Astaxanthin Attenuates Fish Oil-Related Hepatotoxicity and Oxidative Insult in Juvenile Pacific White Shrimp (Litopenaeus vannamei)
Abstract
1. Introduction
2. Results
2.1. Growth Performance and Feed Utilization
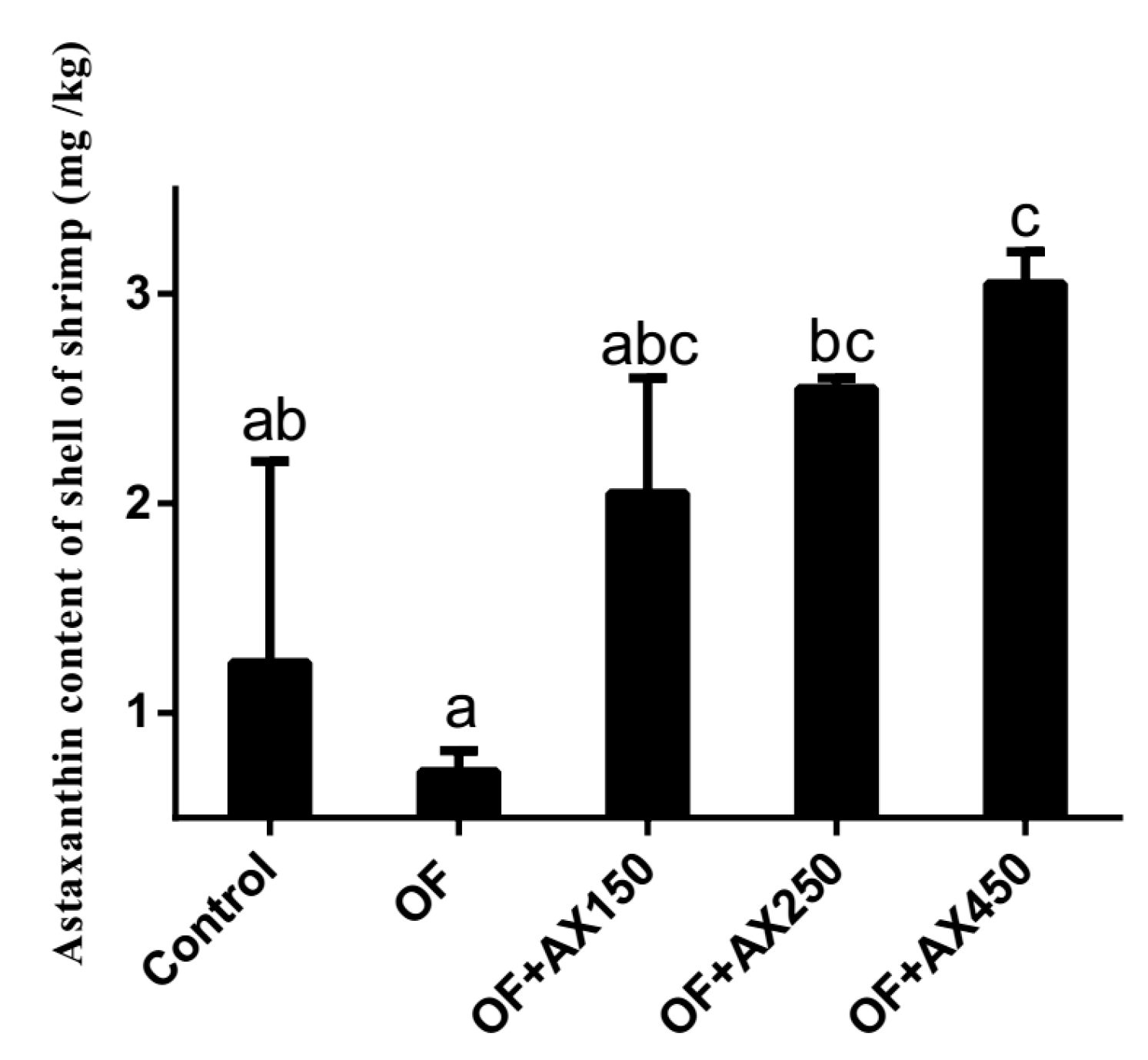
2.2. Shell Astaxanthin Concentration
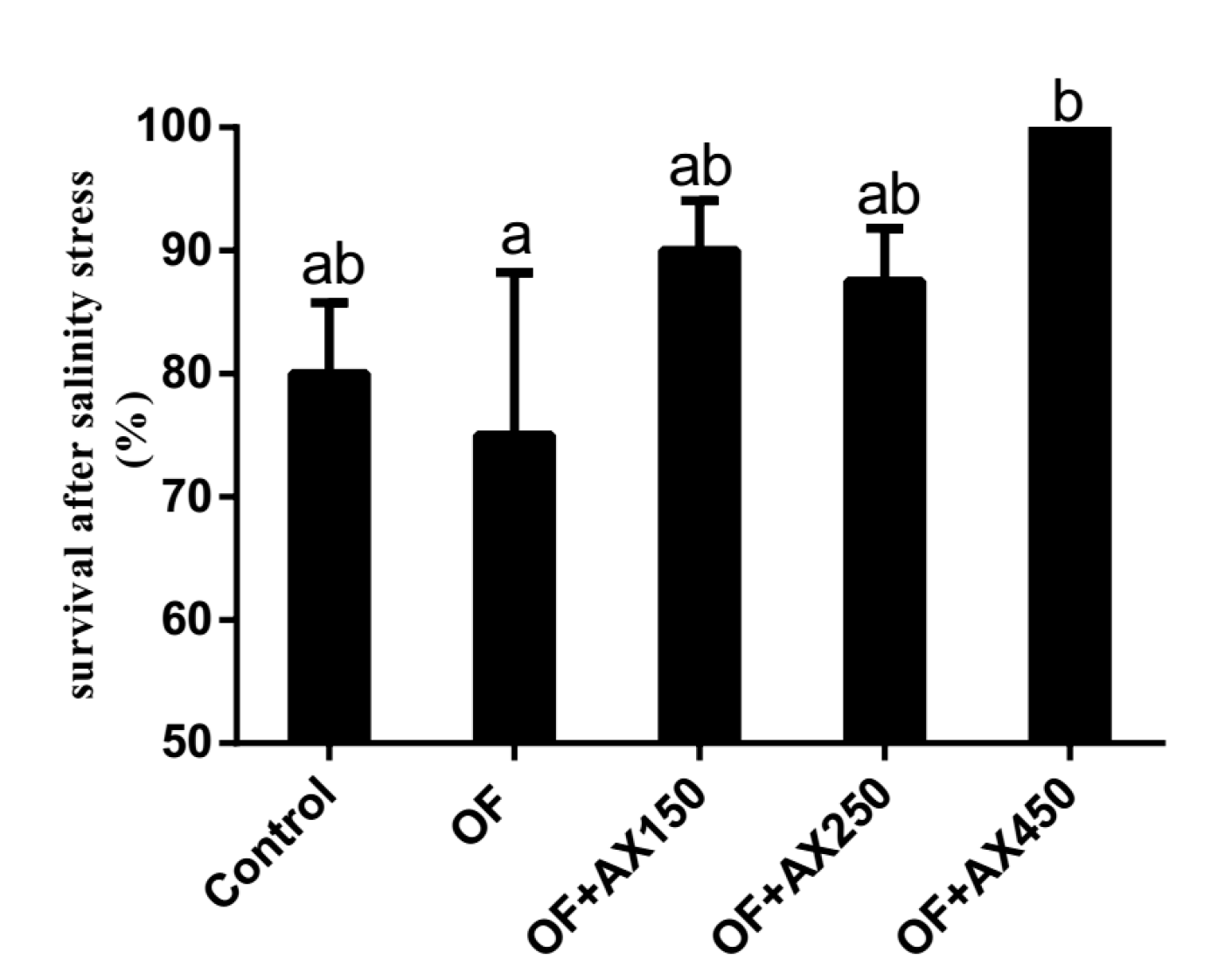
2.3. Survival Rate of Shrimp after the Acute Salinity Change Test
2.4. Hepatopancreatic and Hemolymph Immune Parameters
2.5. Muscle Fatty Acids Composition
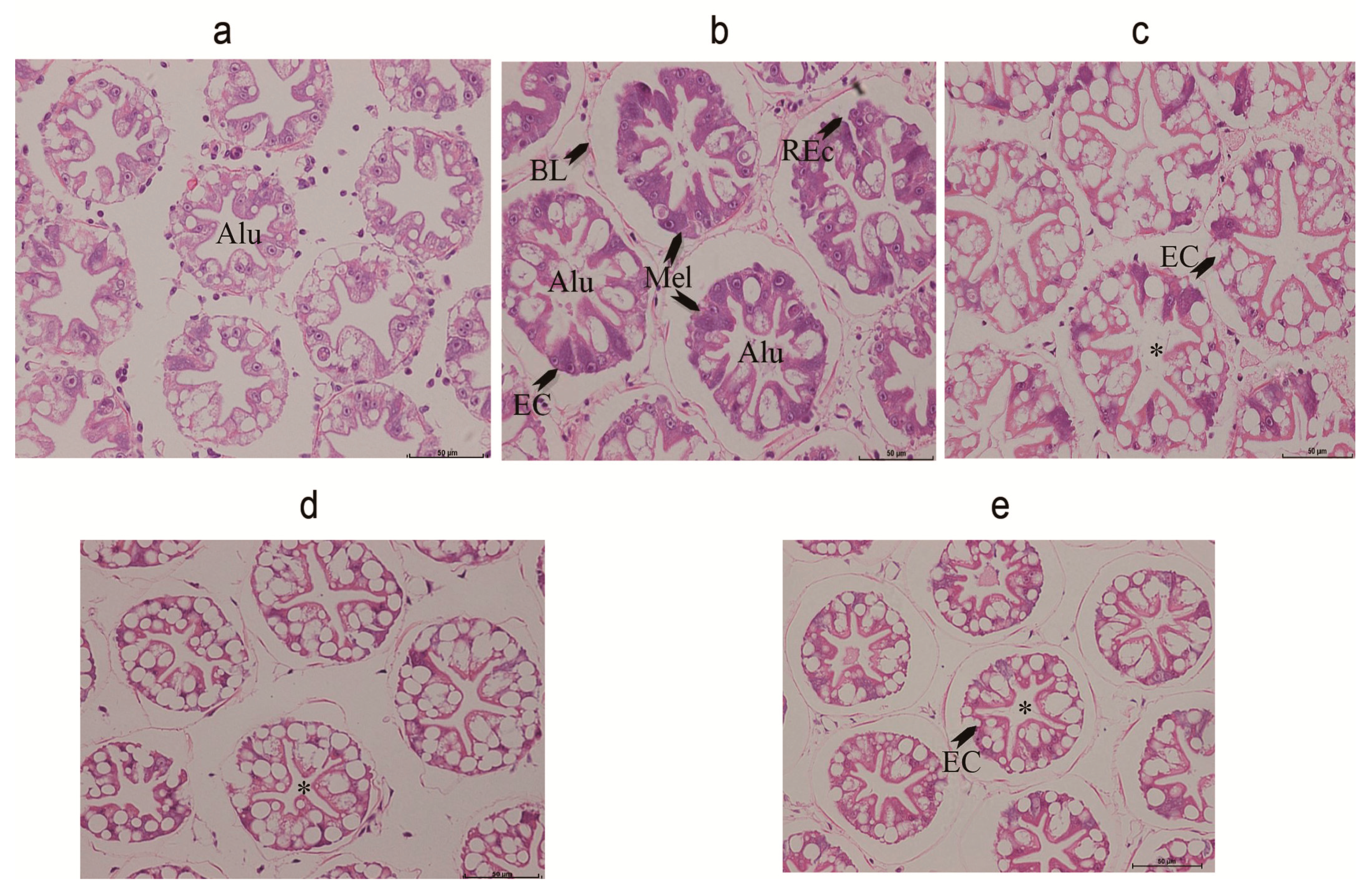
2.6. Hepatopancreas Histology
3. Disscussion
4. Materials and Methods
4.1. Diet Preparation
4.2. Shrimp and Experimental Conditions
4.3. Sample Collection
4.4. Histopathological Studies
4.5. Fatty Acid Composition
4.6. Astaxanthin Content of the Shell
4.7. Lipid Peroxidation and Enzyme Activity Assays
4.8. Acute Salinity Change Experiment
4.9. Calculations and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albrektsen, S.; Østbye, T.-K.; Pedersen, M.; Ytteborg, E.; Ruyter, B.; Ytrestøyl, T. Dietary impacts of sulphuric acid extracted fish bone compounds on astaxanthin utilization and muscle quality in Atlantic salmon (Salmo salar). Aquaculture 2018, 495, 255–266. [Google Scholar] [CrossRef]
- Mattei, R.; Polotow, T.G.; Vardaris, C.V.; Guerra, B.A.; Leite, J.R.; Otton, R.; Barros, M.P. Astaxanthin limits fish oil-related oxidative insult in the anterior forebrain of Wistar rats: Putative anxiolytic effects? Pharmacol. Biochem. Behav. 2011, 99, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.M.; Reza, H.M.; Saadi, H.M.; Mahmud, W.; Ibrahim, A.A.; Alam, M.M.; Kabir, N.; Saifullah, A.R.M.; Tropa, S.T.; Quddus, A.H.M.R. Astaxanthin ameliorates aluminum chloride-induced spatial memory impairment and neuronal oxidative stress in mice. Eur. J. Pharmacol. 2016, 777, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.-K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Pan, C.H.; Hunter, B. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture 2003, 216, 177–191. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.-J.; Tian, L.-X.; Yang, H.-J.; Liang, G.-Y.; Yue, Y.-R.; Xu, D.-H. Effects of dietary astaxanthin on growth, antioxidant capacity and gene expression in Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2013, 19, 917–927. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Li, X.; Chen, Z.; Liang, G.; Leng, X. Dietary astaxanthin improved the body pigmentation and antioxidant function, but not the growth of discus fish (Symphysodon spp.). Aquac. Res. 2017, 48, 1359–1367. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Dietary administration of astaxanthin improves feed utilization, growth performance and survival of Asian seabass, Lates calcarifer (Bloch, 1790). Aquac. Nutr. 2019, 25, 1410–1421. [Google Scholar] [CrossRef]
- Lam, H.S.; Ngoc, P.T. Effect of dietary astaxanthin on pigment accumulation in the muscle-skin, resistance to salinity and copper toxicity of commercial clownfish, Amphiprion ocellaris. Vietnam J. Mar. Sci. Technol. 2018, 18, 60–69. [Google Scholar]
- Niu, J.; Tian, L.X.; Liu, Y.J.; Yang, H.J.; Ye, C.X.; Gao, W.; Mai, K. Sen Effect of dietary astaxanthin on growth, survival, and stress tolerance of postlarval shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2009, 40, 795–802. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Guo, Z.-X.; Ye, C.-X.; Wang, A.-L. Effect of dietary astaxanthin on the growth performance, non-specific immunity, and antioxidant capacity of pufferfish (Takifugu obscurus) under high temperature stress. Fish Physiol. Biochem. 2018, 44, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mourente, G.; Diaz-Salvago, E.; Bell, J.G.; Tocher, D.R. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: Attenuation by dietary vitamin E. Aquaculture 2002, 214, 343–361. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid oxidation. Prog. Lipid Res. 1980, 19, 1–22. [Google Scholar] [CrossRef]
- Howell, B.R.; Day, O.J.; Ellis, T.; Baynes, S.M. Early life stages of farmed fish. In Biology of Farmed Fish; Black, K.D., Pickering, A.D., Eds.; Academic Press: Sheffield, UK, 1998; pp. 27–66. [Google Scholar]
- Hamre, K. Metabolism, interactions, requirements and functions of vitamin E in fish. Aquac. Nutr. 2011, 17, 98–115. [Google Scholar] [CrossRef]
- Kanazawa, K. Hepatotoxicity caused by dietary secondary products originating from lipid peroxidation. In Nutritional and Toxicological Consequences of Food Processing; Springer: Boston, MA, USA, 1991; pp. 237–253. [Google Scholar]
- Hang, T.N.A. Effects of Dietary Oxidation Status and Vitamin E Level on Performance, Fillet Quality and Robustness to Acute Stress in Atlantic Salmon (Salmo salar L.); Norwegian University of Life Sciences: Ås, Norway, 2012. [Google Scholar]
