Advances in Studies on the Pharmacological Activities of Fucoxanthin
Abstract
:1. Introduction
2. Different Sources of Fucoxanthin and Stability Improvement
2.1. Source of Fucoxanthin
2.2. Improvement of the Stability of Fucoxanthin
2.2.1. Use of Emulsifier
2.2.2. Encapsulation of Fucoxanthin
3. Pharmacological Activities
3.1. Anticancer Activity
3.1.1. Anti-Liver Cancer Activity
3.1.2. Anti-Gastric Cancer Activity
3.1.3. Anti-Leukemia Activity
3.1.4. Anti-Breast Cancer Activity
3.1.5. Glioma
3.1.6. Anti-Colon Cancer Activity
3.1.7. Anti-Cervical Cancer Activity
3.1.8. Other Anticancer Activities
3.2. Anti-Obesity Activity and Its Mechanism
3.2.1. Regulation of UCP1
3.2.2. Inhibition of α-amylase and α-glucosidase
3.3. Effect on the Intestinal Flora
3.4. Reduction in Oxidative Stress and Its Mechanism
3.5. Anti-Fibrotic Activity of Fucoxanthin
3.6. Fucoxanthin Anti-Inflammatory Activity
3.6.1. Inflammation Caused by Lipopolysaccharide
3.6.2. Anti-Dermatitis Activity
3.7. Other Activities
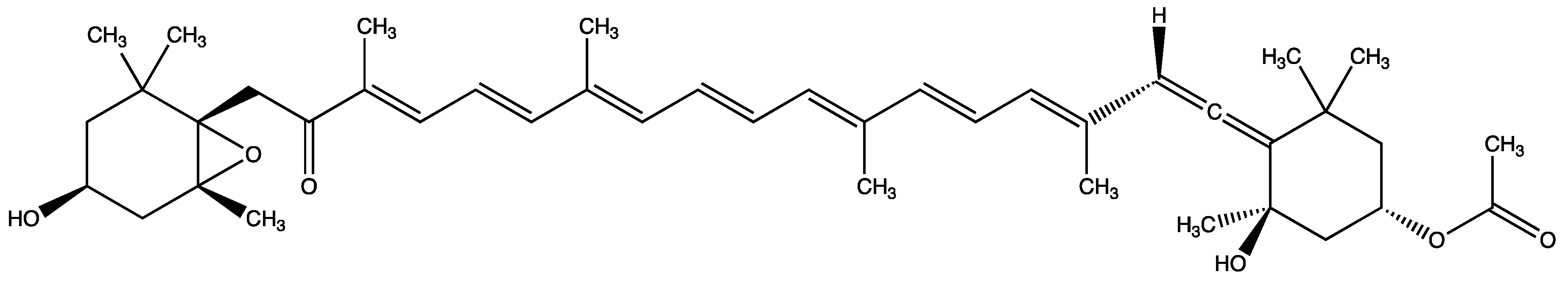
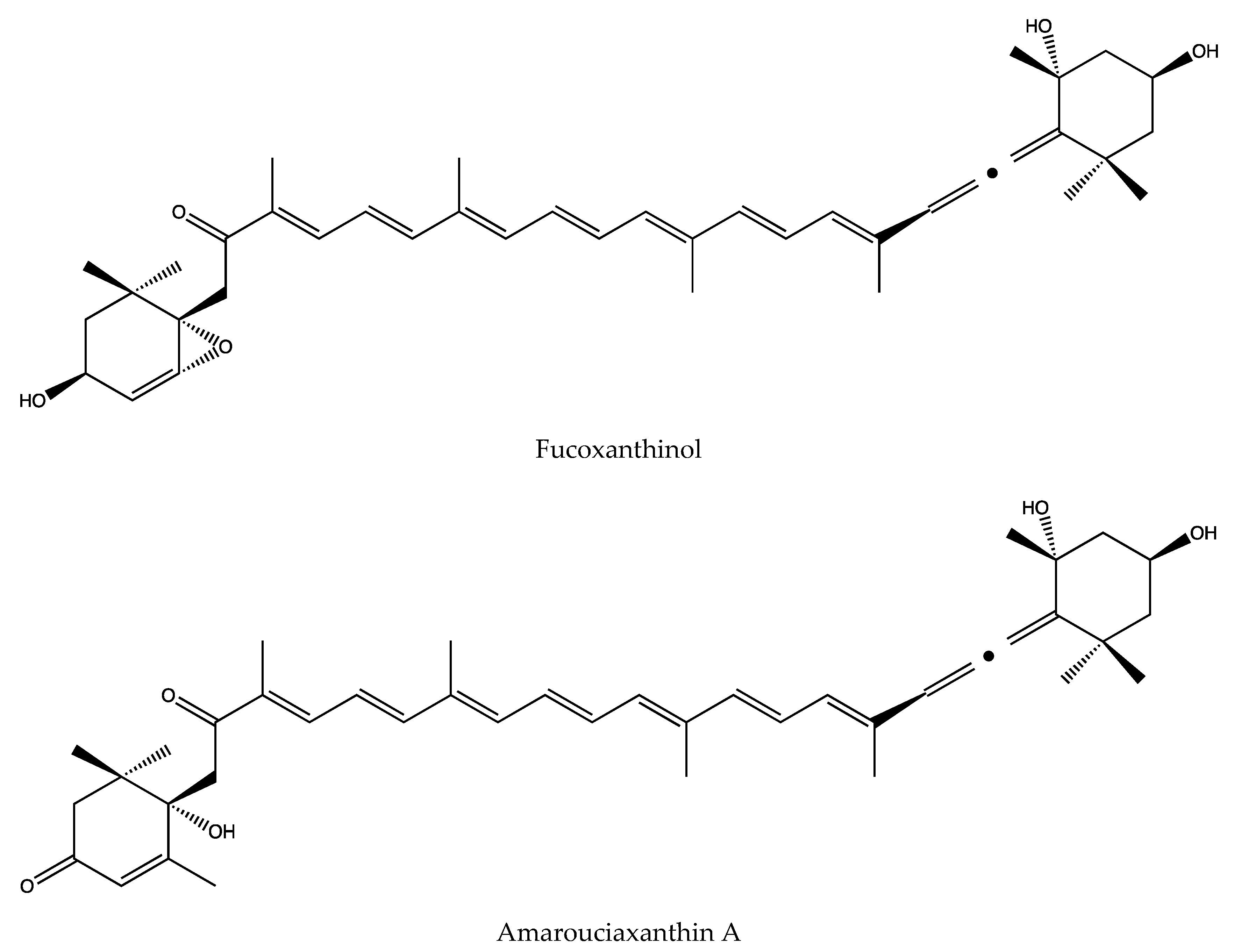
3.8. Main Metabolites and Their Activities of Fucoxanthin
| Mechanism | Source | Experimental Application | Reference |
|---|---|---|---|
| Purchased from BGG Japan 11 Nichiyu 10 Mitsubishi Chemical Food | Emulsified powder containing 1.1% (W/W) fucoxanthin | [12] | |
| Purchased from BGG-Japan Co., Ltd. (Tokyo, Japan) | Investigated the formulation and stability characteristics of monodisperse oil-in-water (O/W) emulsions encapsulating fucoxanthin | [13] | |
| Purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China) | To prepare the complex of whey protein and monomer fucoxanthin | [14] | |
| Extract from dry wakame from Shandong Jiayi Aquatic Food Co. LTD | Preparation of fucoxanthin nanocomposite | [15] | |
| Enhanced fucoxanthin stability | Extract from Sphagnum angustifolium, purchased from Algae Resource Development Technology Company (Shiraz, Iran) | Encapsulated fucoxanthin with PS, HNT | [16] |
| Purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA) | Encapsulate fucoxanthin (FX) | [17] | |
| Purchased from YIGEDA Bio-Tech Co., Ltd. (Beijing, China) | To determine the activity of fucoxanthin in gastric acid | [18] | |
| Extracted from dried Undaria pinnatifida was supplied by Jiayi Aquatic Products Co. Ltd. (Shandong, China) | In order to spray-dry the fucoxanthin coating | [19] | |
| Purchased from Wako Pure Chemical Co., Ltd. (Japan) | It was given intraperitoneally to the rats | [23] | |
| Extracted from the Chaetoceros calcitrans | Deal with HepG2 cancer cell | [24] | |
| Extracted from the brown sea algae Lonicera aponica (kombu) | Deal with HepG2 cancer cell | [25] | |
| Extracted from the brown sea algae Undaria pinnatifida (Wakame), provided by the company of Wuhan Heli | Deal with human gastric adenocarcinoma MGC-803 cells | [28] | |
| Extracted from the Japanese brown algae Undaria pinnatifida, supplied by Nippon Seisakusho Co. Ltd. (Beijing, China) | Deal with human gastric cancer SGC-7901cells | [29] | |
| Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with human leukemia cell lines, K562 and TK6 | [30] | |
| It did not publicly identify the source | Deal with human promyelocytic leukemia cells | [31] | |
| Antitumor | Extracted from the freeze-dried powder of marine alga, Undaria pinnatifida and Codium fragile, respectively | Deal with human umbilical vein endothelial cells (HUVECs) | [32] |
