Enhanced Biomass and Astaxanthin Production of Haematococcus pluvialis by a Cell Transformation Strategy with Optimized Initial Biomass Density
Abstract
1. Introduction
2. Results
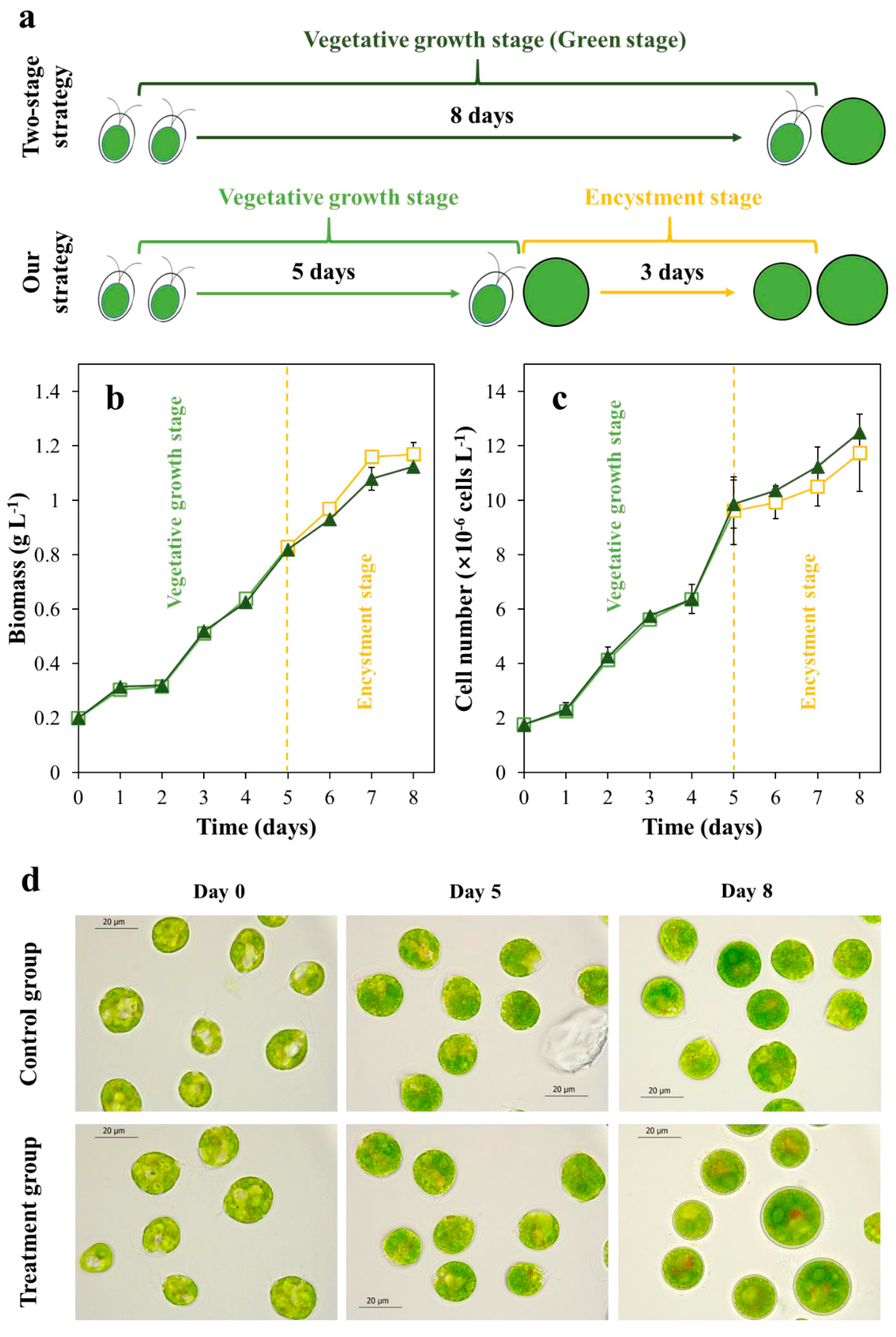
2.1. The Cell Morphology and Growth in the Vegetative Growth Stage
2.2. The Biomass, Astaxanthin Concentration, and Cell Morphology in the Red Stage
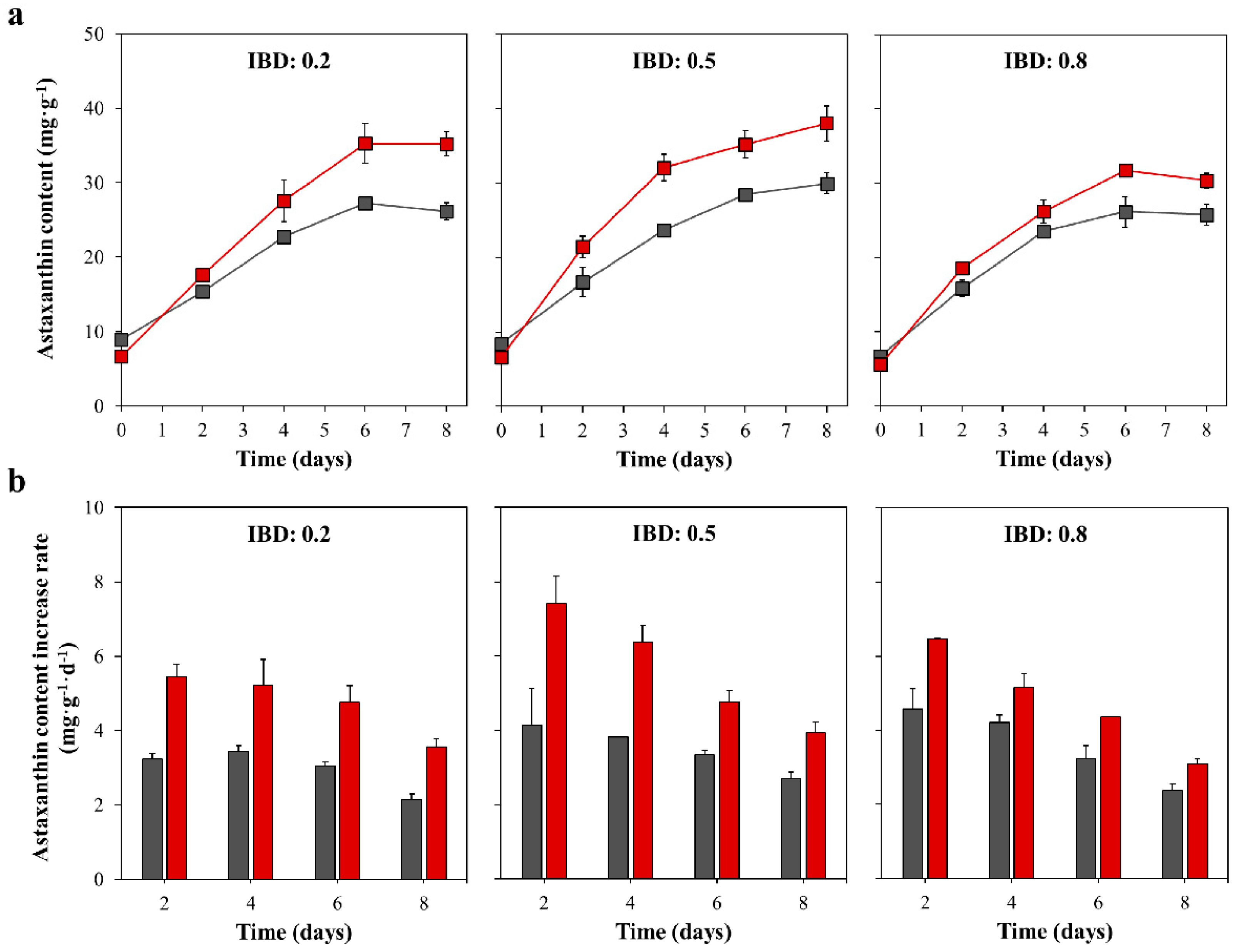
2.3. The Astaxanthin Content and Astaxanthin Content Increase Rate in the Red Stage
3. Discussion
4. Material and Methods
4.1. Strain and Culture Condition
4.2. Analytical Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, G.F.; Kancheva, V.D.; Menshov, V.A.; Naumov, V.V.; Vasil’ev, R.F.; Veprintsev, T.L.; Trofimov, A.V.; Tsaplev, Y.B.; Yablonskaya, O.I. Exogenous and endogenous mediators of oxygen metabolism: Alternatives for chemical and biological activity. Stud. Nat. Prod. Chem. 2016, 47, 357–385. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.K.; Angelova, S.E.; Veprintsev, T.L.; Denev, P.; Fabbri, D.; Dettori, M.A.; Kratchanova, M.; Naumov, V.V.; Trofimov, A.V.; Vasil’ev, R.F.; et al. Antioxidant potential of curcumin-related compounds studied by chemiluminescence kinetics, chain-breaking efficiencies, scavenging activity (ORAC) and DFT calculations. Beilstein J. Org. Chem. 2015, 11, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Sun, S.; Gross, M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: Astaxanthin, a carotenoid without vitamin an activity, enhances in vitro immunoglobulin production in response to at-dependent stimulant and antigen. Nutr. Cancer 1995, 23, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential anti-atherosclerotic properties of astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.L.; Niu, J.F.; Shen, S.D.; Wang, G.C. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Droop, M.R. Some factors governing encystment in Haematococcus pluvialis. Arch. für Mikrobiol. 1955, 21, 267–272. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nagai, S. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 1993, 59, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Kakizono, T.; Kobayashi, M.; Nagai, S. Effect of carbon/nitrogen ratio on encystment accompanied with astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 1992, 74, 403–405. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kurimura, Y.; Kakizono, T.; Nishio, N.; Tsuji, Y. Morphological changes in the life cycle of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 1997, 84, 94–97. [Google Scholar] [CrossRef]
