Bioactive Steroids from the Red Sea Soft Coral Sinularia polydactyla
Abstract
1. Introduction
2. Results and Discussion
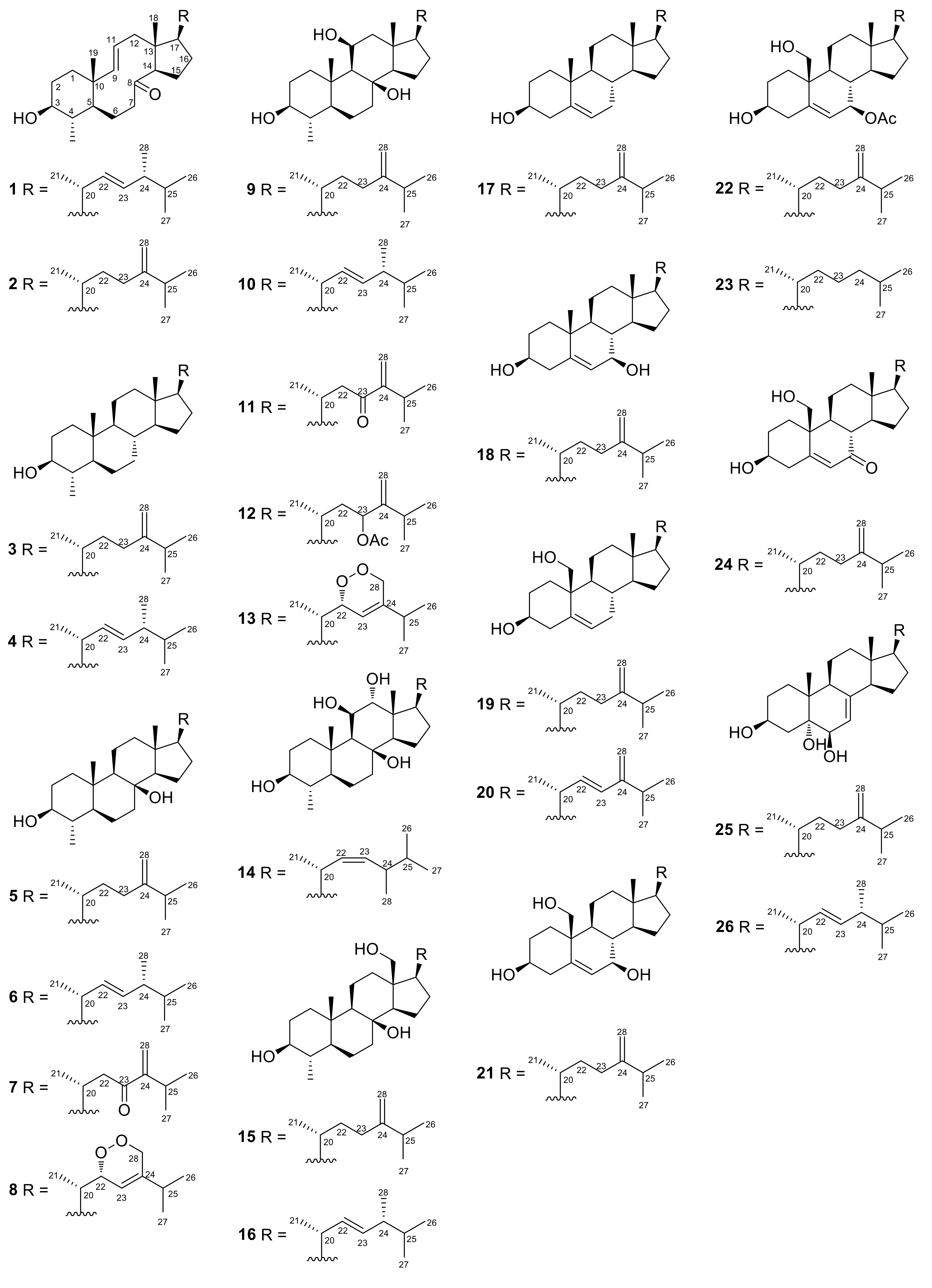
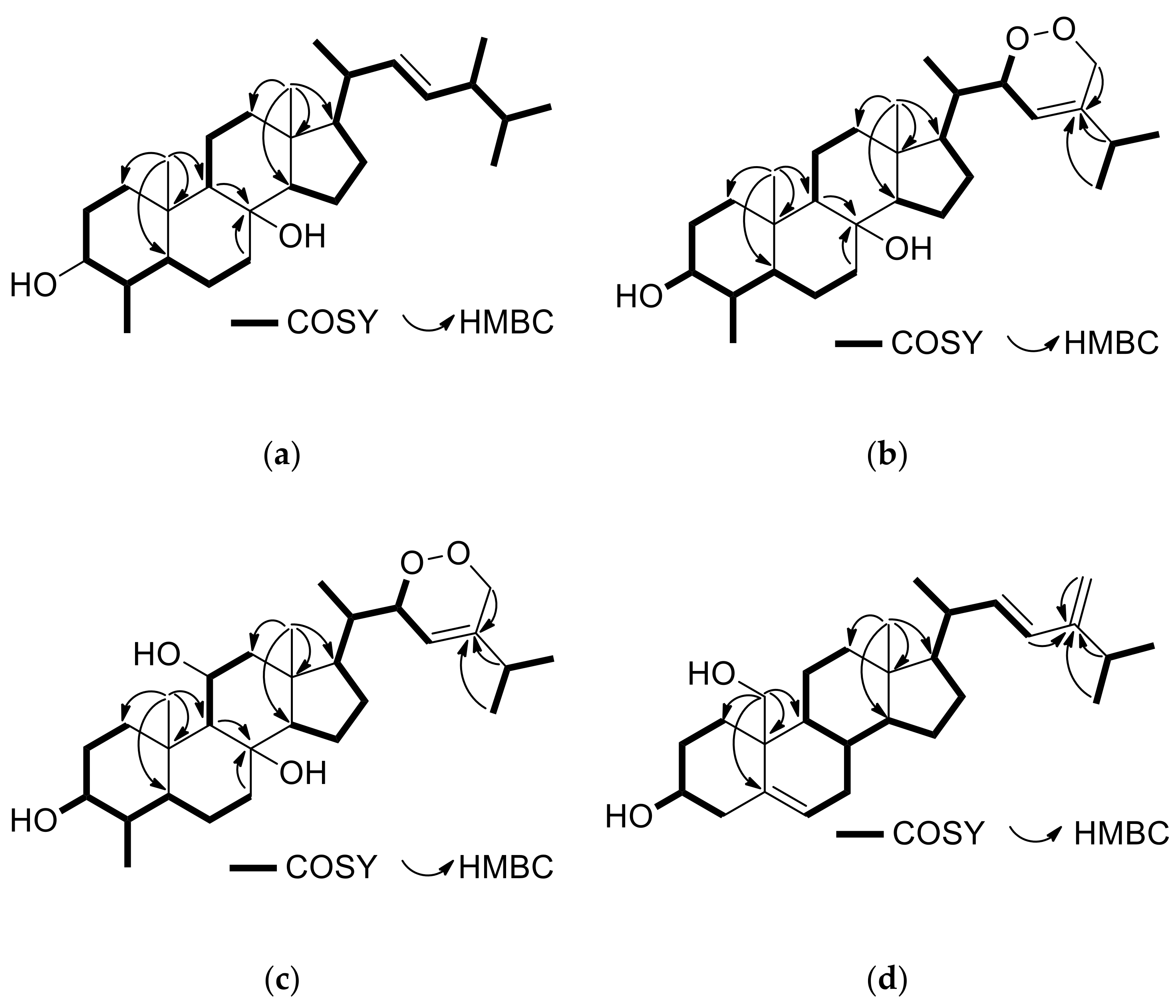
2.1. Structure Elucidation of the Isolated Metabolites
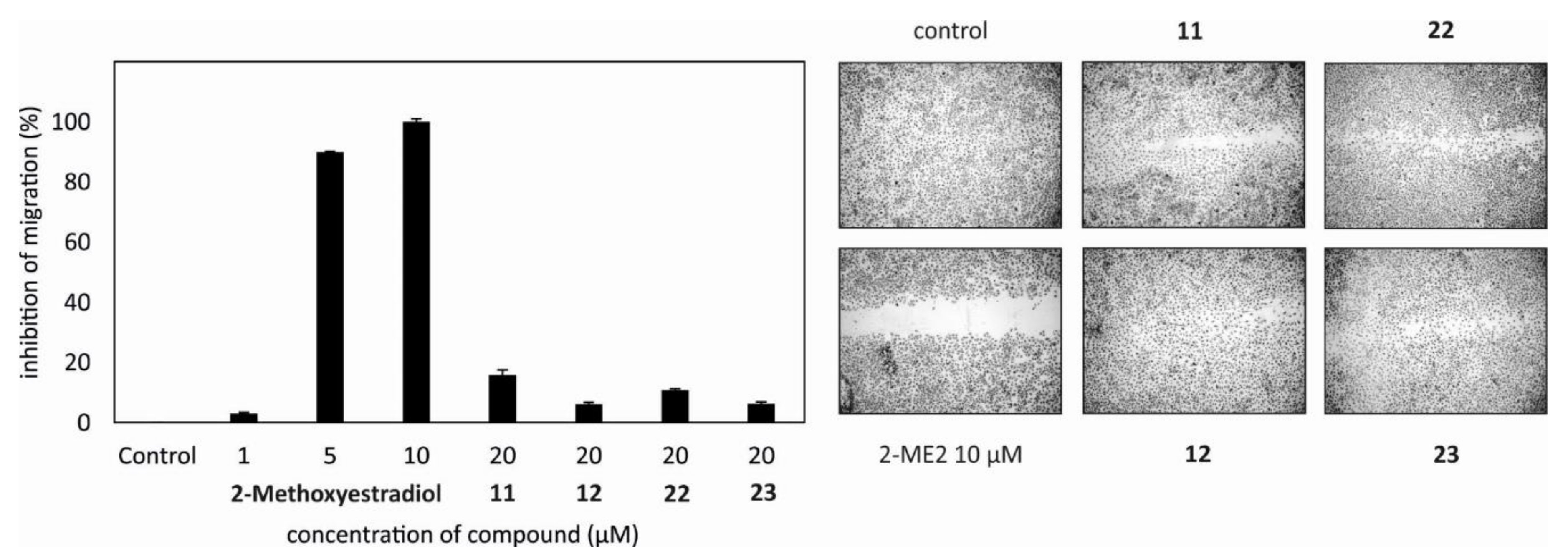
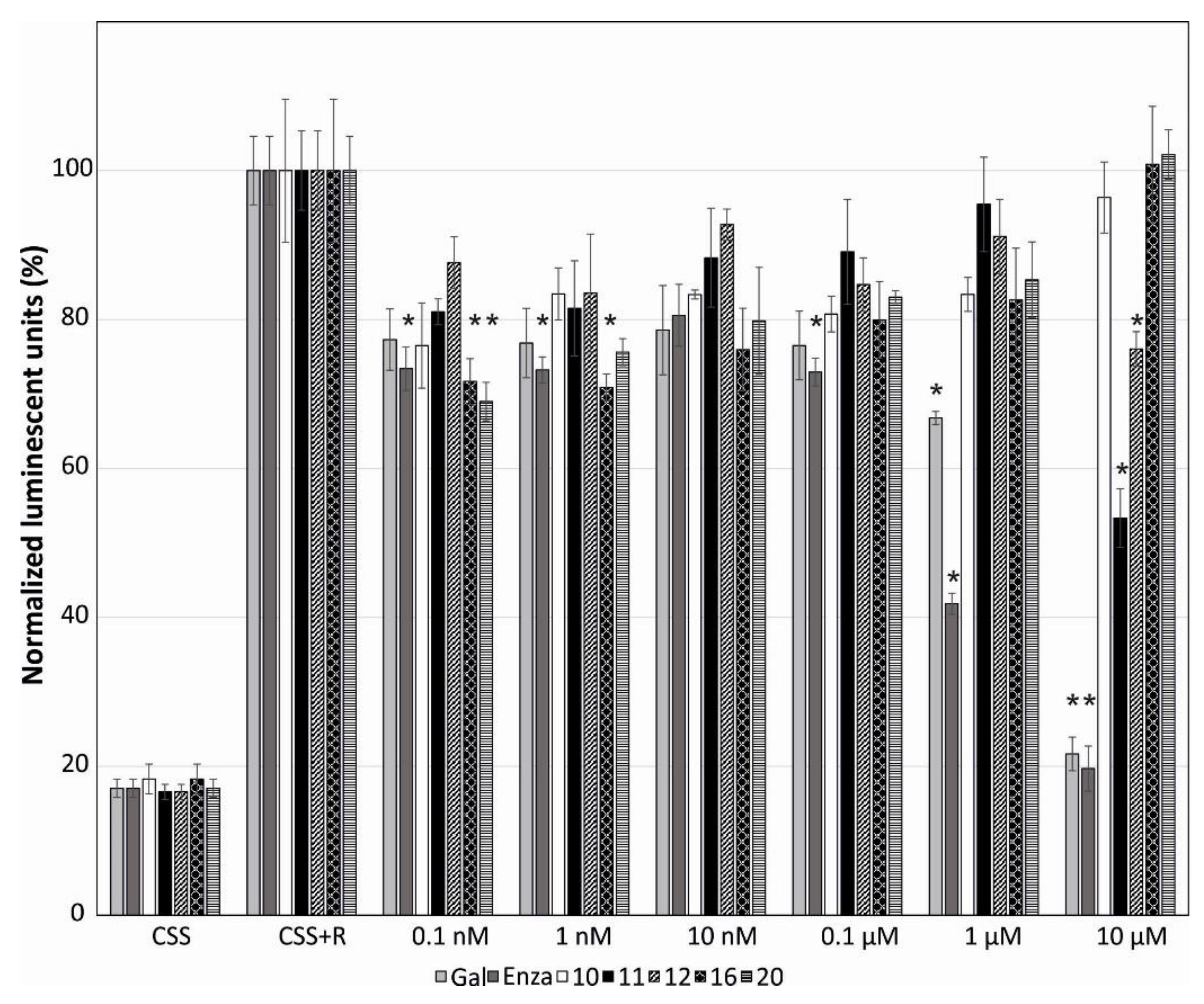
