Halosmysin A, a Novel 14-Membered Macrodiolide Isolated from the Marine-Algae-Derived Fungus Halosphaeriaceae sp.
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Culturing and Isolation of Metabolites
3.4. Alkaline Hydrolysis of 1
3.5. Assay for Cytotoxicity
3.6. The Origin of the Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicoletti, R.; Vinale, F. Bioactive Compounds from Marine-Derived Aspergillus, Penicillium, Talaromyces and Trichoderma Species. Mar. Drugs 2018, 16, 408. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural Products from Marine Fungi—Still an Underrepresented Resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural 369 products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kitada, H.; Kajimoto, T.; Numata, A.; Tanaka, R. The relationship between the CD Cotton effect and the absolute configuration of FD-838 and its seven stereoisomers. J. Org. Chem. 2010, 75, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, J.; Simpson, T.J. Fungal Products. Part V. The absolute stereochemistry of colletodiol and the structures of related metabolites of Colletotrichum capsici. J. Chem. Soc. Perkin Trans. 1 1973, 1487–1493. [Google Scholar] [CrossRef]
- Ronald, R.C.; Gurusiddaiah, S. Grahamimycin A1: A novel dilactone antibiotic from Cytospora. Tetrahedron Lett. 1980, 21, 681–684. [Google Scholar] [CrossRef]
- Gurusiddaiah, S.; Ronald, R.C. Grahamimycins: Antibiotics from Cytospora sp. Ehrenb. W.F.P.L. 13A. Antimicrob. Agents Chemother. 1981, 19, 681–684. [Google Scholar] [CrossRef]