- Chen, S.; Zhuang, Z.; Yin, P.; Chen, X.; Zhang, Y.; Tian, L.; Niu, J.; Liu, Y. Changes in growth performance, haematological parameters, hepatopancreas histopathology and antioxidant status of pacific white shrimp (Litopenaeus vannamei) fed oxidized fish oil: Regulation by dietary myo-inositol. Fish Shellfish Immunol. 2019, 88, 53–64. [Google Scholar] [CrossRef]
- Fontagné-Dicharry, S.; Larroquet, L.; Dias, K.; Cluzeaud, M.; Heraud, C.; Corlay, D. Effects of dietary oxidized fish oil supplementation on oxidative stress and antioxidant defense system in juvenile rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 74, 43–51. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Huo, Y.; Guo, T.; Fang, H.; Zhang, Y.; Liu, Y.; Tian, L.; Niu, J. Effects of dietary oxidized fish oil on growth performance, antioxidant defense system, apoptosis and mitochondrial function of juvenile largemouth bass (Micropterus salmoides). Aquaculture 2019, 500, 347–358. [Google Scholar] [CrossRef]
- Zhong, Y.; Lall, S.P.; Shahidi, F. Effects of oxidized dietary oil and vitamin E supplementation on lipid profile and oxidation of muscle and liver of juvenile Atlantic cod (Gadus morhua). J. Agric. Food Chem. 2007, 55, 6379–6386. [Google Scholar] [CrossRef]
- Koshio, S.; Teshima, S.; Kanazawa, A. Effects of dietary oxidized oil for Penaeus japonicus. Fish. Sci. 1994, 60, 283–288. [Google Scholar] [CrossRef]
- Laohabanjong, R.; Tantikitti, C.; Benjakul, S.; Supamattaya, K.; Boonyaratpalin, M. Lipid oxidation in fish meal stored under different conditions on growth, feed efficiency and hepatopancreatic cells of black tiger shrimp (Penaeus monodon). Aquaculture 2009, 286, 283–289. [Google Scholar] [CrossRef]
- Wang, L.G.; Li, E.C.; Qin, J.G.; Du, Z.Y.; Yu, N.; Kong, Y.Q.; Feng, D.X.; Chen, L.Q. Effect of oxidized fish oil and $α$-tocopherol on growth, antioxidation status, serum immune enzyme activity and resistance to A eromonas hydrophila challenge of Chinese mitten crab Eriocheir sinensis. Aquac. Nutr. 2015, 21, 414–424. [Google Scholar] [CrossRef]
- Sirirustananun, N.; Chen, J.C.; Lin, Y.C.; Yeh, S.T.; Liou, C.H.; Chen, L.L.; Sim, S.S.; Chiew, S.L. Dietary administration of a Gracilaria tenuistipitata extract enhances the immune response and resistance against Vibrio alginolyticus and white spot syndrome virus in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 31, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.W. Major viral diseases of the black tiger prawn (Penaeus monodon) in Thailand. World J. Microbiol. Biotechnol. 1997, 13, 433–442. [Google Scholar] [CrossRef]
