Marine Organisms from the Yucatan Peninsula (Mexico) as a Potential Natural Source of Antibacterial Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Animal Material Studies
2.2. Antibacterial Activity and Bioassay-Guided Isolation of A. compressa Crude Extract
2.3. Antibacterial Activity and Bioassay-Guided Isolation of A. citrina Crude Extract
3. Material and Methods
3.1. General Experimental Procedures
3.2. Animal Collection and Identification
3.3. Preparation of the Organic Extracts
3.4. Bioassay-Guided Isolation of the A. compressa Crude Extract
3.5. Bioassay-Guided Isolation of the A. citrina Crude Extract
3.6. Antimicrobial Activity Assays
3.6.1. Bacterial Strains and Culture Preparation
3.6.2. Microdilution Method: Minimum Inhibitory Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maxwell, D. Beyond Maritime Symbolism: Toxic marine objects from ritual contexts at Tikal. Anc. Mesoam. 2000, 11, 91–98. [Google Scholar] [CrossRef]
- Voultsiadou, E. Therapeutic properties and uses of marine invertebrates in the ancient Greek world and early Byzantium. J. Ethnopharmacol. 2010, 130, 237–247. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally; World Health Organization (WHO): Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization (WHO). WHO Priority Pathogens List for R&D of New Antibiotics. 2017. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 17 June 2020).
- Maragakis, L.L.; Perl, T.M. Acinetobacter baumannii: Epidemiology, Antimicrobial Resistance, and Treatment Options. Clin. Infect. Dis. 2008, 46, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2015, 3, 15–21. [Google Scholar] [CrossRef]
- Gales, A.C.; Jones, R.N.; Sader, H.S. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: Results from the SENTRY Antimicrobial Surveillance Program (2006-09). J. Antimicrob. Chemother. 2011, 66, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options Against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2019, 63, 1–18. [Google Scholar]
- Kong, D.X.; Jiang, Y.Y.; Zhang, H.Y. Marine natural products as sources of novel scaffolds: Achievement and concern. Drug Discov. Today 2010, 15, 884–886. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef]
- Cuevas, C.; Francesch, A. Development of Yondelis® (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep. 2009, 26, 322–337. [Google Scholar] [CrossRef]
- Katz, J.; Janik, J.E.; Younes, A. Brentuximab Vedotin (SGN-35). Clin. Cancer Res. 2011, 17, 6428–6436. [Google Scholar] [CrossRef] [PubMed]
- Altmann, K.H. Drugs from the Oceans: Marine Natural Products as Leads for Drug Discovery. Chimia 2017, 71, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Bye, R.; Linares, E.; Estrada, E. Biological diversity of medicinal plants in Mexico. In Phytochemistry of Medicinal Plants, Recent Advances in Phytochemistry; Arnason, J.T., Mata, R., Romeo, J.T., Eds.; Plenum Press: New York, NY, USA, 1995; Volume 29, p. 65. [Google Scholar]
- Hernández-Bolio, G.I.; Ruiz-Vargas, J.A.; Peña-Rodríguez, L.M. Natural Products from the Yucatecan Flora: Structural Diversity and Biological Activity. J. Nat. Prod. 2019, 82, 647–656. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Pérez-Povedano, M.; Lenis-Rojas, O.A.; Rodríguez, J.; Jiménez, C. Marine Natural Products from the Yucatan Peninsula. Mar. Drugs 2020, 18, 59. [Google Scholar] [CrossRef]
- Morales, J.L.; Cantillo-Ciau, Z.; Sánchez-Molina, I.; Mena-Rejón, G. Screening of Antibacterial and Antifungal Activities of Six Marine Macroalgae from Coast of Yucatan Peninsula. Pharm. Biol. 2006, 4, 632–635. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Morales, J.L. Antibacterial activity in marine algae from the coast of Yucatan, Mexico. Bot. Mar. 2004, 47, 140–146. [Google Scholar] [CrossRef]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J. Appl. Phycol. 2007, 19, 49–458. [Google Scholar] [CrossRef]
- Morales-Landa, J.L.; Zapata-Pérez, O.; Cedillo-Rivera, R.; Segura-Puertas, L.; Simá-Álvarez, R.; Sánchez-Rodríguez, J. Antimicrobial, Antiprotozoal, and Toxic Activities of Cnidarian Extracts from the Mexican Caribbean Sea. Pharm. Biol. 2007, 45, 37–43. [Google Scholar] [CrossRef]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrin, Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J. Ethnopharmacol. 2008, 120, 92–97. [Google Scholar] [CrossRef]
- De Lara-Issasi, G.; Álvarez-Hernández, S.; Collado-Vides, L. Ichtyotoxic activity of extracts from Mexican marine macroalgae. J. Appl. Phycol. 2000, 12, 45–52. [Google Scholar] [CrossRef]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrín, Y. In vitro cytotoxic and antiproliferative activities of marine macroalgae from Yucatan, Mexico. Cienc. Mar. 2009, 35, 35–358. [Google Scholar] [CrossRef]
- Bohlin, L.; Sjöstrand, U.; Djerassi, C.; Sullivan, B. Minor and Trace Sterols in Marine Invertebrates. Part 20. 3β-Hydroxy-methyl-A-nor-Patinosterol and 3β-Hydroxymethyl-A-nor-dinosterol. Two New Sterols with Modified Nucleus and Side-Chain from the Sponge Teichaxinella morchella. J. Chem. Soc. Perkin Trans. 1981, 1023–1028. [Google Scholar] [CrossRef]
- García-Arredondo, A.; Rojas-Molina, A.; Ibara-Alvarado, C.; Lazcano-Pérez, F.; Arreguín-Espinosa, R.; Sánchez-Rodríguez, J. Composition and biological activities of the aqueous extracts of three scleractinian corals from the Mexican Caribbean: Pseudodiploria stigosa, Porites astreoides and Siderastrea siderea. J. Venom. Anim. Toxins 2016, 22, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Pech-Puch, D.; Rodríguez, J.; Cautain, B.; Sandoval-Castro, C.A.; Jiménez, C. Cytotoxic Furanoditerpenes from the Sponge Spongia tubulifera Collected in the Mexican Caribbean. Mar. Drugs 2019, 17, 416. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Clinical Breakpoints and Dosing of Antibiotics. 2019. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 17 June 2020).
