Application of Gelatin Decorated with Allura Red as Resonance Rayleigh Scattering Sensor to Detect Chito-Oligosaccharides
Abstract
1. Introduction
2. Results and Discussion
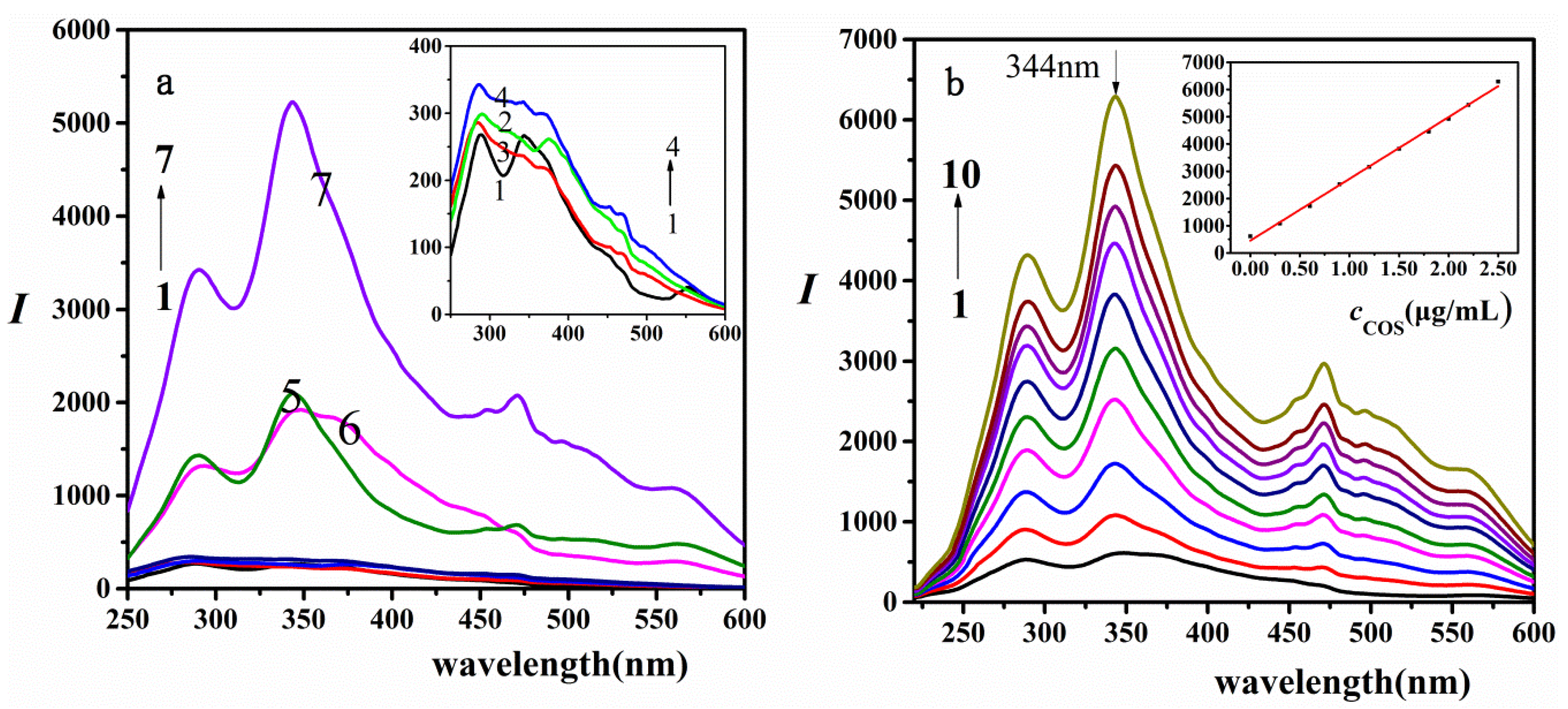
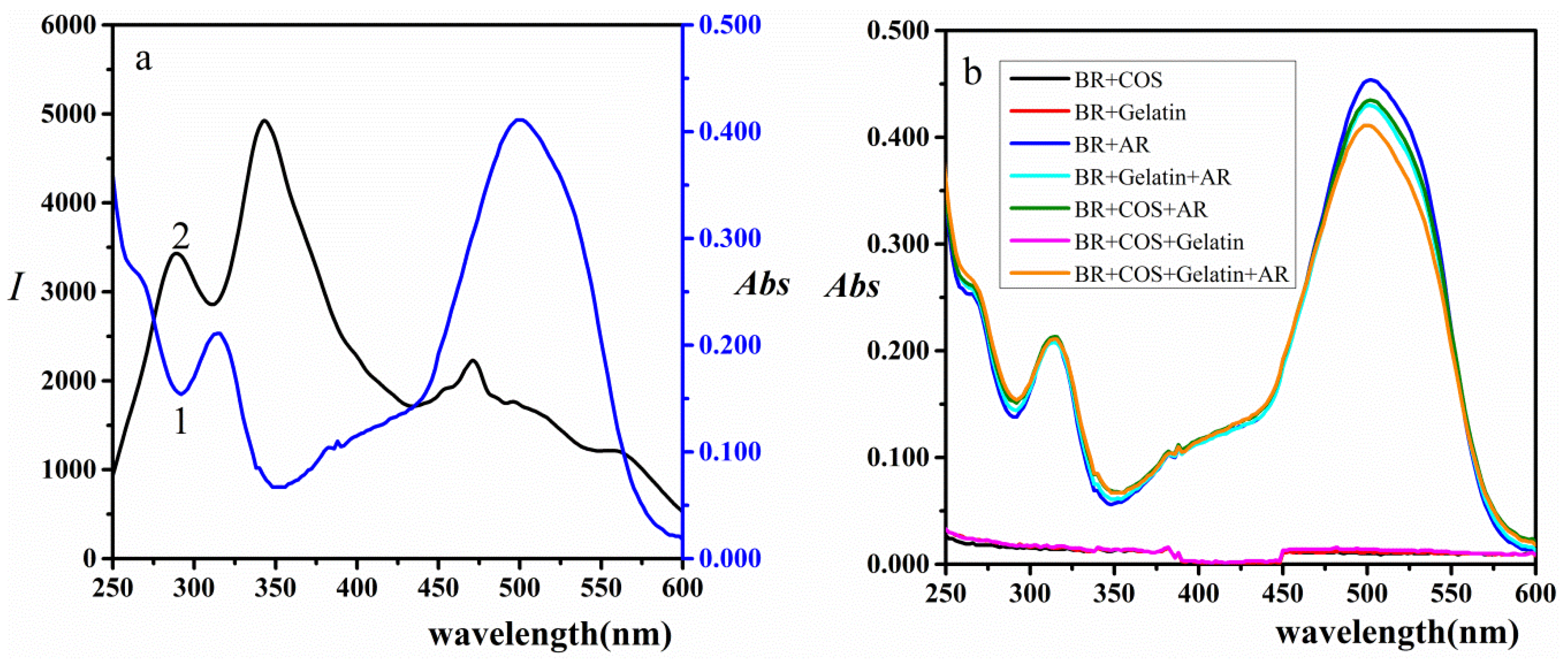
2.1. RRS Characteristics
2.2. Optimization of the Experimental Conditions
2.2.1. Effects of Acidity
2.2.2. Effect of the Concentration of AR
2.2.3. Effects of Gelatin
2.2.4. Effects of Temperature and Interaction Time
2.2.5. Effects of Sequence and Standing Time
2.3. Orthogonal Experimental Design Optimization
2.4. Effects of Ionic Strength
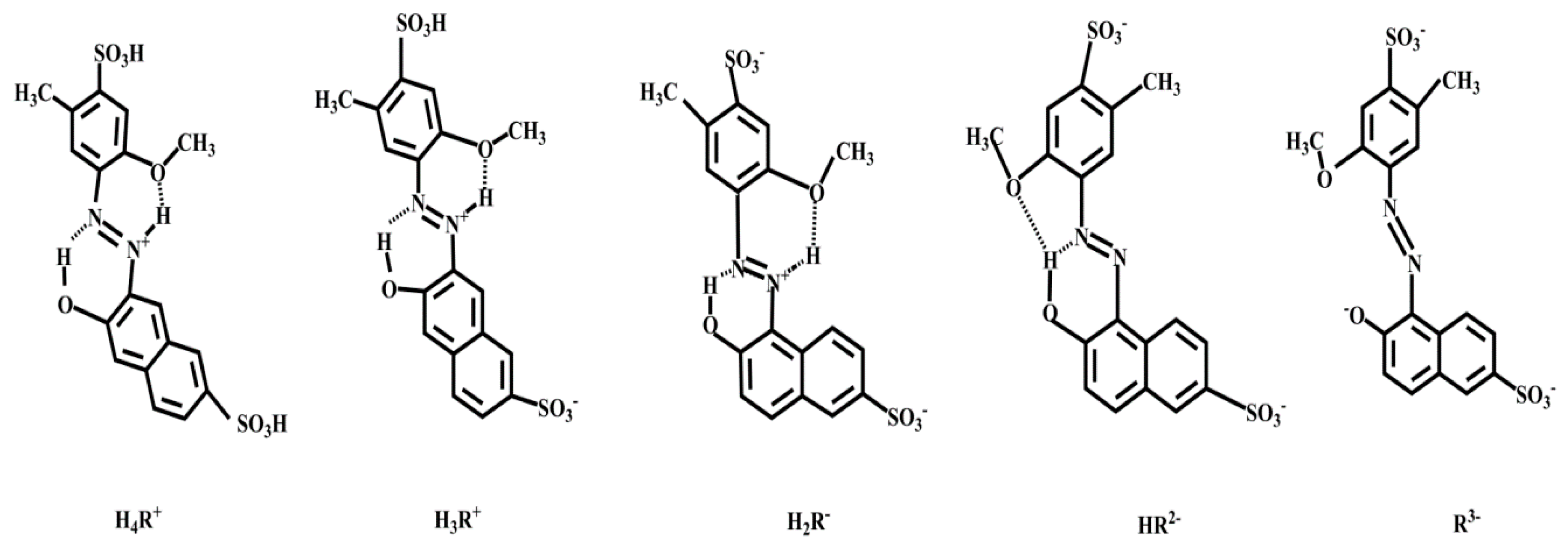
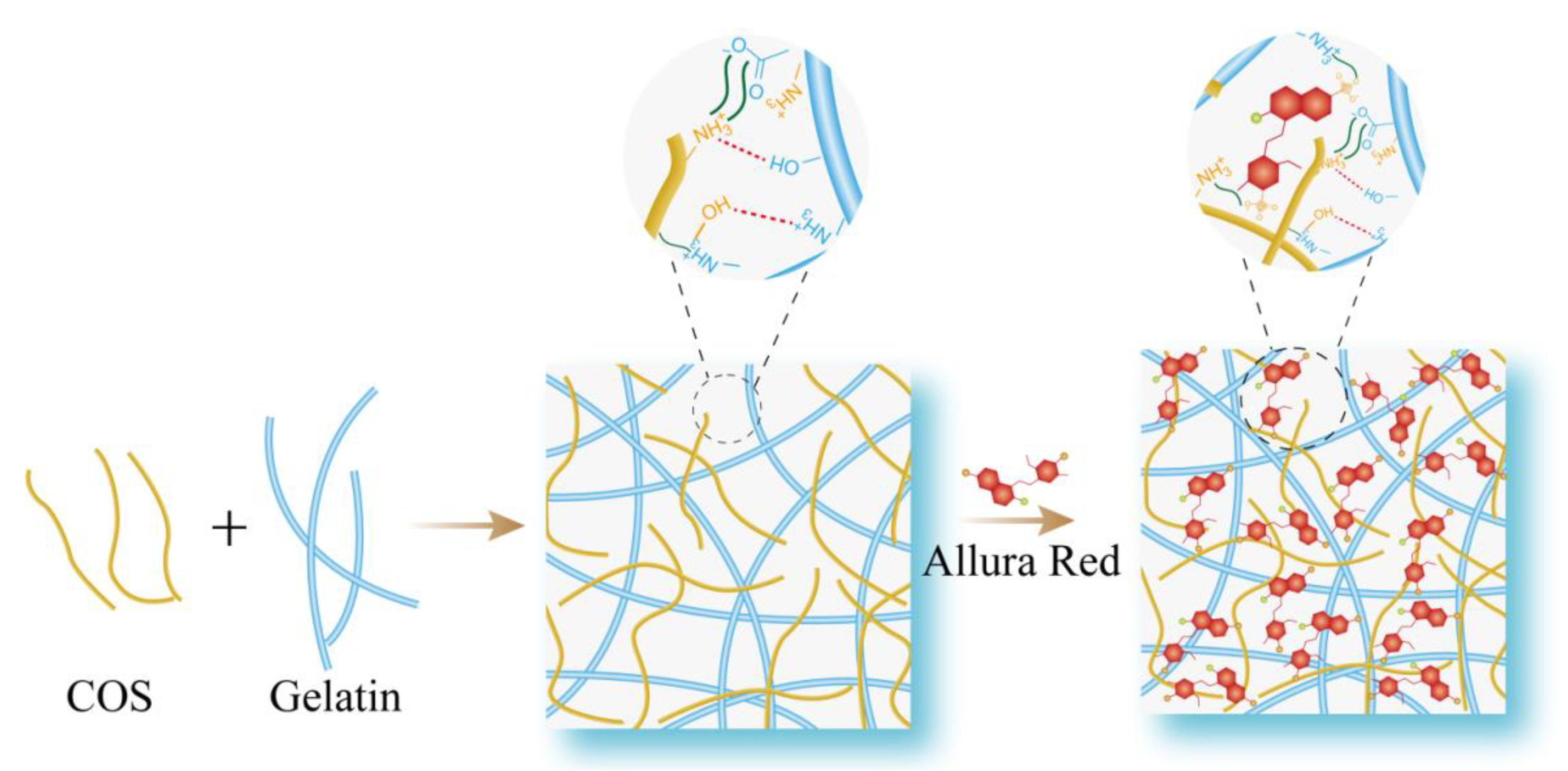
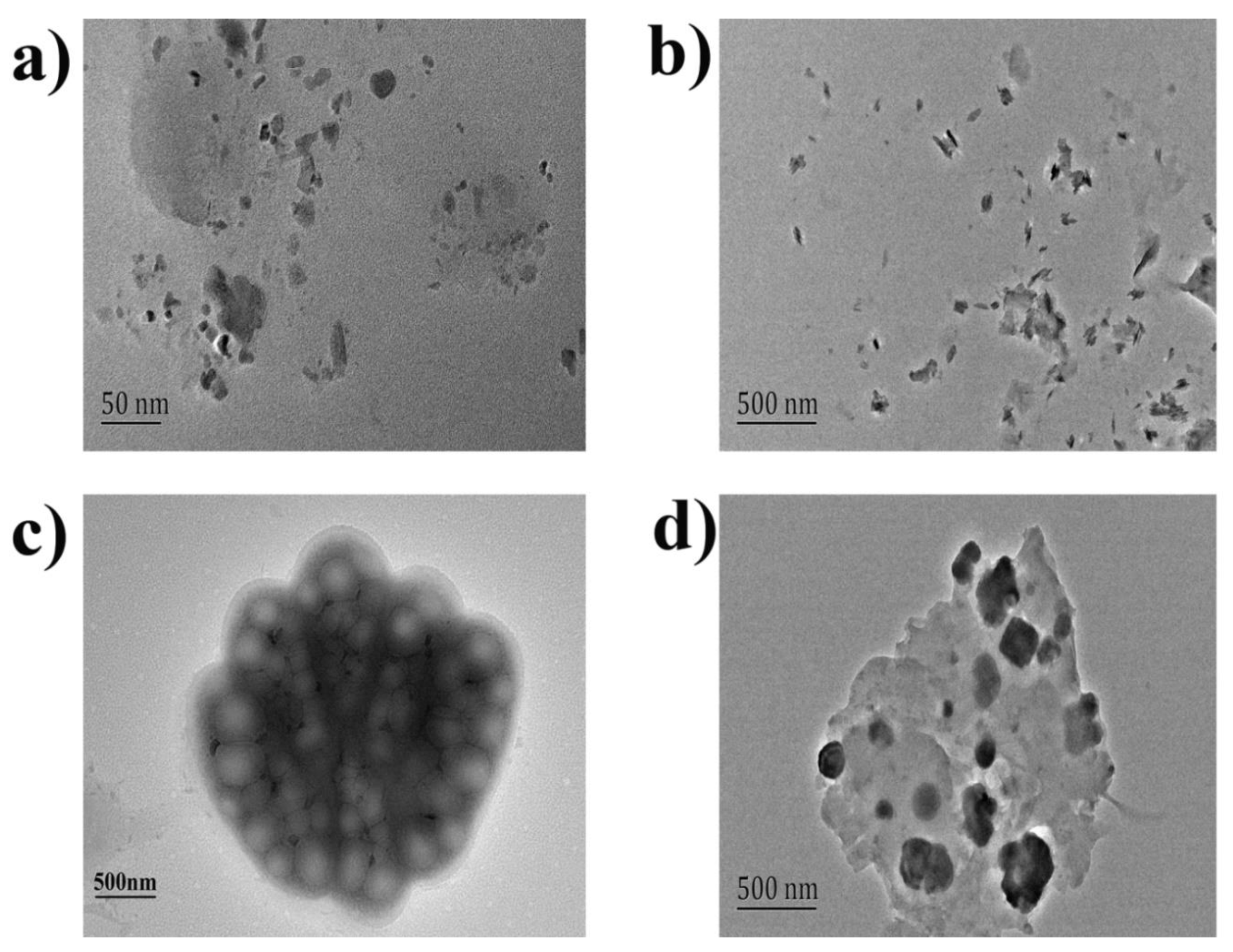
3. Mechanism for Molecular Interaction and Reasons for RRS Enhancement
3.1. Mechanism
3.2. The Reasons for RRS Enhancement
3.2.1. Increased Rigid Plane
3.2.2. Scattering–Absorbing–Rescattering
3.2.3. Enhancement of Hydrophobicity
4. Selectively and Analytical Applications
4.1. Selectively
4.2. Linear Range and Detection Limit
4.3. Application
4.3.1. Sample Pretreatment
4.3.2. Detection of the Samples
5. Materials and Procedure
5.1. Material and Reagents
5.2. Procedure
6. Conclusions
7. Highlights
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zou:, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef]
- Lee, J.Y.; Termsarasab, U.; Lee, M.Y.; Kim, D.H.; Kim, J.S.; Cho, H.J.; Kim, D.D. Chemosensitizing indomethacin-conjugated chitosan oligosaccharide nanoparticles for tumor-targeted drug delivery. Acta Biomater. 2017, 57, 262–273. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Husain, T.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xing, R.; Liu, S.; Qin, Y.; Li, B.; Wang, X.; Li, P. Separation and scavenging superoxide radical activity of chitooligomers with degree of polymerization 6-16. Int. J. Biol. Macromol. 