OctoPartenopin: Identification and Preliminary Characterization of a Novel Antimicrobial Peptide from the Suckers of Octopus vulgaris
Abstract
1. Introduction
2. Results
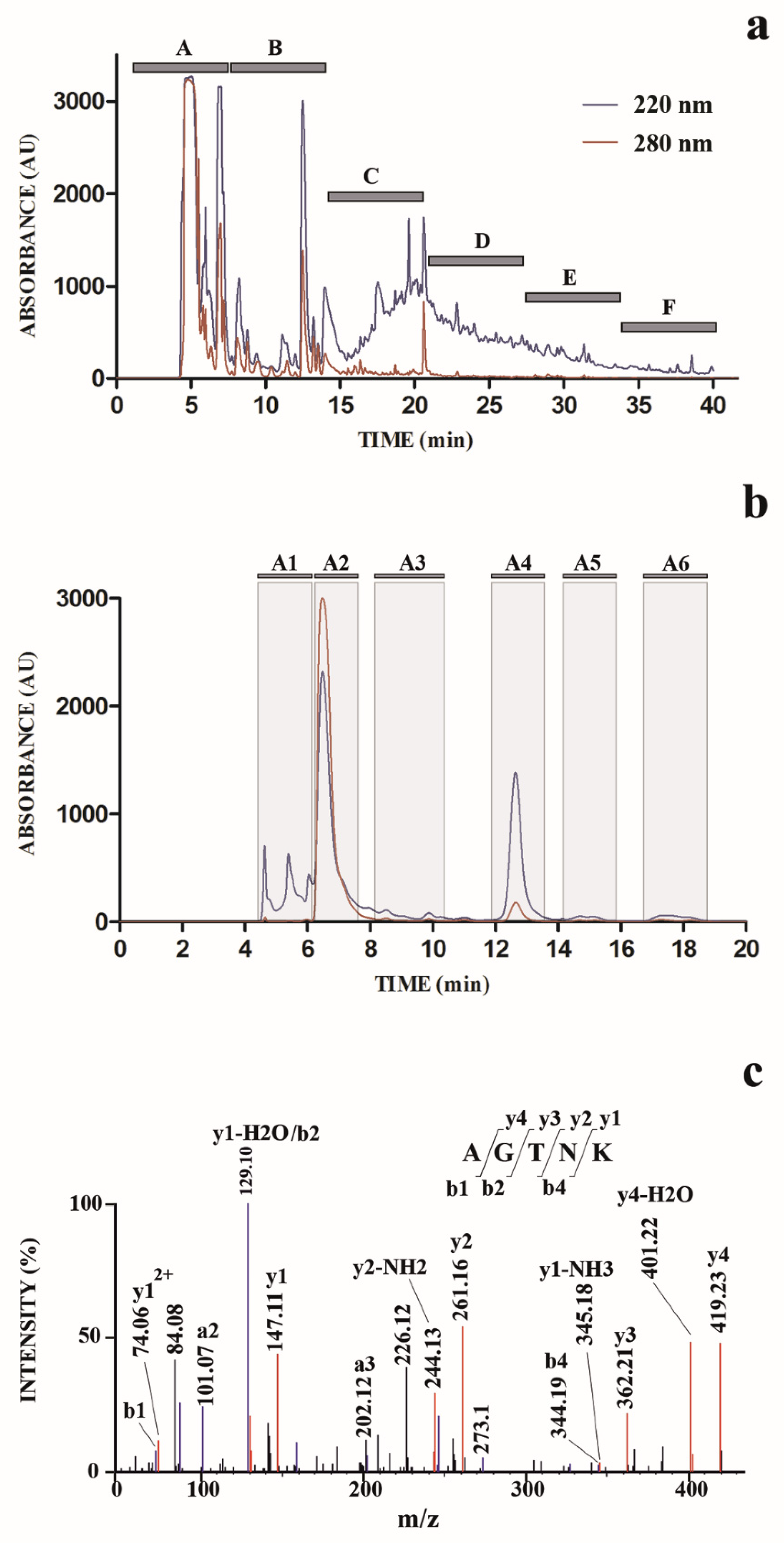
2.1. Antimicrobial Activity of the Sucker Extract and Purified Fraction
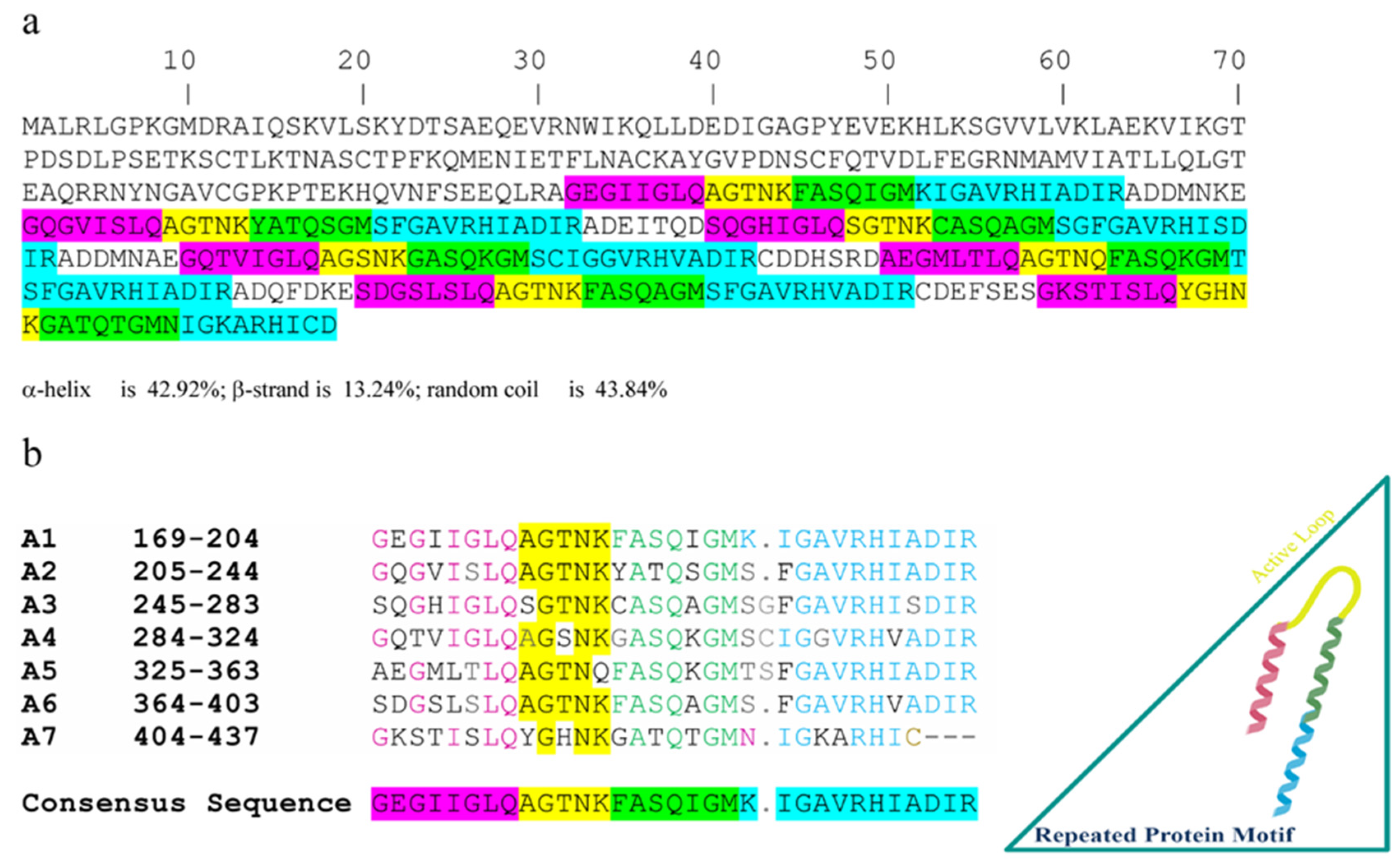
2.2. Peptide Identification by Mass Spectrometry
2.3. MIC Determination of the Synthetized AGTNK Peptide
2.4. Rational Design of Peptide Analogues
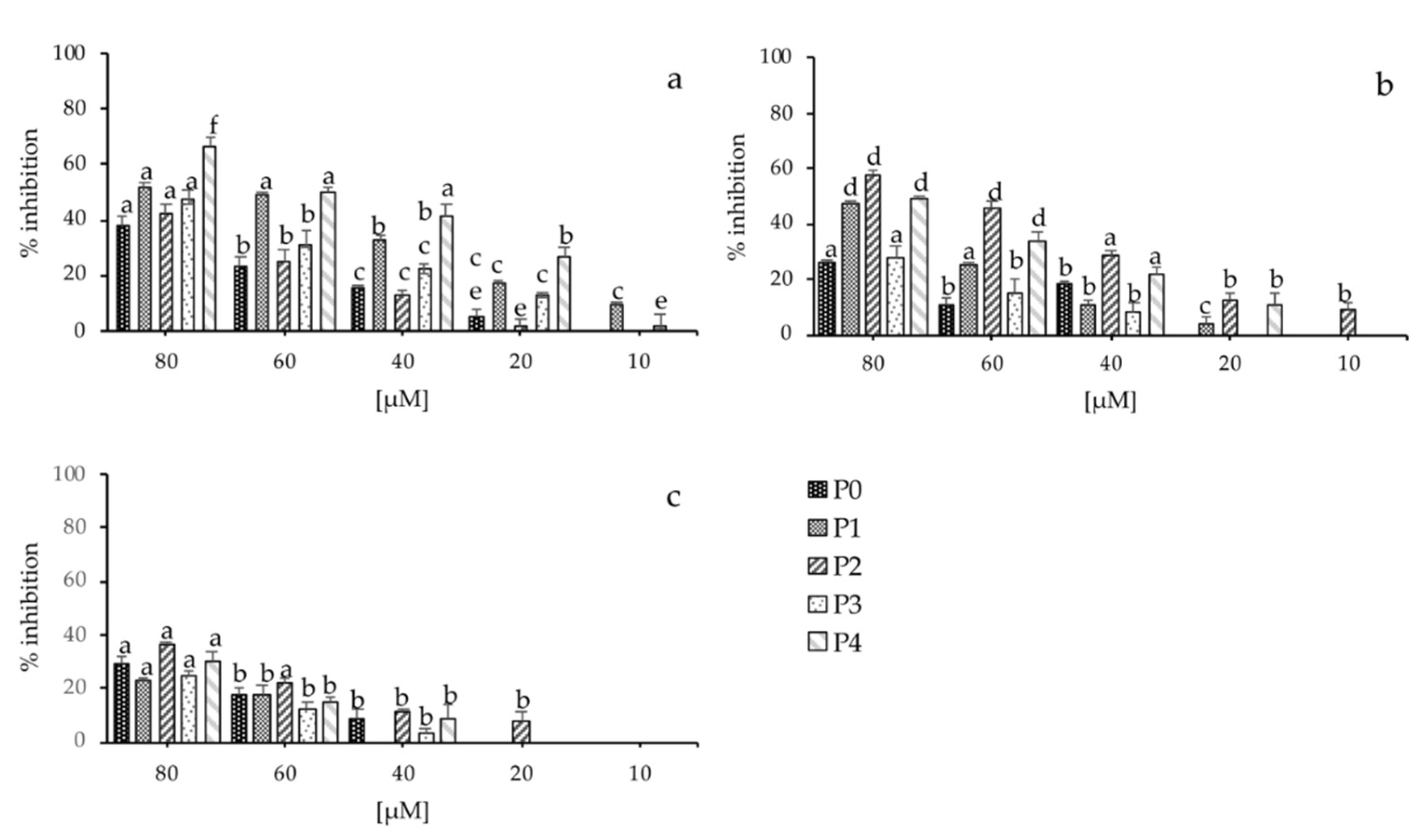
2.5. Antimicrobial Activity of the Peptide Analogues
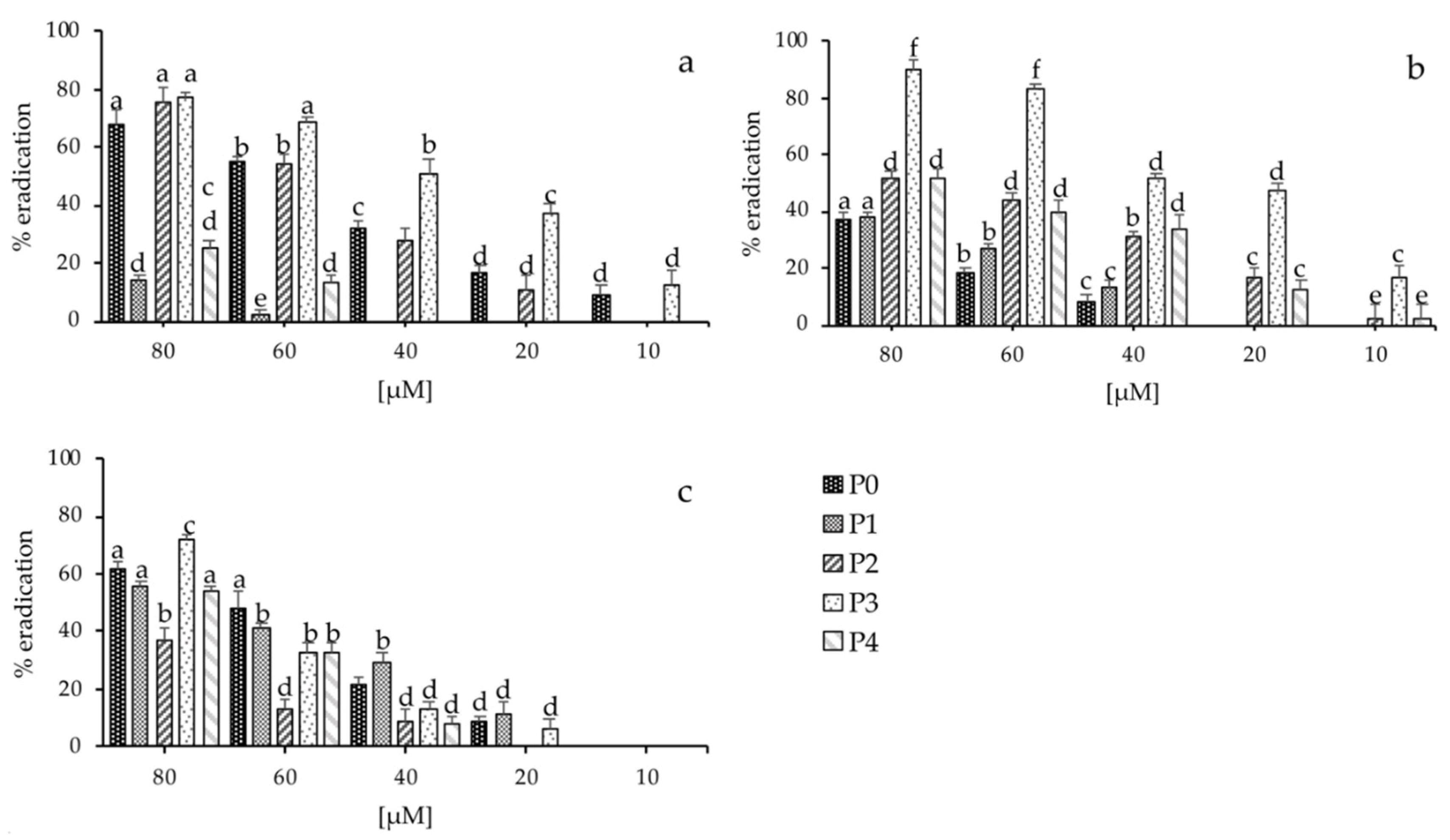
2.6. Biofilm Inhibition and Eradication Assay
3. Discussion
4. Materials and Methods
4.1. Octopus Collection
4.2. Methanolic Extraction of Peptides from Octopus Suckers
4.3. Bacterial Strain, Media, and Culture Conditions
4.4. Antimicrobial Agar Diffusion Assay of the Extract
4.5. Sucker Extract (SE) Purification and Characterization
4.6. Peptide Characterization of Extract Fractions
4.7. Peptide Synthesis and Purification
4.8. Antimicrobial Assay (MIC Determination) of the Purified Fraction and Synthetic Peptides
4.9. Biofilm Inhibition and Eradication Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ambrose, R.F. Food preferences, prey availability, and the diet of Octopus bimaculatus Verrill. J. Exp. Mar. Biol. Ecol. 1984, 77, 29–44. [Google Scholar] [CrossRef]
- Mather, J. Navigation by spatial memory and use of visual landmarks in octopuses. J. Comp. Physiol. A 1991, 168, 491–497. [Google Scholar] [CrossRef]
- Nixon, M.; Young, J.Z. The Brain and Lives of Cephalopods; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Yarnall, J.L. Aspects of the behaviour of Octopus cyanea gray. Anim. Behav. 1969, 17, 747–754. [Google Scholar] [CrossRef]
- Forsythe, J.W.; Hanlon, R.T. Foraging and associated behavior by Octopus cyanea Gray, 1849 on a coral atoll, French Polynesia. J. Exp. Mar. Biol. Ecol. 1997, 209, 15–31. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Verriopoulos, G. Modelling the effect of temperature on hatching and settlement patterns of meroplanktonic organisms: The case of octopus. Sci. Mar. 2006, 70, 699–708. [Google Scholar] [CrossRef]
- Balguerias, E.; Hernandez–Gonzalez, C.; Perales–Raya, C. On the identity of Octopus vulgaris Cuvier, 1797 stocks in the Saharan Bank (Northwest Africa) and their spatio-temporal variations in abundance in relation to some environmental factors. Bull. Mar. Sci. 2002, 71, 147–163. [Google Scholar]