| Extracted from Undaria. pinnatifida | Deal with lymphatic endothelial cells (LEC) | [33] | |
| Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with human MCF-7 breast cancer cells | [34] | |
| Purchased from Wako Chemicals (Richmond, VA, USA) | Deal with human breast cancer line MCF-7 and MDA-MB-231 | [35] | |
| Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with human leukemia cancer cell line U937 and K562 | [37] | |
| Purchased from Sigma Chemical Company (St. Louis, MO) | Deal with human glioma cancer cell line U87 and U251 | [38] | |
| Extract from the edible seaweed Undaria pinnatifida | Deal with human colon cancer cell lines, Caco-2, HT-29, and DLD-1 | [42] | |
| Purchased from the Oryza Oil & Fat Chemical Co. Ltd. (Aichi, Japan) and Dr. Hayato Maeda (Hirosaki University, Japan) | Deal with azoxymethane-dextrane sodium sulfate (AOM/DSS) carcinogenic model mice | [43] | |
| Extract from the chloroplasts of brown sea-weeds | Deal with human cervical cancer cell lines HeLa, SiHa, and CaSki | [45] | |
| Purchased from Sigma- Aldrich (St. Louis, MO) | Deal with Human NPC cell line C666-1 | [47] | |
| Extract from Laminaria japonica | Deal with human non-small lung cancer cell | [48] | |
| Extract from Undaria pinnatifida | Deal with A549, DLD-1, H1299, MCF7, MDA-MB-231, MRC5, SKOV3, TIG-3, and U2OS cell lines | [49] | |
| Extract from dried powder of seaweed (Undaria pinnatifida) after removing carbohydrate and protein was obtained from Riken Vitamin (Tokyo, Japan) | Fed to Male Wistar rats and female KK-Ay mice | [53] | |
| Extract from the Phaeodactylum tricornutum strain UTEX 640 (SAG 1090-1b),which was obtained from the culture collection of Algae (SAG) from the University of Goettingen (Germany) | Fed to C57BL/6J mice | [54] | |
| Anti-Obesity Effect | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with FL83B cell line | [55] |
| Purchased from Sigma–Aldrich (St. Louis, MO, USA) | Deal with 3T3-L1 preadipocyte cell | [56] | |
| Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with 3T3-L1 preadipocyte cell | [57] | |
| Purchased from Beijing HFK Bioscience Co., 90 Ltd. | Fed to BALB/c mice | [59] | |
| Dried Undaria pinnatifida was supplied by Jiayi Aquatic Products Co. Ltd. (Shandong, China) | Fed to C57BL/6J mice | [60] | |
| Effect on the intestinal flora | Extract from Undaria pinnatifida | Fed to Escherichia coli and lactobacilli | [61] |
| Extract from Diatoma anceps (wet) at the Punta Plaza location—Antarctic Continent | Deal with 3T3 mouse fibroblast | [64] | |
| Reduction in oxidative stress | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with raw 264.7 cell | [65] |
| Purchased from Shandong Jiejing Group Corporation (Rizhao, Shandong, China) | Fed to Male ICR mice | [67] | |
| Purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) | Deal with the human keratinocyte cell line HaCaT | [71] | |
| Purchased from Sigma (St. Louis, MO) | Deal with LX-2 cells | [73] | |
| Anti-fibroti | Purchased from Biopurify (Chengdu, China) | Deal with Glomerular mesangial cells (GMCs) | [79] |
| Extract from dried Chaetoceros calcitrans | Deal with the human liver cancer cells (HepG2) | [85] | |
| Extract from Conticribra weissflogii ND-8 | Deal with C57BL/6 mice and RAW 264.7 cells | [86] | |