- Elliot, A.M. Morphology and life history of Haematococcus pluvialis. Arch. Protistenk. 1934, 82, 250–272. [Google Scholar]
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Eur. J. Phycol. 2002, 37, 217–226. [Google Scholar] [CrossRef]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Factors responsible for astaxanthin formation in the chlorophyte Haematococcus pluvialis. Bioresour. Technol. 1996, 55, 207–214. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae). Eur. J. Phycol. 2008, 43, 365–376. [Google Scholar] [CrossRef]
- Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). J. Appl. Phycol. 2010, 22, 253–263. [Google Scholar] [CrossRef]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Choi, Y.E.; Yun, Y.S.; Park, J.M. Evaluation of factors promoting astaxanthin production by a unicellular green alga, Haematococcus pluvialis, with fractional factorial design. Biotechnol. Prog. 2002, 18, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, J.; Sommerfeld, M.; Hu, Q. Susceptibility and protective mechanisms of motile and nonmotile cells of Haematococcus pluvialis (Chlorophyceae) to photooxidative stress. J. Appl. Phycol. 2012, 48, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Zhang, Z.; Hu, Q.; Sommerfeld, M.; Lu, Y.H.; Han, D.X. Cellular capacities for high-light acclimation and changing lipid profiles across life cycle stages of the green alga Haematococcus pluvialis. PLoS ONE 2014, 9, e106679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Liu, G.X.; Zhang, L.T. Cell cycles and proliferation patterns in Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2017, 35, 1205–1211. [Google Scholar] [CrossRef]
- Shah, M.; Mahfuzur, R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef]
- Del, R.E.; Acién, F.G.; García-Malea, M.C.; Rivas, J.; Molina-Grima, E.; Guerrero, M.G. Efficiency assessment of the one-step production of astaxanthin by the microalga Haematococcus pluvialis. Biotechnol. Bioeng. 2008, 100, 397–402. [Google Scholar] [CrossRef]