- Álvarez-Fraga, L.; Vázquez-Ucha, J.C.; Martínez-Guitián, M.; Vallejo, J.A.; Bou, G.; Beceiro, A.; Poza, M. Pneumonia infection in mice reveals the involvement of the feoA gene in the pathogenesis of Acinetobacter baumannii. Virulence 2018, 9, 496–509. [Google Scholar] [CrossRef]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-spectrum antibacterial agents. Med. Chem. Comm. 2018, 9, 12–21. [Google Scholar] [CrossRef]
- Maxson, T.; Mitchell, D.A. Targeted treatment for bacterial infections: Prospects for pathogen-specific antibiotics coupled with rapid diagnostics. Tetrahedron 2016, 72, 3609–3624. [Google Scholar] [CrossRef]
- Stout, E.P.; Yu, L.C.; Molinski, T.F. Antifungal Diterpene Alkaloids from the Caribbean Sponge Agelas citrina: Unified Configurational Assignments of Agelasidines and Agelasines. Eur. J. Org. Chem. 2012, 27, 5131–5135. [Google Scholar] [CrossRef]
- Cychon, C.; Lichte, E.; Köck, M. The marine sponge Agelas citrina as a source of the new pyrrole–imidazole alkaloids citrinamines A–D and N-methylagelongine. Beilstein J. Org. Chem. 2015, 11, 2029–2037. [Google Scholar] [CrossRef]
- Quintana, J.; Brango-Vanegas, J.; Costa, G.M.; Castellanos, L.; Arévalo, C.; Duque, C. Marine organisms as source of extracts to disrupt bacterial communication: Bioguided isolation and identification of quorum sensing inhibitors from Ircinia felix. Rev. Bras. Farmacogn. 2015, 25, 199–207. [Google Scholar] [CrossRef]
- Mora-Cristancho, J.A.; Arévalo-Ferro, C.; Ramos, F.A.; Tello, E.; Duque, C.; Lhullier, C.; Falkenberg, M.; Schenkel, E.P. Antifouling Activities against Colonizer Marine Bacteria of Extracts from Marine Invertebrates Collected in the Colombian Caribbean Sea and on the Brazilian Coast (Santa Catarina). Z. Naturforsch. C 2011, 66, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.; Lidgard, R.O.; Murphy, P.T.; Wells, R.J.; Blount, J.F. Brominated Tyrosine-Derived Metabolites from the Sponge Ianthella basta. Aust. J. Chem. 1981, 34, 765–786. [Google Scholar] [CrossRef]
- Pettit, G.R.; Butler, M.S.; Williams, M.D.; Filiatrault, M.J.; Pettit, R.K. Isolation and structure of hemibastadinols 1-3 from the Papua New Guinea marine sponge Ianthella basta. J. Nat. Prod. 1996, 59, 927–934. [Google Scholar] [CrossRef]
- Le Norcy, T.; Niemann, H.; Proksch, P.; Tait, K.; Linossier, I.; Réhel, K.; Hellio, C.; Faÿ, F. Sponge-inspired dibromohemibastadin prevents and disrupts bacterial biofilms without toxicity. Mar. Drugs 2017, 15, 222. [Google Scholar] [CrossRef]
- García-Vilas, J.A.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Aeroplysinin-1, a Sponge-Derived Multi-Targeted Bioactive Marine Drug. Mar. Drugs 2016, 14, 1. [Google Scholar] [CrossRef]
- Litaudon, M.; Guyot, M. Ianthelline, Un nouveau derive de la dibromo-3,5-tyrosine, isole de l’eponge Iantella ardis (Bahanas). Tetrahedron Lett. 1986, 4455–4456. [Google Scholar] [CrossRef]
- Xu, N.J.; Sun, X.; Yan, X.J. A new cyclostellettamine from sponge Amphimedon compressa. Chin. Chem. Lett. 2007, 18, 947–950. [Google Scholar] [CrossRef]
- Kelly, S.R.; Garo, E.; Jensen, P.R.; Fenical, W.; Pawlik, J.R. Effects of Caribbean sponge secondary metabolites on bacterial surface colonization. Aquat. Microb. Ecol. 2005, 40, 191–203. [Google Scholar] [CrossRef]
- Lhullier, C.; Moritz, M.I.G.; Tabalipa, E.O.; Sardá, F.N.; Schneider, N.F.Z.; Moraes, M.H.; Constantino, L.; Reginatto, F.H.; Steindel, M.; Pinheiro, U.S.; et al. Biological activities of marine invertebrates extracts from the northeast brazilian coast. Brazilian J. Biol. 2020, 80. in press. [Google Scholar] [CrossRef]
- Arevabini, C.; Crivelenti, Y.D.; de Abreu, M.H.; Bitencourt, T.A.; Santos, M.F.; Berlinck, R.G.; Hajdu, E.; Beleboni, R.O.; Fachin, A.L.; Marins, M. Antifungal activity of metabolites from the marine sponges Amphimedon sp. and Monanchora arbuscula against Aspergillus flavus strains isolated from peanuts (Arachis hypogaea). Nat. Prod. Commun. 2014, 9, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.M.; Peng, J.; Dunbar, D.C.; Schinazi, R.F.; de Castro Andrews, A.G.; Cuevas, C.; Garcia-Fernandez, L.F.; Kelly, M.; Hamann, M.T. Batzelladine alkaloids from the caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron 2007, 63, 11179–11188. [Google Scholar] [CrossRef]
