Fucoidan Isolated from Saccharina japonica Inhibits LPS-Induced Inflammation in Macrophages via Blocking NF-κB, MAPK and JAK-STAT Pathways
Abstract
1. Introduction
2. Results
2.1. Yield and Physicochemical Properties of Polysaccharides Isolated from S. japonica
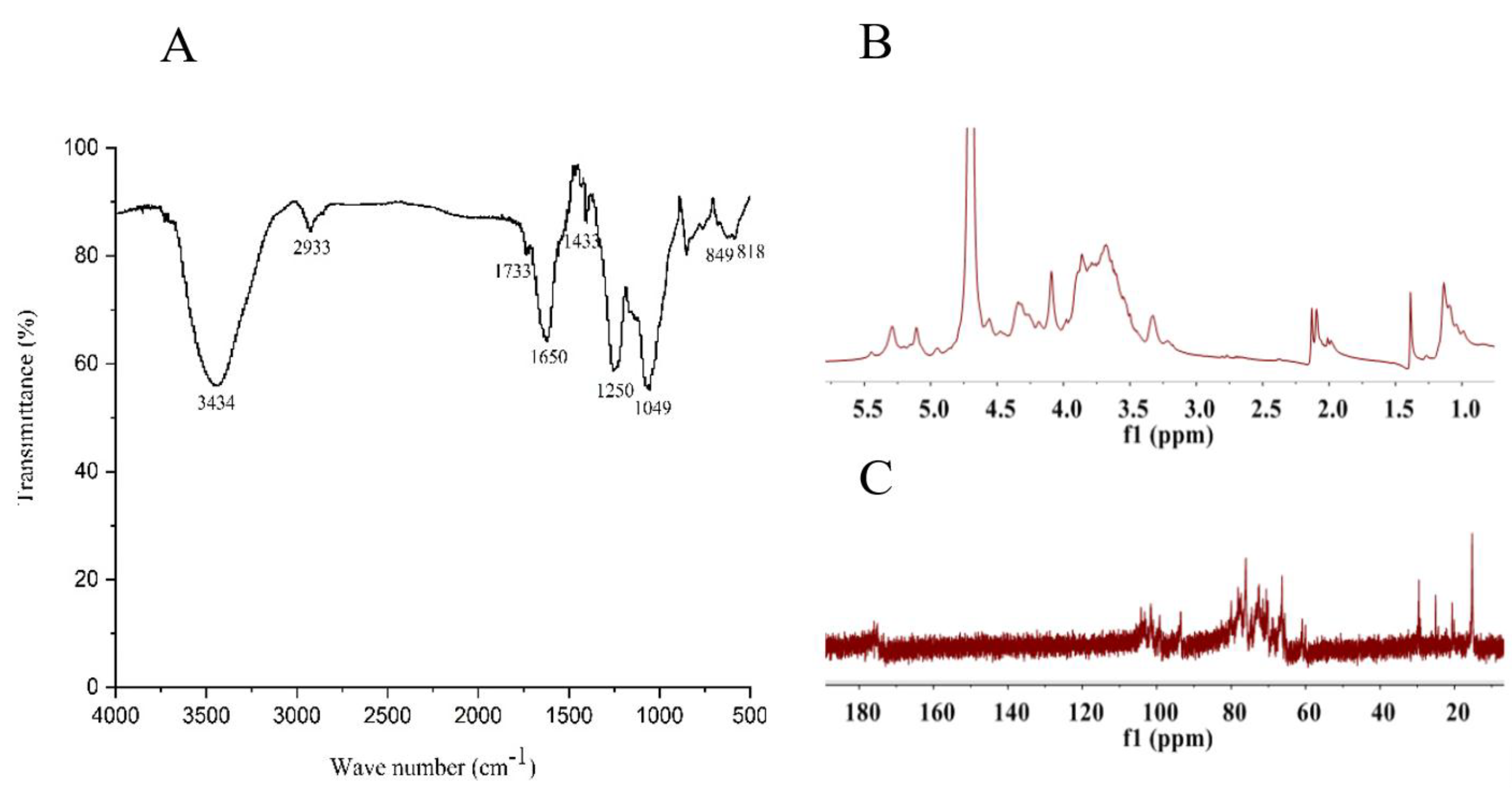
2.2. Structural Feature of SF6
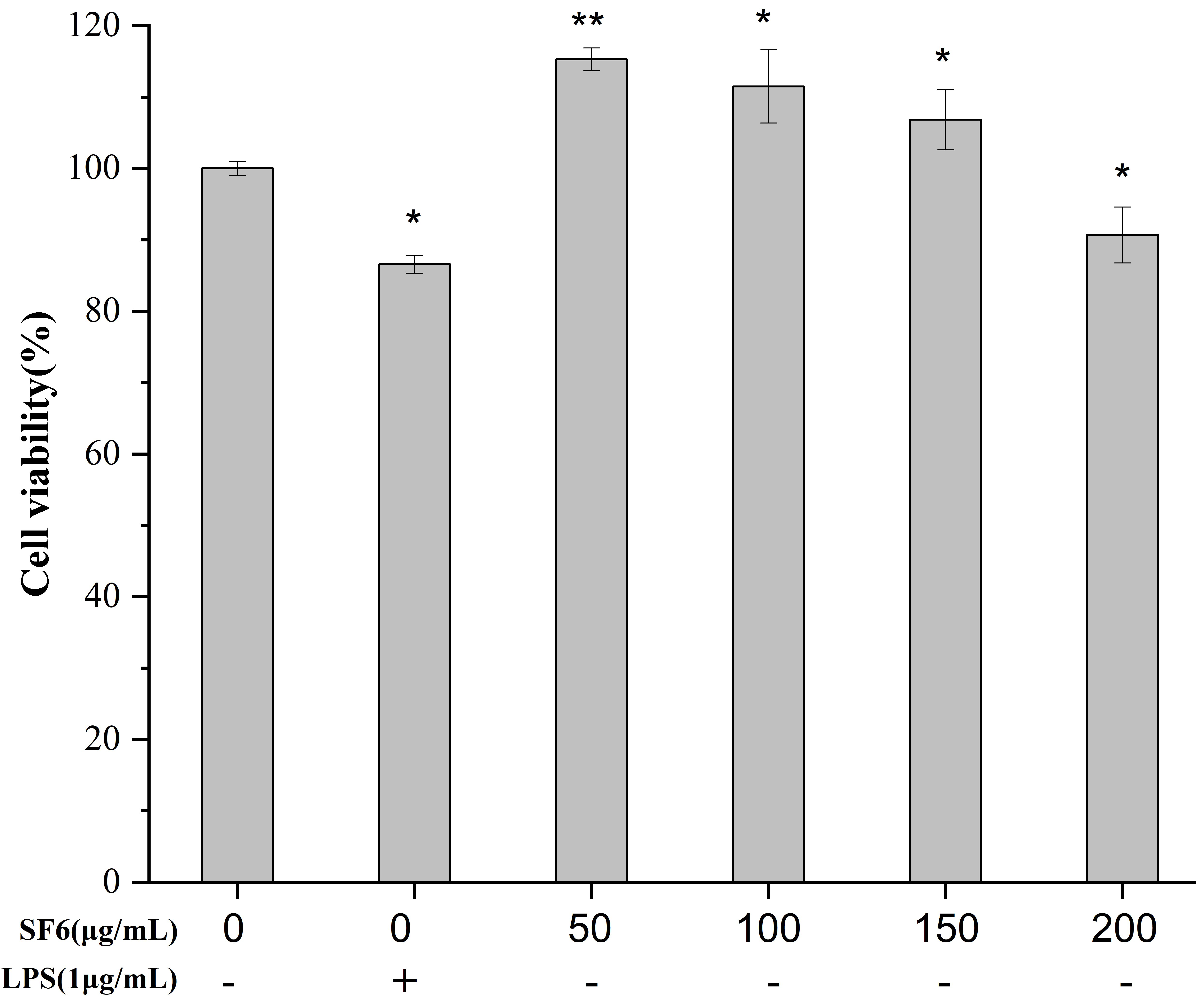
2.3. Effect of SF6 on Macrophage Viability
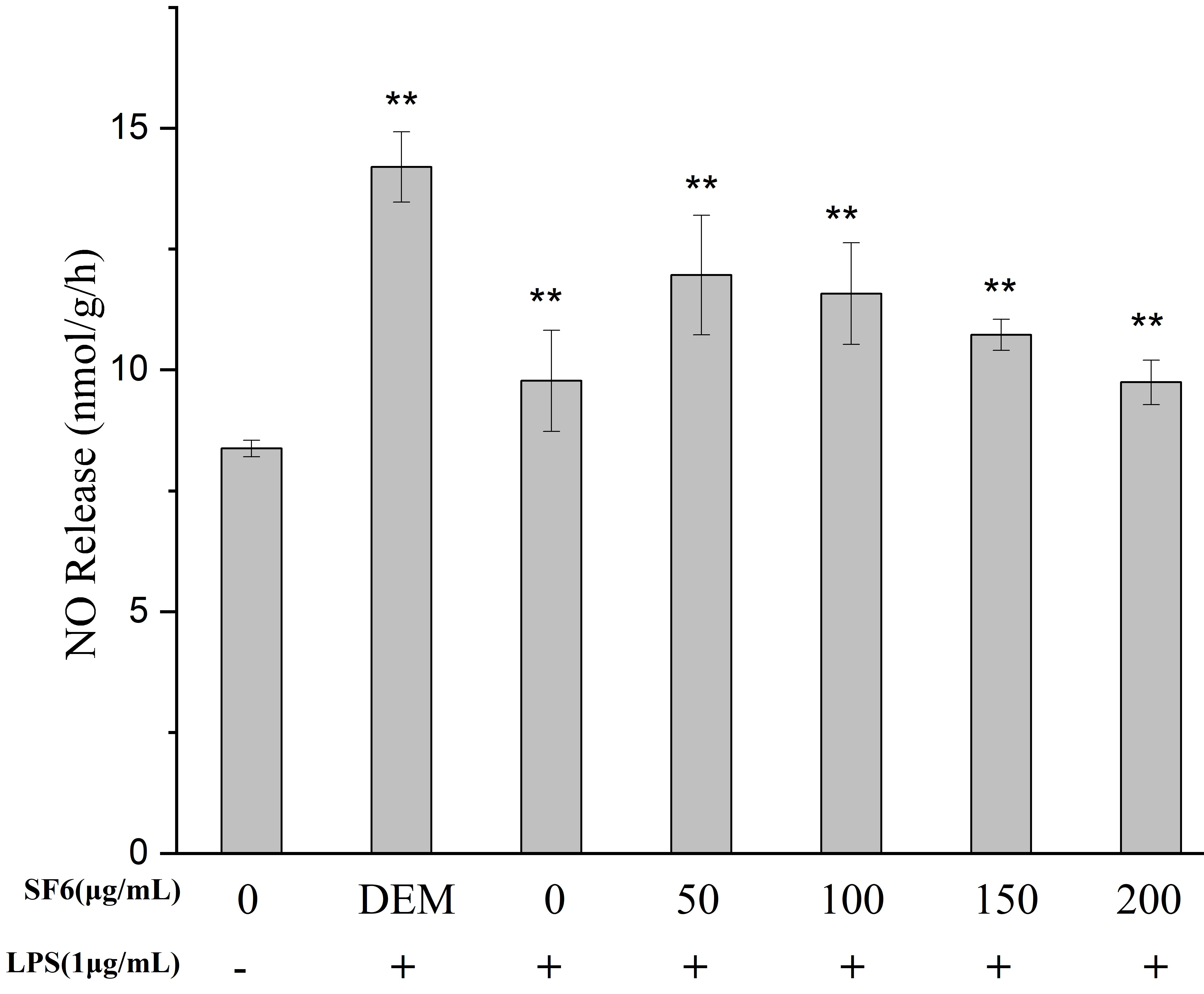
2.4. Effect of SF Fractions on NO Release in LPS-Stimulated RAW 264.7 Macrophage
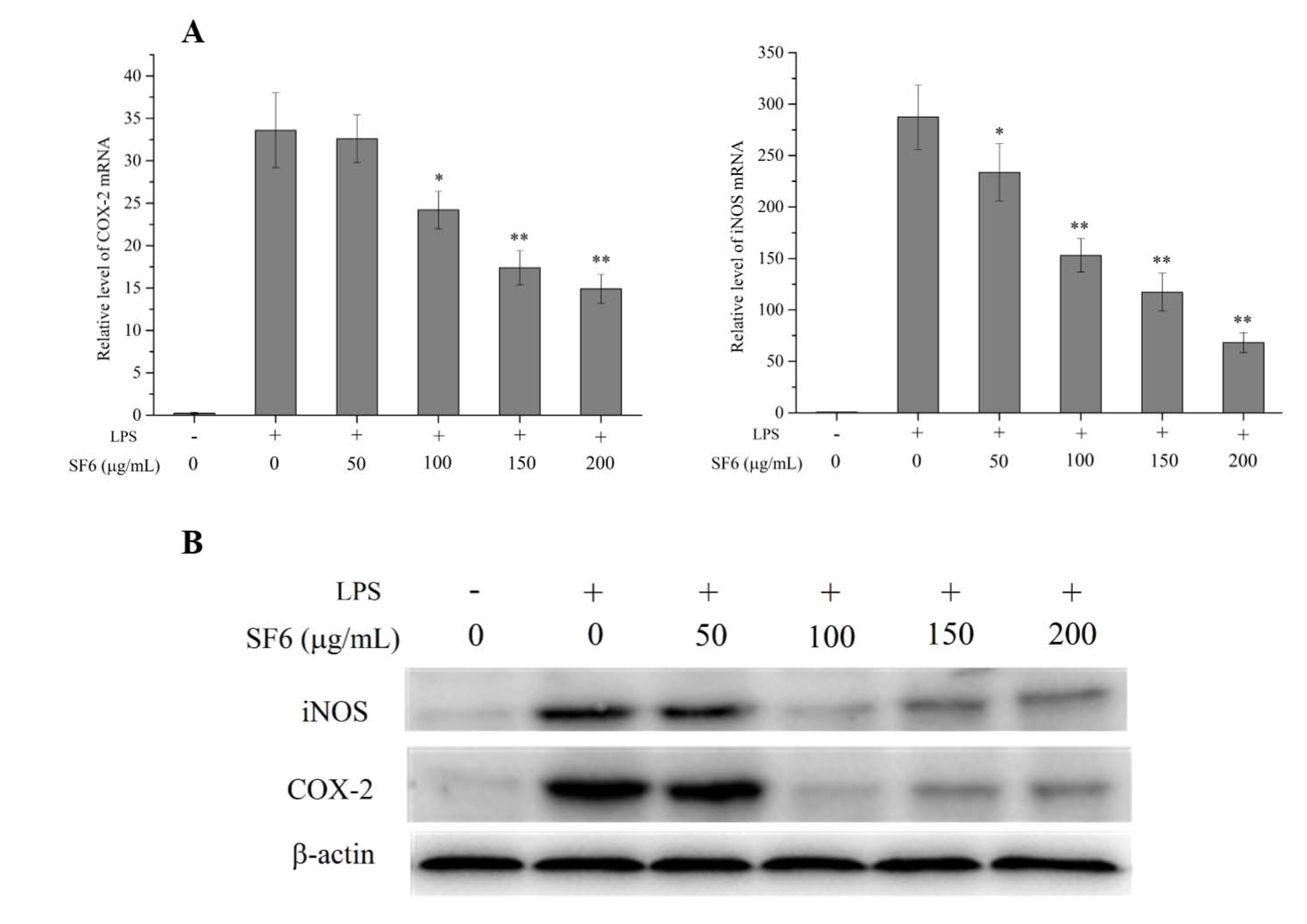
2.5. Effect of SF6 on iNOS and COX-2 mRNA and Protein Expression in LPS-Induced RAW 264.7 Cells
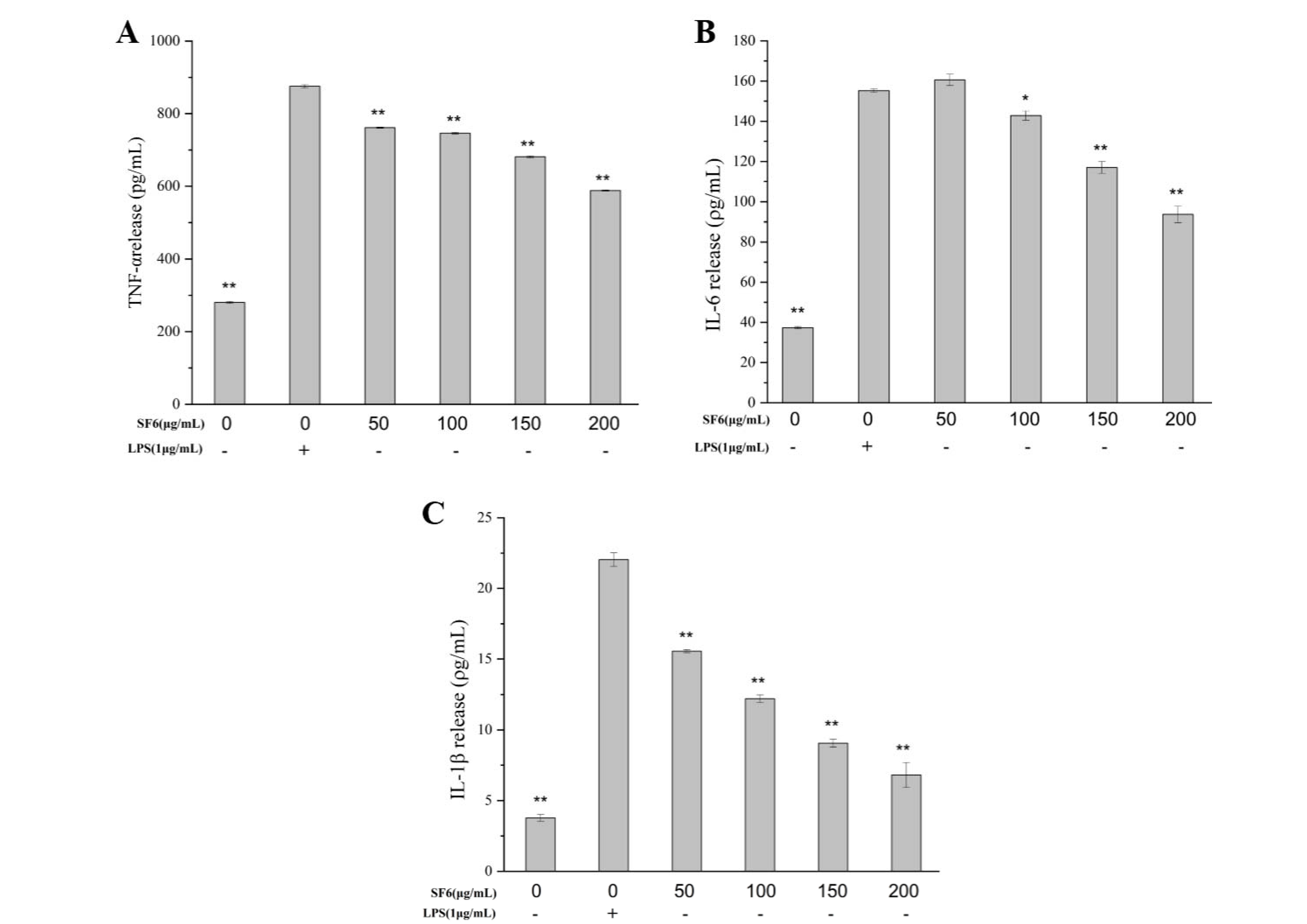
2.6. Inhibitory Effect of SF6 on Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW 264.7 Cells
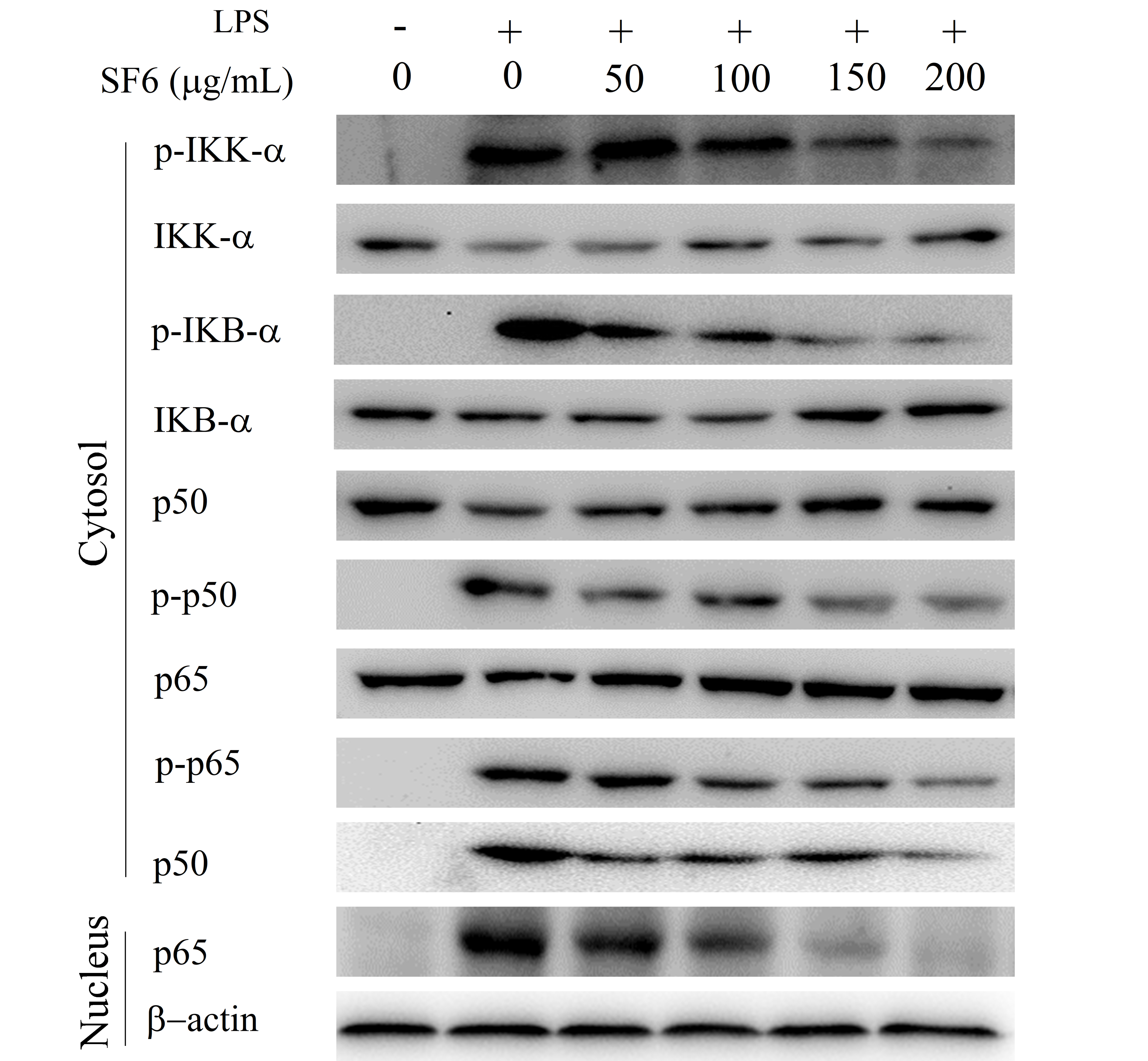
2.7. Effect of SF6 on NF-κB Activation in LPS-Induced RAW 264.7 Cells
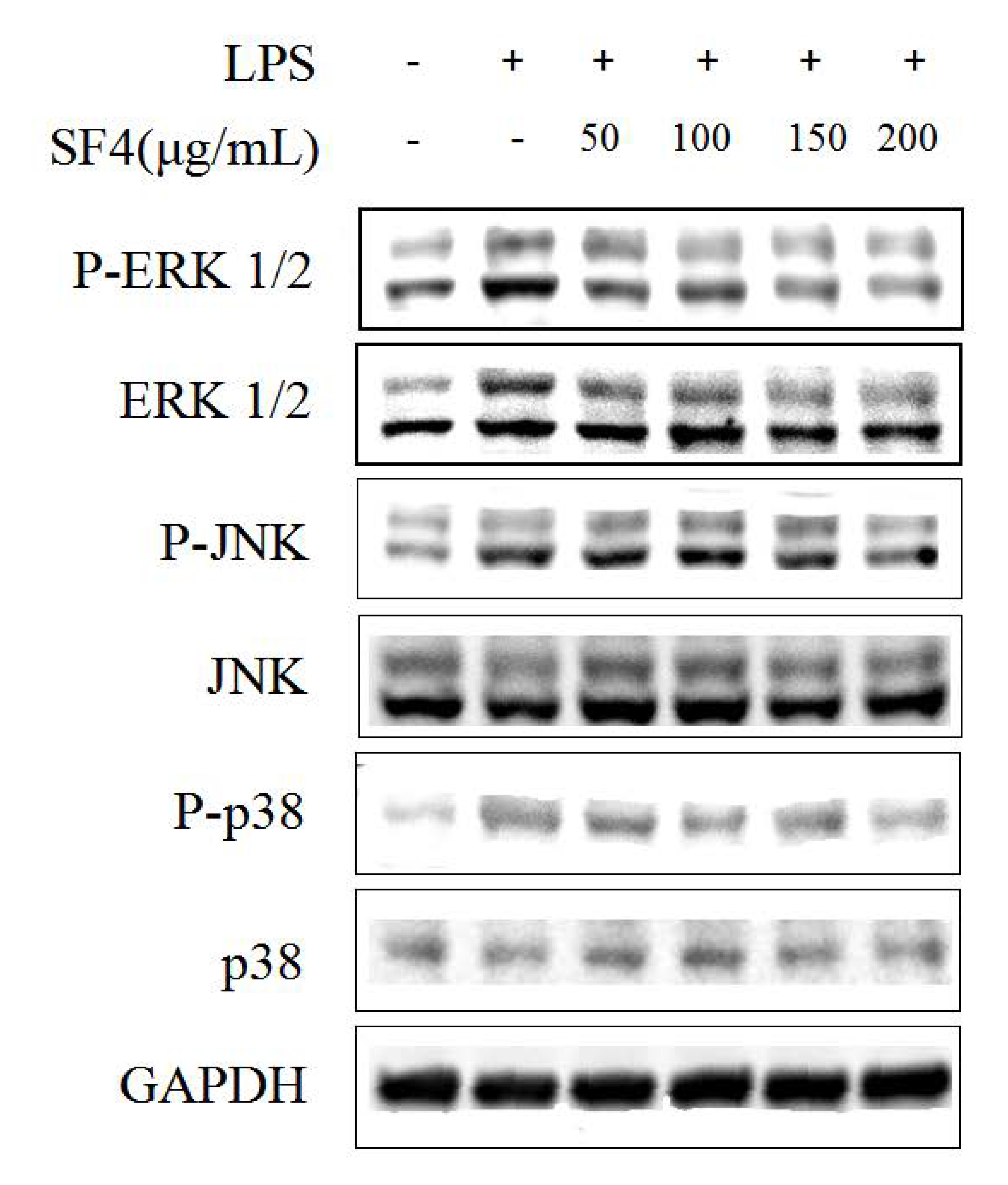
2.8. Effect of SF6 on the Phosphorylation of MAPKs in LPS-Stimulated RAW 264.7 Macrophages
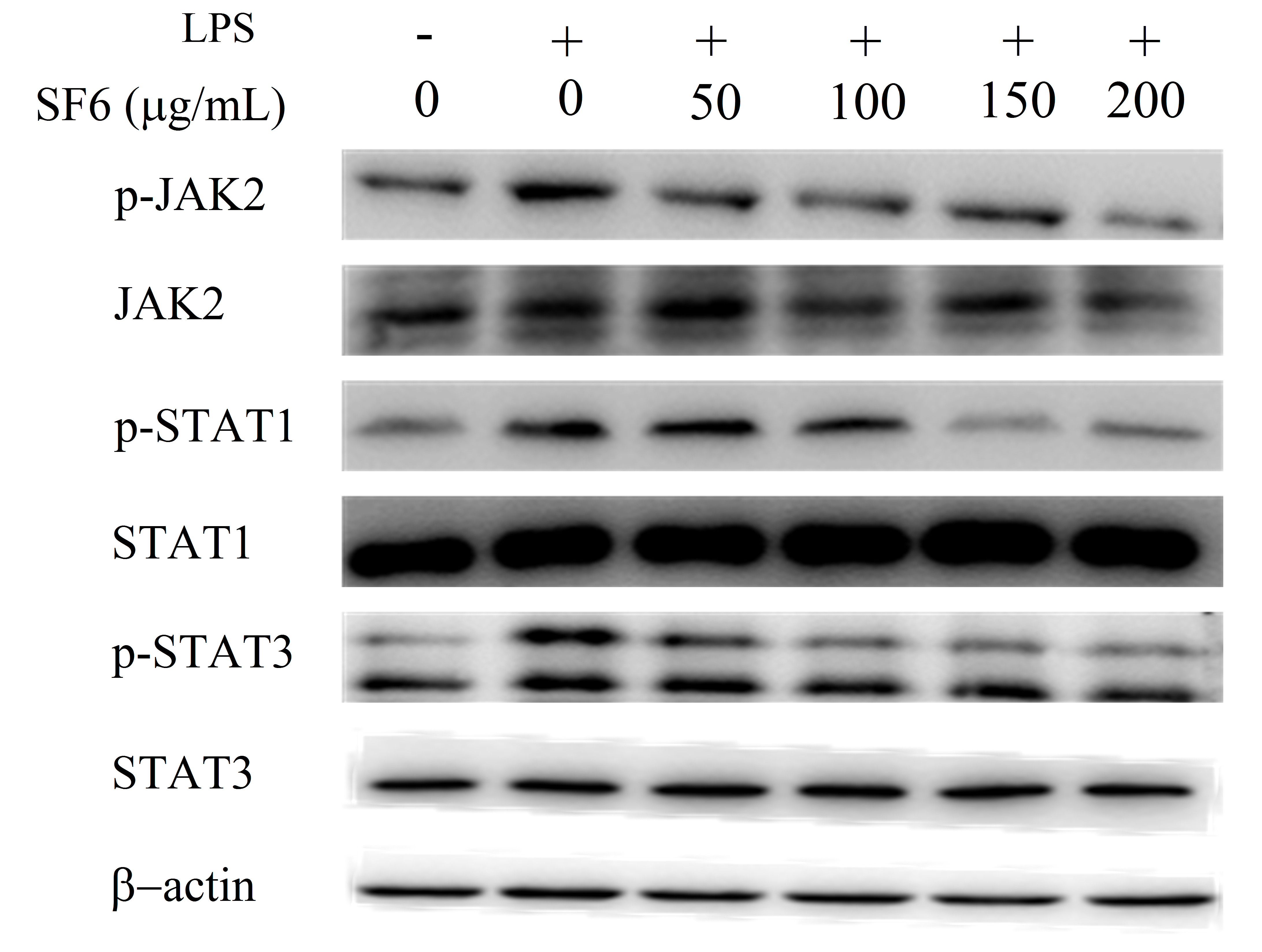
2.9. Effect of SF6 on JAK2-STAT1/3 Pathway Activation in LPS-Induced RAW 264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation and Purification of the Polysaccharide from Saccharina japonica
4.3. Homogeneity and Molecular Weight of the Purified Fucoidan
4.4. Composition Analysis
4.5. Fourier Transform Infrared Characterization
4.6. NMR Spectroscopy
4.7. Biological Assays
4.7.1. Cell Culture
4.7.2. Measurement of Cell Viability of RAW 264.7 Macrophages
4.7.3. Measurement of NO Production
4.7.4. Measurement of Pro-Inflammatory Cytokines Production
4.7.5. Preparation of Cell Lysates
