Functional Characterization of a New GH107 Endo-α-(1,4)-Fucoidanase from the Marine Bacterium Formosa haliotis
Abstract
1. Introduction
2. Results and Discussion
2.1. Primary Structure of Fhf1
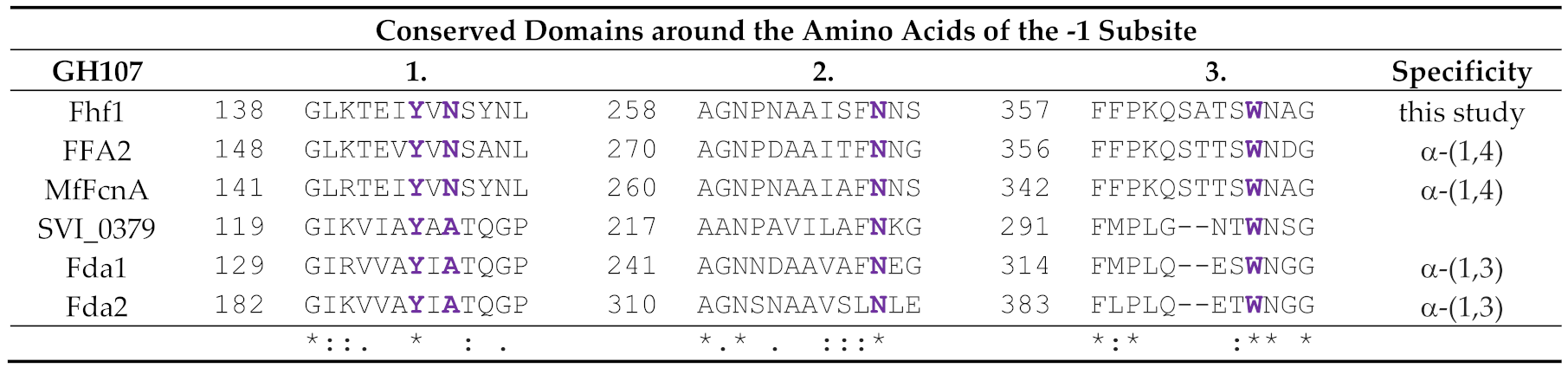
2.2. Sequence Comparisons of Fhf1 to Other Fucoidanases
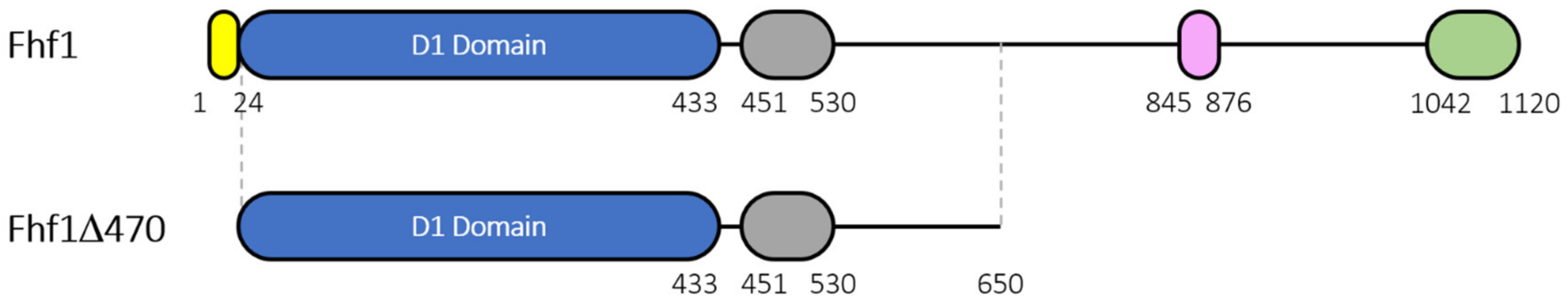
2.3. Construction of a Truncated Version and Recombinant Expression and Purification of Fhf1
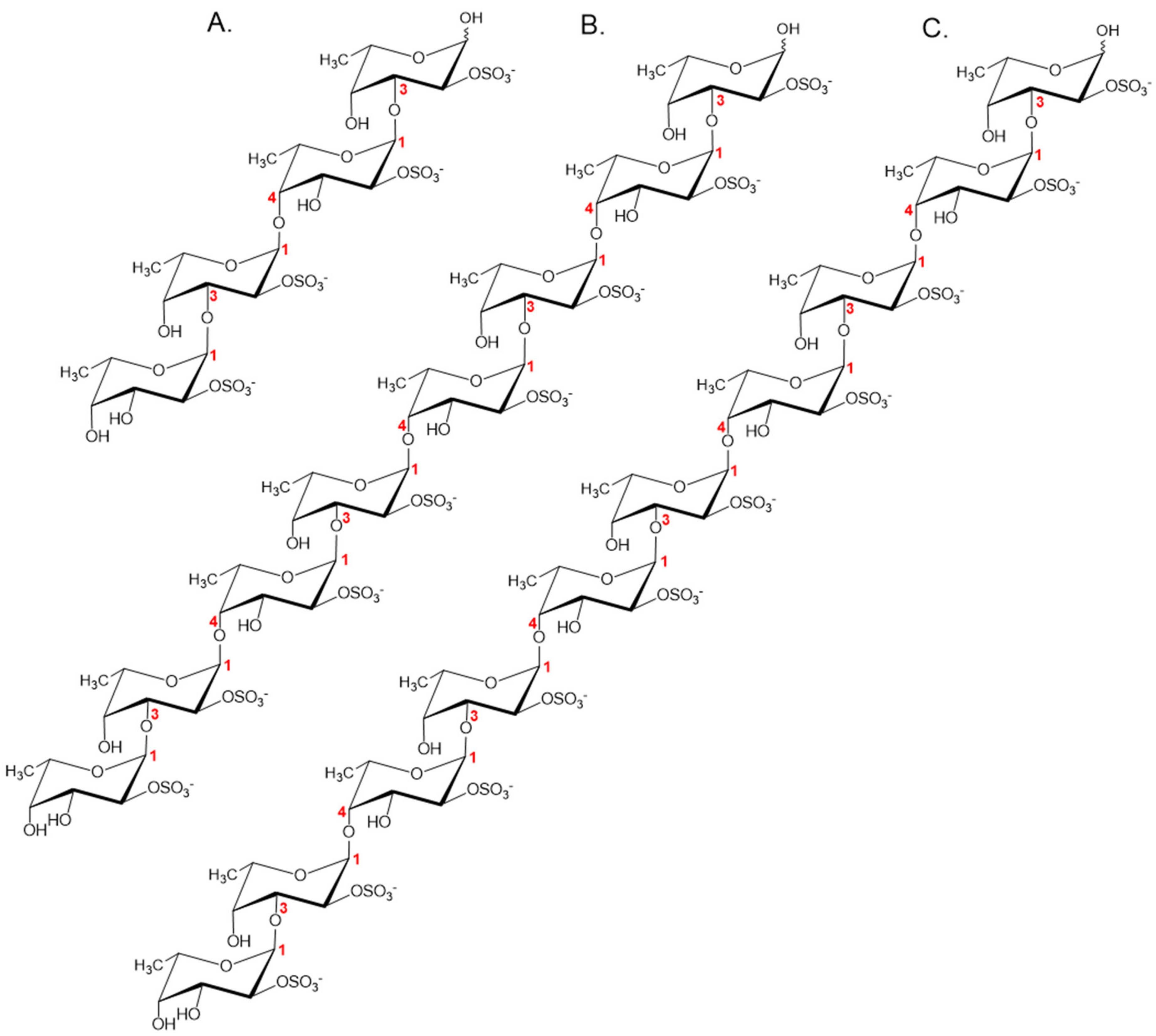
2.4. Substrate Specificity of the Fhf1Δ470 Fucoidanase
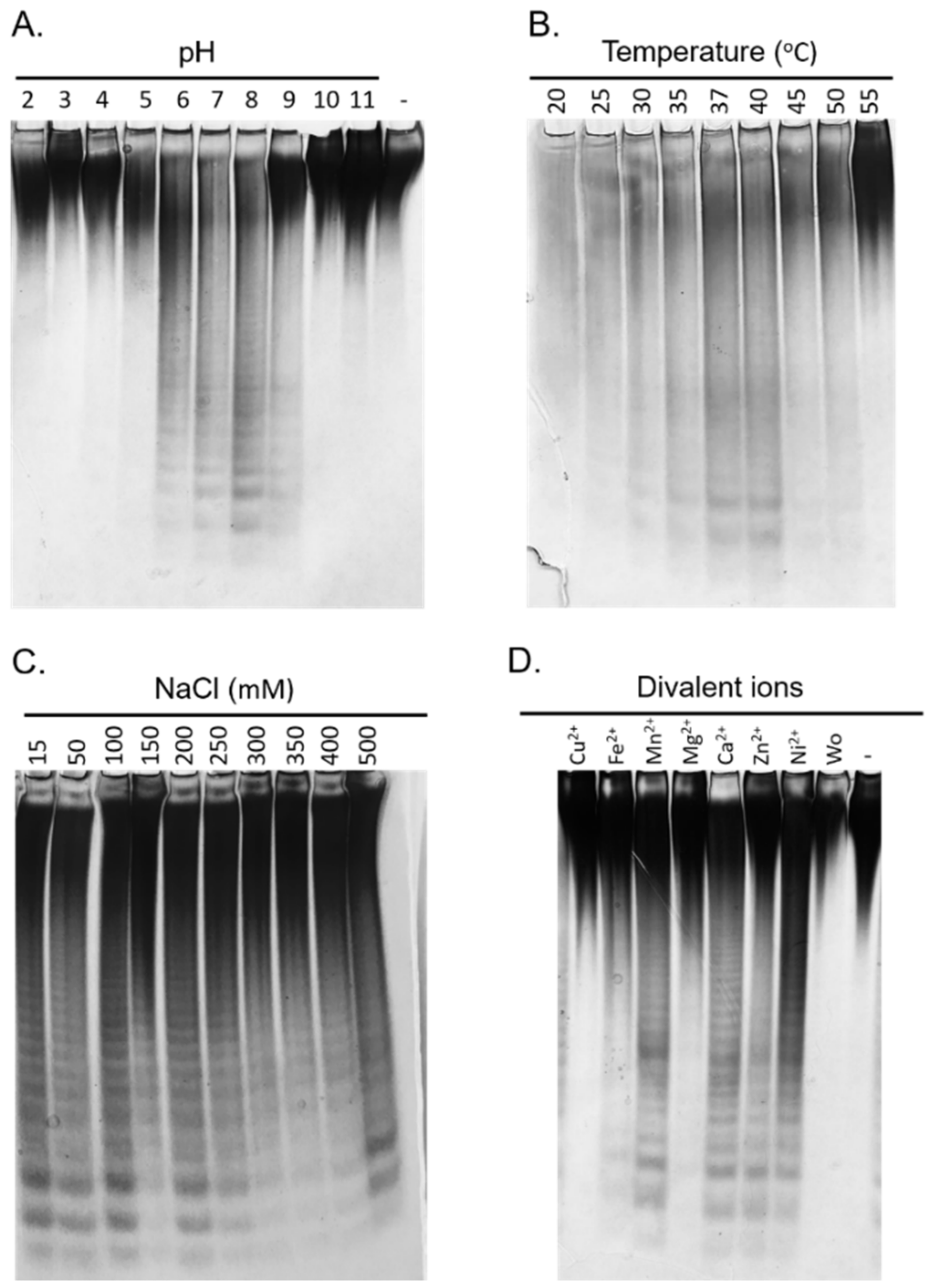
2.5. Biochemical Characterization of the Fhf1Δ470 Fucoidanase
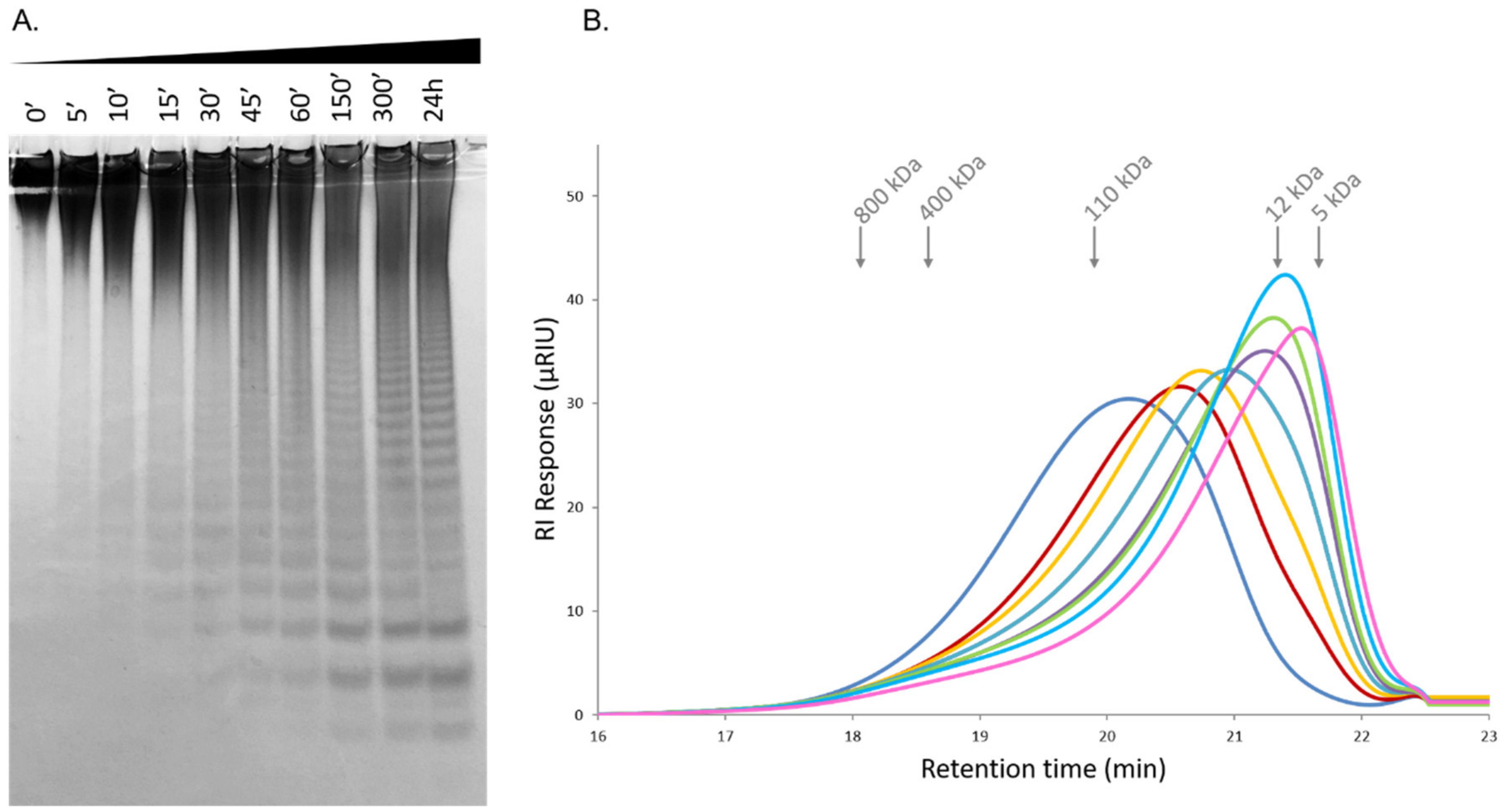
2.6. Time-Course Hydrolysis of F. evanescens Fucoidan Using Fhf1Δ470
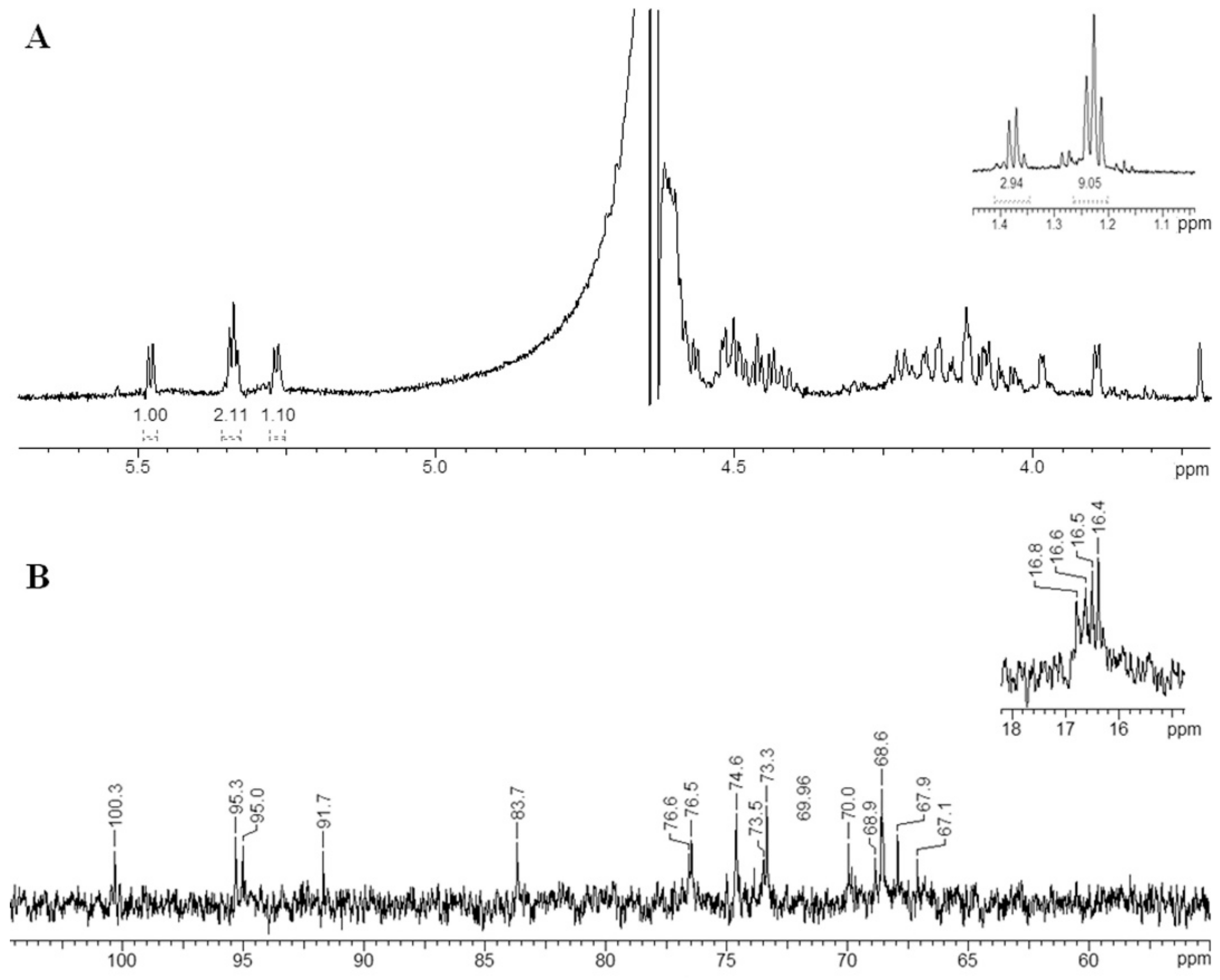
2.7. Structural Determination of the Fucoidanase Hydrolysis Products
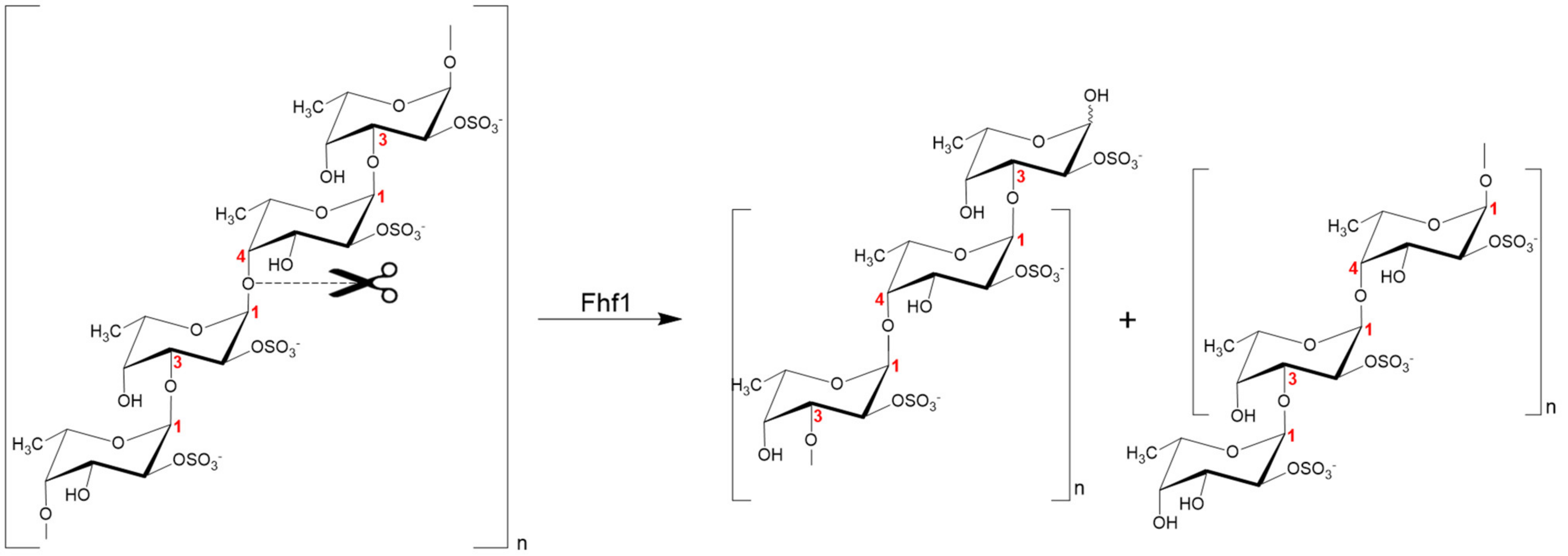
2.8. Proposed Fhf1 Mode of Action
3. Materials and Methods
3.1. Fucoidan Substrates