- Fontagné, S.; Bazin, D.; Brèque, J.; Vachot, C.; Bernarde, C.; Rouault, T.; Bergot, P. Effects of dietary oxidized lipid and vitamin A on the early development and antioxidant status of Siberian sturgeon (Acipenser baeri) larvae. Aquaculture 2006, 257, 400–411. [Google Scholar] [CrossRef]
- Dong, G.F.; Huang, F.; Zhu, X.M.; Zhang, L.; Mei, M.X.; Hu, Q.W.; Liu, H.Y. Nutriphysiological and cytological responses of juvenile channel catfish (Ictalurus punctatus) to dietary oxidized fish oil. Aquac. Nutr. 2012, 18, 673–684. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; López-Varela, S.; Garrido-Polonio, M.C.; Cuesta, C. Dietary effects on growth, liver peroxides, and serum and lipoprotein lipids in rats fed a thermoxidised and polymerised sunflower oil. J. Sci. Food Agric. 1998, 76, 364–372. [Google Scholar] [CrossRef]
- Lewis-McCrea, L.M.; Lall, S.P. Effects of moderately oxidized dietary lipid and the role of vitamin E on the development of skeletal abnormalities in juvenile Atlantic halibut (Hippoglossus hippoglossus). Aquaculture 2007, 262, 142–155. [Google Scholar] [CrossRef]
- Fontagné, S.; Lataillade, E.; Breque, J.; Kaushik, S. Lipid peroxidative stress and antioxidant defence status during ontogeny of rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2008, 100, 102–111. [Google Scholar] [CrossRef]
- Fatima, M.; Afzal, M.; Shah, S.Z.H. Effect of dietary oxidized oil and vitamin E on growth performance, lipid peroxidation and fatty acid profile of Labeo rohita fingerlings. Aquac. Nutr. 2019, 25, 281–291. [Google Scholar] [CrossRef]
- Gao, J.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Nguyen, B.T.; Mamauag, R.E. Effect of dietary oxidized fish oil and vitamin C supplementation on growth performance and reduction of oxidative stress in Red Sea Bream Pagrus major. Aquac. Nutr. 2013, 19, 35–44. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, Y.J.; Tian, L.X.; Niu, J.; Liang, G.; Yang, H.; Yuan, Y.; Zhang, Y.Q. Effect of dietary vitamin E and selenium supplementation on growth, body composition, and antioxidant defense mechanism in juvenile largemouth bass (Micropterus salmoide) fed oxidized fish oil. Fish Physiol. Biochem. 2013, 39, 593–604. [Google Scholar] [CrossRef]