- Hanson, K.; O’neill, J.A.; Simpson, T.J.; Willis, C.L. Bartanol and bartallol, novel macrodiolides from Cytospora sp. ATCC 20502. J. Chem. Soc. Perkin Trans. 1 1994, 2493–2497. [Google Scholar] [CrossRef]
- Höller, U.; König, G.M.; Wright, A.D. A new tyrosine kinase inhibitor from a marine isolate of Ulocladium botrytis and new metabolites from the marine fungi Asteromyces cruciatus and Varicosporina ramulosa. Eur. J. Org. Chem. 1999, 2949–2955. [Google Scholar] [CrossRef]
- Grabley, S.; Hammann, P.; Thiericke, R.; Wink, J. Secondary metabolites by Chemical screening. 21 Clonostachydiol, a novel anthelmintic macrolide from the fungus Clonostachys cylindrospora (strain FH-A 6607). J. Antibiot. 1992, 46, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Mitova, M.I.; Ellis, G.; van der Sar, S.; Phipps, R.K.; Blunt, J.W.; Cummings, N.J.; Cole, A.L.J.; Munro, M.H.G. Bioactivity profiling using HPLC/microtiter-plate analysis: Application to a New Zealand marine alga-derived fungus, Gliocladium sp. J. Nat. Prod. 2006, 69, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Ojima, K.; Yangchum, A.; Laksanacharoen, P.; Tasanathai, K.; Tanakitpipattana, D.; Tokuyama, H.; Isaka, M. Cordybislactone, a stereoisomer of the 14-membered bislactoneclonostachydiol, from the hopper pathogenic fungus Cordyceps sp. BCC 49294: Revision of the absolute configuration of clonostachydiol. J. Antibiot. 2018, 71, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Yangchum, A.; Auncharoen, P.; Srichomthong, K.; Srikitikulchai, P. Ring B aromatic norpimarane glucoside from a Xylaria sp. J. Nat. Prod. 2011, 74, 300–302. [Google Scholar] [CrossRef]