4.7.6. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duan, M.; Sun, X.; Ma, N.; Liu, Y.; Luo, T.; Song, S.; Ai, C. Polysaccharides from Laminaria japonica alleviated metabolic syndrome in BALB/c mice by normalizing the gut microbiota. Int. J. Biol. Macromol. 2019, 121, 996–1004. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jager, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of alpha-glucosidase inhibitors by combined use of high-resolution alpha-glucosidase inhibition profiling and HPLC-HRMS-SPE-NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobinski, M.; Okla, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Cordoba, O.; Damonte, E.B.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 2019, 478, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Anastyuk, S.D.; Shevchenko, N.M.; Usoltseva Menshova, R.V.; Silchenko, A.S.; Zadorozhny, P.A.; Dmitrenok, P.S.; Ermakova, S.P. Structural features and anticancer activity in vitro of fucoidan derivatives from brown alga Saccharina cichorioides. Carbohydr. Polym. 2017, 157, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Thinh, P.D.; Ly, B.M.; Usoltseva, R.V.; Shevchenko, N.M.; Rasin, A.B.; Anastyuk, S.D.; Malyarenko, O.S.; Zvyagintseva, T.N.; San, P.T.; Ermakova, S.P. A novel sulfated fucan from Vietnamese sea cucumber Stichopus variegatus: Isolation, structure and anticancer activity in vitro. Int. J. Biol. Macromol. 2018, 117, 1101–1109. [Google Scholar] [CrossRef]
- Asanka Sanjeewa, K.K.; Jayawardena, T.U.; Kim, H.S.; Kim, S.Y.; Shanura Fernando, I.P.; Wang, L.; Abetunga, D.T.U.; Kim, W.S.; Lee, D.S.; Jeon, Y.J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-kappaB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.S.; Lee, D.S.; Jeon, Y.J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef]