2012, 51, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y.I. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Yuan, S.; Yang, X.; Zou, P.; Li, L.; Li, G.; Shao, Y.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Wang, J. Chitosan Oligosaccharides Induce Apoptosis in Human Renal Carcinoma via Reactive-Oxygen-Species-Dependent Endoplasmic Reticulum Stress. J. Agric. Food Chem. 2019, 67, 1691–1701. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransfor. 2017, 36, 57–67. [Google Scholar] [CrossRef]
- Huang, B.; Xiao, D.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, J.; Huang, R.; Yang, C.; Yin, Y. Chitosan Oligosaccharide Reduces Intestinal Inflammation That Involves Calcium-Sensing Receptor (CaSR) Activation in Lipopolysaccharide (LPS)-Challenged Piglets. J. Agric. Food Chem. 2016, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Jimtaisong, A.; Saewan, N. Utilization of carboxymethyl chitosan in cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21. [Google Scholar] [CrossRef]
- Swiatkiewicz, S.; Swiatkiewicz, M.; Arczewska-Wlosek, A.; Jozefiak, D. Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core-Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(lactic- co-glycolic acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Huang, X. Study on determination of total chitooligosaccharides by Imoto olorimetry. Sci. Technol. Food Ind. 2012, 33, 71–77. [Google Scholar]
- Hu Zuyan, R.S.; Lu, Y. Determination of the Content of Chitosan Oligosaccharide in Moringa Leaves Chitosan Tea. Mod. Food 2017, 21, 90–95. [Google Scholar]
