Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima
Abstract
1. Introduction
2. Results
2.1. Monosaccharide Composition of the Brown Seaweeds F. evanescens and S. latissima
2.2. Enzyme-Assisted Fucoidan Extraction from F. evanescens and S. latissima
2.2.1. Yields of Crude Fucoidans Extracted from F. evanescens and S. latissima
2.2.2. Yields and Chemical Compositions of Fucoidan IEX Fractions of F. evanescens and S. latissima
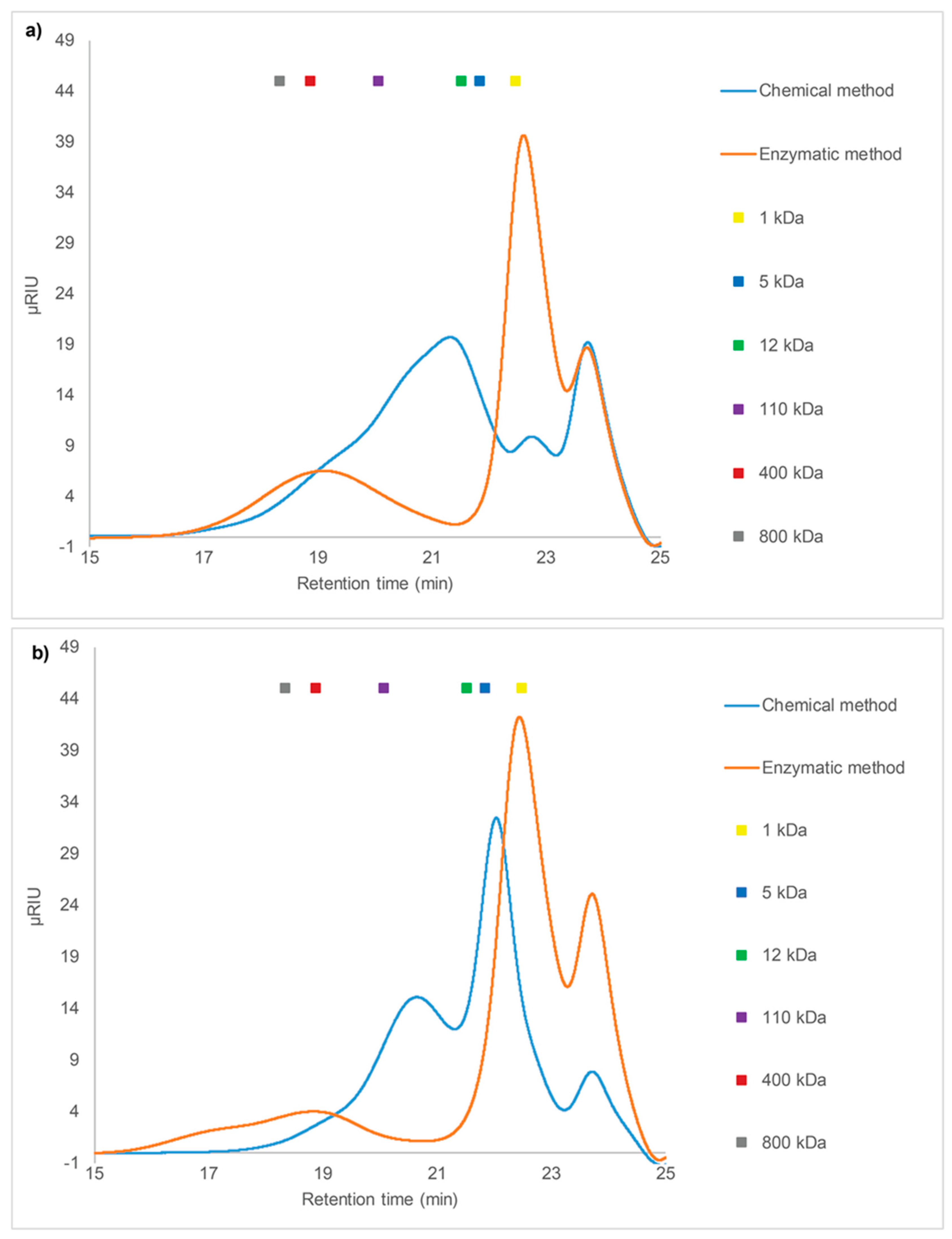
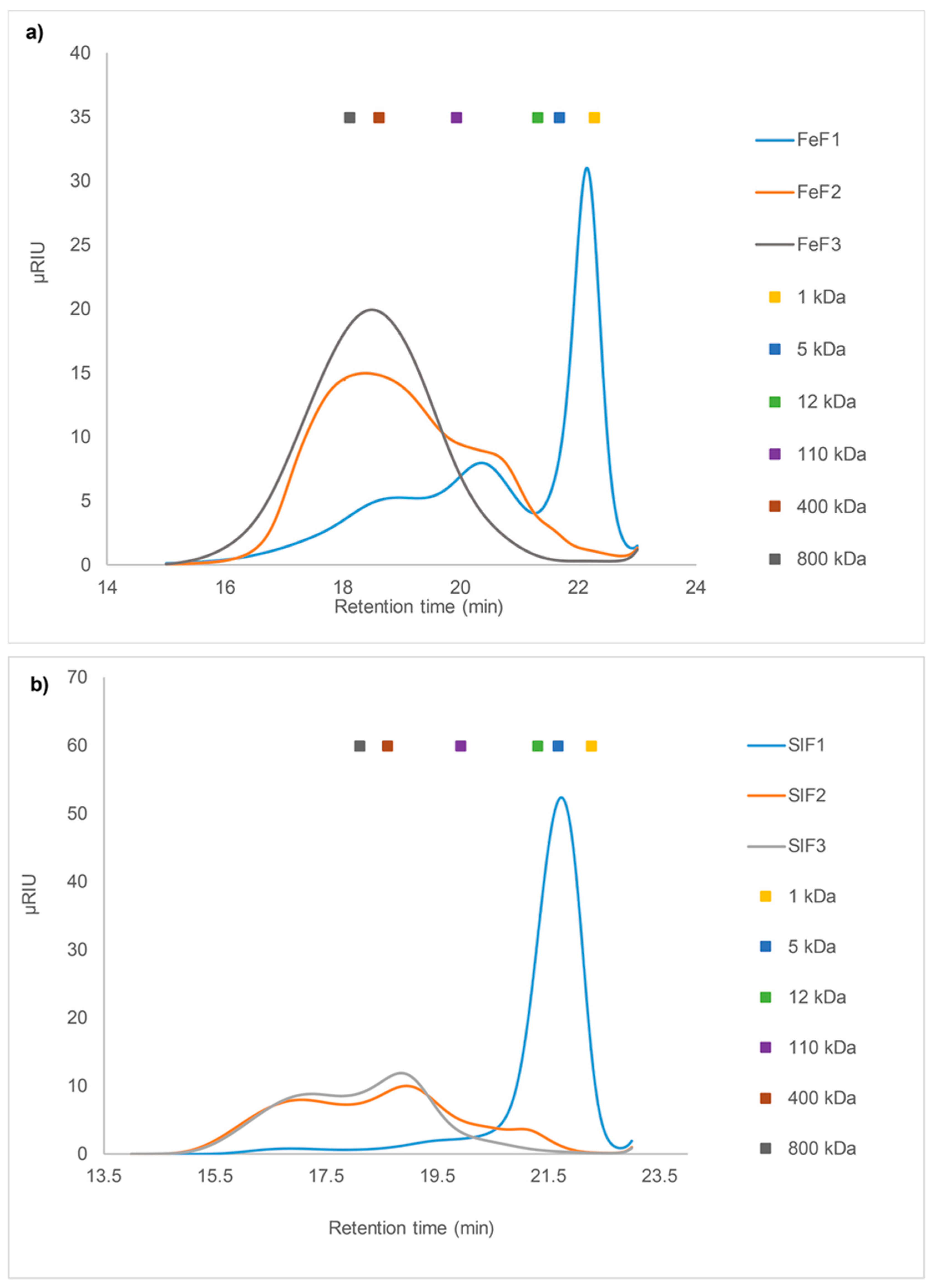
2.3. Size Exclusion Chromatography (SEC) Analysis
2.3.1. SEC of Crude Fucoidans
2.3.2. SEC of Fucoidan Fractions from F. evanescens and S. latissima
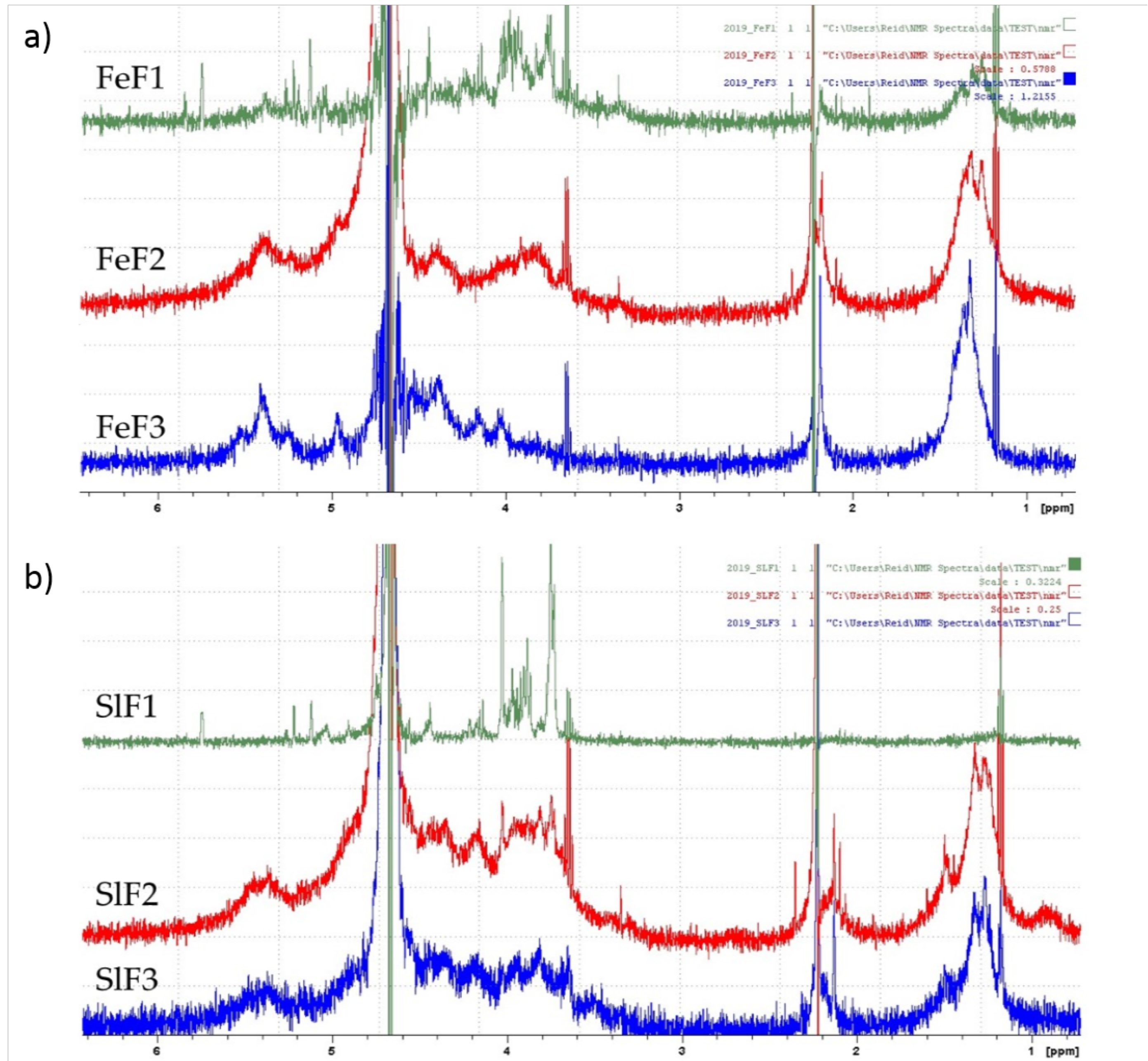
2.4. 1H NMR Spectrum of Fucoidan Fractions from F. evanescens and S. latissima
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Alginate Lyase Expression and purification
4.3. Fucoidan Extraction from Brown Seaweeds
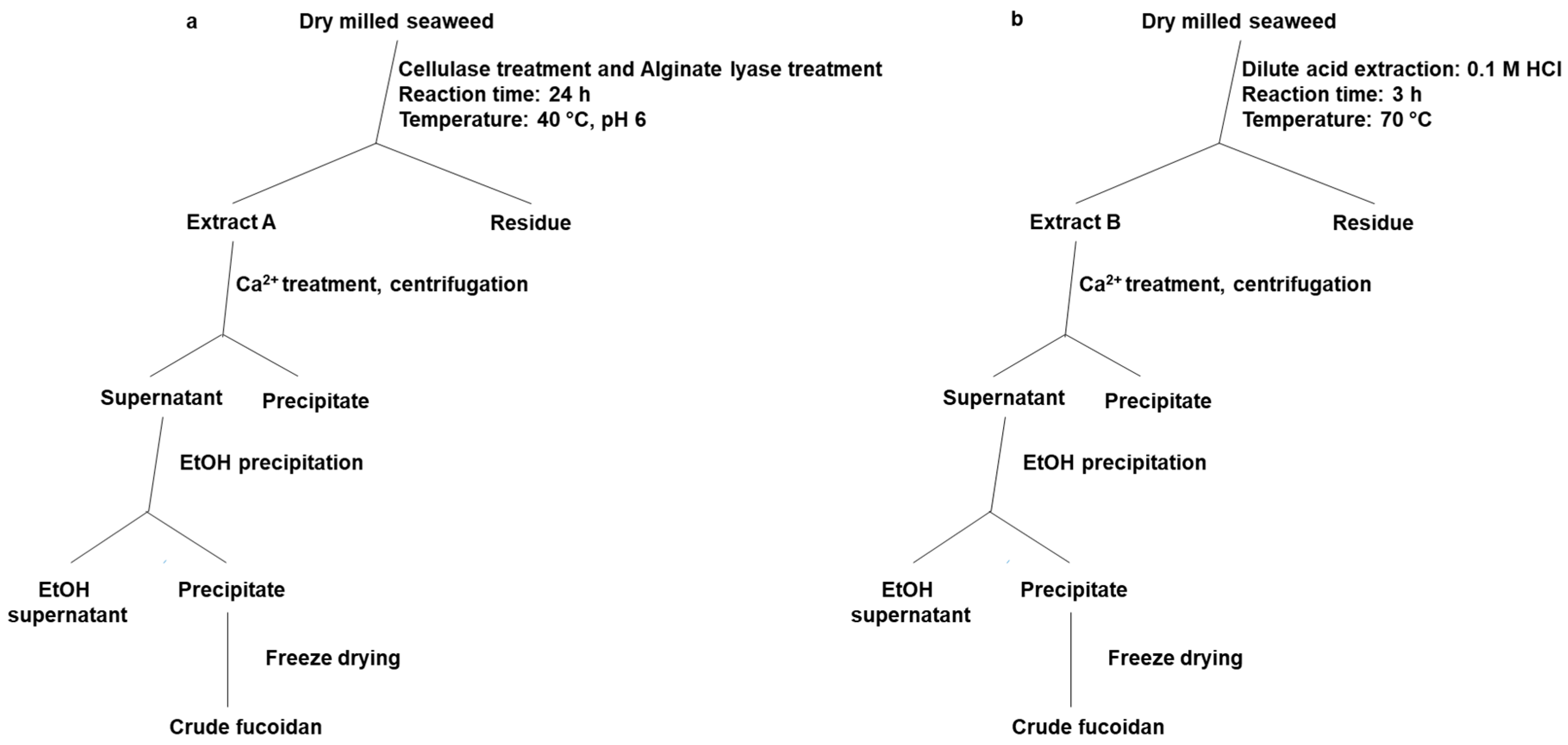
4.3.1. Chemical Extraction Method
4.3.2. Enzyme-Assisted Extraction Method
4.4. Fucoidan Fractionation by Anion-Exchange Chromatography
4.5. Chemical Composition Analysis
4.5.1. Two-Step Acid Hydrolysis
4.5.2. Chemical Composition Analysis
4.5.3. TFA Hydrolysis and Sulfate Content Analysis
4.6. Determination of Molecular Weight by Size Exclusion Chromatography
4.7. 1H NMR Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.-D.; Lin, H.Y.; Hwang, P.A. The anti-tumor activity of brown seaweed oligo-fucoidan via lncRNA expression modulation in HepG2 cells. Cytotechnology 2019, 71, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Liu, B.; Li, S.; Chen, J.; Tang, H.; Jiang, D.; Zhang, Q.; Zhong, W. The structural features of the sulfated heteropolysaccharide (ST-1) from Sargassum thunbergii and its neuroprotective activities. Int. J. Biol. Macromol. 