- Liscovitch-Brauer, N.; Alon, S.; Porath, H.T.; Elstein, B.; Unger, R.; Ziv, T.; Admon, A.; Levanon, E.Y.; Rosenthal, J.J.; Eisenberg, E. Trade-off between Transcriptome Plasticity and Genome Evolution in Cephalopods. Cell 2017, 169, 191–202. [Google Scholar] [CrossRef]
- Garrett, S.; Rosenthal, J.J. RNA editing underlies temperature adaptation in K+ channels from polar octopuses. Science 2012, 335, 848–851. [Google Scholar] [CrossRef]
- Vaz–Pires, P.; Seixas, P.; Barbosa, A. Aquaculture potential of the common octopus (Octopus vulgaris Cuvier, 1797): A review. Aquaculture 2004, 238, 221–238. [Google Scholar] [CrossRef]
- Di Cosmo, A.; Bertapelle, C.; Porcellini, A.; Polese, G. Magnitude Assessment of Adult Neurogenesis in the Octopus vulgaris Brain Using a Flow Cytometry–Based Technique. Front. Physiol. 2018, 9, 1050. [Google Scholar] [CrossRef]
- Di Cosmo, A.; Maselli, V.; Polese, G. Octopus vulgaris: An Alternative in Evolution. Results Probl. Cell Differ. 2018, 65, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Castellanos–Martinez, S.; Arteta, D.; Catarino, S.; Gestal, C. De novo transcriptome sequencing of the Octopus vulgaris hemocytes using Illumina RNA–Seq technology: Response to the infection by the gastrointestinal parasite Aggregata octopiana. PLoS ONE 2014, 9, e107873. [Google Scholar] [CrossRef] [PubMed]
- Albertin, C.B.; Simakov, O.; Mitros, T.; Wang, Z.Y.; Pungor, J.R.; Edsinger–Gonzales, E.; Brenner, S.; Ragsdale, C.W.; Rokhsar, D.S. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 2015, 524, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mao, Y.; Huang, Z.; Qu, M.; Chen, J.; Ding, S.; Hong, J.; Sun, T. Transcriptome analysis of the Octopus vulgaris central nervous system. PLoS ONE 2012, 7, e40320. [Google Scholar] [CrossRef]
- Young, J.Z. The Anatomy of the Nervous System of Octopus vulgaris; Oxford University Press: New York, NY, USA, 1971; p. 690. [Google Scholar]
- Young, J.Z. The organization of a cephalopod ganglion. Phil. Trans. R. Soc. Lond. B 1972, 263. [Google Scholar] [CrossRef]
- Young, J.Z. Brain, behaviour and evolution of cephalopods. Symp. Zool. Soc. Land. 1977, 38, 377–434. [Google Scholar]
- Zullo, L.; Eichenstein, H.; Maiole, F.; Hochner, B. Motor control pathways in the nervous system of Octopus vulgaris arm. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2019, 205, 271–279. [Google Scholar] [CrossRef]
- Graziadei, P. Receptors in the Suckers of Octopus. Nature 1962, 195, 57–59. [Google Scholar] [CrossRef]
- Graziadei, P. Sensory receptor cells and related neurons in cephalopods. Cold Spring Harb. Symp. Quant. Biol. 1965, 30, 45–57. [Google Scholar] [CrossRef]