3.2. Identification of the Fhf1 Gene and Sequence Analysis
3.3. Cloning of the Fhf1 and Fhf1Δ470 Genes
3.4. Production of Recombinant Enzymes
3.5. SDS-PAGE Electrophoresis
3.6. Western Blot Analysis
3.7. Activity Assays
3.8. Carbohydrate–Polyacrylamide Gel Electrophoresis (C-PAGE)
3.9. HPSEC-RI Analysis
3.10. Oligosaccharides Isolation
3.11. Nuclear Magnetic Resonance Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Berteau, O. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Fitton, J.; Stringer, D.; Karpiniec, S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Simón-Vázquez, R.; Giménez-Abián, J.F.; Díaz, J.F.; González-Fernández, Á.; Domínguez, H. Fucoidans: The importance of processing on their anti-tumoral properties. Algal Res. 2020, 45, 101748. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Fucoidans from Brown Seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural Characteristics and Anticancer Activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.-S.; Kim, E. Fucoidan from Seaweed Fucus vesiculosus Inhibits Migration and Invasion of Human Lung Cancer Cell via PI3K-Akt-mTOR Pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.-Y. Fucoidan as a Marine Anticancer Agent in Preclinical Development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.; Ushakova, N.; Zyuzina, K.; Bilan, M.; Elizarova, A.; Somonova, O.; Madzhuga, A.; Krylov, V.; Preobrazhenskaya, M.; Usov, A.; et al. Influence of Fucoidans on Hemostatic System. Mar. Drugs 2013, 11, 2444–2458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef]
- Dörschmann, P.; Mikkelsen, M.D.; Thi, T.N.; Roider, J.; Meyer, A.S.; Klettner, A. Effects of a Newly Developed Enzyme-Assisted Extraction Method on the Biological Activities of Fucoidans in Ocular Cells. Mar. Drugs 2020, 18, 282. [Google Scholar] [CrossRef]