| Anti-inflammatory | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with Raw 264.7 cell | [88] |
| Purchase from HiQ Marine Bio- tech Company in Taiwan | Deal with 70 patients who visited the outpatient department of Gastroenterology and Hepatology, with ages ranging from 20 to 75 years old | [94] | |
| Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with human immortalized keratinocytes HaCaT | [95] | |
| Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with the THP-1 human monocytic leukemia cell line and HaCaT human keratinocytes, and fed with female Swiss CD-1 mice | [96] | |
| Anti-dermatitis | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Fed to male SD rats | [97] |
| Purchased from Phytolox (Okinawa, Japan) | Deal with WEHI-3 cells and fed to male Nc/Nga mice | [98] | |
| Extract from Undaria pinnatifida | Deal with Transfected Chinese hamster ovary (CHO) cells, rat basophil leukemia (RBL) cells, U373 cells, and BA/F3 cells | [100] | |
| Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Deal with Rat pheochromocytoma cells PC-12 | [101] | |
| Other activities | Extract from Laminaria japonica and purchase from Hi-Q Marine Biotech International Ltd. (New Taipei City, Taiwan) | Fed to male Sprague Dawley (SD) rats | [102] |
| Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Fed to Male ICR mice | [103] |
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Liu, M.; Li, W.; Chen, Y.; Wan, X.-Y.; Wang, J. Fucoxanthin: A promising compound for human inflammation -related diseases. Life Sci. 2020, 255. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.N.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schäfer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzal, S.; Garg, S.; Ishida, Y.; Terao, K.; Kaul, S.C.; Wadhwa, R. Rat Glioma Cell-Based Functional Characterization of Anti-Stress and Protein Deaggregation Activities in the Marine Carotenoids, Astaxanthin and Fucoxanthin. Mar. Drugs 2019, 17, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrushkina, M.; Gusev, E.; Sorokin, B.; Zotko, N.; Mamaeva, A.; Filimonova, A.; Kulikovskiy, M.; Maltsev, Y.; Yampolsky, I.; Guglya, E.; et al. Fucoxanthin production by heterokont microalgae. Algal Res. 2017, 24, 387–393. [Google Scholar] [CrossRef]
- Kanazawa, K.; Ozaki, Y.; Hashimoto, T.; Das, S.K.; Matsushita, S.; Hirano, M.; Okada, T.; Komoto, A.; Mori, N.; Nakatsuka, M. Commercial-scale Preparation of Biofunctional Fucoxanthin from Waste Parts of Brown Sea Algae Laminalia japonica. Food Sci. Technol. Res. 2008, 14, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Cabanelas, I.I.T.; Wijffels, R.H.; Barbosa, M.J. Barbosa. Process optimization of fucoxanthin production with Tisochrysis lutea. Bioresour. Technol. 2020, 315. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Purification and Formulation of Xanthophyll for Pharmaceutical Use: Current Strategies and Future Trends. ChembioEng Rev. 2015, 2, 393–405. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C.-W. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a Major Carotenoid in Isochrysis aff. galbana: Characterization of Extraction for Commercial Application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Ma, Z.; Khalid, N.; Shu, G.; Zhao, Y.; Kobayashi, I.; Neves, M.A.; Tuwo, A.; Nakajima, M. Fucoxanthin-Loaded Oil-in-Water Emulsion-Based Delivery Systems: Effects of Natural Emulsifiers on the Formulation, Stability, and Bioaccessibility. ACS Omega 2019, 4, 10502–10509. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Nebashi, N.; Muromachi, A.; Nakano, Y.; Ito, Y.; Nagasawa, T. Emulsified Fucoxanthin Increases Stability and Absorption in Rats. J. Jpn. Soc. Food Sci. 2018, 65, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Zhao, Y.; Khalid, N.; Shu, G.; Neves, M.A.; Kobayashi, I.; Nakajima, M. Comparative study of oil-in-water emulsions encapsulating fucoxanthin formulated by microchannel emulsification and high-pressure homogenization. Food Hydrocolloid 2020, 108. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C.; Gao, J.; Wu, H.; Sun, Q. Aggregation of Fucoxanthin and Its Effects on Binding and Delivery Properties of Whey Proteins. J. Agr. Food Chem. 2019, 67, 10412–10422. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, H.; Zhao, L.; Xu, Y.; Wang, J. Preparation and Antioxidative, Antitumor Activity of Fucoflavin Nanocomposite; Oceans and Lakes: Ixelles, Belgium, 2019; Volume 50, pp. 77–84. [Google Scholar]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.-M.; Fazaeli, M. Encapsulation of fucoxanthin in binary matrices of porous starch and halloysite. Food Hydrocolloid 2020, 100. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.; Wang, J.; Zhu, J.; Wang, D.; Zhao, L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocolloid 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Li, Y.; Dou, X.; Pang, J.; Liang, M.; Feng, C.; Kong, M.; Liu, Y.; Cheng, X.; Wang, Y.; Chen, X. Improvement of fucoxanthin oral efficacy via vehicles based on gum Arabic, gelatin and alginate hydrogel Delivery system for oral efficacy enhancement of functional food ingredients. J. Funct. Foods 2019, 63. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RSC Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.; Flórez-Fernández, N.; Simón-Vázquez, R.; Giménez-Abián, J.; Díaz, J.; González-Fernández, Á.; Domínguez, H. Fucoidans: The importance of processing on their anti-tumoral properties. Algal Res. 2020, 45. [Google Scholar] [CrossRef]
- Banakar, M.C.; Paramasivan, S.K.; Chattopadhyay, M.B.; Datta, S.; Chakraborty, P.; Chatterjee, M.; Kannan, K.; Thygarajan, E. 1 alpha, 25-dihydroxyvitamin D-3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J. Gastroenterol. 2004, 10, 1268–1275. [Google Scholar] [CrossRef]
- De Zwart, L.L.; Meerman, J.H.; Commandeur, J.N.; Vermeulen, N.P. Biomarkers of free radical damage applications in experimental animals and in humans. Free. Radic. Biol. Med. 1999, 26, 202–226. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, T.; Shi, D.; Ye, M.B.; Yi, Q. Protective role of fucoxanthin in diethylnitrosamine-induced hepatocarcinogenesis in experimental adult rats. Drug Dev. Res. 2019, 80, 209–217. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Imam, M.U.; Foo, J.B.; Ismail, N.; Azmi, N.H.; Tor, Y.S.; Khong, N.M.; Ismail, M. Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways. Biotechnol. Rep. 2019, 21, e00296. [Google Scholar] [CrossRef]
- Das, S.K.; Hashimoto, T.; Kanazawa, K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. BBA Gen. Subj. 2008, 1780, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Shen, J. Anti-apoptotic effect of McL-1 protein and cancer therapy. Chem. Life 2011, 31, 000863–000867. [Google Scholar]
- Weng, C. Molecular mechanism of McL-1 regulating apoptosis. 2005.