- Graziadei, P.P.C.; Gagne, H.T. Sensory innervation in the rim of the octopus sucker. J. Morphol. 1976, 150, 639–680. [Google Scholar] [CrossRef]
- Levy, G.; Hochner, B. Embodied Organization of Octopus vulgaris Morphology, Vision, and Locomotion. Front. Physiol. 2017, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, M.; Legrand, B.; Duval, E.; Henry, J.; Baudy-Floc’h, M.; Zatylny-Gaudin, C.; Bondon, A. From a Marine Neuropeptide to Antimicrobial Pseudopeptides Containing Aza–β3–Amino Acids: Structure and Activity. J. Med. Chem. 2012, 55, 2025–2034. [Google Scholar] [CrossRef][Green Version]
- Houyvet, B.; Zanuttini, B.; Corre, E.; Le Corguille, G.; Henry, J.; Zatylny-Gaudin, C. Design of antimicrobial peptides from a cuttlefish database. Amino Acids 2018, 50, 1573–1582. [Google Scholar] [CrossRef]
- Destoumieux–Garzón, D.; Rosa, R.D.; Schmitt, P.; Barreto, C.; Vidal–Dupiol, J.; Mitta, G.; Gueguen, Y.; Bachère, E. Antimicrobial peptides in marine invertebrate health and disease. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Di Cosmo, A.; Polese, G. Neuroendocrine-Immune Systems Response to Environmental Stressors in the Cephalopod Octopus vulgaris. Front. Physiol. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Gestal, C.; Castellanos-Martinez, S. Understanding the cephalopod immune system based on functional and molecular evidence. Fish. Shellfish Immunol. 2015, 46, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.P.; Beard, T.D.; Bennett, E.M.; Cumming, G.S.; Cork, S.J.; Agard, J.; Dobson, A.P.; Peterson, G.D. Trade-offs across space, time, and ecosystem services. Ecol. Soc. 2006, 11, 28. [Google Scholar] [CrossRef]
- Castillo, M.G.; Salazar, K.A.; Joffe, N.R. The immune response of cephalopods from head to foot. Fish. Shellfish Immunol. 2015, 46, 145–160. [Google Scholar] [CrossRef]
- Troncone, L.; De Lisa, E.; Bertapelle, C.; Porcellini, A.; Laccetti, P.; Polese, G.; Di Cosmo, A. Morphofunctional characterization and antibacterial activity of haemocytes from Octopus vulgaris. J. Nat. Hist. 2015, 49, 1457–1475. [Google Scholar] [CrossRef]
- Warr, G.W. Immunity in invertebrates. J. Invertebr. Pathol. 1981, 38, 311–314. [Google Scholar] [CrossRef]
- Marchalonis, J.J. Immunity in Evolution; Harvard University Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Iwanaga, S.; Lee, B.L. Recent advances in the innate immunity of invertebrate animals. J. Biochem. Mol. Biol. 2005, 38, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Tomonaga, S. Morphology of Octopus haemocytes. J. Nat. Fish. Univ. 2003, 51, 157–164. [Google Scholar]
