Natarajan, R.; Clark, L.C.; Marshall, J.R. A report of high-dose selenium supplementation: Response and toxicities. J. Trace Elem. Med. Biol. 2004, 18, 69–74. [Google Scholar] [CrossRef]
- Kuriki, K.; Nagaya, T.; Tokudome, Y.; Imaeda, N.; Fujiwara, N.; Sato, J.; Goto, C.; Ikeda, M.; Maki, S.; Tajima, K.; et al. Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: A cross-sectional study. J. Nutr. 2003, 133, 3643–3650. [Google Scholar] [CrossRef]
- Cohen, M.H.; Williams, G.A.; Sridhara, R.; Chen, G.; McGuinn, W.D., Jr.; Morse, D.; Abraham, S.; Rahman, A.; Liang, C.; Lostritto, R.; et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin. Cancer Res. 2004, 10, 1212–1218. [Google Scholar] [CrossRef]
- Weng, C.H.; Chen, L.Y.; Lin, Y.C.; Shih, J.Y.; Tseng, R.Y.; Chiu, A.C.; Yeh, Y.H.; Liu, C.; Lin, Y.T.; Fang, J.M.; et al. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene 2019, 38, 455–468. [Google Scholar] [CrossRef]
- Ware, K.E.; Hinz, T.K.; Kleczko, E.; Singleton, K.R.; Marek, L.A.; Helfrich, B.A.; Cummings, C.T.; Graham, D.K.; Astling, D.; Tan, A.C.; et al. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis 2013, 2, e39. [Google Scholar] [CrossRef]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W. Overcoming acquired resistance of gefitinib in lung cancer cells without T790M by AZD9291 or Twist1 knockdown in vitro and in vivo. Arch. Toxicol. 2019, 93, 1555–1571. [Google Scholar] [CrossRef] [PubMed]
- Lelj-Garolla, B.; Kumano, M.; Beraldi, E.; Nappi, L.; Rocchi, P.; Ionescu, D.N.; Fazli, L.; Zoubeidi, A.; Gleave, M.E. Hsp27 Inhibition with OGX-427 Sensitizes Non-Small Cell Lung Cancer Cells to Erlotinib and Chemotherapy. Mol. Cancer Ther. 2015, 14, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Avril, T.; Vauleon, E.; Chevet, E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 2017, 6, e373. [Google Scholar] [CrossRef]
- Lin, Y.C.; Shih, J.Y.; Huang, W.J.; Chao, S.W.; Chang, Y.L.; Chen, C.C. DUSP1 expression induced by HDAC1 inhibition mediates gefitinib sensitivity in non-small cell lung cancers. Clin. Cancer Res. 2015, 21, 428–438. [Google Scholar] [CrossRef]
- Casas, C. GRP78 at the Centre of the Stage in Cancer and Neuroprotection. Front. Neurosci. 2017, 11, 177. [Google Scholar] [CrossRef]
- Kwon, D.; Koh, J.; Kim, S.; Go, H.; Min, H.S.; Kim, Y.A.; Kim, D.K.; Jeon, Y.K.; Chung, D.H. Overexpression of endoplasmic reticulum stress-related proteins, XBP1s and GRP78, predicts poor prognosis in pulmonary adenocarcinoma. Lung Cancer 2018, 122, 131–137. [Google Scholar] [CrossRef]
- Van Lidth de Jeude, J.F.; Spaan, C.N.; Meijer, B.J.; Smit, W.L.; Soeratram, T.T.D.; Wielenga, M.C.B.; Westendorp, B.F.; Lee, A.S.; Meisner, S.; Vermeulen, J.L.M.; et al. Heterozygosity of Chaperone Grp78 Reduces Intestinal Stem Cell Regeneration Potential and Protects against Adenoma Formation. Cancer Res. 2018, 78, 6098–6106. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Brandhorst, S.; Rangel, D.F.; Navarrete, G.; Cohen, P.; Longo, V.D.; Chen, J.; Groshen, S.; Morgan, T.E.; Dubeau, L. Effects of Prolonged GRP78 Haploinsufficiency on Organ Homeostasis, Behavior, Cancer and Chemotoxic Resistance in Aged Mice. Sci. Rep. 2017, 7, 40919. [Google Scholar] [CrossRef]
- Dadey, D.Y.A.; Kapoor, V.; Hoye, K.; Khudanyan, A.; Collins, A.; Thotala, D.; Hallahan, D.E. Antibody Targeting GRP78 Enhances the Efficacy of Radiation Therapy in Human Glioblastoma and Non-Small Cell Lung Cancer Cell Lines and Tumor Models. Clin. Cancer Res. 2017, 23, 2556–2564. [Google Scholar] [CrossRef]
- Cook, K.L.; Clarke, R. Role of GRP78 in promoting therapeutic-resistant breast cancer. Future Med. Chem. 2015, 7, 1529–1534. [Google Scholar] [CrossRef]