- Fábregas, J.; Otero, A.; Maseda, A.; Domínguez, A. Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2001, 89, 65–71. [Google Scholar] [CrossRef]
- Orosa, M.; Franqueira, D.; Cid, A.; Abalde, J. Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour. Technol. 2005, 96, 373–378. [Google Scholar] [CrossRef]
- He, P.; Duncan, J.; Barber, J. Astaxanthin accumulation in the green alga Haematococcus pluvialis: Effects of cultivation parameters. J. Integr. Plant Biology. 2007, 49, 447–451. [Google Scholar] [CrossRef]
- Choi, Y.E.; Yun, Y.S.; Park, J.M.; Yang, J.W. Determination of the time transferring cells for astaxanthin production considering two-stage process of Haematococcus pluvialis cultivation. Bioresour. Technol. 2011, 102, 11249–11253. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cai, M.G.; Lin, M.W.; Huang, X.H.; Wang, J.; Ke, H.W.; Zheng, X.H.; Chen, D.; Wang, C.H.; Wu, S.T.; et al. Differences between motile and nonmotile cells of Haematococcus pluvialis in the production of astaxanthin at different light intensities. Mar. Drugs. 2019, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.H.; Braune, W.; Greulich, F. Functional aspects of secondary carotenoids in Haematococcus lacustris [Girod] Rostafinski (Volvocales) IV. Protection from photodynamic damage. J. Photochem. Photobiol. B 1993, 20, 153–160. [Google Scholar] [CrossRef]
- Hagen, C.; Braune, W.; Björn, L.O. Functional aspects of secondary carotenoids in Haematococcus lacustris (Volvocales) III. Action as a “sunshade”. J. Phycol. 1994, 30, 241–248. [Google Scholar] [CrossRef]
- Wang, J.; Han, D.; Sommerfeld, M.R.; Lu, C.; Hu, Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J. Appl. Phycol. 2013, 25, 253–260. [Google Scholar] [CrossRef]
- Han, S.; Bin, L.; Xue, L.; Kawing, C.; Feng, C. Staged cultivation enhances biomass accumulation in the green growth phase of Haematococcus pluvialis. Bioresour. Technol. 2017, 233, 326–331. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chen, W.; Shen, H.; Han, D.X.; Li, Y.T.; Jones, D.T.; Timlin, J.A.; Hu, Q. Basic culturing and analytical measurement techniques. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Blackwell: Hoboken, NJ, USA, 2013; pp. 37–68. [Google Scholar]

| Parameters | IBD: 0.2 | IBD: 0.5 | IBD: 0.8 | |||
|---|---|---|---|---|---|---|