- Bidder, A.; Boletzky, M.; Boletzky, S.; Hochberg, F.; Mangold, K.; Marthy, H.-J.; Portmann, A.; Teichert, C.; Worms, J. Cephalopodes; Masson: Paris, France, 1989; Vol. V. [Google Scholar]
- Novoa, B.; Tafalla, C.; Guerra, A.; Figueras, A. Cellular immunological parameters of the octopus, Octopus vulgaris. J. Shellfish Res. 2002, 21, 243–248. [Google Scholar]
- Fisher, W.; Dinuzzo, A. Agglutination of bacteria and erythrocytes by serum from 6 species of marine mollusks. J. Invertebr. Pathol. 1991, 57, 380–394. [Google Scholar] [CrossRef]
- Rogener, W.; Renwrantz, L.; Uhlenbruck, G. Isolation and characterization of a lectin from the hemolymph of the cephalopod Octopus vulgaris (Lam.) inhibited by alpha-D–lactose and N–acetyl–lactosamine. Dev. Comp. Immunol. 1985, 9, 605–616. [Google Scholar] [CrossRef]
- Thøgersen, I.B.; Salvesen, G.; Brucato, F.H.; Pizzo, S.V.; Enghild, J.J. Purification and characterization of an alpha–macroglobulin proteinase inhibitor from the mollusc Octopus vulgaris. Biochem. J. 1992, 285, 521–527. [Google Scholar] [CrossRef]
- Rajanbabu, V.; Chen, J.Y. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 2011, 32, 415–420. [Google Scholar] [CrossRef]
- Ismail, M.; Riad, R. Screening the Antimicrobial Activity of Different Sepia officinalis (Cephalopoda: Sepioidea) Parts Collected from Alexandria Mediterranean Waters, Egypt Against Some Human Pathogens. Singap. J. Sci. Res. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Derby, C.D. Cephalopod ink: Production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700–2730. [Google Scholar] [CrossRef]
- Besednova, N.N.; Kovalev, N.N.; Zaporozhets, T.S.; Kuznetsova, T.A.; Gazha, A.K. Cephalopods as a Source of New Antimicrobial Substances. Antibiot. Khimioter. 2016, 61, 32–42. [Google Scholar]
- Besednova, N.N.; Zaporozhets, T.S.; Kovalev, N.N.; Makarenkova, I.D.; Yakovlev, Y.M. Cephalopods: The potential for their use in medicine. Russ. J. Mar. Biol. 2017, 43, 101–110. [Google Scholar] [CrossRef]
- Monolisha, S.; Aswathi, E.; Patterson, J.; Patterson, J. Molecular characterization and antimicrobial activity of Octopus aegina and Octopus dolfusii in Gulf of Mannar Coast. Int. J. Pharm. Sci. Res. 2013, 4, 3582–3587. [Google Scholar]
- Gogineni, V.; Hamann, M.T. Marine natural product peptides with therapeutic potential: Chemistry, biosynthesis, and pharmacology. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 81–196. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. Int. J. Mol. Sci. 2016, 17, 785. [Google Scholar] [CrossRef] [PubMed]