- Han, J.; Su, Y.; Xu, Y.; Huo, X.; She, X. Asymmetric total synthesis and revision of the absolute configuration of 4-Keto-clonostachydiol. J. Org. Chem. 2009, 74, 3930–3932. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Notni, J.; Dorfelt, H.; Grafe, U. Acremonol and acremodiol, new fungal bislactones. J. Antibiot. 2002, 55, 660–662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.T.; Wei, Y.J.; Ge, H.M.; Tan, R.X. Acaulide, an osteogenic macrodiolide from Acaulium sp. H-JQSF, an isopod-associated fungus. Org. Lett. 2018, 20, 1007–1010. [Google Scholar] [CrossRef]
- Amstutz, R.; Hungerbühler, E.; Seebach, D. Revidierte struktur des makrodiolids colletodiol. Helv. Chim. Acta 1981, 6, 1796–1799. [Google Scholar] [CrossRef]
- Khomane, N.B.; Kumar, R.N.; Mali, P.R.; Shirsat, P.K.; Meshram, H.M. Formal synthesis of 14-membered unsymmetrical bis-macrolactone, (–)-colletodiol. Tetrahedron Lett. 2017, 58, 4687–4690. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Murthy, V.S.; Sharma, G.V.M. Studies directed towards the synthesis of clonostachydiol-Part I. Tetrahedron Lett. 1995, 36, 139–142. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Murthy, V.S.; Sharma, G.V.M. The first synthesis and determination of absolute stereochemistry of clonostachydiol-Part II. Tetrahedron Lett. 1995, 36, 143–146. [Google Scholar] [CrossRef]