- Yu, R.-X.; Hu, X.; Xu, S.-Q.; Jiang, Z.-J.; Yang, W. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur. J. Pharmacol. 2011, 657, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cheng, J.; Min, Z.; Yin, T.; Zhang, R.; Zhang, W.; Hu, L.; Cui, Z.; Gao, C.; Xu, S.; et al. Effects of fucoxanthin on autophagy and apoptosis in SGC-7901cells and the mechanism. J. Cell. Biochem. 2018, 119, 7274–7284. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.P.; Ferreira, J.; Vettorazzi, A.; Azqueta, A.; Rocha, E.; Ramos, A.A. Cytotoxic activity of fucoxanthin, alone and in combination with the cancer drugs imatinib and doxorubicin, in CML cell lines. Environ. Toxicol. Pharmacol. 2018, 59, 24–33. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Terasaki, M.; Nagao, A. Characterization of apoptosis induced by fucoxanthin in human promyelocytic leukemia cells. Biosci. Biotechnol. Biochem. 2005, 69, 224–227. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.-L.; Xu, Q.-J.; Hu, G.-Q.; Xie, S.-Q. Fucoxanthin Induces Human MCF-7 Breast Cancer Cells Apoptosis via Endoplasmic Reticulum Pathway; Institute of Chemical Biology, Henan University: Henan, China, 2014; Volume 49, pp. 117–120. [Google Scholar]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Inhibitory effects of fucoxanthinol on the viability of human breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with modulation of the NF-kappaB pathway. Cell. Biol. Toxicol. 2014, 30, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K.; Sowmya, P.R.-R.; Arathi, B.P.; Shilpa, S.; Shwetha, H.J.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. 2018, 118, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Zhao, G.-X.; Chen, Y.; Sun, K.-L.; Wang, B. Fucoxanthin attenuates doxorubicin-induced cardiotoxicity via anti-oxidant and anti-apoptotic mechanisms associated with p38, JNK and p53 pathways. J. Funct. Foods 2019, 62. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Zhang, Y.; Wang, Z.; Yang, Y.; Bai, M.; Dai, Y. Fucoxanthin Activates Apoptosis via Inhibition of PI3K/Akt/mTOR Pathway and Suppresses Invasion and Migration by Restriction of p38-MMP-2/9 Pathway in Human Glioblastoma Cells. Neurochem. Res. 2016, 41, 2728–2751. [Google Scholar] [CrossRef]
- Dunbar, E.; Yachnis, A.T. Glioma Diagnosis: Immunohistochemistry and Beyond. Adv. Anat. Pathol. 2010, 17, 187–201. [Google Scholar] [CrossRef]
- Liang, C.; Yang, L.; Guo, S. All-trans retinoic acid inhibits migration, invasion and proliferation, and promotes apoptosis in glioma cells in vitro. Oncol. Lett. 2015, 9, 2833–2838. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.S. Molecular mechanisms of glioma invasiveness: The role of proteases. Nat. Rev. Cancer 2003, 3, 489–501. [Google Scholar] [CrossRef]
- Hosokawa, M.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPAR gamma ligand, troglitazone, on colon cancer cells. BBA Gen. Subj. 2004, 1675, 113–119. [Google Scholar] [CrossRef]
- Terasaki, M.; Ikuta, M.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Dietary Fucoxanthin Induces Anoikis in Colorectal Adenocarcinoma by Suppressing Integrin Signaling in a Murine Colorectal Cancer Model. J. Clin. Med. 2020, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.X. Advances in the research on the pathogenesis and nursing of cervical cancer. World Latest Med. Inf. 2019, 19, 97–99. [Google Scholar]
- Jin, Y.; Qiu, S.; Shao, N.; Zheng, J. Fucoxanthin and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Synergistically Promotes Apoptosis of Human Cervical Cancer Cells by Targeting PI3K/Akt/NF-kappa B Signaling Pathway. Med. Sci. Monit. 2018, 24, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Laussmann, M.A.; Passante, E.; Hellwig, C.T.; Tomiczek, B.; Flanagan, L.; Prehn, J.H.M.; Huber, H.J.; Rehm, M. Proteasome Inhibition Can Impair Caspase-8 Activation upon Submaximal Stimulation of Apoptotic Tumor Necrosis Factor-related Apoptosis Inducing Ligand (TRAIL) Signaling. J. Biol. Chem. 2012, 287, 14402–14411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, Y.; Cao, X.; Zhao, R.; Gong, S.; Jin, L.; Feng, C. Fucoxanthin treatment inhibits nasopharyngeal carcinoma cell proliferation through induction of autophagy mechanism. Environ. Toxicol. 2020, 35, 1082–1090. [Google Scholar] [CrossRef]
- Mei, C.; Zhou, S.; Zhu, L.; Ming, J.; Zeng, F.-D.; Xu, R. Antitumor Effects of Laminaria Extract Fucoxanthin on Lung Cancer. Mar. Drugs 2017, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine Carotenoid Fucoxanthin Possesses Anti-Metastasis Activity: Molecular Evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Fu, C.; Huang, L.; Jiang, Y.; Deng, X.; Guo, J.; Guo, J. Anti-Obesity Effect of Chitosan Oligosaccharide Capsules (COSCs) in Obese Rats by Ameliorating Leptin Resistance and Adipogenesis. Mar. Drugs 2018, 16, 198. [Google Scholar] [CrossRef] [Green Version]