- Challinor, V.L.; Bode, H.B. Bioactive natural products from novel microbial sources. Ann. N. Y. Acad. Sci. 2015, 1354, 82–97. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Wittine, K.; Saftić, L.; Peršurić, Ž.; Kraljević Pavelić, S. Novel Antiretroviral Structures from Marine Organisms. Molecules 2019, 24, 3486. [Google Scholar] [CrossRef]
- Prasasty, V.; Radifar, M.; Istyastono, E. Natural Peptides in Drug Discovery Targeting Acetylcholinesterase. Molecules 2018, 23, 2344. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine Natural Peptides: Determination of Absolute Configuration Using Liquid Chromatography Methods and Evaluation of Bioactivities. Molecules 2018, 23, 306. [Google Scholar] [CrossRef]
- Semreen, M.H.; El-Gamal, M.I.; Abdin, S.; Alkhazraji, H.; Kamal, L.; Hammad, S.; El-Awady, F.; Waleed, D.; Kourbaj, L. Recent updates of marine antimicrobial peptides. Saudi Pharm. J. 2018, 26, 396–409. [Google Scholar] [CrossRef]
- Zannella, C.; Mosca, F.; Mariani, F.; Franci, G.; Folliero, V.; Galdiero, M.; Tiscar, P.G.; Galdiero, M. Microbial diseases of bivalve mollusks: Infections, immunology and antimicrobial defense. Mar. drugs 2017, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; De Moro, G.; Manfrin, C.; Venier, P.; Pallavicini, A. Big defensins and mytimacins, new AMP families of the Mediterranean mussel Mytilus galloprovincialis. Dev. Comp. Immunol. 2012, 36, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Bachère, E.; Gueguen, Y.; Gonzalez, M.; de Lorgeril, J.; Garnier, J.; Romestand, B. Insights into the anti- microbial defense of marine invertebrates: The penaeid shrimps and the oyster Crassostrea gigas. Immunol. Rev. 2004, 198, 149–168. [Google Scholar] [CrossRef] [PubMed]
- De Zoysa, M.; Whang, I.; Lee, Y.; Lee, S.; Lee, J.-S.; Lee, J. Defensin from disk abalone Haliotis discus discus: Molecular cloning, sequence characterization and immune response against bacterial infection. Fish. Shellfish Immunol. 2010, 28, 261–266. [Google Scholar] [CrossRef]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef]
- Cornet, V.; Henry, J.; Goux, D.; Duval, E.; Bernay, B.; Le Corguillé, G.; Corre, E.; Zatylny–Gaudin, C. How Egg Case Proteins Can Protect Cuttlefish Offspring? PLoS ONE 2015, 10, e0132836. [Google Scholar] [CrossRef][Green Version]