- Yadav, J.S.; Swamy, T.; Subba Reddy, B.V. A stereoselective approach for the total synthesis of clonostachydiol. Synlett 2008, 2773–2776. [Google Scholar] [CrossRef]
- Ramulu, U.; Ramesh, D.; Rajaram, S.; Reddy, S.P.; Venkatesham, K.; Venkateswarlu, Y. Stereoselective total synthesis of clonostachydiol. Tetrahedron Asymmetry 2012, 23, 117–123. [Google Scholar] [CrossRef]
- Chu, M.; Mierzwa, R.; Truumees, I.; Gentile, F.; Patel, M.; Gullo, V.; Chan, T.-M.; Puar, M.S. Two novel diketopiperazines isolated from the fungus Tolypocladium sp. Tetrahedron Lett. 1993, 34, 7537–7540. [Google Scholar] [CrossRef]
- Usami, Y.; Aoki, S.; Hara, T.; Numata, A. New dioxopiperazine metabolites from a Fusarium species separated from a marine alga. J. Antibiot. 2002, 55, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, D.O.; Borges, W.S.; Vieira, N.J.; de Oliveira, L.F.; da Silva, C.H.T.P.; Lopes, N.P.; Dias, L.G.; Duran-Patron, R.; ColladoI., G.; Pupo, M.T. Diketopiperazines produced by endophytic fungi found in association with two Asteraceae species. Phytochemisry 2010, 71, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Andolfi, A. Occurrence and properties of thiosilvatins. Mar. Drugs 2019, 17, 664. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.J.; Stevenson, G.I. Studies of polyketide chain-assembly processes: Origins of the hydrogen and oxygen atoms in colletodiol. J. Chem. Soc. Chem. Commun. 1985, 1822–1824. [Google Scholar] [CrossRef]
- O’neill, J.A.; Simpson, T.J.; Willis, C.L. Biosynthesis of colletodiol and related polyketide macrodiolides in Cytospora sp. ATCC 20502: Synthesis and metabolism of advanced intermediates. J. Chem. Soc. Chem. Commun. 1993, 738–740. [Google Scholar] [CrossRef]