- Lowell, B.B.; S-Susulic, V.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Maratos-Flier, E. Development of Obesity in Transgenic Mice after Genetic Ablation of Brown Adipose-Tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef]
- Kogure, A.; Sakane, N.; Takakura, Y.; Umekawa, T.; Yoshioka, K.; Nishino, H.; Yamamoto, T.; Kawada, T.; Yoshikawa, T.; Yoshida, T. Effects of caffeine on the uncoupling protein family in obese yellow KK mice. Clin. Exp. Pharmacol. Physiol. 2002, 29, 391–394. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef]
- Gille, A.; Stojnic, B.; Derwenskus, F.; Trautmann, A.; Schmid-Staiger, U.; Posten, C.; Briviba, K.; Palou, A.; Bonet, M.L.; Ribot, J. A Lipophilic Fucoxanthin-Rich Phaeodactylum tricornutum Extract Ameliorates Effects of Diet-Induced Obesity in C57BL/6J Mice. Nutrients 2019, 11, 796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-H.; Chen, Y.-L.; Huang, W.-C.; Liou, C.-J. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 495, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, J.-H.; Yang, S.-H.; Um, J.-I.; Hong, K.W.; Kang, K.; Pan, C.-H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawee-Ai, A.; Kim, A.T.; Kim, S.M. Inhibitory activities of microalgal fucoxanthin against alpha-amylase, alpha-glucosidase, and glucose oxidase in 3T3-L1 cells linked to type 2 diabetes. J. Oceanol. Limnol. 2019, 37, 928–937. [Google Scholar] [CrossRef]
- Ahuja, S.; Shankar, P. Association between westernized diets, gut microbiome, and obesity and related disorders. Agro Food Ind. Hi-Tech 2015, 26, 28–32. [Google Scholar]
- Guo, B.; Yang, B.; Pang, X.; Chen, T.; Chen, F.; Cheng, K.-W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of Gut Microbiota by Fucoxanthin During Alleviation of Obesity in High-Fat Diet-Fed Mice. J. Agr. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Sun, X.; Wang, S.; Xu, Y. Fucoxanthin Isolated from Undaria pinnatifida Can Interact with Escherichia coli and lactobacilli in the Intestine and Inhibit the Growth of Pathogenic Bacteria. J. Ocean Univ. China 2019, 18, 926–932. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Mallett, A.; Bearne, C.; Rowland, I. The Influence of Incubation Ph on the Activity of Rat and Human Gut Flora Enzymes. J. Appl. Bacteriol. 1989, 66, 433–437. [Google Scholar] [CrossRef]
- Tavares, R.S.N.; Kawakami, C.M.; Pereira, K.D.C.; Amaral, G.T.D.; Benevenuto, C.G.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Fucoxanthin for Topical Administration, a Phototoxic vs. Photoprotective Potential in a Tiered Strategy Assessed by In Vitro Methods. Antioxidants 2020, 9, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, E.J.; Kim, S.C.; Lee, J.-H.; Lee, J.R.; Kim, I.K.; Baek, S.Y.; Kim, Y.W. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress. BMC Complemt. Altern. Med. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Lin, Y.A. Molecular regulatory mechanism of Nrf2 antioxidant. 2018. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-XXSW201801001.htm (accessed on 11 December 2020).
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective Effects of Fucoxanthin against Alcoholic Liver Injury by Activation of Nrf2-Mediated Antioxidant Defense and Inhibition of TLR4-Mediated Inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Dou, X.; Li, S.; Zhang, X.; Sun, X.; Zhou, Z.; Song, Z. Nuclear Factor (Erythroid-Derived 2)-Like 2 Activation-Induced Hepatic Very-Low-Density Lipoprotein Receptor Overexpression in Response to Oxidative Stress Contributes to Alcoholic Liver Disease in Mice. Hepatology 2014, 59, 1381–1392. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, C.D.; Reisman, S.A. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol. Appl. Pharm. 2010, 244, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Nioi, P.; McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H: Quinone oxidoreductase 1 gene: Reassessment of the ARE consensus sequence. Biochem. J. 2003, 374, 337–348. [Google Scholar] [CrossRef]