2018, 108, 307–313. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Faustino Alves, M.G.D.C.; Will, L.S.E.P.; Costa, T.G.; Sabry, D.A.; De Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, A.; Janicek, T.; Manns, D.; Nielsen, M.M.; Balsby, T.J.S.; Meyer, A.S.; Rasmussen, M.B.; Hou, X.; Saake, B.; Göke, C.; et al. Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata—Seasonal variation and impact of environmental factors. J. Appl. Phycol. 2017, 29, 3121–3137. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.G.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Chizhov, A.O.; Krupnova, T.N.; Sundukova, E.V.; Isakov, V.V. Water-soluble polysaccharides of some far-eastern brown seaweeds. Distribution, structure, and their dependence on the developmental conditions. J. Exp. Mar. Bio. Ecol. 2003, 294, 1–13. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Malyarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from brown alga Fucus evanescens: Structure and biological activity. Front. Mar. Sci. 2016, 3, 129. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Ishina, I.A.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Zadorozhny, P.A.; Dmitrenok, P.S.; Ermakova, S.P. Structural characteristics and anticancer activity in vitro of fucoidan from brown alga Padina boryana. Carbohydr. Polym. 2018, 184, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Surits, V.V.; Usoltseva, R.V.; Shevchenko, N.M.; Thinh, P.D.; Ermakova, S.P. Structural characteristics and anticancer activity in vitro of fucoidans from brown seaweeds Sargassum miyabei and S. oligocystum. Chem. Nat. Compd. 2020, 56, 34–38. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.Y.; You, S. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2011, 16, 291–297. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 2327–2346. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Jin, W.; Zhang, H.; Zhang, Q. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012, 87, 153–159. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Woo, H.C.; Chun, B.S. Subcritical water extraction of fucoidan from Saccharina japonica: Optimization, characterization and biological studies. J. Appl. Phycol. 2018, 30, 579–590. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; López-García, M.; González-Muñoz, M.J.; Vilariño, J.M.L.; Domínguez, H. Ultrasound-assisted extraction of fucoidan from Sargassum muticum. J. Appl. Phycol. 2017, 29, 1553–1561. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2018, 31, 1391–1402. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Athukorala, Y.; Jeon, Y.J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar] [CrossRef]
- Hammed, A.M.; Jaswir, I.; Simsek, S.; Alam, Z.; Amid, A. Enzyme aided extraction of sulfated polysaccharides from Turbinaria turbinata brown seaweed. Int. Food Res. J. 2017, 24, 1660–1666. [Google Scholar]
- Mabeau, S.; Kloareg, B.; Joseleau, J.P. Fractionation and analysis of fucans from brown algae. Phytochemistry 1990, 29, 2441–2445. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Manns, D.; Nyffenegger, C.; Saake, B.; Meyer, A.S. Impact of different alginate lyases on combined cellulase-lyase saccharification of brown seaweed. RSC Adv. 2016, 6, 45392–45401. [Google Scholar] [CrossRef]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1453–1476. [Google Scholar] [CrossRef]