- Cao, L.; Wu, J.; Li, X.; Zheng, L.; Wu, M.; Liu, P.; Huang, Q. Validated Hpaec-Pad method for the determination of fully deacetylated chitooligosaccharides. Int. J. Mol. Sci. 2016, 17, 1699. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shon, D.H.; Lee, K.H. Enzyme-linked immunosorbent assay for detection of chitooligosaccharides. Biosci. Bitechnol. Biochem. 2000, 64, 696–701. [Google Scholar] [CrossRef]
- Hattori, T.; Anraku, N.; Kato, R. Capillary electrophoresis of chitooligosaccharides in acidic solution: Simple determination using a quaternary-ammonium-modified column and indirect photometric detection with crystal violet. J. Chromatogr. B 2010, 878, 477–480. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Q.; Liu, S.; Yang, J.; Teng, P.; Zhu, J.; Qiao, M.; Shi, Y.; Duan, R.; Hu, X. Study on erythrosine-phen-Cd(II) systems by resonance Rayleigh scattering, absorption spectra and their analytical applications. Spectrochim. Acta A 2015, 140, 15–20. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Su, Z.; Bai, Y. Resonance Rayleigh scattering method for the determination of chitosan using erythrosine B as a probe and PVA as sensitization. Food Chem. 2018, 239, 126–131. [Google Scholar] [CrossRef]
- Luo, Y.; He, L.; Zhan, S.; Wu, Y.; Liu, L.; Zhi, W.; Zhou, P. Ultrasensitive resonance scattering (RS) spectral detection for trace tetracycline in milk using aptamer-coated nanogold (ACNG) as a catalyst. J. Agric. Food Chem. 2014, 62, 1032–1037. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. Hydrogel based aptasensor for thrombin sensing by Resonance Rayleigh Scattering. Anal. Chim. Acta 2019, 1079, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, F.; Liu, Z.; Hu, X.; Yi, A.; Duan, H. Resonance Rayleigh scattering spectra for studying the interaction of anthracycline antineoplastic antibiotics with some anionic surfactants and their analytical applications. Anal. Chim. Acta 2007, 601, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, Y.; Fan, Y.Z.; Dong, J.X.; Li, N.B.; Luo, H.Q. A resonance Rayleigh scattering sensor for detection of Pb(2+) ions via cleavage-induced G-wire formation. J. Hazard. Mater. 