- Dore, C.M.; das, C.F.A.M.G.; Will, L.S.; Costa, T.G.; Sabry, D.A.; de Souza Rego, L.A.; Accardo, C.M.; Rocha, H.A.; Filgueira, L.G.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Wang, Q.; Luo, X.; He, Y.; Song, Y. Hypolipidemic effects of sulfated fucoidan from Kjellmaniella crassifolia through modulating the cholesterol and aliphatic metabolic pathways. J. Funct. Foods 2018, 51, 8–15. [Google Scholar] [CrossRef]
- Ren, D.; Wang, Q.; Yang, Y.; Hu, Y.; Song, Y.; He, Y.; Liu, S.; Wu, L. Hypolipidemic effects of fucoidan fractions from Saccharina sculpera (Laminariales, Phaeophyceae). Int. J. Biol. Macromol. 2019, 140, 188–195. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Purification, molecular properties, structural characterization, and immunomodulatory activities of water soluble polysaccharides from Sargassum angustifolium. Int. J. Biol. Macromol. 2018, 109, 793–802. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Kim, J.; Jeon, Y.-J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.; Fernando, I.P.; Kim, E.A.; Ahn, G.; Jee, Y.; Jeon, Y.J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef]
- Park, J.; Cha, J.D.; Choi, K.M.; Lee, K.Y.; Han, K.M.; Jang, Y.S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017, 43, 91–98. [Google Scholar] [CrossRef]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-kappaB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry 2010, 71, 1586–1595. [Google Scholar] [CrossRef]