- Yang, S.-P.; Liu, H.-L.; Wang, C.-G.; Yang, P.; Sun, C.-B.; Chan, S.-M. Effect of oxidized fish oil on growth performance and oxidative stress of Litopenaeus vannamei. Aquac. Nutr. 2015, 21, 121–127. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, C.; Cao, X.; Zhu, J.; He, J.; Wu, P.; Ye, Y. Supplementation of dietary astaxanthin alleviated oxidative damage induced by chronic high pH stress, and enhanced carapace astaxanthin concentration of Chinese mitten crab Eriocheir sinensis. Aquaculture 2018, 483, 230–237. [Google Scholar] [CrossRef]
- Niu, J.; Li, C.H.; Liu, Y.J.; Tian, L.X.; Chen, X.; Huang, Z.; Lin, H.Z. Dietary values of astaxanthin and canthaxanthin in Penaeus monodon in the presence and absence of cholesterol supplementation: Effect on growth, nutrient digestibility and tissue carotenoid composition. Br. J. Nutr. 2012, 108, 80–91. [Google Scholar] [CrossRef]
- Segner, H.; Arend, P.; Von Poeppinghausen, K.; Schmidt, H. The effect of feeding astaxanthin to Oreochromis niloticus and Colisa labiosa on the histology of the liver. Aquaculture 1989, 79, 381–390. [Google Scholar] [CrossRef]
- Wang, W.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dawood, M.A.O.; Hossain, M.S.; Moss, A.S. Effects of dietary astaxanthin and vitamin E and their interactions on the growth performance, pigmentation, digestive enzyme activity of kuruma shrimp (Marsupenaeus japonicus). Aquac. Res. 2019, 50, 1186–1197. [Google Scholar] [CrossRef]
- Li, M.-Y.; Gao, C.-S.; Du, X.-Y.; Zhao, L.; Niu, X.-T.; Wang, G.-Q.; Zhang, D.-M. Effect of sub-chronic exposure to selenium and astaxanthin on Channa argus: Bioaccumulation, oxidative stress and inflammatory response. Chemosphere 2020, 244, 125546. [Google Scholar] [CrossRef]
- Chien, Y.H.; Shiau, W.C. The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus Bate. J. Exp. Mar. Bio. Ecol. 2005, 318, 201–211. [Google Scholar] [CrossRef]
- Pan, C.H.; Chien, Y.H.; Hunter, B. The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. J. Exp. Mar. Bio. Ecol. 2003, 297, 107–118. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, Y.J.; Yang, H.J.; Yuan, Y.; Liu, F.J.; Tian, L.X.; Liang, G.Y.; Yuan, R.M. Effect of dietary oxidized fish oil on growth performance, body composition, antioxidant defence mechanism and liver histology of juvenile largemouth bass Micropterus salmoides. Aquac. Nutr. 2012, 18, 321–331. [Google Scholar] [CrossRef]
- Bhavan, P.S.; Geraldine, P. Histopathology of the hepatopancreas and gills of the prawn Macrobrachium malcolmsonii exposed to endosulfan. Aquat. Toxicol. 2000, 50, 331–339. [Google Scholar] [CrossRef]