| Control Group | Treatment Group | Control Group | Treatment Group | Control Group | Treatment Group | |
| Biomass (g L−1) | 0.58 ± 0.06 | 0.92 ± 0.06 | 1.24 ± 0.13 | 2.02 ± 0.03 | 1.66 ± 0.08 | 2.62 ± 0.06 |
| Biomass productivity (g L−1 d−1) | 0.34 ± 0.04 | 0.44 ± 0.49 | 0.50 ± 0.08 | 0.66 ± 0.04 | 0.62 ± 0.04 | 0.72 ± 0.05 |
| Astaxanthin concentration (mg L−1) | 15.62 ± 1.35 | 32.26 ± 2.76 | 34.67 ± 2.91 | 72.51 ± 0.82 | 43.63 ± 3.03 | 87.35 ± 0.40 |
| Astaxanthin productivity (mg L−1 d−1) | 2.76 ± 0.33 | 5.42 ± 0.59 | 5.42 ± 0.05 | 11.36 ± 0.83 | 6.68 ± 0.66 | 12.37 ± 0.02 |
| Astaxanthin content (mg g−1) | 27.29 ± 0.67 | 35.29 ± 2.66 | 29.99 ± 1.43 | 38.02 ± 2.40 | 26.19 ± 0.59 | 33.35 ± 0.87 |
| Astaxanthin content increase rate (mg g-1 d−1) | 0.34 ± 0.01 | 0.54 ± 0.03 | 0.41 ± 0.10 | 0.74 ± 0.07 | 0.46 ± 0.06 | 0.65 ± 0.00 |
| Cell mortality rate (ca.%) | 25.85 ± 1.20 | 7.63 ± 3.35 | 23.14 ± 5.22 | 4.56 ± 0.27 | 19.08 ± 0.88 | 3.51 ± 2.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Cai, M.; Lin, M.; Huang, X.; Wang, J.; Ke, H.; Wang, C.; Zheng, X.; Chen, D.; Yang, S. Enhanced Biomass and Astaxanthin Production of Haematococcus pluvialis by a Cell Transformation Strategy with Optimized Initial Biomass Density. Mar. Drugs 2020, 18, 341. https://doi.org/10.3390/md18070341
Li F, Cai M, Lin M, Huang X, Wang J, Ke H, Wang C, Zheng X, Chen D, Yang S. Enhanced Biomass and Astaxanthin Production of Haematococcus pluvialis by a Cell Transformation Strategy with Optimized Initial Biomass Density. Marine Drugs. 2020; 18(7):341. https://doi.org/10.3390/md18070341
Chicago/Turabian StyleLi, Feng, Minggang Cai, Mingwei Lin, Xianghu Huang, Jun Wang, Hongwei Ke, Chunhui Wang, Xuehong Zheng, Ding Chen, and Shihui Yang. 2020. "Enhanced Biomass and Astaxanthin Production of Haematococcus pluvialis by a Cell Transformation Strategy with Optimized Initial Biomass Density" Marine Drugs 18, no. 7: 341. https://doi.org/10.3390/md18070341
APA StyleLi, F., Cai, M., Lin, M., Huang, X., Wang, J., Ke, H., Wang, C., Zheng, X., Chen, D., & Yang, S. (2020). Enhanced Biomass and Astaxanthin Production of Haematococcus pluvialis by a Cell Transformation Strategy with Optimized Initial Biomass Density. Marine Drugs, 18(7), 341. https://doi.org/10.3390/md18070341