- Kremer, N.; Schwartzman, J.; Augustin, R.; Zhou, L.; Ruby, E.; Hourdez, S.; McFall-Ngai, M. The dual nature of haemocyanin in the establishment and persistence of the squid—vibrio symbiosis the dual nature of haemocyanin in the establishment and persistence of the squid—vibrio symbiosis. Proc. R Soc. Biol. Sci. 2014, 281. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Arena, S.; Renzone, G.; Scaloni, A. A multi–approach peptidomic analysis of hen egg white reveals novel putative bioactive molecules. J. Proteom. 2020, 215. [Google Scholar] [CrossRef]
- Arena, S.; Scaloni, A. An Extensive Description of the Peptidomic Repertoire of the Hen Egg Yolk Plasma. J. Agric. Food Chem. 2018, 66, 3239–3255. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio–preservative to enhance the shelf–life of food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef] [PubMed]
- Nikapitiya, C.; Dananjaya, S.H.S.; Chandrarathna, H.; De Zoysa, M.; Whang, I. Octominin: A Novel Synthetic Anticandidal Peptide Derived from Defense Protein of Octopus minor. Mar. Drugs 2020, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Nagahawatta, D.P.; Yang, H.-W.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; De Zoysa, M.; Whang, I.; Ryu, B. Octominin Inhibits LPS-Induced Chemokine and Pro–inflammatory Cytokine Secretion from RAW 264.7 Macrophages via Blocking TLRs/NF-κB Signal Transduction. Biomolecules 2020, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Harris, F.; Bhatt, T.; Singh, J.; Phoenix, D.A. The effect of C–terminal amidation on the efficacy and selectivity of antimicrobial and anticancer peptides. Mol. Cell. Biochem. 2009, 332, 43. [Google Scholar] [CrossRef]
- Strandberg, E.; Tiltak, D.; Ieronimo, M.; Kanithasen, N.; Wadhwani, P.; Ulrich, A.S. Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic α-helical peptides. Pure Appl. Chem. 2007, 79, 717–728. [Google Scholar] [CrossRef]
- Xie, C.; Zeng, P.; Ericksen, B.; Wu, Z.; Lu, W.Y.; Lu, W. Effects of the terminal charges in human neutrophil alpha-defensin 2 on its bactericidal and membrane activity. Peptides 2005, 26, 2377–2383. [Google Scholar] [CrossRef]
- Mehta, N.M.; Carpenter, S.E.; Consalvo, A.P. C–Terminal α–Amidation. In Post–translational Modification of Protein Biopharmaceuticals; Walsh, G., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 253–276. [Google Scholar]
- Hooper, S.L.; Thuma, J.B. Invertebrate Muscles: Muscle Specific Genes and Proteins. Physiol. Rev. 2005, 85, 1001–1060. [Google Scholar] [CrossRef]
- Garnier, J.; Gibrat, J.F.; Robson, B. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzym. 1996, 266, 540–553. [Google Scholar] [CrossRef]
- Martin, D. Samuel Karlin, Versatile Mathematician, Dies at 83.2008. Available online: https://www.nytimes.com/2008/02/21/us/21karlin.html (accessed on 21 February 2008).