- Caceci, T.; Neck, K.F.; Lewis, D.D.H.; Sis, R.F. Ultrastructure of the hepatopancreas of the pacific white shrimp, Penaeus vannamei (Crustacea: Decapoda). J. Mar. Biol. Assoc. 1988, 68, 323–337. [Google Scholar] [CrossRef]
- Bautista, M.N.; Lavilla-Pitogo, C.R.; Subosa, P.F.; Begino, E.T. Aflatoxin B1 contamination of shrimp feeds and its effect on growth and hepatopancreas of pre-adult Penaeus monodon. J. Sci. Food Agric. 1994, 65, 5–11. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011.
- Yu, Y.Y.; Chen, S.J.; Chen, M.; Tian, L.X.; Niu, J.; Liu, Y.J.; Xu, D.H. Effect of cadmium-polluted diet on growth, salinity stress, hepatotoxicity of juvenile Pacific white shrimp (Litopenaeus vannamei): Protective effect of Zn(II)-curcumin. Ecotoxicol. Environ. Saf. 2016, 125, 176–183. [Google Scholar] [CrossRef]
- Yuan, J.P.; Gong, X.D.; Chen, F. Separation and identification of astaxanthin esters and chlorophylls in haematococcus lacustris by HPLC. Biotechnol. Tech. 1996, 10, 655–660. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. In Methods in Enzymology; Lester Packer, A.N.G., Ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 407–421. [Google Scholar]

| Items | D1 | D2 | D3 | D4 | D5 |
|---|---|---|---|---|---|
| Control | OFO | OFO + AX150 | OFO + AX250 | OFO + AX450 | |
| Initial body weight (g) | 0.53 ± 0.02 | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.53 ± 0.02 | 0.53 ± 0.01 |
| Final body weight (g) | 9.62 ± 1.42 a,b | 8.31 ± 0.33 a | 8.78 ± 0.23 a,b | 10.46 ± 1.77 a,b | 11.09 ± 2.13 b |
| Weight gain (%) | 1674.2 ± 106.8 a,b | 1457.7 ± 62.2 a | 1548.7 ± 24.2 a,b | 1866.7 ± 335.9 a,b | 1980.8 ± 399.9 b |
| SGR (% day−1) | 5.13 ± 0.10 | 4.90 ± 0.04 | 5.00 ± 0.03 | 5.30 ± 0.17 | 5.40 ± 021 |
| Survival (%) | 87.78 ± 7.78 | 75.83 ± 6.85 | 81.11 ± 6.76 | 88.89 ± 9.50 | 86.67 ± 8.39 |
| FCR | 1.37 ± 0.07 | 1.42 ± 0.13 | 1.48 ± 0.02 | 1.18 ± 0.12 | 1.21 ± 0.26 |
| Items | D1 | D2 | D3 | D4 | D5 |
|---|---|---|---|---|---|
| Control | Oxidized Fish Oil (OFO) | OFO + AX150 | OFO + AX250 | OFO + AX450 | |
| Hemolymph malondialdehyde (MDA) (mmol L−1) | 4.40 ± 0.40 a | 6.76 ± 0.75 b | 4.78 ± 0.29 a,b | 5.22 ± 0.22 a,b | 6.01 ± 0.36 a,b |
| Hemolymph catalase (CAT) (U L−1) | 1.07 ± 0.06 b,c | 0.54 ± 0.26 a | 0.90 ± 0.19 a,b | 1.17 ± 0.14 b,c | 1.50 ± 0.13 c |