2017, 336, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.H.; Pi, J.; Lin, X.; Li, B.; Li, A.; Yang, P.H.; Cai, J. Gold nanoprobes-based resonance Rayleigh scattering assay platform: Sensitive cytosensing of breast cancer cells and facile monitoring of folate receptor expression. Biosens. Bioelectron. 2015, 74, 165–169. [Google Scholar] [CrossRef]
- Liang, A.; Peng, J.; Liu, Q.; Wen, G.; Lu, Z.; Jiang, Z. Highly sensitive and selective determination of fluorine ion by graphene oxide/nanogold resonance Rayleigh scattering-energy transfer analytical platform. Food Chem. 2015, 181, 38–42. [Google Scholar] [CrossRef]
- Liang, A.; Wang, Y.; Wen, G.; Zhang, X.; Luo, Y.; Jiang, Z. A silver nanorod resonance rayleigh scattering-energy transfer analytical platform for trace tea polyphenols. Food Chem. 2016, 197, 395–399. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Z.; Liu, G.; Su, Z.; Bai, Y. Study on Brilliant Blue-chitosan System by Dual-wavelength Overlapping Resonance Rayleigh Scattering Method and its Analytical Applications. Spectrochim. Acta Part A 2018, 191, 463–468. [Google Scholar] [CrossRef]
- Tan, X.; Yang, J.; Li, Q.; Yang, Q.; Shen, Y. Double-wavelength overlapping resonance Rayleigh scattering technique for the simultaneous quantitative analysis of three beta-adrenergic blockade. Spectrochim. Acta Part A 2016, 161, 19–26. [Google Scholar] [CrossRef]