- Bernan, V.S.; Roll, D.M.; Ireland, C.M.; Greenstein, M.; Maiese, W.M.; Steinberg, D.A. A study on the mechanism of action of sceptrin, an antimicrobial agent isolated from the South Pacific sponge Agelas mauritiana. J. Antimicrob. Chemoth. 1993, 32, 539–550. [Google Scholar] [CrossRef]
- Walker, R.P.; Faulkner, D.J.; Van Engen, D.; Clardy, J. Sceptrin, an antimicrobial agent from the sponge Agelas sceptrum. J. Am. Chem. Soc. 1981, 103, 6772–6773. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.-Y.; Huang, A.M.; Wang, L.; Wang, Q.; Cao, P.-Y.; Yang, P.M. Antibacterial Meroterpenoids from the South China Sea Sponge Dysidea sp. Chem. Pharm. Bull. 2016, 64, 1036–1042. [Google Scholar] [CrossRef]
- Albrizio, S.; Ciminiello, P.; Fattorusso, E.; Magno, S.; Pawlik, J.R. Amphitoxin, a new high molecular weight antifeedant pyridinium salt from the Caribbean sponge Amphimedon compressa. J. Nat. Prod. 1995, 58, 647–652. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Hollenbeak, K.H.; Campbell, D.C. Marine natural products: Halitoxin, toxic complex of several marine sponges of the genus Haliclona. J. Org. Chem. 1978, 43, 3916–3922. [Google Scholar] [CrossRef]
- Turk, T.; Sepčić, K.; Mancini, I.; Guella, G. 3-Akylpyridinium and 3-alkylpyridine compounds from marine sponges, their synthesis, biological activities and potential use. Stud. Nat. Prod. Chem. 2008, 35, 355–397. [Google Scholar]
- Kelman, D.; Kashman, Y.; Hill, R.T.; Rosenberg, E.; Loya, Y. Chemical warfare in the sea: The search for antibiotics from Red Sea corals and sponges. Pure Appl. Chem. 2009, 81, 1113–1121. [Google Scholar] [CrossRef]
- Kelman, D.; Kashman, Y.; Rosenberg, E.; Ilan, M.; Ifrach, I.; Loya, Y. Antimicrobial activity of the reef sponge Amphimedon viridis from the Red Sea: Evidence for selective toxicity. Aquat. Microb. Ecol. 2001, 24, 9–16. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Ogawa, C.A.; Almeida, A.M.P.; Sanchez, M.A.A.; Malpezzi, E.L.A.; Costa, L.V.; Hadju, E.; Freitas, J.C. Chemical and Pharmacological Characterization of Halitoxin from Amphimedon viridis (Porifera) from the southeastern Brazilian Coast. Comp. Biochem. Physiol. 1996, 115C, 155–163. [Google Scholar] [CrossRef]
- Kelly, S.R.; Jensen, P.R.; Henkel, T.P.; Fenical, W.; Pawlik, J.R. Effects of Caribbean sponge extracts on bacterial attachment. Aquat. Microb. Ecol. 2003, 31, 175–182. [Google Scholar] [CrossRef]
- Anta, C.; González, N.; Santafé, G.; Rodríguez, J.; Jiménez, C. New Xenia Diterpenoids from the Indonesian Soft Coral Xenia sp. J. Nat. Prod. 2002, 65, 766–768. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A, -B, -C and -D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on Na, K-atpase from the okinawa sea sponge Agelas sp. Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar] [CrossRef]
- Wu, H.; Nakamura, H.; Kobayashi, J. Structures of agelasines, diterpenes having a 9-methyladeninium chromophore isolated from the Okinawan marine sponge Agelas nakamurai Hoshino. Bull. Chem. Soc. Jpn. 1986, 59, 2495–2504. [Google Scholar] [CrossRef]
- Fu, X.; Schmitz, F.J.; Tanner, R.S.; Kelly-Borges, M. Agelasines H and I, 9-methyladenine-containing diterpenoids from an Agelas sponge. J. Nat. Prod. 1998, 61, 548–550. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kaneko, M.; Okamura, H.; Nakatani, M.; Van Soest, R.W.M. New alkaloids from the Papua New Guinean sponge Agelas nakamurai. J. Nat. Prod. 1998, 61, 1310–1312. [Google Scholar] [CrossRef]
- Arai, M.; Yamano, Y.; Setiawan, A.; Kobayashi, M. Identification of the target protein of agelasine D, a marine sponge diterpene alkaloid, as an anti-dormant mycobacterial substance. Chem. Bio. Chem. 2014, 15, 117–123. [Google Scholar] [CrossRef]
- Abdjul, D.B.; Yamazaki, H.; Kanno, S.I.; Takahashi, O.; Kirikoshi, R.; Ukai, K.; Namikoshi, M. Structures and Biological Evaluations of Agelasines Isolated from the Okinawan Marine Sponge Agelas nakamurai. J. Nat. Prod. 2015, 78, 1428–1433. [Google Scholar] [CrossRef]
- Chu, M.J.; Tang, X.L.; Qin, G.F.; Sun, Y.T.; Li, L.; de Voogd, N.J.; Li, P.L.; Li, G.Q. Pyrrole Derivatives and Diterpene Alkaloids from the South China Sea Sponge Agelas nakamurai. Chem. Biodivers. 2017, 14, e1600446. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.L.; Sun, J.B.; Yang, F.; Liu, M.; Tang, J.; Sun, F.; Jiao, W.H.; Wang, S.P.; Zhang, W.; Lin, H.W. New diterpene alkaloids from the marine sponge Agelas mauritiana. RSC Adv. 2017, 7, 23970–23976. [Google Scholar] [CrossRef]