- Pereira, M.S.; Mulloy, B.; Mourao, P.A. Structure and anticoagulant activity of sulfated fucans. Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J. Biol. Chem. 1999, 274, 7656–7667. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Q.; Wang, Q.; He, Y.; Ren, D.; Liu, S.; Wu, L. Structural characterization and antitumor effects of fucoidans from brown algae Kjellmaniella crassifolia farmed in northern China. Int. J. Biol. Macromol. 2018, 119, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Duan, M.; Liu, Y.; Luo, T.; Ma, N.; Song, S.; Ai, C. The beneficial effects of Gracilaria lemaneiformis polysaccharides on obesity and the gut microbiota in high fat diet-fed mice. J. Funct. Foods 2018, 46, 48–56. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.M.; Anastyuk, S.D.; Menshova, R.V.; Vishchuk, O.S.; Isakov, V.I.; Zadorozhny, P.A.; Sikorskaya, T.V.; Zvyagintseva, T.N. Further studies on structure of fucoidan from brown alga Saccharina gurjanovae. Carbohydr. Polym. 2015, 121, 207–216. [Google Scholar] [CrossRef]
- Naggi, A.; Torri, G.; Iacomini, M.; Colombo Castelli, G.; Reggi, M.; Fermani, S.; Dubinsky, Z.; Goffredo, S.; Falini, G. Structure and Function of Stony Coral Intraskeletal Polysaccharides. ACS Omega 2018, 3, 2895–2901. [Google Scholar] [CrossRef]
- Chale-Dzul, J.; Moo-Puc, R.; Robledo, D.; Freile-Pelegrín, Y. Hepatoprotective effect of the fucoidan from the brown seaweed Turbinaria tricostata. J. Appl. Phycol. 2014, 27, 2123–2135. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Popivnich, I.B.; Isakov, V.V.; Scobun, A.S.; Sundukova, E.V.; Elyakova, L.A. A new procedure for the separation of water-soluble polysaccharides from brown seaweeds. Carbohydr. Res. 1999, 332, 32–39. [Google Scholar] [CrossRef]