- Hu, M.Y.; Yan, H.Y.; Chung, W.S.; Shiao, J.C.; Hwang, P.P. Acoustically evoked potentials in two cephalopods inferred using the auditory brainstem response (ABR) approach. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 278–283. [Google Scholar] [CrossRef]
- Baik, S.; Kim, D.W.; Park, Y.; Lee, T.J.; Ho Bhang, S.; Pang, C. A wet–tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 2017, 546, 396–400. [Google Scholar] [CrossRef]
- Cianchetti, M.; Calisti, M.; Margheri, L.; Kuba, M.; Laschi, C. Bioinspired locomotion and grasping in water: The soft eight–arm OCTOPUS robot. Bioinspiration Biomim. 2015, 10, 035003. [Google Scholar] [CrossRef] [PubMed]
- Laschi, C.; Cianchetti, M.; Mazzolai, B.; Margheri, L.; Follador, M.; Dario, P. Soft Robot Arm Inspired by the Octopus. Adv. Robot. 2012, 26, 709–727. [Google Scholar] [CrossRef]
- Yekutieli, Y.; Sagiv–Zohar, R.; Aharonov, R.; Engel, Y.; Hochner, B.; Flash, T. Dynamic model of the octopus arm. I. Biomechanics of the octopus reaching movement. J. Neurophysiol. 2005, 94, 1443–1458. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, P.; Young, J.Z. The nervous system of the arms. In The Anatomy of the Nervous System of Octopus Vulgaris; Clarendon Press: Oxford, UK, 1971. [Google Scholar]
- Graziadei, P.P.C.; Gagne, H.T. Neural components in octopus sucker. J. Cell Biol. 1973, 59, A21–A121. [Google Scholar]
- Packard, A. The skin of cephalopods (Coleoids): General and special adaptations. In The Mollusca, Form and Function; Trueman, E.R., Clarke, M.R., Eds.; Academic Press Inc.: Berkeley, CA, USA, 1988; Volume 11, pp. 37–67. [Google Scholar]
- Maselli, V.; Al–Soudy, A.S.; Buglione, M.; Aria, M.; Polese, G.; Di Cosmo, A. Sensorial Hierarchy in Octopus vulgaris’s Food Choice: Chemical vs. Visual. Animals 2020, 10, 457. [Google Scholar] [CrossRef]
- Kier, W.M.; Stella, M.P. The arrangement and function of octopus arm musculature and connective tissue. J. Morphol. 2007, 268, 831–843. [Google Scholar] [CrossRef]
- Kier, W.M.; Smith, A.M. The Structure and Adhesive Mechanism of Octopus Suckers1. Integr. Comp. Biol. 2002, 42, 1146–1153. [Google Scholar] [CrossRef]
- Stanley, D.W.; Hultin, H.O. Protoelytic Activity in North American Squid and Its Relation to Quality. Can. Inst. Food Sci. Technol. J. 1984, 17, 163–167. [Google Scholar] [CrossRef]
- Sakai, J.; Matsumoto, J.J. Proteolytic enzymes of squid mantle muscle. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 68, 389–395. [Google Scholar] [CrossRef]
- Hurtado, J.L.; Borderìas, J.; Montero, P.; An, H. Characterization of proteolytic activity in octopus (Octopus vulgaris) arm muscle. J. Food Biochem. 1999, 23, 469–483. [Google Scholar] [CrossRef]
- Hurtado, J.L.; Montero, P.; Borderías, J.; An, H. Properties of Proteolytic Enzymes from Muscle of Octopus (Octopus vulgaris) and Effects of High Hydrostatic Pressure. J. Food Sci. 2002, 67, 2555–2564. [Google Scholar] [CrossRef]
- Uribe, R.; Jay, D. A review of actin binding proteins: New perspectives. Mol. Biol. Rep. 2009, 36, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, E.; de Alteriis, E.; De Natale, A.; D’Alterio, A.; Siciliano, A.; Guida, M.; Lombardi, L.; Falanga, A.; Galdiero, S. Eradication of Candida albicans persister cell biofilm by the membranotropic peptide gH625. Sci. Rep. 2020, 10, 5780. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Lee, M.Y.; Kim, J.Y.; Kim, H.; Jung, M.; Shin, M.K.; Lee, W.K.; Cheong, G.W.; Lee, J.R.; Jang, M.K. Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug–Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm. Molecules 2019, 24, 4560. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Neundorf, I. Antimicrobial Peptides: Basics for Clinical Application; Springer: Singapore, 2019. [Google Scholar]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro–Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, Z.; Yu, Z.; Wang, H.; Liu, Y.; Li, H.; Wang, L.; Li, C.; Song, L. A tandem-repeat galectin–1 from Apostichopus japonicus with broad PAMP recognition pattern and antibacterial activity. Fish. Shellfish Immunol. 2020, 99, 167–175. [Google Scholar] [CrossRef]
- Tazato, S.; Conlon, J.M.; Iwamuro, S. Cloning and expression of genes enocoding antimicrobial peptides and bradykinin from the skin and brain of Oki Tago’s brown frog, Rana tagoi okiensis. Peptides 2010, 31, 1480–1487. [Google Scholar] [CrossRef]
- König, E.; Zhou, M.; Wang, L.; Chen, T.; Bininda-Emonds, O.R.; Shaw, C. Antimicrobial peptides and alytesin are co–secreted from the venom of the Midwife toad, Alytes maurus (Alytidae, Anura): Implications for the evolution of frog skin defensive secretions. Toxicon 2012, 60, 967–981. [Google Scholar] [CrossRef]
- Rončević, T.; Gajski, G.; Ilić, N.; Goić-Barišić, I.; Tonkić, M.; Zoranić, L.; Simunić, J.; Benincasa, M.; Mijaković, M.; Tossi, A.; et al. PGLa-H tandem-repeat peptides active against multidrug resistant clinical bacterial isolates. Biochim. Biophys. Acta Biomembr. 2017, 1859, 228–237. [Google Scholar] [CrossRef]