- Raub, M.; Cardellina, J.; Spande, T. The piclavines, antimicrobial indolizidines from the tunicate Clavelina picta. Tetrahedron Lett. 1992, 33, 2257–2260. [Google Scholar] [CrossRef]
- Bianco, É.M.; Krug, J.L.; Zimath, P.L.; Kroger, A.; Paganelli, C.J.; Boeder, A.M.; Dos Santos, L.; Tenfen, A.; Ribeiro, S.M.; Kuroshima, K.N.; et al. Antimicrobial (including antimollicutes), antioxidant and anticholinesterase activities of Brazilian and Spanish marine organisms evaluation of extracts and pure compounds. Rev. Bras. Farmacogn. 2015, 25, 668–676. [Google Scholar] [CrossRef]
- Arumugam, V.; Venkatesan, M.; Ramachandran, K.; Ramachandran, S.; Palanisamy, S.K.; Sundaresan, U. Purification, Characterization and Antibacterial Properties of Peptide from Marine Ascidian Didemnum sp. Int. J. Pept. Res. Ther. 2020, 26, 201–208. [Google Scholar] [CrossRef]
- Jaffarali, H.A.; Tamilselvi, M.; Sivakumar, V. Antibacterial activity of the marine ascidians Phallusia nigra and Herdmania pallida from the Tuticorin coast, India. J. Biol. Res.Thessal. 2008, 10, 171–179. [Google Scholar]
- Zidar, N.; Montalvão, S.; Hodnik, Ž.; Nawrot, D.; Žula, A.; Ilaš, J.; Kikelj, D.; Tammela, P.; Mašič, L.P. Antimicrobial Activity of the Marine Alkaloids, Clathrodin and Oroidin, and Their Synthetic Analogues. Mar. Drugs 2014, 12, 940–963. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Jamison, T.F. Total Synthesis of (±)-Sceptrin. Org. Lett. 2020. Article ASAP. [Google Scholar] [CrossRef]
- Marques, D.N.; De Almeida, A.S.; de Oliveira Sousa, A.R.; Pereira, R.; Andrade, A.L.; Chaves, R.P.; Carneiro, R.F.; De Vasconcelos, M.A.; Do Nascimiento-Neto, L.G.; Pinheiro, U.; et al. Antibacterial activity of a new lectin isolated from the marine sponge Chondrilla caribensis. Int. J. Biol. Macromol. 2018, 109, 1292–1301. [Google Scholar] [CrossRef]
- Sepčić, K.; Kauferstein, S.; Mebs, D.; Turk, T. Biological Activities of Aqueous and Organic Extracts from Tropical Marine Sponges. Mar. Drugs 2010, 8, 1550–1566. [Google Scholar] [CrossRef]
- Arai, M.; Yamano, Y.; Kamiya, K.; Setiawan, A.; Kobayashi, M. Anti-dormant mycobacterial activity and target molecule of melophlins, tetramic acid derivatives isolated from a marine sponge of Melophlus sp. J. Nat. Med. 2016, 70, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Laport, M.S.; Marinho, P.R.; da Silva Santos, O.C.; de Almeida, P.; Romanos, M.T.V.; Muricy, G.; Brito, M.A.V.P.; Giambiagi-deMarval, M. Antimicrobial activity of marine sponges against coagulase-negative staphylococci isolated from bovine mastitis. Vet. Microbiol. 2012, 155, 362–368. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.F.; De Oliveira, J.H.; Galetti, F.C.; De Souza, A.O.; Silva, C.L.; Hajdu, E.; Peixinho, S.; Berlinck, R.G. Antimycobacterial Brominated Metabolites from Two Species of Marine Sponges. Planta Med. 2006, 72, 437–441. [Google Scholar] [CrossRef] [PubMed]
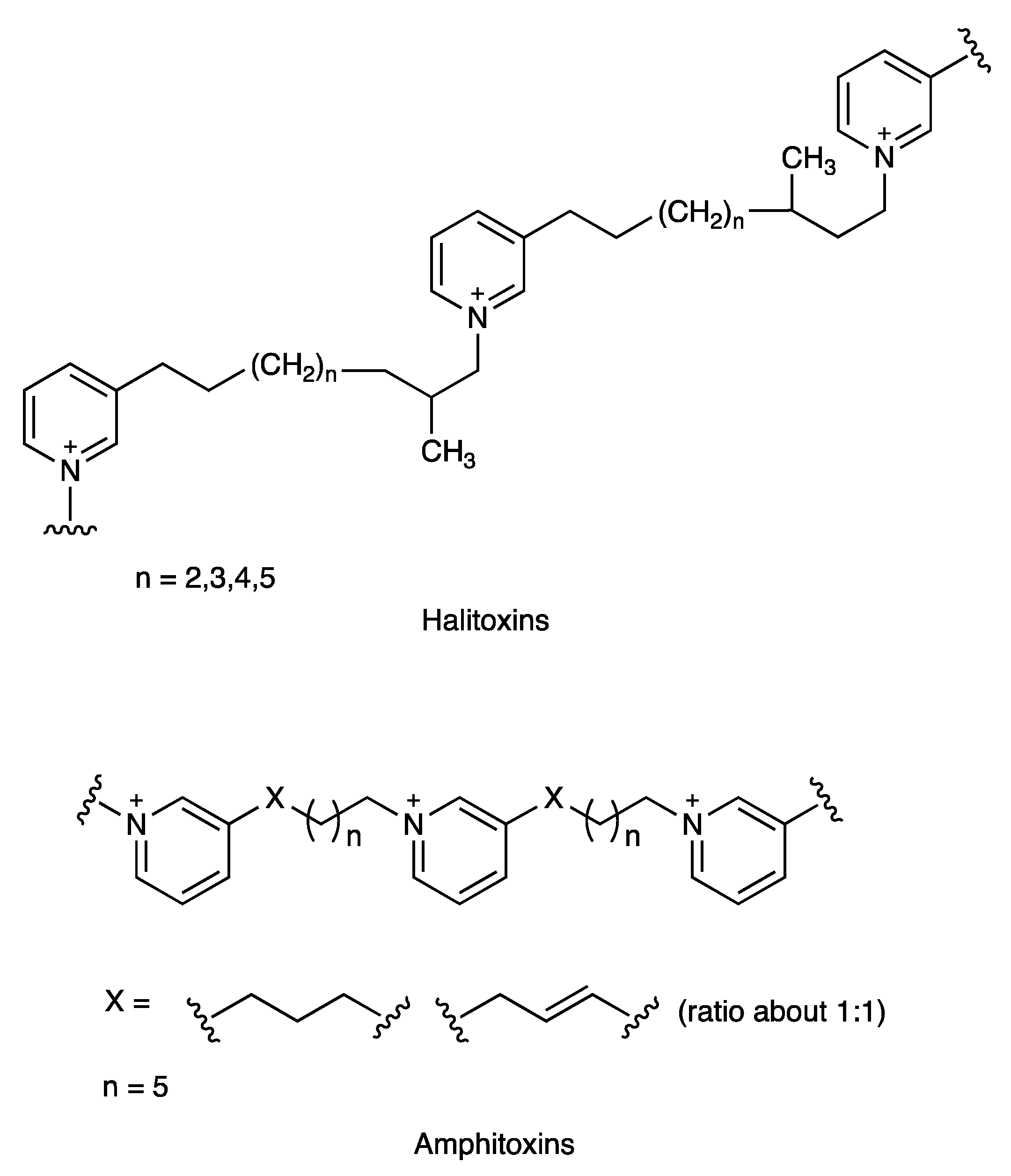
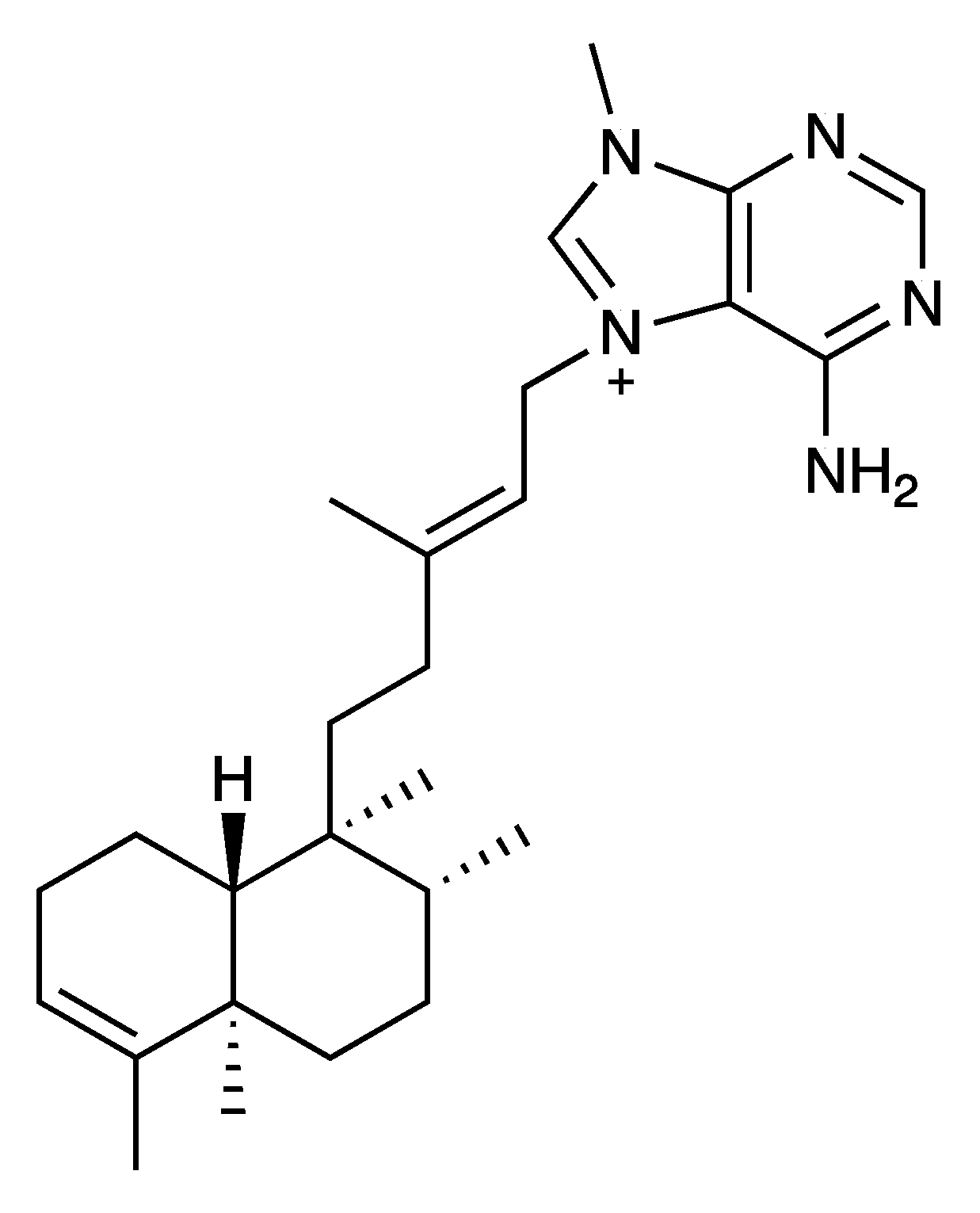
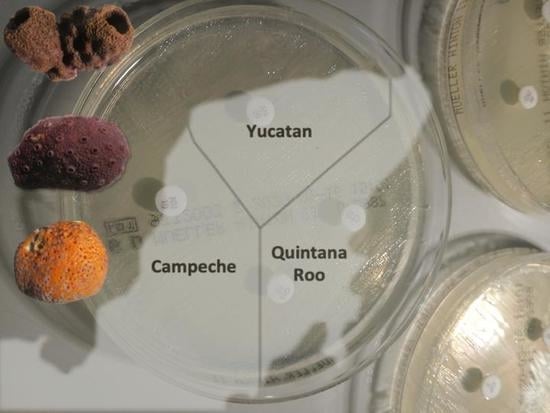
| Phylum | Order | Family, Species (Code) | A. baumanni ATCC 17978 | K. pneumonia ATCC 700603 | P. aeruginosa ATCC 27823 | S. aureus ATCC 29213 |
|---|---|---|---|---|---|---|
| Chordata | Aplousobranchia | Clavelinidae Clavelina sp. (T18-M1) | ≥512 | ≥512 | ≥512 | ≥512 |
| Didemnidae Didemnum perlucidum (E8-2) | ≥512 | ≥512 | ≥512 | >512 | ||
| Didemnum sp. (T18-M4) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Didemnum sp. (E01) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Trididemnum solidum (E7-2) | ≥512 | ≥512 | >512 | >512 | ||
| Polysyncraton sp. (EY18-8) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Polycitoridae Eudistoma amanitum (RIO18-T1) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Eudistoma sp. (TY18-2) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Polyclinidae Polyclinum sp. (T18-M5) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Phlebobranchia | Ascidiidae Phallusia nigra (TY18-1) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Perophoridae Ecteinascidia sp. (T18-M2) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Stolidobranchia | Molgulidae Molgula sp. (T18-M6) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Styelidae Polycarpa sp. (E41) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Porifera | Agelasida | Agelasidae Agelas citrina (CZE56) | 8 | 8 | 8 | 0.5 |