- Hao, X.L.; Li, N.B.; Luo, H.Q. Determination of dextran sulfate sodium with crystal violet by triple-wavelength overlapping resonance Rayleigh scattering. Spectrochim. Acta Part A 2009, 71, 1673–1677. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Liu, S.; Liu, Z.; Yang, J.; Zhu, J.; Qiao, M.; Duan, R.; Hu, X. A novel method for detecting allura red based on triple-wavelength overlapping resonance Rayleigh scattering. RSC Adv. 2014, 4, 37100–37106. [Google Scholar] [CrossRef]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Esteghlal, S.; Niakousari, M.; Hosseini, S.M.H. Physical and mechanical properties of gelatin-CMC composite films under the influence of electrostatic interactions. Int. J. Biol. Macromol. 2018, 114, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, C.; Pei, Y.; Liu, X.; Tang, K. Preparation and properties of microfibrillated chitin/gelatin composites. Int. J. Biol. Macromol. 2019, 130, 715–719. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R. Comparative study on the behaviour of Chitosan-Gelatin based Hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohyd. Polym. 2019, 207, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Wongputtaraksa, T.; Ratanavaraporn, J.; Pichyangkura, R.; Damrongsakkul, S. Surface modification of Thai silk fibroin scaffolds with gelatin and chitooligosaccharide for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. B 2012, 100, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Bevziuk, K.; Chebotarev, A.; Snigur, D.; Bazel, Y.; Fizer, M.; Sidey, V. Spectrophotometric and theoretical studies of the protonation of Allura Red AC and Ponceau 4R. J. Mol. Struct. 2017, 1144, 216–224. [Google Scholar] [CrossRef]
- Derkatch, S.R.; Kolotova, D.S.; Milyaeva, O.Y.; Noskov, B.A. Dynamic properties of gelatin/surfactant adsorption layers. Colloids Surf. A 2016, 508, 251–256. [Google Scholar] [CrossRef]