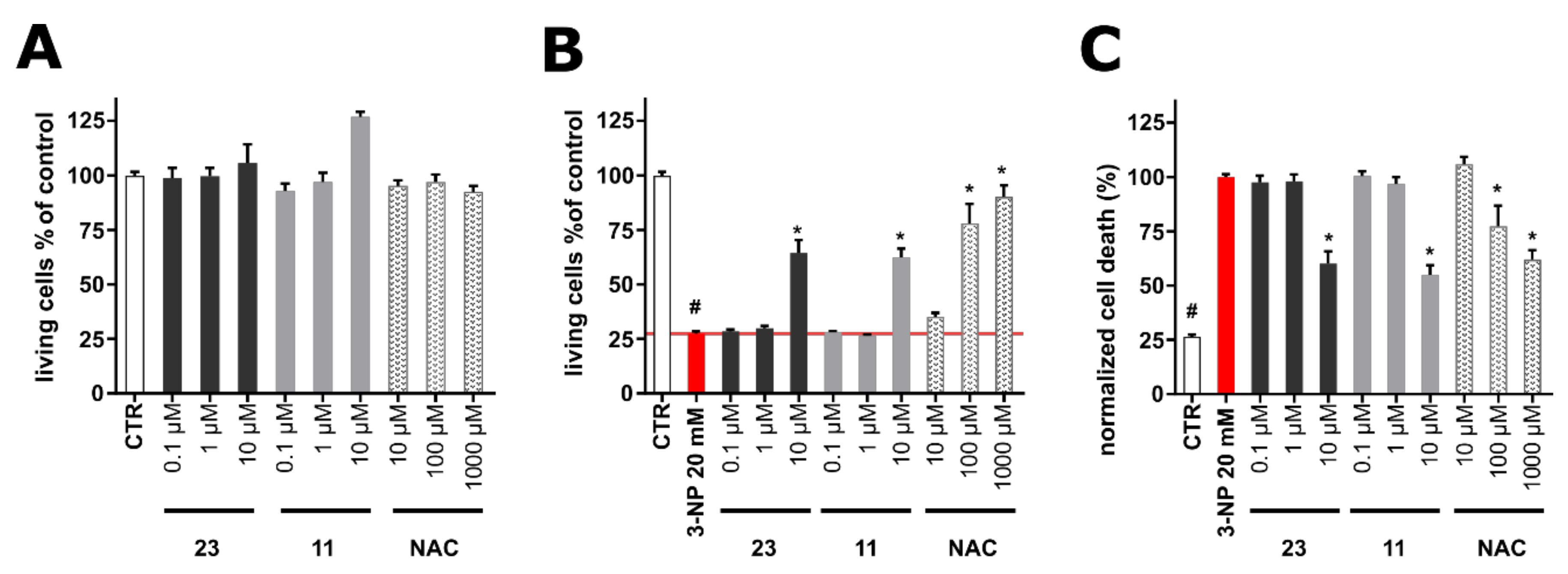
2.2. Evaluation of the Biological Activity of the Isolated Metabolites
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. Evaluation of Cytotoxicity
3.6. Cell-Surface ELISA CD62E (E-Selectin, ELAM)
3.7. Migration Scratch Assay
3.8. AR-Transcriptional Reporter Assay
3.9. SH-SY5Y Cell Treatments and Evaluation of Cell Viability/Cytotoxicity
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kotb, M.M.A.; Hanafy, M.H.; Rirache, H.; Matsumura, S.; Al-Sofyani, A.A.; Ahmed, A.G.; Bawazir, G.; Al-Horani, F.A. Status of coral reefs in the Red Sea and Gulf of Aden region. In Status of Coral Reefs of the World: 2008; Wilkinson, C., Ed.; Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre: Townsville, Australia, 2008; pp. 67–78. [Google Scholar]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- MarinLit. A Database of the Marine Natural Products Literature. Available online: http://pubs.rsc.org/marinlit/ (accessed on 30 September 2020).