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Hyun, J.W. Fucoxanthin Enhances the Level of Reduced Glutathione via the Nrf2-Mediated Pathway in Human Keratinocytes. Mar. Drugs 2014, 12, 4214–4230. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.M.; Kim, S.G. Inhibition of Arachidonic Acid and Iron-Induced Mitochondrial Dysfunction and Apoptosis by Oltipraz and Novel 1,2-Dithiole-3-thione Congeners. Mol. Pharmacol. 2009, 75, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-B.; Bae, M.; Hu, S.; Kang, H.; Park, Y.-K.; Lee, J. Fucoxanthin exerts anti-fibrogenic effects in hepatic stellate cells. Biochem. Biophys. Res. Commun. 2019, 513, 657–662. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, S.; Yu, F.; Li, H.; Guo, C.; Fan, X. Ghrelin Attenuates Liver Fibrosis through Regulation of TGF-beta 1 Expression and Autophagy. Int. J. Mol. Sci. 2015, 16, 21911–21930. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Bae, M.; Park, Y.-K.; Lee, Y.; Pham, T.X.; Rudraiah, S.; Manautou, J.; Koo, S.I.; Lee, J. Histone deacetylase 9 plays a role in the antifibrogenic effect of astaxanthin in hepatic stellate cells. J. Nutr. Biochem. 2017, 40, 172–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jee, M.H.; Hong, K.Y.; Park, J.H.; Lee, J.S.; Kim, H.S.; Lee, S.H.; Jang, S.K. New Mechanism of Hepatic Fibrogenesis: Hepatitis C Virus Infection Induces Transforming Growth Factor beta 1 Production through Glucose-Regulated Protein 94. J. Virol. 2016, 90, 3044–3055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.-L.; Chen, J.; Mu, Y.; Zhang, H.; Chen, G.; Liu, P.; Liu, W. The effects of inhibiting the activation of hepatic stellate cells by lignan components from the fruits of Schisandra chinensis and the mechanism of schisanhenol. J. Nat. Med. 2020, 74, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef]
- Yang, G.; Jin, L.; Zheng, D.; Tang, X.; Yang, J.; Fan, L.; Xie, X. Fucoxanthin Alleviates Oxidative Stress through Akt/Sirt1/FoxO3 alpha Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs. Mar. Drugs 2019, 17, 702. [Google Scholar] [CrossRef] [Green Version]
- Accili, D.; Arden, K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Furukawa-Hibi, Y.; Chen, C.; Horio, Y.; Isobe, K.; Ikeda, K.; Motoyama, N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int. J. Mol. Med. 2005, 16, 237–243. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Cao, Q.; Wang, F.; Huang, L.-Y.; Sang, T.-T.; Liu, F.; Chen, S. SIRT1 Protects Against Oxidative Stress-Induced Endothelial Progenitor Cells Apoptosis by Inhibiting FOXO3a via FOXO3a Ubiquitination and Degradation. J. Cell. Physiol. 2015, 230, 2098–2107. [Google Scholar] [CrossRef]
- Essers, M.A.G.; Weijzen, S.; De Vries-Smits, A.M.M.; Saarloos, I.; De Ruiter, N.D.; Bos, J.L.; Burgering, B. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004, 23, 4802–4812. [Google Scholar] [CrossRef] [Green Version]
- Akasaki, Y.; Alvarez-Garcia, O.; Saito, M.; Caramés, B.; Iwamoto, Y.; Lotz, M.K. FoxO Transcription Factors Support Oxidative Stress Resistance in Human Chondrocytes. Arthritis Rheumatol. 2014, 66, 3349–3358. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.-L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a Marine Xanthophyll Isolated From Conticribra weissflogii ND-8: Preventive Anti-Inflammatory Effect in a Mouse Model of Sepsis. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Z.; Sudirman, S.; Hsu, Y.-C.; Su, C.-Y.; Kuo, H.-P. Fucoxanthin-Rich Brown Algae Extract Improves Male Reproductive Function on Streptozotocin-Nicotinamide-Induced Diabetic Rat Model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Ren, X.; Wang, Y.; Hu, J.; Wu, H.-T.; Song, S.; Yan, C. Fucoxanthin alleviates palmitate-induced inflammation in RAW 264.7 cells through improving lipid metabolism and attenuating mitochondrial dysfunction. Food Funct. 2020, 11, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, M.-S.; Choi, H.-J.; Choi, J.-W.; Shin, T.; Woo, H.-C.; Kim, J.-I.; Kim, H.-R. Hexane fraction from Laminaria japonica exerts anti-inflammatory effects on lipopolysaccharide-stimulated RAW 264.7 macrophages via inhibiting NF-kappaB pathway. Eur. J. Nutr. 2013, 52, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-R.; Lee, M.-S.; Shin, T.-S.; Hua, H.; Jang, B.-C.; Choi, J.S.; Byun, D.-S.; Utsuki, T.; Ingram, N.; Kim, H.-R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-kappa B, Akt, and p38 MAPK. Toxicol. Vitr. 2011, 25, 1789–1795. [Google Scholar] [CrossRef]
- Wang, M.; Yao, J.; Zhang, J.; Zheng, Y.; Song, J.; Wang, M. Protective effects of Shanxi aged vinegar against hydrogen peroxide-induced oxidative damage in LO2 cells through Nrf2-mediated antioxidant responses. Rsc. Adv. 2017, 7, 17377–17386. [Google Scholar] [CrossRef] [Green Version]