- Chen, A.; Lan, Y.; Liu, J.; Zhang, F.; Zhang, L.; Li, B.; Zhao, X. The structure property and endothelial protective activity of fucoidan from Laminaria japonica. Int. J. Biol. Macromol. 2017, 105 Pt 2, 1421–1429. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, Y.; Xing, Y.; Zhu, H.; Shen, J.; Tian, J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia-reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011, 49, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Mao, G.; Wu, T.; Hu, Y.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. A fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated structure alleviates gut microbiota dysbiosis and metabolic syndromes in HFD-fed mice. Food Funct. 2018, 9, 5371–5380. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.L.; Jiang, Y.F.; Lee, H.G.; Jeon, Y.J.; Kang, M.C. Characterization and screening of anti-tumor activity of fucoidan from acid-processed hijiki (Hizikia fusiforme). Int. J. Biol. Macromol. 2019, 139, 170–180. [Google Scholar] [CrossRef]
- Kazłowski, B.; Chiu, Y.-H.; Kazłowska, K.; Pan, C.-L.; Wu, C.-J. Prevention of Japanese encephalitis virus infections by low-degree-polymerisation sulfated saccharides from Gracilaria sp. and Monostroma nitidum. Food Chem. 2012, 133, 866–874. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, W.; Fang, F.; Li, H.; Sun, H.; Chen, Y.; Qi, X. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr. Polym. 2008, 71, 428–434. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S. Bioactivities of Nizamuddinia zanardinii sulfated polysaccharides extracted by enzyme, ultrasound and enzyme-ultrasound methods. J. Food Sci. Technol. 2019, 56, 1212–1220. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Fukuda, T.; Majumder, K.; Zhang, H.; Matsui, T.; Mine, Y. Adenine has an anti-inflammatory effect through the activation of adenine receptor signaling in mouse macrophage. J. Funct. Foods 2017, 28, 235–239. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Li, Z.; Sang, Y.; Niu, Y.; Zhang, Q.; Ding, H.; Yin, S. Fucoidan from Undaria pinnatifida prevents vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in the l-NAME-induced hypertensive rat model. Food Funct. 2016, 7, 2398–2408. [Google Scholar] [CrossRef]
- Chang, K.C.; Ko, Y.S.; Kim, H.J.; Nam, D.Y.; Lee, D.U. 13-Methylberberine reduces HMGB1 release in LPS-activated RAW264.7 cells and increases the survival of septic mice through AMPK/P38 MAPK activation. Int. Immunopharmacol. 2016, 40, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xiao, X.H.; Hu, L.B.; Jie, H.Y.; Wang, Y.; Ye, W.C.; Li, M.M.; Liu, Z. Anhuienoside C Ameliorates Collagen-Induced Arthritis through Inhibition of MAPK and NF-kappaB Signaling Pathways. Front. Pharmacol. 2017, 8, 299. [Google Scholar] [CrossRef]
- Delma, C.R.; Thirugnanasambandan, S.; Srinivasan, G.P.; Raviprakash, N.; Manna, S.K.; Natarajan, M.; Aravindan, N. Fucoidan from marine brown algae attenuates pancreatic cancer progression by regulating p53—NFkappaB crosstalk. Phytochemistry 2019, 167, 112078. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Choi, Y.S.; Kim, Y.K.; Rahman, M.S.; Pradeep, G.C.; Yoo, J.C.; Suh, J.W. A multifunctional alanine-rich anti-inflammatory peptide BCP61 showed potent inhibitory effects by inhibiting both NF-kappaB and MAPK expression. Inflammation 2017, 40, 688–696. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Ye, J.; Zhai, X.; Song, J.; Jiang, C.; Wang, J.; Zhang, H.; Jia, X.; Zhu, F. Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-kappaB and MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2017, 196, 66–74. [Google Scholar] [CrossRef]