- Batham, E.J. Care of eggs by Octopus maorum. Trans. R. Soc. N. Z. 1957, 84, 629–638. [Google Scholar]
- Robison, B.; Seibel, B.; Drazen, J. Deep-Sea Octopus (Graneledone boreopacifica) Conducts the Longest-Known Egg-Brooding Period of Any Animal. PLoS ONE 2014, 9, e103437. [Google Scholar] [CrossRef] [PubMed]
- Boletztky, S. Embryonic development of cephalopods at low temperatures. Antarct. Sci. 1994, 6, 139–142. [Google Scholar] [CrossRef]
- Wodinsky, J. Hormonal Inhibition of Feeding and Death in Octopus: Control by Optic Gland Secretion. Science 1977, 198, 948. [Google Scholar] [CrossRef]
- Di Cosmo, A.; Polese, G.; Bertapelle, C.; Palumbo, A.L.Z.; Zullo, L. Cefalopodi. Benessere ed Animal Care dell’Animale da laboratorio; Le Point Veterinaire Italie: Milano, Italy, 2015. [Google Scholar]
- Polese, G.; Winlow, W.; Di Cosmo, A. Dose-dependent effects of the clinical anesthetic isoflurane on Octopus vulgaris: A contribution to cephalopod welfare. J. Aquat. Anim. Health 2014, 26, 285–294. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty–Fifth Informational Supplement; CLSI: Wayne, PA, USA, 2012; pp. 23–25. [Google Scholar]
- Lonoce, C.; Salem, R.; Marusic, C.; Jutras, P.V.; Scaloni, A.; Salzano, A.M.; Lucretti, S.; Steinkellner, H.; Benvenuto, E.; Donini, M. Production of a tumour-targeting antibody with a human-compatible glycosylation profile in N. benthamiana hairy root cultures. Biotechnol. J. 2016, 11, 1209–1220. [Google Scholar] [CrossRef]
- Galdiero, S.; Capasso, D.; Vitiello, M.; D’Isanto, M.; Pedone, C.; Galdiero, M. Role of surface-exposed loops of Haemophilus influenzae protein P2 in the mitogen–activated protein kinase cascade. Infect. Immun. 2003, 71, 2798–2809. [Google Scholar] [CrossRef]
- Di Onofrio, V.; Gesuele, R.; Maione, A.; Liguori, G.; Liguori, R.; Guida, M.; Nigro, R.; Galdiero, E. Prevention of Pseudomonas aeruginosa Biofilm Formation on Soft Contact Lenses by Allium sativum Fermented Extract (BGE) and Cannabinol Oil Extract (CBD). Antibiotics 2019, 8, 258. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Di Bonaventura, G.; Djukic, S.; Cirkovic, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2007, 115, 891–899. [Google Scholar] [CrossRef]
| Strain | SE | HPLC Fraction | Positive Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | AMP | G | AMPH-B | ||
| Mean ± SD (Ø mm) | ||||||||||
| Gram-positive | ||||||||||
| S. aureus ATCC 6538 | 11.0 ± 1.2 | 12.0 ± 2.0 | - | 8.0 ± 1.5 | - | 6.0 ± 2.8 | - | S | S | NT |
| Gram-negative | ||||||||||
| P. aeruginosa ATCC 9027 | 8.0 ± 2.3 | 10.0 ± 3.1 | - | 6.0 ± 3.0 | - | - | - | R | S | NT |
| Yeast | ||||||||||
| C. albicans ATCC 90028 | - | 7.0 ± 1.8 | - | - | - | - | - | NT | NT | S |
| Strain | MIC80 (µg/mL) HPLC Fraction | |||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | |
| S. aureus ATCC 6538 | 150 ± 1 | 120 ± 5 | 180 ± 4 | 120 ± 2 | 120 ± 5 | 200 ± 6 |
| P. aeruginosa ATCC 9027 | >300 | 200 ± 2 | >300 | 180 ± 5 | >300 | 200 ± 5 |
| C. albicans ATCC 90028 | >300 | >300 | >300 | 300 ± 2 | >300 | >300 |
| Sequence | MIC80 (µM) | ||||
|---|---|---|---|---|---|
| Mass Value | S. aureus ATCC 6538 | P. aeruginosa ATCC 9027 | C. albicans ATCC 90028 | ||
| P0 | NH2-AGTNK-CONH2 | 488.53 | 150 ± 2 | 200 ± 3 | 200 ± 3 |
| P1 | NH2-QAGTNK-CONH2 | 616.66 | 150 ± 3 | 100 ± 5 | 200 ± 5 |
| P2 | NH2-QAGSNKGASQKGMS-CONH2 | 1349.47 | 50 ± 5 | 50 ± 2 | 100 ± 3 |
| P3 | NH2-EGQGVISLQAGTNK-CONH2 | 1400.54 | 80 ± 1 | 50 ± 3 | 100 ± 4 |
| P4 | NH2-GEGIIGLQAGTNKFASQIGMKIGAVRHIADIR-CONH2 | 3321.88 | 80 ± 5 | 50 ± 2 | 180 ± 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maselli, V.; Galdiero, E.; Salzano, A.M.; Scaloni, A.; Maione, A.; Falanga, A.; Naviglio, D.; Guida, M.; Di Cosmo, A.; Galdiero, S. OctoPartenopin: Identification and Preliminary Characterization of a Novel Antimicrobial Peptide from the Suckers of Octopus vulgaris. Mar. Drugs 2020, 18, 380. https://doi.org/10.3390/md18080380
Maselli V, Galdiero E, Salzano AM, Scaloni A, Maione A, Falanga A, Naviglio D, Guida M, Di Cosmo A, Galdiero S. OctoPartenopin: Identification and Preliminary Characterization of a Novel Antimicrobial Peptide from the Suckers of Octopus vulgaris. Marine Drugs. 2020; 18(8):380. https://doi.org/10.3390/md18080380
Chicago/Turabian StyleMaselli, Valeria, Emilia Galdiero, Anna Maria Salzano, Andrea Scaloni, Angela Maione, Annarita Falanga, Daniele Naviglio, Marco Guida, Anna Di Cosmo, and Stefania Galdiero. 2020. "OctoPartenopin: Identification and Preliminary Characterization of a Novel Antimicrobial Peptide from the Suckers of Octopus vulgaris" Marine Drugs 18, no. 8: 380. https://doi.org/10.3390/md18080380
APA StyleMaselli, V., Galdiero, E., Salzano, A. M., Scaloni, A., Maione, A., Falanga, A., Naviglio, D., Guida, M., Di Cosmo, A., & Galdiero, S. (2020). OctoPartenopin: Identification and Preliminary Characterization of a Novel Antimicrobial Peptide from the Suckers of Octopus vulgaris. Marine Drugs, 18(8), 380. https://doi.org/10.3390/md18080380