- Končić, M.; Ioannou, E.; Sawadogo, W.; Abdel-Razik, A.; Vagias, C.; Diederich, M.; Roussis, V. 4a-Methylated steroids with cytotoxic activity from the soft coral Litophyton mollis. Steroids 2016, 115, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. 5,8-Epidioxysterols and related derivatives from a Chinese soft coral Sinularia flexibilis. Steroids 2006, 71, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Kokke, W.; Bohlin, L.; Fenical, W.; Djerassi, C. Novel dinoflagellate 4α-methylated sterols from four caribbean gorgonians. Phytochemistry 1982, 21, 881–887. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishizaka, T.; Mitsuhashi, H. Marine sterols X. Minor constituents of the sterols of the soft coral Sarcophyton glaucum. Steroids 1982, 40, 209–221. [Google Scholar] [CrossRef]
- Mehta, G.; Venkateswarlu, Y.; Rama, R.M.; Uma, R. A novel 4α-methyl sterol from the soft coral Nephthea chabroli. J. Chem. Res. 1999, 23, 628–629. [Google Scholar] [CrossRef]
- Bortolotto, M.; Braekman, J.; Daloze, D.; Tursch, B. Chemical studies of marine invertebrates. XXIX. 4α-methyl-3β,8β-dihydroxy-5α-ergost-24(28)-en-23-one, a novel polyhydroxygenated sterol from the soft coral litophyton viridis. Steroids 1977, 30, 159–164. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, Y.; Wen, Z.; Hsu, C.; Wang, S.; Dai, C.; Duh, C. New 19-oxygenated and 4-methylated steroids from the formosan soft coral Nephthea chabroli. Steroids 2009, 74, 543–547. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, Z.; Wang, S.; Hsu, C.; Duh, C. New anti-inflammatory 4-methylated steroids from the formosan soft coral Nephthea chabroli. Steroids 2008, 73, 1181–1186. [Google Scholar] [CrossRef]
- Viegelmann, C.; Parker, J.; Ooi, T.; Clements, C.; Abbott, G.; Young, L.; Kennedy, J.; Dobson, A.; Edrada-Ebel, R. Isolation and identification of antitrypanosomal and antimycobacterial active steroids from the sponge Haliclona simulans. Mar. Drugs 2014, 12, 2937–2952. [Google Scholar] [CrossRef]
- Riccardis, F.; Minale, L. Marine sterols side-chain-oxygenated sterols, possibly of abiotic origin, from the new caledonian sponge stelodoryx chlorophylla. J. Nat. Prod. 1993, 56, 282–287. [Google Scholar] [CrossRef]
- Iguchi, K.; Saitou, S.; Yamada, Y. Novel 19-oxygenated sterols from the okinawan soft coral Litophyton viridis. Chem. Pharm. Bull. 1989, 37, 2553–2554. [Google Scholar] [CrossRef]
- Cheng, S.; Dai, C.; Duh, C. New 4-methylated and 19-oxygenated steroids from the formosan soft coral Nephthea erecta. Steroids 2007, 72, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Ellithey, M.; Lall, N.; Hussein, A.; Meyer, D. Cytotoxic, cytostatic and HIV-1 PR inhibitory activities of the soft coral Litophyton arboretum. Mar. Drugs 2013, 11, 4917–4936. [Google Scholar] [CrossRef]
- Duh, C.; Wang, S.; Chu, M.; Sheu, J. Cytotoxic sterols from the soft coral Nephthea erecta. J. Nat. Prod. 1998, 61, 1022–1024. [Google Scholar] [CrossRef]
- Iorizzi, M.; Minale, L.; Riccio, R. Polar steroids from the marine scallop Patinopecten yessoensis. J. Nat. Prod. 1988, 51, 1098–1103. [Google Scholar] [CrossRef]