- Qu, T.; Wang, E.; Jin, B.; Li, W.; Liu, R.; Zhao, Z.B. 5-Aminosalicylic acid inhibits inflammatory responses by suppressing JNK and p38 activity in murine macrophages. Immunopharmacol. Immunotoxicol. 2017, 39, 45–53. [Google Scholar] [CrossRef]
- Ren, D.Y.; Xu, T.; Li, R.; Huang, C.; Huang, Y.; Li, R.Q.; Li, H.Y.; Li, J. 5,7,3′-Triacetyl hesperetin suppresses adjuvant-induced arthritis in rats through modulating JAK2/STAT3 pathway. Am. J. Chin. Med. 2013, 41, 601–614. [Google Scholar] [CrossRef]
- Lu, W.; Ding, Z.; Liu, F.; Shan, W.; Cheng, C.; Xu, J.; He, W.; Huang, W.; Ma, J.; Yin, Z. Dopamine delays articular cartilage degradation in osteoarthritis by negative regulation of the NF-kappaB and JAK2/STAT3 signaling pathways. Biomed. Pharmacother. 2019, 119, 109419. [Google Scholar] [CrossRef]
- Yu, Q.; Zeng, K.; Ma, X.; Song, F.; Jiang, Y.; Tu, P.; Wang, X. Resokaempferol-mediated anti-inflammatory effects on activated macrophages via the inhibition of JAK2/STAT3, NF-kappaB and JNK/p38 MAPK signaling pathways. Int. Immunopharmacol. 2016, 38, 104–114. [Google Scholar] [CrossRef]
- Pielesz, A.; Binias, W. Cellulose acetate membrane electrophoresis and FTIR spectroscopy as methods of identifying a fucoidan in Fucus vesiculosus Linnaeus. Carbohydr. Res. 2010, 345, 2676–2682. [Google Scholar] [CrossRef]
- Michel, D.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.L.; Minchin, F.R. Modification of the Lowry assay to measure proteins and phenols in covalently bound complexes. Anal. Biochem. 2005, 346, 43–48. [Google Scholar] [CrossRef]
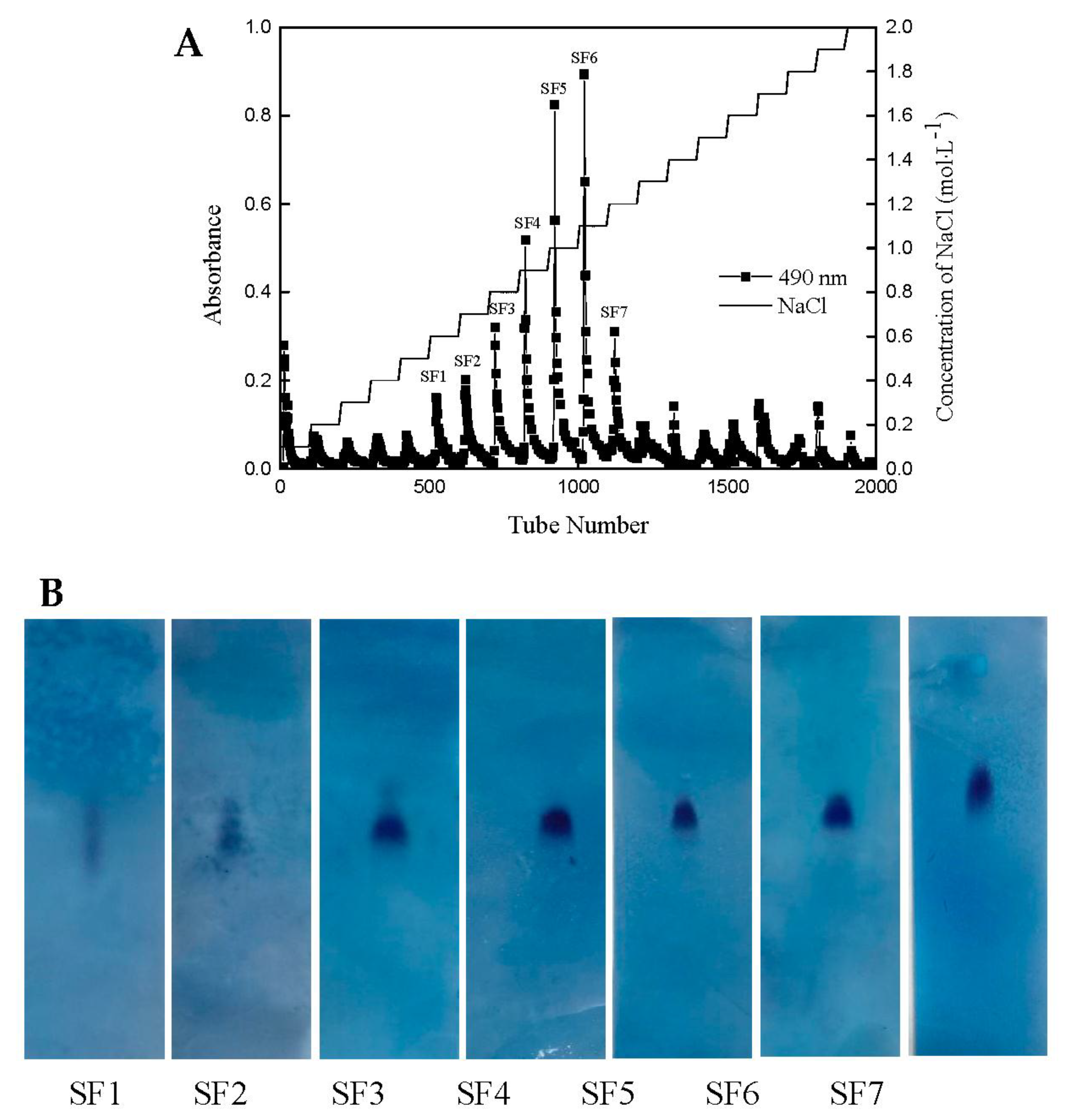
| Sample | Total Sugar (%) | Sulfate (%) | Protein (%) | Monosaccharide Composition (%) | Molecular Weight (KDa) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mannose | Rhamnose | Galactose | Xylose | Fucose | |||||