| Agelas clathrodes (E27-2) | ≥512 | ≥512 | ≥512 | >512 | ||
| Agelas clathrodes (MA18-10) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Agelas dilatata (E25-1) | 128 | 64 | 32 | 64 | ||
| Agelas sceptrum (E26-2) | ≥512 | 256 | 64 | >512 | ||
| Axinellida | Heteroxyidae Myrmekioderma gyroderma (CZE18) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Raspailiidae Ectyoplasia ferox (MA18-9) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Ectyoplasia sp. (MA18-13) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Chondrillida | Chondrillidae Chondrilla caribensis f. hermatypica (MA18-6) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Chondrilla sp. (RIO18-1) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Clathrinida | Clathrinidae Clathrina sp. (EY18-10) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Leucettidae Leucetta floridana (E2-2) | 128 | 256 | ≥512 | 128 | ||
| Clionaida | Clionaidae Cliona delitrix (EY18-1) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Cliona varians (EY18-3) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Dictyoceratida | Dysideidae Dysidea sp. (EY18-12) | 16 | ≥512 | ≥512 | 32 | |
| Irciniidae Ircinia felix (E9-2) | ≥512 | ≥512 | ≥512 | >512 | ||
| Ircinia felix (MA18-11) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Ircinia strobilina (E24-2) | ≥512 | ≥512 | ≥512 | >512 | ||
| Ircinia strobilina (E52) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Spongiidae Spongia tubulifera (E11-2) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Haplosclerida | Callyspongiidae Callyspongia longissima (E28) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Callyspongia plicifera (E31) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Callyspongia vaginalis (E16) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Chalinidae Haliclona (Rhizoniera) curacaoensis (EY18-4) | 4 | 16 | 32 | 4 | ||
| Niphatidae Amphimedon compressa (E29) | 32 | 32 | 32 | 32 | ||
| Niphates digitalis (E15) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Niphates erecta (E49) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Niphates erecta (MA18-7) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Niphates erecta (MA18-12) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Petrosiidae Xestospongia muta (EP) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Homoscleropho-rida | Plakinidae Plakinastrella onkodes (E3) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Poecilosclerida | Crambeidae Monanchora arbuscula (E35) | ≥512 | ≥512 | ≥512 | 16 | |
| Microcionidae Clathria gomezae (EY18-11) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Clathria virgultosa (E7-E34) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Mycalidae Mycale laevis (MA18-1) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Mycale laevis (MA18-5) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Scopalinida | Scopalinidae Scopalina ruetzleri (MA18-5) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Scopalina ruetzleri (E53) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Scopalina ruetzleri (EY18-7) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Suberitida | Halichondriidae Halichondria melanadocia (E18-M1) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Suberitidae Aaptos sp. (E38) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Tethyida | Tethyidae Tethya sp. (E20) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Tetractinellida | Geodiidae Melophlus hajdui (E4) | ≥512 | ≥512 | ≥512 | ≥512 | |
| Tetillidae Cinachyrella kuekenthali (MA18-2) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Verongiida | Aplysinidae Aiolochroia crassa (E50) | 64 | 128 | >128 | 32 | |
| Aiolochroia crassa (MA18-4) | 32 | 128 | 128 | 64 | ||