- Flórez-Méndez, J.; González, L. Role of the consumption of fucoidans and beta-glucans on human health: An update of the literature. Rev. Chil. Nutr. 2019, 46, 768–775. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, L.; Wang, F.; Wang, Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohydr. Polym. 2019, 225, 115211. [Google Scholar] [CrossRef]
- Song, M.Y.; Ku, S.K.; Kim, H.J.; Han, J.S. Low molecular weight fucoidan ameliorating the chronic cisplatin-induced delayed gastrointestinal motility in rats. Food Chem. Toxicol. 2012, 50, 4468–4478. [Google Scholar] [CrossRef]
- Kusaykin, M.I.; Silchenko, A.S.; Zakharenko, A.M.; Zvyagintseva, T.N. Fucoidanases. Glycobiology 2015, cwv072. [Google Scholar] [CrossRef] [PubMed]
- Hifney, A.F.; Gomaa, M.; Fawzy, M.A.; Abdel-Gawad, K.M. Optimizing a Low-Cost Production Process of Crude Fucoidanase by Dendryphiella arenaria Utilizing Cystoseira trinodis (Phaeophyceae) and Enzymatic Hydrolysis of the Brown Algal Biomass. Waste Biomass Valor 2019, 10, 2773–2781. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucl. Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Arai, Y.; Yamaoka, M.; Komatsu, F.; Yagi, H.; Suzuki, H.; Ohshiro, T. Identification and characterization of the fucoidanase gene from Luteolibacter algae H18. J. Biosci. Bioeng. 2018, 126, 567–572. [Google Scholar] [CrossRef]
- Shen, J.; Chang, Y.; Zhang, Y.; Mei, X.; Xue, C. Discovery and Characterization of an Endo-1,3-Fucanase From Marine Bacterium Wenyingzhuangia fucanilytica: A Novel Glycoside Hydrolase Family. Front. Microbiol. 2020, 11, 1674. [Google Scholar] [CrossRef]
- Masanori, T.; Nobuto, K.; Takeshi, S.; Kato, I. Enzymes Capable of Degrading a Sulfated-Fucose-Containing Polysaccharide and Their Encoding Genes. U.S. Patent 6489155B1, 3 December 2002. [Google Scholar]
- Colin, S.; Deniaud, E.; Jam, M.; Descamps, V.; Chevolot, Y.; Kervarec, N.; Yvin, J.-C.; Barbeyron, T.; Michel, G.; Kloareg, B. Cloning and biochemical characterization of the fucanase FcnA: Definition of a novel glycoside hydrolase family specific for sulfated fucans. Glycobiology 2006, 16, 1021–1032. [Google Scholar] [CrossRef]