| Hepatopancreas MDA (nmol mg−1prot ) | 1.96 ± 0.50 | 3.32 ± 1.10 | 3.06 ± 0.81 | 2.11 ± 0.15 | 1.97 ± 0.12 |
| Hepatopancreas iNOS (U mg−1prot) | 1.35 ± 0.11 a,b | 1.28 ± 0.09 a | 1.39 ± 0.10 a,b | 1.54 ± 0.15 a,b | 1.62 ± 0.10 b |
| Metabolite a. | D1 | D2 | D3 | D4 | D5 |
|---|---|---|---|---|---|
| 14:0 | 0.17 ± 0.03 | 0.33 ± 0.14 | 0.23 ± 0.04 | 0.19 ± 0.05 | 0.19 ± 0.04 |
| 15:0 | 0.18 ± 0.02 | 0.19 ± 0.01 | 0.20 ± 0.00 | 0.17 ± 0.02 | 0.18 ± 0.01 |
| 16:0 | 16.83 ± 1.23 | 18.60 ± 1.90 | 18.27 ± 0.55 | 15.10 ± 1.5 | 16.03 ± 1.09 |
| 16:1 | 1.05 ± 0.15 | 1.86 ± 0.66 | 0.98 ± 0.17 | 1.06 ± 0.28 | 0.80 ± 0.11 |
| 17:0 | 0.94 ± 0.02 | 0.93 ± 0.02 | 0.95 ± 0.02 | 0.90 ± 0.01 | 0.95 ± 0.02 |
| 17:1 | 0.71 ± 0.21 | 0.45 ± 0.15 | 0.75 ± 0.09 | 0.87 ± 0.23 | 0.68 ± 0.11 |
| 18:0 | 12.07 ± 0.47 | 11.20 ± 0.79 | 12.00 ± 0.38 | 12.17 ± 0.39 | 12.63 ± 0.34 |
| 18:1 | 20.27 ± 0.78 | 20.30 ± 0.90 | 20.63 ± 0.59 | 19.67 ± 1.60 | 20.03 ± 0.87 |
| 18:2 n-6 | 10.80 ± 0.78 | 11.23 ± 0.62 | 10.97 ± 0.28 | 10.50 ± 0.46 | 10.25 ± 0.46 |
| 18:3 n-3 | 0.58 ± 0.03 | 0.59 ± 0.03 | 0.55 ± 0.06 | 0.52 ± 0.02 | 0.54 ± 0.01 |
| 20:0 | 0.22 ± 0.03 | 0.18 ± 0.06 | 0.25 ± 0.04 | 0.18 ± 0.09 | 0.27 ± 0.12 |
| 20:1 | 1.04 ± 0.08 | 0.98 ± 0.12 | 0.96 ± 0.04 | 1.06 ± 0.04 | 1.10 ± 0.00 |
| C20:2 | 1.77 ± 0.17 | 1.47 ± 0.31 | 1.77 ± 0.09 | 1.97 ± 0.12 | 1.98 ± 0.08 |
| C22:0 | 0.30 ± 0.02 | 0.33 ± 0.04 | 0.30 ± 0.02 | 0.25 ± 0.13 | 0.33 ± 0.03 |
| C 20:4 n-6 | 3.03 ± 0.30 | 2.68 ± 0.54 | 3.13 ± 0.12 | 3.70 ± 0.40 | 3.33 ± 0.19 |
| C 20:5 n-3 | 12.97 ± 1.03 | 9.98 ± 0.79 | 12.00 ± 0.38 | 12.17 ± 0.39 | 12.63 ± 0.34 |
| C24:0 | 0.37 ± 0.04 a,b | 0.26 ± 0.01 a | 0.40 ± 0.05 a,b | 0.54 ± 0.07 b | 0.45 ± 0.07 a,b |
| C24:1 | 0.66 ± 0.33 | 0.37 ± 0.22 | 0.49 ± 0.19 | 0.95 ± 0.53 | 0.85 ± 0.36 |
| C 22:5 n-6 (EPA) | 1.63 ± 0.12 | 1.18 ± 0.40 | 1.17 ± 0.12 | 1.53 ± 0.32 | 1.65 ± 0.14 |
| C 22:6 n-3 (DHA) | 14.27 ± 0.64 | 13.47 ± 0.75 | 13.47 ± 0.87 | 14.17 ± 0.69 | 14.80 ± 0.83 |
| EPA+DHA | 27.23 ± 1.55 | 25.73 ± 1.94 | 25.80 ± 1.20 | 28.73 ± 2.36 | 28.18 ± 1.55 |
| DHA/EPA | 1.11 ± 0.06 | 1.10 ± 0.03 | 1.09 ± 0.04 | 0.99 ± 0.07 | 1.11 ± 0.03 |
| SAFA 1 | 31.10 ± 0.85 | 31.72 ± 0.94 | 32.60 ± 0.90 | 29.44 ± 0.90 | 31.00 ± 1.16 |
| UFA 2 | 68.76 ± 0.87 a,b | 66.11 ± 2.08 a | 66.88 ± 1.20 a,b | 70.56 ± 0.89 b | 68.92 ± 1.16 a,b |
| n-3 PUFA 3 | 29.44 ± 1.56 | 22.69 ± 5.37 | 27.52 ± 1.16 | 30.79 ± 2.62 | 30.37 ± 1.63 |