| Aplysina cauliformis (E36) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Aplysina fistularis (E46) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Aplysina fulva (E42) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Aplysina fulva (EY18-5) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Aplysina muricyanna (E47) | ≥512 | ≥512 | ≥512 | ≥512 | ||
| Imipenem positive control Vancomycin positive control | 0.5 | 0.25 | 2 | nt | ||
| nt | nt | nt | 1 | |||
| Fraction | A. baumannii ATCC 17978 | K. pneumoniae ATCC 700603 | P. aeruginosa ATCC 27823 | S. aureus ATCC 29213 |
|---|---|---|---|---|
| R1 | 128 | 256 | 64 | nt * |
| R2 | 128 | 256 | 256 | Nt |
| R3 | 8 | 16 | 16 | 2 |
| R4 | 8 | 16 | 16 | 2 |
| R5 | >512 | >512 | >512 | Nt |
| R6 | >512 | >512 | >512 | Nt |
| R7 | >512 | >512 | >512 | Nt |
| Bacterial Strain | R3 | R4 | R4H2 | |
|---|---|---|---|---|
| A. baumannii | ATCC 17978 | 8 | 8 | 4 |
| ABRIM | 8 | 8 | 4 | |
| K. pneumoniae | ATCC 700603 | 16 | 16 | 4 |
| Kp3380 | 8 | 8 | 2 | |
| P. aeruginosa | ATCC 27853 | 16 | 16 | 2 |
| PAO1 | 16 | 16 | 4 | |
| S. aureus | ATCC 29213 | 2 | 2 | 1 |
| USA 300 | 1 | 1 | 1 |
| Fraction | A. baumannii ATCC 17978 | K. pneumoniae ATCC 700603 | P. aeruginosa ATCC 27823 | S. aureus ATCC 29213 |
|---|---|---|---|---|
| WW | >128 | >128 | >128 | >128 |
| WB | >128 | >128 | >128 | >128 |
| FH | >128 | >128 | >128 | >128 |
| FM | 64 | 64 | 32 | 4 |
| FD | 16 | 32 | 32 | 2 |
| Bacterial Strain | R1 | R2 | R3 | R4 | R5 | R6 | R7 | (-)-agelasine B | |
|---|---|---|---|---|---|---|---|---|---|
| A. baumannii | ATCC 17978 | >64 | 64 | 16 | >64 | 16 | >64 | 32 | >128 |
| ABRIM | >64 | 64 | 16 | >64 | 16 | >64 | 32 | >128 | |
| K. pneumoniae | ATCC 700603 | >64 | >64 | 32 | >64 | >64 | >64 | 32 | >128 |
| Kp3380 | 64 | 64 | 8 | 64 | 16 | >64 | 16 | >128 | |
| P. aeruginosa | ATCC 27853 | >64 | 64 | 16 | >64 | >64 | >64 | 32 | >128 |
| PAO1 | >64 | 64 | 16 | >64 | >64 | >64 | 64 | >128 | |
| S. aureus | ATCC 29213 | 8 | 4 | 0.5 | 8 | 2 | 8 | 2 | 2 |
| USA 300 | 16 | 8 | 1 | 8 | 4 | 16 | 4 | 2 |
| Family | Species, (Code) | Site | Reported Antibacterial Activity | References |
|---|---|---|---|---|
| Phylum: Chordata Order: Aplousobranchia | ||||
| Clavelinidae | Clavelina sp. (T18-M1) | Progreso, Yucatan (Mangrove) | Example of species of this genus: C. pictus. Stereoisomers of piclavins A2 to A4 displayed low activity against Gram-positive bacteria (S.aureus, B. cereus and C. michiganensis). | [67] |
| Didemnidae | Didemnum perlucidum (E8-2) | Rio Indio, Quintana Roo | Low activity against S. aureus, and not active against E. coli and P. aeruginosa. | [68] |
| Didemnum sp. (T18-M4) | Progreso, Yucatan (Mangrove) | Example of species of this genus: Antimicrobial activity of an unidentified Didemnum species against E. faecalis, S. aureus, S. typhimurium, S. marcescens and P. aeruginosa. | [69] | |
| Didemnum sp. (E01) | Bermejo, Quintana Roo | |||
| Trididemnum solidum (E7-2) | Rio Indio, Quintana Roo | No previous reports for this species. | ||
| Polysyncraton sp. (EY18-8) | Progreso, Yucatan | No previous reports for this genus. | ||
| Polycitoridae | Eudistoma amanitum (RIO18-T1) | Río Indio, Quintana Roo | No previous reports for this genus. | |
| Eudistoma sp. (TY18-2) | Progreso, Yucatan | No previous reports for this genus. | ||
| Polyclinidae | Polyclinum sp. (T18-M5) | Progreso, Yucatan (Mangrove) | The extract of a Polyclinum sp. yielded MICs of > 1000 mg/L against S. aureus, E. coli and P. aeruginosa. | [68] |
| Order: Phlebobranchia | ||||
| Ascidiidae | Phallusia nigra (TY18-1) | Progreso, Yucatan | Low antimicrobial activity against B. subtilis, S. aureus, E. aerogenes, E. coli, K. pneumoniae, P. aeruginosa, S. paratyphii, S. typhii and V. cholera. | [70] |
| Perophoridae | Ecteinascidia sp. (T18-M2) | Progreso, Yucatan (Mangrove) | No previous reports for this genus. | |
| Order: Stolidobranchia | ||||
| Molgulidae | Molgula sp. (T18-M6) | Progreso, Yucatan (Mangrove) | No previous reports for this genus. | |
| Styelidae | Polycarpa sp. (E41) | Alacranes Reef, Yucatan | No previous reports for this genus. | |
| Phylum: Porifera Order: Agelasida | ||||
| Agelasidae | Agelas citrina (CZE56) | Cozumel, Quintana Roo | Antimicrobial activity against E. coli and inhibition of its quorum sensing. No antimicrobial activity against C. violaceum. Inhibition of quorum sensing at high concentrations. | [37] |