- Mengyang Chai, Y.X.; Lai, S.; Chen, F. The research methods of the molecular mtructure ofgelatin and its development. Food Ind. 2017, 38, 253–257. [Google Scholar]
- Liu, J.F.; Li, N.B.; Luo, H.Q. Resonance Rayleigh scattering, second-order scattering and frequency doubling scattering spectra for studying the interaction of erythrosine with Fe(phen)3(2+) and its analytical application. Spectrochim. Acta Part A 2011, 79, 631–637. [Google Scholar] [CrossRef]
- Voron’ko, N.G.; Derkach, S.R.; Kuchina, Y.A.; Sokolan, N.I. The chitosan–gelatin (bio)polyelectrolyte complexes formation in an acidic medium. Carbohydr. Polym. 2016, 138, 265–272. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Rao, C.M. Structural studies on aqueous gelatin solutions: Implications in designing a thermo-responsive nanoparticulate formulation. Int. J. Biol. Macromol. 2017, 95, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qi, P.; Ju, J.; Wang, Q.; Hao, L.; Wang, R.; Sui, K.; Tan, Y. Gelatin/alginate composite nanofiber membranes for effective and even adsorption of cationic dyes. Compos. Part B Eng. 2019, 162, 671–677. [Google Scholar] [CrossRef]
- Lu, W.; Shang, J. A resonance light-scattering (RLS) serving for various quantitative events since 1995: A comment proposed towards how to apprehend well the meaning of RLS and its corresponding guiding role. Spectrochim. Acta Part A 2009, 74, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, M.I.; Dlugach, J.M. Scattering and extinction by spherical particles immersed in an absorbing host medium. J. Quant. Spectrosc. Radiat. 2018, 211, 179–187. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition—Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-enhanced fluorescence and plasmon emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef]
| Method | Compound | Linear Range | Limit of Detection (LOD) | References |