| SF3 | 82.52 ± 2.42 | 12.05 ± 0.13 | 1.42 ± 0.28 | 24.22 | 2.34 | 32.81 | 1.56 | 39.06 | 222.2 |
| SF4 | 79.63 ± 2.03 | 15.00 ± 0.07 | 1.17 ± 0.18 | 17.67 | 28.62 | 16.61 | 1.77 | 35.33 | 228.8 |
| SF5 | 72.45 ± 1.98 | 23.79 ± 0.11 | 0.99 ± 0.06 | 11.02 | 24.80 | 22.83 | 1.97 | 39.37 | 231.5 |
| SF6 | 58.17 ± 1.34 | 36.94 ± 0.08 | 1.02 ± 0.02 | 17.87 | 16.42 | 41.54 | n.d. | 24.15 | 246.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Chen, D.; Ye, Z.; Huang, Y.; Zhang, N.; Lui, E.M.K.; Xue, C.; Xiao, M. Fucoidan Isolated from Saccharina japonica Inhibits LPS-Induced Inflammation in Macrophages via Blocking NF-κB, MAPK and JAK-STAT Pathways. Mar. Drugs 2020, 18, 328. https://doi.org/10.3390/md18060328
Ye J, Chen D, Ye Z, Huang Y, Zhang N, Lui EMK, Xue C, Xiao M. Fucoidan Isolated from Saccharina japonica Inhibits LPS-Induced Inflammation in Macrophages via Blocking NF-κB, MAPK and JAK-STAT Pathways. Marine Drugs. 2020; 18(6):328. https://doi.org/10.3390/md18060328
Chicago/Turabian StyleYe, Jing, Donghui Chen, Zhicheng Ye, Yayan Huang, Na Zhang, Edmund M. K. Lui, Changhu Xue, and Meitian Xiao. 2020. "Fucoidan Isolated from Saccharina japonica Inhibits LPS-Induced Inflammation in Macrophages via Blocking NF-κB, MAPK and JAK-STAT Pathways" Marine Drugs 18, no. 6: 328. https://doi.org/10.3390/md18060328
APA StyleYe, J., Chen, D., Ye, Z., Huang, Y., Zhang, N., Lui, E. M. K., Xue, C., & Xiao, M. (2020). Fucoidan Isolated from Saccharina japonica Inhibits LPS-Induced Inflammation in Macrophages via Blocking NF-κB, MAPK and JAK-STAT Pathways. Marine Drugs, 18(6), 328. https://doi.org/10.3390/md18060328