| Agelas clathrodes (E27-2) | Cozumel, Quintana Roo | Clathrodin did not display antimicrobial activity against E. faecalis, S. aureus and E. col, but displayed low antifungal activity against C. albicans. | [71] | |
| Agelas clathrodes (MA18-10) | Mahahual, Quintana Roo | |||
| Agelas dilatate(E25-1) | Cozumel, Quintana Roo | No previous reports for this species. | ||
| Agelas sceptrum (E26-2) | Cozumel, Quintana Roo | Sceptrin displayed antimicrobial activity against S.aureus, B. subtilis and P. aeruginosa. | [49,72] | |
| Order: Axinellida | ||||
| Heteroxyidae | Myrmekioderma gyroderma (CZE18) | Cozumel, Quintana Roo | No previous reports for this species. | |
| Raspailiidae | Ectyoplasia ferox (MA18-9) | Mahahual, Quintana Roo | No previous reports for this species. | |
| Ectyoplasia sp. (MA18-13) | Mahahual, Quintana Roo | No previous reports for this genus. | ||
| Order: Chondrillida | ||||
| Chondrillidae | Chondrilla caribensis f. hermatypica (MA18-6) | Mahahual, Quintana Roo | No inhibition of S. aureus, S. epidermidis or E. coli growth. | [73] |
| Chondrilla sp. (RIO18-1) | Río Indio, Quintana Roo | No previous reports for this genus. | ||
| Order: Clathrinida | ||||
| Clathrinidae | Clathrina sp. (EY18-10) | Progreso, Yucatan | No previous reports for this genus. | |
| Leucettidae | Leucetta floridana (E2-2) | Bermejo, Quintana Roo | No previous reports for this species. | |
| Order: Clionaida | ||||
| Clionaidae | Cliona delitrix (EY18-1) | Progreso, Yucatan | Quorum sensing inhibition in E.coli. | [37] |
| Cliona varians (EY18-3) | Progreso, Yucatan | No observed antibacterial activity against E. coli or C. violaceum. | [37] | |
| Order: Dictyoceratida | ||||
| Dysideidae | Dysidea sp. (EY18-12) | Progreso, Yucatan | ||
| Irciniidae | Ircinia felix (E9-2) | Rio Indio, Quintana Roo | Quorum sensing inhibition in C. violaceum. No effects on bacterial growth observed for this species. Antibacterial activity against B. subtilis. | [37,74] |
| Ircinia felix (MA18-11) | Mahahual, Quintana Roo | |||
| Ircinia strobilina (E24-2) | Cozumel, Quintana Roo | Antibacterial activity against B. subtilis. No inhibition of E. coli growth. | [74] | |
| Ircinia strobilina (E52) | Bermejo, Quintana Roo | |||
| Spongiidae | Spongia tubulifera (E11-2) | Rio Indio, Quintana Roo | No previous reports for this species. | |
| Order: Haplosclerida | ||||
| Callyspongiidae | Callyspongia longissima (E28) | Alacranes Reef, Yucatan | No previous reports for this species. | |
| Callyspongia plicifera (E31) | Alacranes Reef, Yucatan | Antibacterial activity against E. coli. | [74] | |
| Callyspongia vaginalis (E16) | Cozumel, Quintana Roo | Antibacterial activity against B. subtilis | [74] | |
| Chalinidae | Haliclona (Rhizoniera) curacaoensis(EY18-4) | Progreso, Yucatan | No previous reports for this species. | |
| Niphatidae | Amphimedon compressa (E29) | Alacranes Reef, Yucatan | Antibacterial activity of extracts against marine bacteria strains, E. faecalis, P. aeruginosa and E. coli. 8,8′-dienecyclostellettamine showed a potent antibacterial activity against E. coli, P. aeruginosa and MRSA with IC50 values of 1.3, 2.1, 0.25 mg/L respectively. | [43,44,45,46] |
| Niphates digitalis (E15) | Cozumel, Quintana Roo | No previous reports for this species. | ||
| Niphates erecta (E49) | Alacranes Reef, Yucatan | No previous reports for this species. | ||
| Niphates erecta (MA18-7) | Mahahual, Quintana Roo | |||
| Niphates erecta (MA18-12) | Mahahual, Quintana Roo | |||
| Petrosiidae | Xestospongia muta (EP) | Alacranes Reef, Yucatan | No growth or quorum sensing inhibition of E. coli or C. violaceum. | [37] |
| Order: Homosclerophorida | ||||
| Plakinidae | Plakinastrella onkodes (E3) | Bermejo, Quintana Roo | No previous reports for this species. | |
| Order: Poecilosclerida | ||||
| Crambeidae | Monanchora arbuscula(E35) Synonymised names: M. unguifera | Alacranes Reef, Yucatan | Ptilomycalin A, batzelladines L, M, C, dehydrobatzelladine C, crambescidine 800 and 16β-hidroxycrambescidin 359 were isolated of this species and showed MIC of between 0.31–20.0 mg/L against S. aureus, methicillin-resistant S. aureus (MRSA), P. aeruginosa and M. intracellulare. | [47] |