|---|---|---|---|---|
| Spectrophotometry | 0–60 μg/mL | [13] | ||
| HPLC | 9.13–91.3 μg/mL | [14] | ||
| ion chromatography | (GlcN)1 a | 0.2–11.5 mg/L | 3 mg/mL | [15] |
| (GlcN)2 | 0.2–9.8 mg/L | 8 mg/mL | ||
| (GlcN)3 | 0.2–10.1 mg/L | 8 mg/mL | ||
| (GlcN)4 | 0.2–10.6 mg/L | 11 mg/mL | ||
| (GlcN)5 | 0.2–10.6 mg/L | 13 mg/mL | ||
| (GlcN)6 | 0.2–9.9 mg/L | 16 mg/mL | ||
| ELISA | [16] | |||
| capillary electrophoresis | (GlcN)2 | 2 μmol/L | [17] | |
| (GlcN)3 | 2 μmol/L | |||
| (GlcN)4 | 2 μmol/L | |||
| (GlcN)5 | 3 μmol/L | |||
| (GlcN)6 | 5 μmol/L |
| Controlling Factor | A | B | C | D |
|---|---|---|---|---|
| Lever 1 | 269 | 2583 | 2395 | 1139 |
| Lever 2 | 2228 | 2303 | 1897 | 1985 |
| Lever 3 | 3658 | 1270 | 1863 | 3032 |
| difference | 3389 | 1313 | 532 | 1893 |
| Order of difference | 1 | 3 | 4 | 2 |
| Factor | DF | SS | MS | *F | p |
|---|---|---|---|---|---|
| Total | 8 | 26,163,949 | |||
| A | 2 | 17,367,881 | 8,683,941 | 3.219 | >0.05 |
| B | 2 | 2,867,371 | 1,433,685 | 0.531 | >0.05 |
| C | 2 | 533,159 | 266,579 | 0.099 | >0.05 |
| Residual error | 2 | 5,395,539 | 2,697,769 |
| Coexistent | Tolerance (mol/L) | Relative (%) | Coexistent | Tolerance (mol/L) | Relative (%) |
|---|---|---|---|---|---|
| Oxalic acid | 1.98 × 10−4 | −4.5 | Starch | 40.0 µg/mL | 4.28 |
| Citric acid | 4.86 × 10−4 | −4.66 | Thiourea | 4.00 × 10−3 | −0.34 |
| EDTA | 2.00 × 10−4 | −5.61 | MgSO4 | 1.00 × 10−4 | −2.5 |
| Ascorbic acid | 4.54 × 10−3 | 3.31 | NH4Cl | 3.71 × 10−5 | 3.11 |
| Glycine | 5.33 × 10−3 | 3.06 | ZnSO4 | 3.48 × 10−5 | −3.63 |
| L-lysine | 6.84 × 10−4 | 2.03 | FeCl3 | 1.79 × 10−5 | −4.3 |
| L-leucine | 1.91 × 10−3 | −3.32 | AlCl3 | 3.71 × 10−5 | 3.11 |
| L-aspartic acid | 5.26 × 10−4 | −4.78 | CaCl2 | 2.50 × 10−5 | 4.24 |