- Draget, K.I. 29-Alginates BT—Handbook of Hydrocolloids. In Woodhead Publishing Series in Food Science, Technology and Nutrition, 2nd ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2009; ISBN 978-1-84569-414-2. [Google Scholar]
- Manns, D.; Andersen, S.K.; Saake, B.; Meyer, A.S. Brown seaweed processing: Enzymatic saccharification of Laminaria digitata requires no pre-treatment. J. Appl Phycol. 2016, 28, 1287–1294. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Potential of intensification techniques for the extraction and depolymerization of fucoidan. Algal Res. 2018, 30, 128–148. [Google Scholar] [CrossRef]
- Shiroma, R.; Konishi, T.; Uechi, S.; Tako, M. Structural study of fucoidan from the brown seaweed Hizikia Fusiformis. Food Sci. Technol. Res. 2008, 14, 176–182. [Google Scholar] [CrossRef]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Daniel, R.; Berteau, O.; Jozefonvicz, J.; Goasdoue, N. Degradation of algal (Ascophyllum nodosum) fucoidan by an enzymatic activity contained in digestive glands of the marine mollusc Pecten maximus. Carbohydr. Res. 1999, 322, 291–297. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Inoue, A.; Ojima, T. Functional identification of alginate lyase from the brown alga Saccharina japonica. Sci. Rep. 2019, 9, 4937. [Google Scholar] [CrossRef]
- Pei, X.; Chang, Y.; Shen, J. Cloning, expression and characterization of an endo-acting bifunctional alginate lyase of marine bacterium Wenyingzhuangia fucanilytica. Protein Expr. Purif. 2019, 154, 44–51. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, F.; Sun, Y.; Ning, L.; Yao, Z. Elucidation of degrading pattern and substrate recognition of a novel bifunctional alginate lyase from Flammeovirga sp. NJ-04 and its use for preparation alginate oligosaccharides. Biotechnol. Biofuels 2019, 12, 13. [Google Scholar] [CrossRef]