| Microcionidae | Clathria gomezae (EY18-11) | Progreso, Yucatan | No previous reports for this species. | |
| Clathria virgultosa (E7-E34) | Alacranes Reef, Yucatan | No previous reports for this species. | ||
| Mycalidae | Mycale laevis (MA18-1) | Mahahual, Quintana Roo | No previous reports for this species. | |
| Mycale laevis (MA18-5) | Mahahual, Quintana Roo | |||
| Order: Scopalinida | ||||
| Scopalinidae | Scopalina ruetzleri (DNY) | Rio Indio, Quintana Roo | No previous reports for this species. | |
| Scopalina ruetzleri (E53) | Cozumel, Quintana Roo | |||
| Scopalina ruetzleri (EY18-7) | Progreso, Yucatan | |||
| Order: Suberitida | ||||
| Halichondriidae | Halichondria melanadocia (E18-M1) | Progreso, Yucatan (Mangrove) | No previous reports for this species. | |
| Suberitidae | Aaptos sp. (E38) | Alacranes Reef, Yucatan | No previous reports for this species. | |
| Order: Tethyida | ||||
| Tethyidae | Tethya sp. (E20) | Cozumel, Quintana Roo | No previous reports for this species. | |
| Order: Tetractinellida | ||||
| Geodiidae | Melophlus hajdui (E4) | Bermejo, Quintana Roo | Antibacterial activity against Mycobacterium sp. | [75] |
| Tetillidae | Cinachyrella kuekenthali (MA18-2) | Mahahual, Quintana Roo | Antibacterial activity of aqueous and ethanolic extracts against different species of the genus Staphylococcus sp. | [76] |
| Order: Verongiida | ||||
| Aplysinidae | Aiolochroia crassa(E50) Synonymised names: Pseudoceratina crassa, Ianthella basta and Ianthella ardis | Alacranes Reef, Yucatan | Antibacterial activity of extracts against marine bacteria strains and B. subtilis with MIC of 0.4 mg/L. Bastadin 1-6, hemibastadins 1-3 and hemibastadinols 1-3 showed antibacterial activity againts Neisseria gonorrhoeae, E. faecalis and S. aureus. Dibromohemibastadin-1 showed potent antibacterial activity against the biofilm formation of Paracoccus sp. 4M6 and P. aeruginosa PAO1 (10 µM) and quorum sensing inhibition of E. coli pSB401 (8–16 µM) assays. (-)-Aeroplysinin-1 showed antibacterial activity. Ianthelline showed antibacterial activity against S. aureus. | [38,39,40,41,42,44,74] |
| Aiolochroia crassa (MA18-4) | Mahahual, Quintana Roo | |||
| Aplysina cauliformis (E36) | Alacranes Reef, Yucatan | Antibacterial activity against M. tuberculosis H37Rv. | [77] | |
| Aplysina fistularis (E46) | Alacranes Reef, Yucatan | No previous reports for this species. | ||
| Aplysina fulva (E42) | Alacranes Reef, Yucatan | Antibacterial activity against marine bacteria strains. | [44] | |
| Aplysina fulva (EY18-5) | Progreso, Yucatan | |||
| Aplysina muricyanna (E47) | Alacranes Reef, Yucatan | No previous reports for this species. | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pech-Puch, D.; Pérez-Povedano, M.; Gómez, P.; Martínez-Guitián, M.; Lasarte-Monterrubio, C.; Vázquez-Ucha, J.C.; Novoa-Olmedo, M.L.; Guillén-Hernández, S.; Villegas-Hernández, H.; Bou, G.; et al. Marine Organisms from the Yucatan Peninsula (Mexico) as a Potential Natural Source of Antibacterial Compounds. Mar. Drugs 2020, 18, 369. https://doi.org/10.3390/md18070369
Pech-Puch D, Pérez-Povedano M, Gómez P, Martínez-Guitián M, Lasarte-Monterrubio C, Vázquez-Ucha JC, Novoa-Olmedo ML, Guillén-Hernández S, Villegas-Hernández H, Bou G, et al. Marine Organisms from the Yucatan Peninsula (Mexico) as a Potential Natural Source of Antibacterial Compounds. Marine Drugs. 2020; 18(7):369. https://doi.org/10.3390/md18070369
Chicago/Turabian StylePech-Puch, Dawrin, Mar Pérez-Povedano, Patricia Gómez, Marta Martínez-Guitián, Cristina Lasarte-Monterrubio, Juan Carlos Vázquez-Ucha, María Lourdes Novoa-Olmedo, Sergio Guillén-Hernández, Harold Villegas-Hernández, Germán Bou, and et al. 2020. "Marine Organisms from the Yucatan Peninsula (Mexico) as a Potential Natural Source of Antibacterial Compounds" Marine Drugs 18, no. 7: 369. https://doi.org/10.3390/md18070369
APA StylePech-Puch, D., Pérez-Povedano, M., Gómez, P., Martínez-Guitián, M., Lasarte-Monterrubio, C., Vázquez-Ucha, J. C., Novoa-Olmedo, M. L., Guillén-Hernández, S., Villegas-Hernández, H., Bou, G., Rodríguez, J., Beceiro, A., & Jiménez, C. (2020). Marine Organisms from the Yucatan Peninsula (Mexico) as a Potential Natural Source of Antibacterial Compounds. Marine Drugs, 18(7), 369. https://doi.org/10.3390/md18070369