| Glucosamine | 3.60 µg/mL | 4.24 | HgNO3 | 1.50 × 10−6 | −3.18 |
| Glucose | 5.55 × 10−4 | 1.11 | KOH | 5.12 × 10−5 | −4.37 |
| Method | λ (nm) | Regression Equation (μg/mL) | Linear Range (μg/mL) | R2 | LOD (μg/mL) |
|---|---|---|---|---|---|
| RRS | 288 | ΔI = 1505.4c − 43.967 | 0.30–2.50 | 0.9985 | 0.087 |
| RRS | 344 | ΔI = 2318.1c − 245.39 | 0.30–2.50 | 0.9985 | 0.089 |
| RRS | 471 | ΔI = 1109.6c − 148.19 | 0.30–2.50 | 0.9935 | 0.095 |
| TWO-RRS | 288 + 344 + 471 | ΔI = 4933.2c − 446.21 | 0.30–2.50 | 0.9980 | 0.0478 |
| Simple | Found (μg/mL) | Added (μg/mL) | Total Found (μg/mL) | RSD (%, n = 9) | Recovery (%, n = 9) |
|---|---|---|---|---|---|
| Keer | 0.65 | 0.80 | 1.45 | 2.89 | 100.3 |
| 1.10 | 1.75 | 1.59 | 100.7 | ||
| Longma | 0.78 | 0.80 | 1.56 | 2.59 | 97.3 |
| 1.00 | 1.78 | 3.00 | 100.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Sun, Z.; Su, Z.; Bai, Y. Application of Gelatin Decorated with Allura Red as Resonance Rayleigh Scattering Sensor to Detect Chito-Oligosaccharides. Mar. Drugs 2020, 18, 146. https://doi.org/10.3390/md18030146
Zou W, Sun Z, Su Z, Bai Y. Application of Gelatin Decorated with Allura Red as Resonance Rayleigh Scattering Sensor to Detect Chito-Oligosaccharides. Marine Drugs. 2020; 18(3):146. https://doi.org/10.3390/md18030146
Chicago/Turabian StyleZou, Weiling, Zijun Sun, Zhengquan Su, and Yan Bai. 2020. "Application of Gelatin Decorated with Allura Red as Resonance Rayleigh Scattering Sensor to Detect Chito-Oligosaccharides" Marine Drugs 18, no. 3: 146. https://doi.org/10.3390/md18030146
APA StyleZou, W., Sun, Z., Su, Z., & Bai, Y. (2020). Application of Gelatin Decorated with Allura Red as Resonance Rayleigh Scattering Sensor to Detect Chito-Oligosaccharides. Marine Drugs, 18(3), 146. https://doi.org/10.3390/md18030146