- Li, J.W.; Dong, S.; Song, J.; Li, C.B.; Chen, X.L.; Xie, B.-B.; Zhang, Y.Z. Purification and characterization of a bifunctional alginate lyase from Pseudoalteromonas sp. SM0524. Mar. Drugs 2011, 9, 109–123. [Google Scholar] [CrossRef]
- Pilgaard, B.; Wilkens, C.; Herbst, F.; Vuillemin, M.; Rhein-Knudsen, N.; Meyer, A.S.; Lange, L. Proteomic enzyme analysis of the marine fungus Paradendryphiella salina reveals alginate lyase as a minimal adaptation strategy for brown algae degradation. Sci. Rep. 2019, 9, 12338–123350. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.Y.; Chen, L.; Li, Q.J.; Shen, Y.Z.; Jin, L.; Zhang, X.; Chen, P.C.; Wu, M.J.; Choi, J.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Haroun-Bouhedja, F.; Ellouali, M.; Sinquin, C.; Boisson-Vidal, C. Relationship between sulfate groups and biological activities of fucans. Thromb. Res. 2000, 100, 453–459. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Neupane, S.; Bittkau, K.S.; Alban, S. Size distribution and chain conformation of six different fucoidans using size-exclusion chromatography with multiple detection. J. Chromatogr. A 2020, 1612, 460658. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural similarities of fucoidans from brown algae Silvetia babingtonii and Fucus evanescens, determined by tandem MALDI-TOF mass spectrometry. Carbohydr. Res. 2012, 358, 78–81. [Google Scholar] [CrossRef]
- Khil’chenko, S.R.; Zaporozhets, T.S.; Shevchenko, N.M.; Zvyagintseva, T.N.; Vogel, U.; Seeberger, P.; Lepenies, B. Immunostimulatory activity of fucoidan from the brown alga Fucus evanescens: Role of sulfates and acetates. J. Carbohydr. Chem. 2011, 30, 291–305. [Google Scholar] [CrossRef]