- Hritz, I.; Velayudham, A.; Dolganiuc, A.; Kodys, K.; Mandrekar, P.; Kurt-Jones, E.; Szabo, G. Bone marrow-derived immune cells mediate sensitization to liver injury in a myeloid differentiation factor 88-dependent fashion. Hepatology 2008, 48, 1342–1347. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Brown, J.; Gao, B.; Liangpunsakul, S.; Ju, C. IL-1 Receptor Like 1 (St2) Protects Against Alcoholic Liver Injury by Limiting Nf-Kappa B Activation in Hepatic Macrophages. Alcohol Clin. Exp. Res. 2017, 41, 328a. [Google Scholar]
- Cheng, I.; Weng, S.; Wu, M.; Suk, F.; Lien, G.; Chen, C. Low-molecular-weight fucoidan and high-stability fucoxanthin decrease serum alanine transaminase in patients with nonalcoholic fatty liver disease-A double-blind, randomized controlled trial. Adv. Dig. Med. 2019, 6, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and Rosmarinic Acid Combination Has Anti-Inflammatory Effects through Regulation of NLRP3 Inflammasome in UVB-Exposed HaCaT Keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin-Containing Cream Prevents Epidermal Hyperplasia and UVB-Induced Skin Erythema in Mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Protective Effects of Fucoxanthin on Ultraviolet B-Induced Corneal Denervation and Inflammatory Pain in a Rat Model. Mar. Drugs 2019, 17, 152. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-J.; Lee, C.-J.; Lin, T.-B.; Peng, H.-Y.; Liu, H.-J.; Chen, Y.-S.; Tseng, K.-W. Fucoxanthin Ameliorates Atopic Dermatitis Symptoms by Regulating Keratinocytes and Regulatory Innate Lymphoid Cells. Int. J. Mol. Sci. 2020, 21, 2180. [Google Scholar] [CrossRef] [Green Version]
- Karpinski, T.M.; Adamczak, A. Fucoxanthin-An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paudel, P.; Seone, S.H.; Jung, H.A.; Choi, J.S. Characterizing fucoxanthin as a selective dopamine D-3/D-4 receptor agonist: Relevance to Parkinson’s disease. Chem Biol. Interact. 2019, 310. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.S.; Smid, I. Musgrave and W. Zhang. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (A beta(1-42)) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, Y.-T.; Chen, H.-Y.; Lin, P.-H.; Hsia, S.-M. Preventive Effects of Fucoidan and Fucoxanthin on Hyperuricemic Rats Induced by Potassium Oxonate. Mar. Drugs 2019, 17, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, G.; Lin, Q.; Tang, Z.; Yan, Q.; Yu, X. Fucoxanthin prevents lipopolysaccharide-induced depressive-like behavior in mice via AMPK- NF-B pathway. Metab. Brain Dis. 2019, 34, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Mol. Cell Biol. Lipids 2020, 1865. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Dasa, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Mizuno, M.; Yoshida, M.; Nishitani, Y.; Azuma, T.; Komoto, A.; Maoka, T.; Tanino, Y.; Kanazawa, K. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br. J. Nutr. 2012, 107, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, G.; Chen, H.; Jin, W.; Fang, H.; Chen, W.; Hong, Z.; Zhang, W. Development of Fucoxanthin Certified Reference Material. Chem. Anal. Metrol. 2018, 27. [Google Scholar] [CrossRef]
- Takahashi, K.; Hosokawa, M.; Kasajima, H.; Hatanaka, K.; Kudo, K.; Shimoyama, N.; Miyashita, K. Anticancer effects of fucoxanthin and fucoxanthinol on colorectal cancer cell lines and colorectal cancer tissues. Oncol. Lett. 2015, 10, 1463–1467. [Google Scholar] [CrossRef] [Green Version]
- Yamano, Y.; Chary, M.V.; Wada, A. Stereocontrolled First Total Syntheses of Amarouciaxanthin A and B. Org. Lett. 2013, 15, 5310–5313. [Google Scholar] [CrossRef]
- Yim, M.-J.; Hosokawa, M.; Mizushina, Y.; Yoshida, H.; Saito, Y.; Miyashita, K. Suppressive Effects of Amarouciaxanthin A on 3T3-L1 Adipocyte Differentiation through Down-regulation of PPAR gamma and C/EBP alpha mRNA Expression. J. Agr. Food Chem. 2011, 59, 1646–1652. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Zhao, J.; Fang, C.; Cao, Q.; Xing, M.; Li, X.; Hou, J.; Ji, A.; Song, S. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Mar. Drugs 2020, 18, 634. https://doi.org/10.3390/md18120634
Xiao H, Zhao J, Fang C, Cao Q, Xing M, Li X, Hou J, Ji A, Song S. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Marine Drugs. 2020; 18(12):634. https://doi.org/10.3390/md18120634
Chicago/Turabian StyleXiao, Han, Jiarui Zhao, Chang Fang, Qi Cao, Maochen Xing, Xia Li, Junfeng Hou, Aiguo Ji, and Shuliang Song. 2020. "Advances in Studies on the Pharmacological Activities of Fucoxanthin" Marine Drugs 18, no. 12: 634. https://doi.org/10.3390/md18120634
APA StyleXiao, H., Zhao, J., Fang, C., Cao, Q., Xing, M., Li, X., Hou, J., Ji, A., & Song, S. (2020). Advances in Studies on the Pharmacological Activities of Fucoxanthin. Marine Drugs, 18(12), 634. https://doi.org/10.3390/md18120634