- Sattler, M.; Quinnan, L.R.; Pride, Y.B.; Gramlich, J.L.; Chu, S.C.; Even, G.C.; Kraeft, S.-K.; Chen, L.B.; Salgia, R. 2-Methoxyestradiol alters cell motility, migration, and adhesion. Blood 2003, 102, 289–296. [Google Scholar] [CrossRef][Green Version]
- Trepels, T.; Zeiher, A.M.; Fichtlscherer, S. The endothelium and inflammation. Endothelium 2006, 13, 423–429. [Google Scholar] [CrossRef]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar] [CrossRef]
- Jorda, R.; Řezníčková, E.; Kiełczewska, U.; Maj, J.; Morzycki, J.W.; Siergiejczyk, L.; Bazgier, V.; Berka, K.; Rárová, L.; Wojtkielewicz, A. Synthesis of novel galeterone derivatives and evaluation of their in vitro activity against prostate cancer cell lines. Eur. J. Med. Chem. 2019, 179, 483–492. [Google Scholar] [CrossRef]
- Rárová, L.; Sedlák, D.; Oklestkova, J.; Steigerová, J.; Liebl, J.; Zahler, S.; Bartůněk, P.; Kolář, Z.; Kohout, L.; Kvasnica, M.; et al. The novel brassinosteroid analog BR4848 inhibits angiogenesis in human endothelial cells and induces apoptosis in human cancer cells in vitro. J. Steroid Biochem. Mol. Biol. 2018, 178, 263–271. [Google Scholar]
- Calderon Guzman, D.; Bratoeff, E.; Chávez-Riveros, A.; Osnaya, N.; Barragan, G.; Hernandez Garcia, E.; Olguín, H.; Garcia, E. Effect of two antiandrogens as protectors of prostate and brain in a Huntington’s animal model. Anticancer Agents Med. Chem. 2014, 14, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Colle, D.; Santos, D.; Hartwig, J.; Godoi, M.; Engel, D.; de Bem, A.; Braga, A.; Farina, M. Succinobucol, a lipid-lowering drug, protects against 3-nitropropionic acid-induced mitochondrial dysfunction and oxidative stress in SH-SY5Y cells via upregulation of glutathione levels and glutamate cysteine ligase activity. Mol. Neurobiol. 2016, 53, 1280–1295. [Google Scholar] [CrossRef] [PubMed]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anticancer Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef]
- Rárová, L.; Steigerová, J.; Kvasnica, M.; Bartůněk, P.; Křížová, K.; Chodounská, H.; Kolář, Z.; Sedlák, D.; Oklestkova, J.; Strnad, M. Structure activity relationship studies on cytotoxicity and the effects on steroid receptor of AB-functionalized cholestanes. J. Steroid Biochem. Mol. Biol. 2016, 159, 154–169. [Google Scholar] [CrossRef]
- Bartonkova, I.; Novotna, A.; Dvorak, Z. Novel stably transfected human reporter cell line AIZ-AR as a tool for an assessment of human androgen receptor transcriptional activity. PLoS ONE 2015, 10, e0121316. [Google Scholar] [CrossRef]
- Cheung, Y.-T.; Lau, W.K.-W.; Yu, M.-S.; Lai, C.S.-W.; Yeung, S.-C.; So, K.-F.; Chang, R.C.-C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef]
- Morrogh-Bernard, H.C.; Foitová, I.; Yeen, Z.; Wilkin, P.; de Martin, R.; Rárová, L.; Doležal, K.; Nurcahyo, W.; Olšanský, M. Self-medication by orang-utans (Pongo pygmaeus) using bioactive properties of Dracaena cantleyi. Sci. Rep. 2017, 7, 16653. [Google Scholar] [CrossRef]
- Stone, W.L.; Qui, M.; Smith, M. Lipopolysaccharide enhances the cytotoxicity of 2-chloroethyl ethyl sulfide. BMC Cell Biol. 2003, 4, 1. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Woo, J.-K.; Yun, J.-H.; Ahn, S.; Sim, C.J.; Noh, M.; Oh, D.-C.; Oh, K.-B.; Shin, J. Dictyoneolone, a B/C ring juncture-defused steroid from a Dictyonella sp. sponge. Tetrahedron Lett. 2018, 59, 2021–2024. [Google Scholar] [CrossRef]