- Hmelkov, A.B.; Zvyagintseva, T.N.; Shevchenko, N.M.; Rasin, A.B.; Ermakova, S.P. Ultrasound-assisted extraction of polysaccharides from brown alga Fucus evanescens. Structure and biological activity of the new fucoidan fractions. J. Appl. Phycol. 2018, 30, 2039–2046. [Google Scholar] [CrossRef]
- Ehrig, K.; Alban, S. Sulfated galactofucan from the brown alga Saccharina latissima-variability of yield, structural composition and bioactivity. Mar. Drugs 2015, 13, 76–101. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. J. Appl. Phycol. 2012, 24, 715–723. [Google Scholar] [CrossRef]
- Manns, D.; Deutschle, A.L.; Saake, B.; Meyer, A.S. Methodology for quantitative determination of the carbohydrate composition of brown seaweeds (Laminariaceae). RSC Adv. 2014, 4, 25736–25746. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.T.T.; Mikkelsen, M.D.; Lezyk, M.J.; Bui, L.M.; Tran, V.T.T.; Silchenko, A.S.; Kusaykin, M.I.; Pham, T.D.; Truong, B.H.; Holck, H.; et al. Novel enzyme actions for sulphated galactofucan depolymerisation and a new engineering strtegy for molecular stabilisation of fucoidan degrading enzymes. Mar. Drugs 2018, 16, 422. [Google Scholar] [CrossRef] [PubMed]

| Monomer Category | Monomer | F. evanescens | S. latissima |
|---|---|---|---|
| Neutral monosaccharides (%) | Mannitol | 2.6 a ± 0.6 | 2.1 a ± 0.5 |
| Fucose | 8.7 a ± 0.9 | 4.7 b ± 0.1 | |
| Rhamnose | 0.1 a ± 0.0 | 0.1 a ± 0.0 | |
| Galactose | 1.5 a ± 0.4 | 0.5 b ± 0.5 | |
| Glucose | 6.7 b ± 0.8 | 12.3 a ± 2.4 | |
| Xylose | 0.8 a ± 0.0 | 0.4 b ± 0.1 | |
| Mannose | 0.9 a ± 0.2 | 1.0 a ± 0.3 | |
| Uronic acids (%) | Guluronic Acid | 8.8 a ± 1.1 | 9.3 a ± 2.4 |
| Glucuronic Acid | 1.2 a ± 0.2 | 1.9 a ± 0.5 | |
| Mannuronic Acid | 28.3 a ± 3.6 | 36.1 a ± 8.8 |
| Content | Monomer | F. evanescens | S. latissima | ||