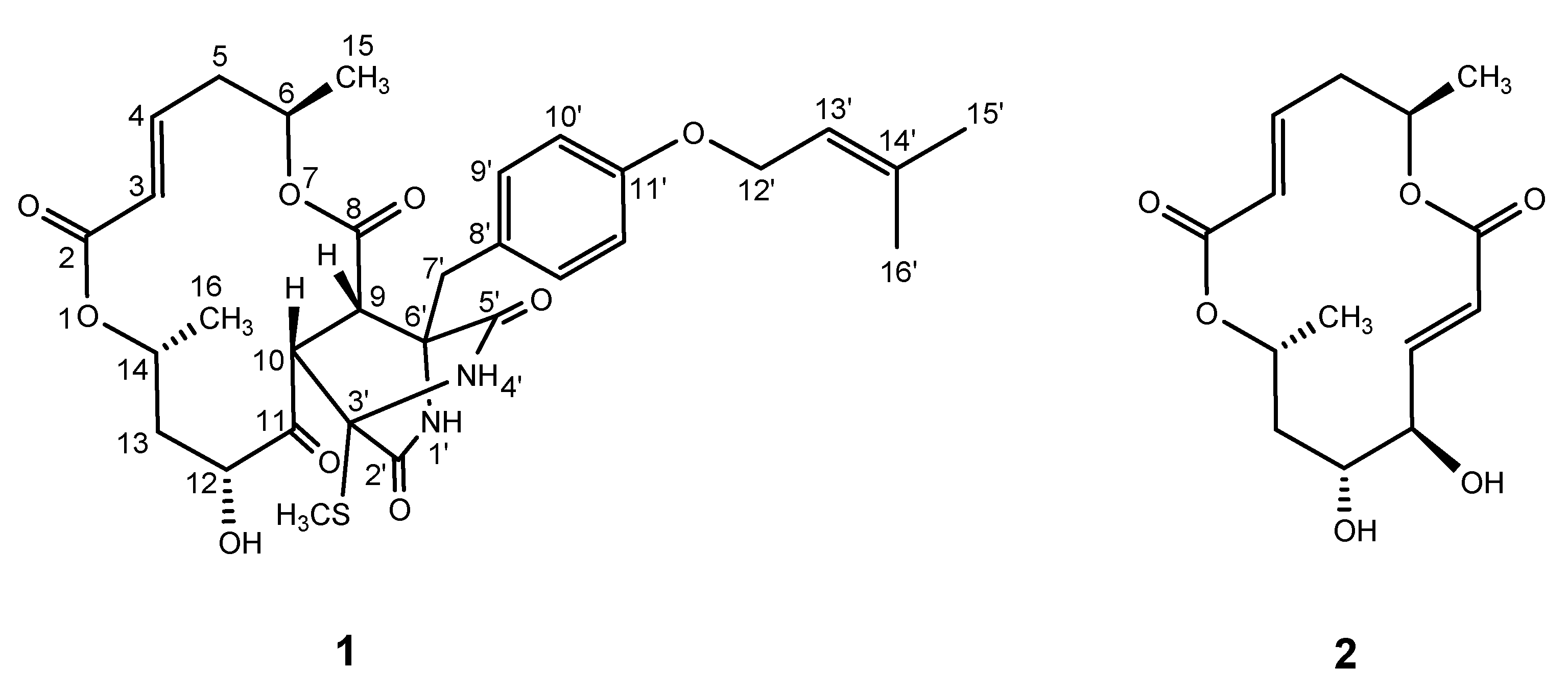
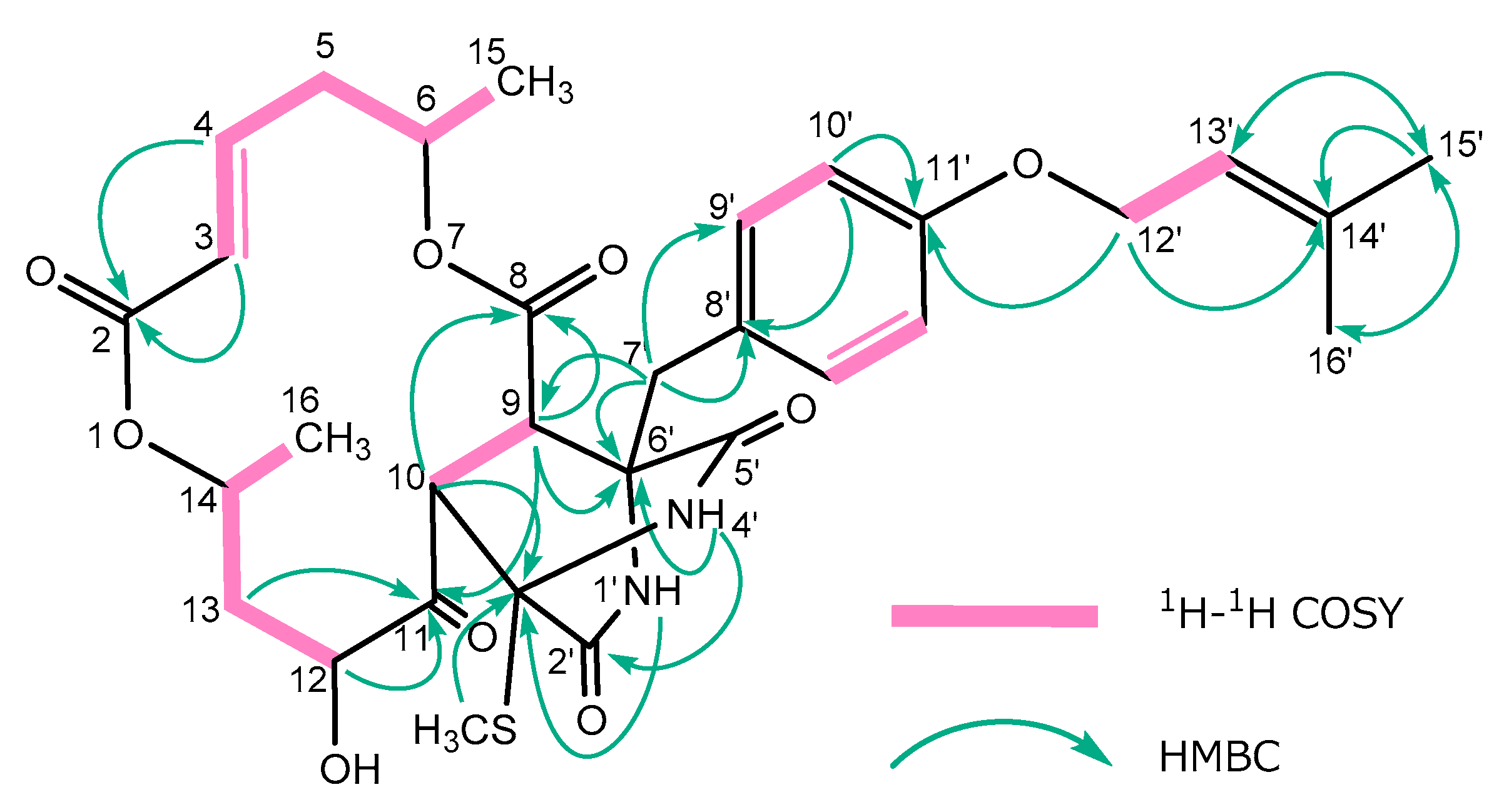
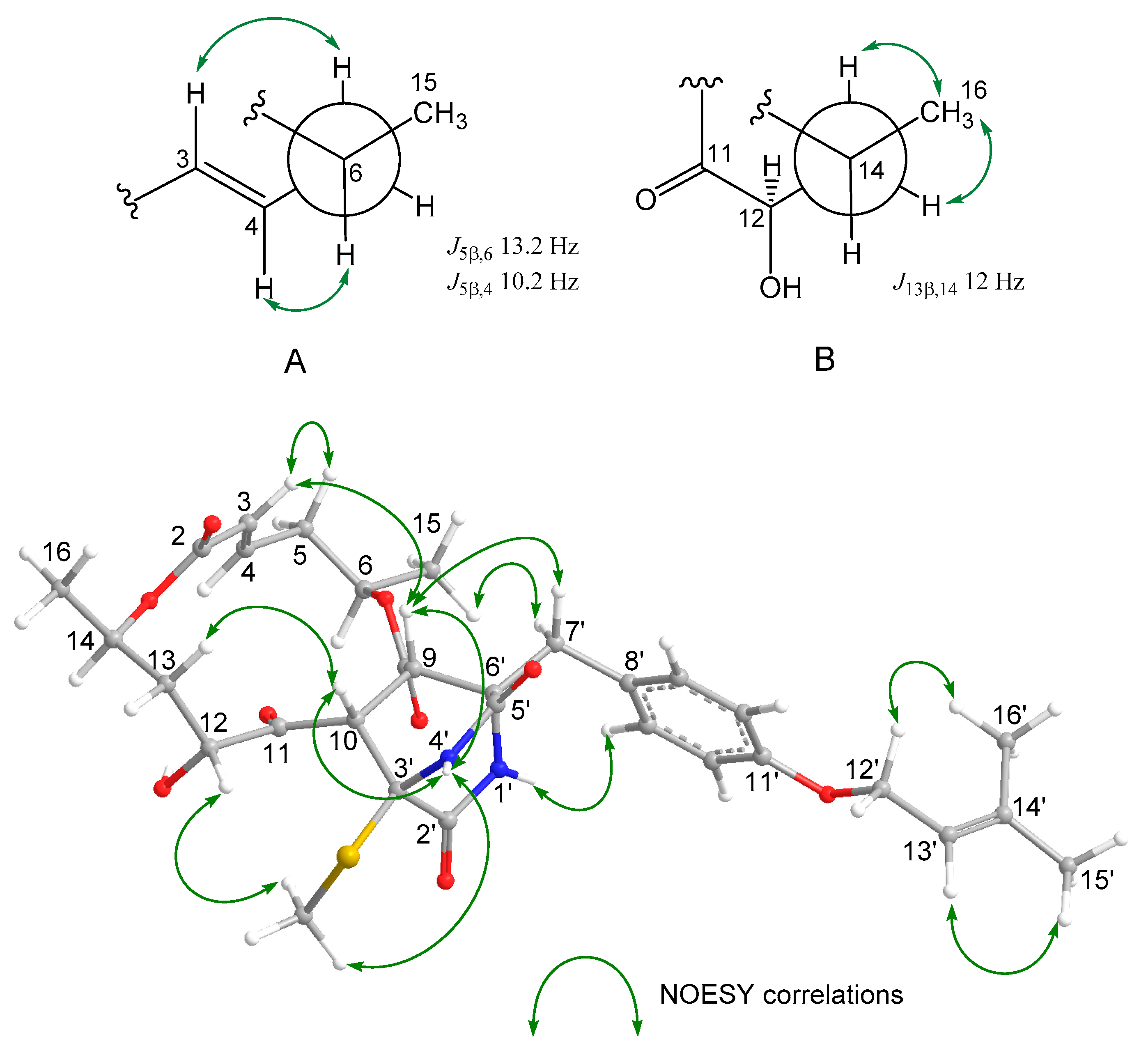

| Position | δH a | J/Hz | δC | Position | δH a | J/Hz | δC | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 165.6 | (s) | 1’ (NH) | 5.73 | s | ||||||
| 3 | 5.64 | d | 16.2 (4) | 125.3 | (d) | 2’ | 165.7 | (s) | |||
| 4 | 6.78 | ddd | 16.2 (3), 10.2 (5β), 6.0 (5α) | 143.5 | (d) | 3’ | 68.6 | (s) | |||
| 5α | 2.48 | ddd | 13.2 (5β), 6.0 (4), 1.2 (6) | 39.0 | (t) | 4’ (NH) | 5.95 | s | |||
| 5β | 2.23 | ddd | 13.2 (5α), 13.2 (6), 10.2 (4) | 5’ | 170.9 | (s) | |||||
| 6 | 5.30 | dqd | 13.2 (5β), 6.0 (15), 1.2 (5α) | 69.9 | (d) | 6’ | 63.7 | (s) | |||
| 8 | 168.9 | (s) | 7’A | 2.83 | d | 14.4 (7’B) | 33.0 | (t) | |||
| 9 | 3.38 | d | 3.0 (10) | 51.9 | (d) | 7’B | 3.88 | d | 14.4 (7’A) | ||
| 10 | 4.57 | d | 3.0 (9) | 51.0 | (d) | 8’ | 125.5 | (s) | |||
| 11 | 208.2 | (s) | 9’ | 6.83 | d | 9.0 (10’) | 131.7 | (d) | |||
| 12 | 4.55 | d | 7.8 (13α) | 75.6 | (d) | 10’ | 7.10 | d | 9.0 (9’) | 115.2 | (d) |
| 13α | 1.96 | ddd | 14.4 (13β), 7.8 (12), 3.0 (14) | 37.4 | (t) | 11’ | 158.5 | (s) | |||
| 13β | 2.64 | ddd | 14.4 (13α), 12.0 (14), 1.2 (12) | 12’ | 4.47 | d | 7.2 (13’) | 64.8 | (t) | ||
| 14 | 5.23 | dqd | 12.0 (13β), 6.0 (16), 3.0 (13α) | 65.5 | (d) | 13’ | 5.47 | br t | 7.2 (12’) | 119.5 | (d) |
| 15 | 1.44 | d | 6.0 (6) | 20.9 | (q) | 14’ | 138.4 | (s) | |||