| Position | 1 1 | 2 1 | 4 2 | 6 2 | 8 1 | 13 1 | 20 3 |
|---|---|---|---|---|---|---|---|
| 1 | 1.33 m, 1.22 m | 1.36 m, 1.24 m | 1.71 m, 0.98 m | 1.72 m, 0.93 m | 1.72 m, 0.96 m | 1.91 m, 1.00 m | 1.91 m, 1.08 m |
| 2 | 1.76 m, 1.48 m | 1.76 m, 1.49 m | 1.77 m, 1.46 m | 1.77 m, 1.52 m | 1.78 m, 1.52 m | 1.79 m, 1.58 m | 1.84 m, 1.40 m |
| 3 | 3.09 td (10.1, 4.8) | 3.10 m | 3.06 td (10.5, 4.9) | 3.04 td (10.2, 4.9) | 3.06 td (10.5, 5.2) | 3.05 td (10.8, 5.0) | 3.56 m |
| 4 | 1.27 m | 1.28 m | 1.27 m | 1.31 m | 1.33 m | 1.41 m | 2.37 m, 2.18 m |
| 5 | 0.91 m | 0.92 m | 0.72 m | 0.68 td (12.4, 2.9) | 0.71 td (12.2, 2.2) | 0.69 td (11.9, 2.2) | - |
| 6 | 1.66 m, 1.60 m | 1.66 m, 1.62 m | 1.64 m, 1.47 m | 1.50 m, 1.33 m | 1.53 m, 1.33 m | 1.56 m, 1.39 m | 5.73 m |
| 7 | 2.29 m, 1.75 m | 2.48 m, 2.24 m | 1.69 m, 1.50 m | 1.63 m, 1.13 m | 1.64 m, 1.19 m | 1.71 m, 1.22 m | 2.01 m, 1.51 m |
| 8 | - | - | 1.28 m | - | - | - | 1.82 m |
| 9 | 5.28 d (15.3) | 5.27 m | 0.60 m | 0.80 m | 0.81 m | 0.88 m | 0.89 m |
| 11 | 5.41 ddd (15.3, 11.1, 3.8) | 5.41 m | 1.47 m, 1.00 m | 1.62 m, 1.49 m | 1.62 m, 1.48 m | 4.43 brd (1.9) | 1.62 m, 1.53 m |
| 12 | 2.47 m, 1.70 m | 2.50 m, 1.72 m | 1.92 m, 1.11 m | 1.95 m, 1.16 m | 1.93 m, 1.17 m | 2.23 m, 1.37 m | 2.01 m, 1.17 m |
| 14 | 2.49 m | 2.50 m | 0.93 m | 1.18 m | 1.23 m | 1.27 m | 0.90 m |
| 15 | 1.64 m, 1.48 m | 1.64 m, 1.50 m | 1.51 m, 1.02 m | 1.45 m, 1.23 m | 1.53 m, 1.33 m | 1.60 m, 1.46 m | 1.50 m, 1.05 m |
| 16 | 1.70 m, 1.47 m | 1.73 m, 1.49 m | 1.63 m, 1.19 m | 1.64 m, 1.23 m | 1.99 m, 1.31 m | 2.00 m, 1.35 m | 1.64 m, 1.26 m |
| 17 | 1.31 m | 1.28 m | 1.09 m | 1.03 m | 1.43 m | 1.43 m | 1.18 m |
| 18 | 1.08 s | 1.09 s | 0.64 s | 0.92 s | 0.89 s | 1.09 s | 0.75 s |
| 19 | 0.94 s | 0.94 s | 0.80 s | 0.97 s | 0.96 s | 1.33 s | 3.81 d (11.5), 3.59 d (11.5) |
| 20 | 2.10 m | 2.11 m | 1.98 m | 1.96 m | 1.56 m | 1.59 m | 2.11 m |
| 21 | 0.99 d (6.8) | 0.93 d (6.1) | 0.97 d (6.6) | 0.94 d (6.4) | 0.83 d (6.9) | 0.85 d (7.0) | 1.03 d (6.5) |
| 22 | 5.15 dd (15.2, 8.1) | 1.53 m, 1.14 m | 5.12 dd (15.1, 7.6) | 5.09 dd (15.2, 8.2) | 4.72 brs | 4.70 brs | 5.56 dd (15.7, 8.7) |
| 23 | 5.20 dd (15.2, 7.3) | 2.08 m, 1.88 m | 5.17 dd (15.1, 7.0) | 5.16 dd (15.2, 7.4) | 5.36 brs | 5.36 brs | 5.92 d (15.7) |
| 24 | 1.83 m | - | 1.83 m | 1.80 m | - | - | - |
| 25 | 1.44 m | 2.21 m | 1.44 m | 1.42 m | 2.24 septet (6.9) | 2.24 septet (6.9) | 2.53 septet (6.8) |
| 26 | 0.81 d (6.8) | 1.00 d (6.8) | 0.81 d (6.8) | 0.81 d (6.8) | 1.05 d (6.9) | 1.05 d (6.9) | 1.06 d (6.8) |
| 27 | 0.80 d (6.8) | 1.01 d (6.9) | 0.79 d (6.8) | 0.79 d (6.8) | 1.05 d (6.9) | 1.06 d (6.9) | 1.04 d (6.8) |
| 28 | 0.89 d (6.8) | 4.70 brs, 4.63 brs | 0.88 d (6.8) | 0.88 d (6.8) | 4.62 d (15.7), 4.20 d (15.7) | 4.59 d (15.7), 4.21 d (15.7) | 4.83 brs, 4.79 brs |
| 29 | 1.04 d (6.1) | 1.04 d (6.1) | 0.92 d (6.3) | 0.95 d (6.8) | 0.95 d (6.4) | 0.96 d (6.4) | - |
| Position | 1 1,2 | 4 3 | 6 1,4 | 8 1,2 | 13 2 | 20 1,5 |
|---|---|---|---|---|---|---|
| 1 | 38.5 | 36.8 | 37.7 | 37.2 | 37.5 | 32.9 |
| 2 | 30.7 | 31.1 | 30.9 | 30.3 | 30.2 | 31.7 |
| 3 | 76.6 | 76.6 | 76.8 | 76.4 | 76.5 | 70.8 |