|---|---|---|---|---|---|
| Enzymatic Method | Chemical Method | Enzymatic Method | Chemical Method | ||
| Neutral monosaccharides (%mol) | Mannitol | 0.2 b ± 0.0 | 0.43 a± 0.0 | 2.1 a ± 0.2 | 2.2 a ± 0.3 |
| Fucose | 24.8 b ± 2.9 | 60.9 a ± 0.9 | 12.6 b ± 0.4 | 31.2 a ± 4.2 | |
| Rhamnose | 0.2 b ± 0.1 | 0.9 a ± 0.2 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | |
| Galactose | 0.9 b± 0.1 | 5.4 a ± 0.1 | 2.3 a ± 0.1 | 2.9 a ± 2.4 | |
| Glucose | 0.7 b ± 0.1 | 6.2 a ± 0.1 | 1.6 b ± 0.0 | 57.7 a ± 3.1 | |
| Xylose | 0.8 b ± 0.1 | 5.8 a ± 0.1 | 0.8 b ± 0.0 | 3.0 a ± 0.0 | |
| Mannose | 0.4 b ± 0.0 | 2.6 a ± 0.1 | 0.9 a ± 0.0 | 0.9 a ± 0.1 | |
| Uronic acid (%mol) | GuluA | 12.6 a ± 1.8 | 0.9 b ± 0.1 | 18.6 a ± 0.9 | 0.2 b ± 0.1 |
| GluA | 1.0 b ± 0.2 | 3.9 a ± 0.1 | 1.3 a ± 0.2 | 0.7 b ± 0.1 | |
| ManA | 58.4 a ± 2.6 | 13.1 b ± 0.4 | 59.6 a ± 1.9 | 1.0 b ± 0.2 | |
| Sulfate (SO42−) (%wt) | 21.4 b ± 0.5 | 38.0 a ± 0.4 | 15.5 b ± 1.7 | 31.6 a ± 0.9 | |
| Degree of sulfation (molar ratio SO42−: Fucose) | 2.1 | 1.9 | 2.5 | 2.1 | |
| Fucoidan yield (fucose extraction %wt) | 40 | 43 | 29 | 29 | |
| Content | Monomer | F. evanescens | S. latissima | ||||
|---|---|---|---|---|---|---|---|
| FeF1 | FeF2 | FeF3 | SlF1 | SlF2 | SlF3 | ||
| Yield, % of crude extract | 4.2 | 7.9 | 18.2 | 3.4 | 6.2 | 3.8 | |
| Neutral monosaccharides (%mol) | Mannitol | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.1 a ± 0.0 | 0.0 a ± 0.0 |
| Fucose | 34 c ± 3.1 | 74.7 b ± 0.8 | 87.8 a ± 1.4 | 5.4 b ± 1.2 | 64.7 a ± 0.3 | 63.3 a ± 0.7 | |
| Rhamnose | 0.3 c ± 0.1 | 0.8 a ± 0.1 | 0.5 b ± 0.1 | 0.1 b ± 0.0 | 0.3 a ± 0.0 | 0.3 a ± 0.0 | |
| Galactose | 4.6 c ± 0.4 | 15.4 a ± 0.4 | 9.0 b ± 0.9 | 0.5 c ± 0.0 | 12.2 b ± 0.1 | 26.9 a ± 0.3 | |