- Gu, Y.J.; Li, H.D.; Zhao, L.; Zhao, S.; He, W.B.; Rui, L.; Su, C.; Zheng, H.C.; Su, R.J. GRP78 confers the resistance to 5-FU by activating the c-Src/LSF/TS axis in hepatocellular carcinoma. Oncotarget 2015, 6, 33658–33674. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.B.; Huang, W.; Zeleniak, A.E.; Hindoyan, A.; Wu, H.; Donahue, T.R.; Hill, R. Expression of GRP78, Master Regulator of the Unfolded Protein Response, Increases Chemoresistance in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2016, 15, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.H.; Tung, C.L.; Su, Y.C.; Tsao, C.J.; Wu, T.F. Proteomic Profile of Sorafenib Resistance in Hepatocellular Carcinoma; GRP78 Expression Is Associated With Inferior Response to Sorafenib. Cancer Genom. Proteom. 2019, 16, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gao, Y.; Liu, A.; Yang, X.; Huang, F.; Xu, L.; Danfeng, X.; Chen, L. EIF3D promotes sunitinib resistance of renal cell carcinoma by interacting with GRP78 and inhibiting its degradation. EBioMedicine 2019, 49, 189–201. [Google Scholar] [CrossRef]
- Dauer, P.; Sharma, N.S.; Gupta, V.K.; Durden, B.; Hadad, R.; Banerjee, S.; Dudeja, V.; Saluja, A. ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness”. Cell Death Dis. 2019, 10, 132. [Google Scholar] [CrossRef]
- Tseng, C.C.; Stanciauskas, R.; Zhang, P.; Woo, D.; Wu, K.; Kelly, K.; Gill, P.S.; Yu, M.; Pinaud, F.; Lee, A.S. GRP78 regulates CD44v membrane homeostasis and cell spreading in tamoxifen-resistant breast cancer. Life Sci. Alliance 2019, 2. [Google Scholar] [CrossRef]
- Sun, L.L.; Chen, C.M.; Zhang, J.; Wang, J.; Yang, C.Z.; Lin, L.Z. Glucose-Regulated Protein 78 Signaling Regulates Hypoxia-Induced Epithelial-Mesenchymal Transition in A549 Cells. Front. Oncol. 2019, 9, 137. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Fan, Y.; Li, H.; Li, Y. Overexpressed GRP78 affects EMT and cell-matrix adhesion via autocrine TGF-beta/Smad2/3 signaling. Int. J. Biochem. Cell Biol. 2015, 64, 202–211. [Google Scholar] [CrossRef]
- Murdolo, G.; Bartolini, D.; Tortoioli, C.; Piroddi, M.; Torquato, P.; Galli, F. Selenium and Cancer Stem Cells. Adv. Cancer Res. 2017, 136, 235–257. [Google Scholar] [CrossRef]
- Hwang, W.; Chiu, Y.F.; Kuo, M.H.; Lee, K.L.; Lee, A.C.; Yu, C.C.; Chang, J.L.; Huang, W.C.; Hsiao, S.H.; Lin, S.E.; et al. Expression of Neuroendocrine Factor VGF in Lung Cancer Cells Confers Resistance to EGFR Kinase Inhibitors and Triggers Epithelial-to-Mesenchymal Transition. Cancer Res. 2017, 77, 3013–3026. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-H.; Tzeng, Y.-T.; Lai, G.-M.; Chang, C.-L.; Hu, M.-H.; Tsai, W.-L.; Liu, Y.-R.; Hsia, S.; Chuang, S.-E.; Chiou, T.-J.; et al. Omega-3 Fatty Acid-Enriched Fish Oil and Selenium Combination Modulates Endoplasmic Reticulum Stress Response Elements and Reverses Acquired Gefitinib Resistance in HCC827 Lung Adenocarcinoma Cells. Mar. Drugs 2020, 18, 399. https://doi.org/10.3390/md18080399
Liao C-H, Tzeng Y-T, Lai G-M, Chang C-L, Hu M-H, Tsai W-L, Liu Y-R, Hsia S, Chuang S-E, Chiou T-J, et al. Omega-3 Fatty Acid-Enriched Fish Oil and Selenium Combination Modulates Endoplasmic Reticulum Stress Response Elements and Reverses Acquired Gefitinib Resistance in HCC827 Lung Adenocarcinoma Cells. Marine Drugs. 2020; 18(8):399. https://doi.org/10.3390/md18080399
Chicago/Turabian StyleLiao, Chien-Huang, Yu-Tien Tzeng, Gi-Ming Lai, Chia-Lun Chang, Ming-Hung Hu, Wei-Lun Tsai, Yun-Ru Liu, Simon Hsia, Shuang-En Chuang, Tzeon-Jye Chiou, and et al. 2020. "Omega-3 Fatty Acid-Enriched Fish Oil and Selenium Combination Modulates Endoplasmic Reticulum Stress Response Elements and Reverses Acquired Gefitinib Resistance in HCC827 Lung Adenocarcinoma Cells" Marine Drugs 18, no. 8: 399. https://doi.org/10.3390/md18080399
APA StyleLiao, C.-H., Tzeng, Y.-T., Lai, G.-M., Chang, C.-L., Hu, M.-H., Tsai, W.-L., Liu, Y.-R., Hsia, S., Chuang, S.-E., Chiou, T.-J., Wang, L.-M., Whang-Peng, J., & Yao, C.-J. (2020). Omega-3 Fatty Acid-Enriched Fish Oil and Selenium Combination Modulates Endoplasmic Reticulum Stress Response Elements and Reverses Acquired Gefitinib Resistance in HCC827 Lung Adenocarcinoma Cells. Marine Drugs, 18(8), 399. https://doi.org/10.3390/md18080399