| n-6 PUFA | 13.83 ± 0.49 | 15.08 ± 0.73 | 14.10 ± 0.29 | 14.20 ± 0.17 | 13.58 ± 0.39 |
| n-3/n-6 ratio | 2.14 ± 0.18 | 1.56 ± 0.41 | 1.95 ± 0.05 | 2.17 ± 0.20 | 2.24 ± 0.14 |
| Items | D1 | D2 | D3 | D4 | D5 |
|---|---|---|---|---|---|
| Control | OFO | OFO + AX150 | OFO + AX250 | OFO + AX450 | |
| Ingredients (g kg−1 diet) | |||||
| Fish meal | 220 | 220 | 220 | 220 | 220 |
| Soybean meal | 210 | 210 | 210 | 210 | 210 |
| Wheat flour | 245 | 245 | 245 | 245 | 245 |
| Peanut meal | 100 | 100 | 100 | 100 | 100 |
| soybean protein concentrate | 60 | 60 | 60 | 60 | 60 |
| Beer yeast | 50 | 50 | 50 | 50 | 50 |
| Chicken meal | 30 | 30 | 30 | 30 | 30 |
| Fresh Fish oil | 30 | 0 | 0 | 0 | 0 |
| Oxidized fish oil | 0 | 30 | 30 | 30 | 30 |
| Soya lecithin | 10 | 10 | 10 | 10 | 10 |
| Vitamin premix a | 10 | 10 | 10 | 10 | 10 |
| Mineral premix b | 10 | 10 | 10 | 10 | 10 |
| Ca(H2PO4)2–H2O | 20 | 20 | 20 | 20 | 20 |
| Vitamin C | 1 | 1 | 1 | 1 | 1 |
| Choline chloride (50%) | 2 | 2 | 2 | 2 | 2 |
| Astaxanthin (10%) | 0 | 0 | 0.15 | 0.25 | 0.45 |
| Cellulose | 2 | 2 | 1.85 | 1.75 | 1.55 |
| Proximate analysis (g kg−1 diet ) | |||||
| Crude protein | 390.1 | 391.1 | 392.6 | 392.6 | 393.1 |
| Crude lipid | 71 | 70 | 71 | 71 | 74 |
| Astaxanthin (mg/kg) | 0.47 | 0 | 57 | 170 | 289 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Liu, Y.; Yin, P.; Zhou, W.; Tian, L.; Liu, Y.; Xu, D.; Niu, J. Astaxanthin Attenuates Fish Oil-Related Hepatotoxicity and Oxidative Insult in Juvenile Pacific White Shrimp (Litopenaeus vannamei). Mar. Drugs 2020, 18, 218. https://doi.org/10.3390/md18040218
Yu Y, Liu Y, Yin P, Zhou W, Tian L, Liu Y, Xu D, Niu J. Astaxanthin Attenuates Fish Oil-Related Hepatotoxicity and Oxidative Insult in Juvenile Pacific White Shrimp (Litopenaeus vannamei). Marine Drugs. 2020; 18(4):218. https://doi.org/10.3390/md18040218
Chicago/Turabian StyleYu, Yingying, Yang Liu, Peng Yin, Weiwen Zhou, Lixia Tian, Yongjian Liu, Donghui Xu, and Jin Niu. 2020. "Astaxanthin Attenuates Fish Oil-Related Hepatotoxicity and Oxidative Insult in Juvenile Pacific White Shrimp (Litopenaeus vannamei)" Marine Drugs 18, no. 4: 218. https://doi.org/10.3390/md18040218
APA StyleYu, Y., Liu, Y., Yin, P., Zhou, W., Tian, L., Liu, Y., Xu, D., & Niu, J. (2020). Astaxanthin Attenuates Fish Oil-Related Hepatotoxicity and Oxidative Insult in Juvenile Pacific White Shrimp (Litopenaeus vannamei). Marine Drugs, 18(4), 218. https://doi.org/10.3390/md18040218