| Glucose | 7.7 a ± 0.7 | 1.4 b ± 0.1 | 0.3 c ± 0.1 | 0.4 b ± 0.0 | 0.6 a ± 0.1 | 0.4 b ± 0.1 | |
| Xylose | 5.3 a ± 0.5 | 2.8 b ± 0.1 | 1.5 c ± 0.3 | 0.8 c ± 0.1 | 4.8 a ± 0.0 | 3.4 b ± 0.2 | |
| Mannose | 3.2 a ± 0.5 | 2.3 b ± 0.1 | 0.3 c ± 0.1 | 0.8 c ± 0.1 | 3.5 a ± 0.2 | 2.1 b ± 0.1 | |
| Uronic acid (%mol) | GuluA | 9.1 a ± 0.5 | 2.2 b ± 0.2 | 0.0 c ± 0.0 | 1.1 c ± 0.1 | 6.9 a ± 0.3 | 2.8 b ± 0.2 |
| GluA | 3.8 a ± 0.3 | 0.3 b ± 0.0 | 0.5 b ± 0.1 | 8.5 a ± 4.7 | 0.0 b ± 0.0 | 0.0 b ± 0.0 | |
| ManA | 32.2 a ± 0.6 | 0.2 b ± 0.0 | 0.0 b ± 0.0 | 82.4 a ± 4.3 | 6.9 b ± 0.1 | 0.8 c ± 0.1 | |
| Sulfate (SO42−) (wt%) | 20.4 b ± 3.4 | 34.8 ab ± 2.0 | 38.7 a ± 1.0 | 6.6 c ± 3.6 | 35.6 b ± 2.5 | 46.4 a ± 3.5 | |
| Degree of sulfation (molar ratio SO42−: Fuc) | 1.3 | 1.7 | 1.6 | 1.8 | 2.4 | 3.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. https://doi.org/10.3390/md18060296
Nguyen TT, Mikkelsen MD, Tran VHN, Trang VTD, Rhein-Knudsen N, Holck J, Rasin AB, Cao HTT, Van TTT, Meyer AS. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Marine Drugs. 2020; 18(6):296. https://doi.org/10.3390/md18060296
Chicago/Turabian StyleNguyen, Thuan Thi, Maria Dalgaard Mikkelsen, Vy Ha Nguyen Tran, Vo Thi Dieu Trang, Nanna Rhein-Knudsen, Jesper Holck, Anton B. Rasin, Hang Thi Thuy Cao, Tran Thi Thanh Van, and Anne S. Meyer. 2020. "Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima" Marine Drugs 18, no. 6: 296. https://doi.org/10.3390/md18060296
APA StyleNguyen, T. T., Mikkelsen, M. D., Tran, V. H. N., Trang, V. T. D., Rhein-Knudsen, N., Holck, J., Rasin, A. B., Cao, H. T. T., Van, T. T. T., & Meyer, A. S. (2020). Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Marine Drugs, 18(6), 296. https://doi.org/10.3390/md18060296