| 16 | 1.26 | d | 6.0 (14) | 20.2 | (q) | 15’ | 1.79 | s | 25.8 | (q) | |
| 12-OH | Not observed | 16’ | 1.74 | s | 18.2 | (q) | |||||
| S-CH3 | 2.25 | s | 13.0 | (q) | |||||||
| Compounds | Cell Line P388 | Cell Line HL-60 | Cell Line L1210 |
|---|---|---|---|
| IC50 (μM) a | IC50 (μM) a | IC50 (μM) a | |
| 1 | 6.8 ± 1.1 | 2.2 ± 3.1 | 11.7 ± 2.8 |
| 2 | >300 | >300 | >300 |
| DMSO (control) | >300 | >300 | >300 |
| 5-fluorouracil b | 3.9 ± 1.8 | 0.2 ± 2.5 | 0.2 ± 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, T.; Kogure, H.; Kataoka, M.; Kikuchi, T.; Hirano, T. Halosmysin A, a Novel 14-Membered Macrodiolide Isolated from the Marine-Algae-Derived Fungus Halosphaeriaceae sp. Mar. Drugs 2020, 18, 320. https://doi.org/10.3390/md18060320
Yamada T, Kogure H, Kataoka M, Kikuchi T, Hirano T. Halosmysin A, a Novel 14-Membered Macrodiolide Isolated from the Marine-Algae-Derived Fungus Halosphaeriaceae sp. Marine Drugs. 2020; 18(6):320. https://doi.org/10.3390/md18060320
Chicago/Turabian StyleYamada, Takeshi, Haruka Kogure, Minami Kataoka, Takashi Kikuchi, and Tomoya Hirano. 2020. "Halosmysin A, a Novel 14-Membered Macrodiolide Isolated from the Marine-Algae-Derived Fungus Halosphaeriaceae sp." Marine Drugs 18, no. 6: 320. https://doi.org/10.3390/md18060320
APA StyleYamada, T., Kogure, H., Kataoka, M., Kikuchi, T., & Hirano, T. (2020). Halosmysin A, a Novel 14-Membered Macrodiolide Isolated from the Marine-Algae-Derived Fungus Halosphaeriaceae sp. Marine Drugs, 18(6), 320. https://doi.org/10.3390/md18060320