| 4 | 39.8 | 39.2 | 39.0 | 38.5 | 38.2 | 41.9 |
| 5 | 53.5 | 51.0 | 51.9 | 51.2 | 52.3 | 134.9 |
| 6 | 20.0 | 24.2 | 20.5 | 19.8 | 20.0 | 126.8 |
| 7 | 47.1 | 32.2 | 40.1 | 39.6 | 39.9 | 30.8 |
| 8 | 213.1 | 36.0 | 73.6 | 73.5 | 75.3 | 33.4 |
| 9 | 142.0 | 54.6 | 56.6 | 56.0 | 57.6 | 49.9 |
| 10 | 39.1 | 34.9 | 36.9 | 36.3 | 36.8 | 41.0 |
| 11 | 127.6 | 21.1 | 18.3 | 18.1 | 69.8 | 21.4 |
| 12 | 46.8 | 40.2 | 41.6 | 40.5 | 49.0 | 39.6 |
| 13 | 55.2 | 42.4 | 43.0 | 42.7 | 41.8 | 42.3 |
| 14 | 62.7 | 56.6 | 59.7 | 59.0 | 60.2 | 57.5 |
| 15 | 26.6 | 24.2 | 19.0 | 18.7 | 19.2 | 24.1 |
| 16 | 28.4 | 28.6 | 28.3 | 27.0 | 26.9 | 28.1 |
| 17 | 56.7 | 56.1 | 57.1 | 52.4 | 53.9 | 55.4 |
| 18 | 13.5 | 12.3 | 14.0 | 12.8 | 15.0 | 12.0 |
| 19 | 15.8 | 13.4 | 13.8 | 13.2 | 15.6 | 62.3 |
| 20 | 39.1 | 40.0 | 39.6 | 39.8 | 40.0 | 39.9 |
| 21 | 21.7 | 20.9 | 21.3 | 12.8 | 12.9 | 20.2 |
| 22 | 135.2 | 135.9 | 135.7 | 79.9 | 79.7 | 135.4 |
| 23 | 132.6 | 131.6 | 131.8 | 119.4 | 119.2 | 128.8 |
| 24 | 43.0 | 42.8 | 43.2 | 142.0 | 142.0 | 153.0 |
| 25 | 33.2 | 33.1 | 33.8 | 30.8 | 31.1 | 28.8 |
| 26 | 19.8 | 19.6 | 19.9 | 21.0 | 21.1 | 21.7 |
| 27 | 20.1 | 19.9 | 20.2 | 21.0 | 21.1 | 22.1 |
| 28 | 17.6 | 17.6 | 18.0 | 70.6 | 70.8 | 109.0 |
| 29 | 16.4 | 15.1 | 16.0 | 14.9 | 15.2 |
| Compound | HeLa | MCF7 | BJ |
|---|---|---|---|
| 3 | >50 | >50 | >50 |
| 4 | >50 | >50 | >50 |
| 5 | >50 | >50 | >50 |
| 6 | >50 | >50 | >50 |
| 7 | >50 | >50 | >50 |
| 9 | 25.9 ± 4.9 | 36.4 ± 5.7 | 18.3 ± 4.0 |
| 10 | 32.9 ± 6.8 | 32.7 ± 1.3 | 4.1 ± 1.9 |
| 11 | 18.8 ± 6.4 | 21.7 ± 1.4 | >50 |
| 12 | 15.7 ± 2.0 | 25.3 ± 6.5 | >50 |
| 14 | >50 | 29.1 ± 5.0 | >50 |
| 15 | 19.0 ± 4.3 | 18.9 ± 0.1 | >50 |
| 16 | 32.9 ± 5.2 | 33.8 ± 1.0 | 23.2 ± 1.4 |
| 17 | >50 | >50 | >50 |
| 18 | 22.6 ± 0.4 | 28.6 ± 5.3 | >50 |
| 19 | >50 | >50 | >50 |
| 20 | >50 | >50 | >50 |
| 22 | 7.5 ± 0.1 | 8.9 ± 0.0 | 14.8 ± 5.8 |
| 23 | 12.0 ± 1.7 | 11.2 ± 0.5 | 14.5 ± 3.9 |
| 24 | 21.4 ± 2.0 | 31.7 ± 0.3 | 45.9 ± 2.8 |
| 25 | >50 | >50 | >50 |
| 26 | >50 | >50 | >50 |
| cisplatin | 11.4 ± 3.8 | 7.7 ± 1.7 | 6.9 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tammam, M.A.; Rárová, L.; Kvasnicová, M.; Gonzalez, G.; Emam, A.M.; Mahdy, A.; Strnad, M.; Ioannou, E.; Roussis, V. Bioactive Steroids from the Red Sea Soft Coral Sinularia polydactyla. Mar. Drugs 2020, 18, 632. https://doi.org/10.3390/md18120632
Tammam MA, Rárová L, Kvasnicová M, Gonzalez G, Emam AM, Mahdy A, Strnad M, Ioannou E, Roussis V. Bioactive Steroids from the Red Sea Soft Coral Sinularia polydactyla. Marine Drugs. 2020; 18(12):632. https://doi.org/10.3390/md18120632
Chicago/Turabian StyleTammam, Mohamed A., Lucie Rárová, Marie Kvasnicová, Gabriel Gonzalez, Ahmed M. Emam, Aldoushy Mahdy, Miroslav Strnad, Efstathia Ioannou, and Vassilios Roussis. 2020. "Bioactive Steroids from the Red Sea Soft Coral Sinularia polydactyla" Marine Drugs 18, no. 12: 632. https://doi.org/10.3390/md18120632
APA StyleTammam, M. A., Rárová, L., Kvasnicová, M., Gonzalez, G., Emam, A. M., Mahdy, A., Strnad, M., Ioannou, E., & Roussis, V. (2020). Bioactive Steroids from the Red Sea Soft Coral Sinularia polydactyla. Marine Drugs, 18(12), 632. https://doi.org/10.3390/md18120632