- Zueva, A.O.; Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Usoltseva, R.V.; Kalinovsky, A.I.; Kurilenko, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Expression and biochemical characterization of two recombinant fucoidanases from the marine bacterium Wenyingzhuangia fucanilytica CZ1127T. Int. J. Biol. Macromol. 2020, 164, 3025–3037. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Ustyuzhanina, N.E.; Kusaykin, M.I.; Krylov, V.B.; Shashkov, A.S.; Dmitrenok, A.S.; Usoltseva, R.V.; Zueva, A.O.; Nifantiev, N.E.; Zvyagintseva, T.N. Expression and biochemical characterization and substrate specificity of the fucoidanase from Formosa algae. Glycobiology 2017. [Google Scholar] [CrossRef]
- Vickers, C.; Liu, F.; Abe, K.; Salama-Alber, O.; Jenkins, M.; Springate, C.M.K.; Burke, J.E.; Withers, S.G.; Boraston, A.B. Endo-fucoidan hydrolases from glycoside hydrolase family 107 (GH107) display structural and mechanistic similarities to α-l-fucosidases from GH29. J. Biol. Chem. 2018, 293, 18296–18308. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Tanaka, R.; Mizutani, Y.; Shibata, T.; Miyake, H.; Iehata, S.; Mori, T.; Kuroda, K.; Ueda, M. Genome Sequence of Formosa haliotis Strain MA1, a Brown Alga-Degrading Bacterium Isolated from the Gut of Abalone Haliotis gigantea. Genome Announc. 2016, 4, e01312-16. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhu, Y.; LaFrentz, B.R.; Evenhuis, J.P.; Hunnicutt, D.W.; Conrad, R.A.; Barbier, P.; Gullstrand, C.W.; Roets, J.E.; Powers, J.L.; et al. The Type IX Secretion System Is Required for Virulence of the Fish Pathogen Flavobacterium columnare. Appl. Environ. Microbiol. 2017, 83, e01769-17. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Dickens, N.J.; Beatson, S.; Ponting, C.P. Cadherin-like domains in α-dystroglycan, α/ε-sarcoglycan and yeast and bacterial proteins. Curr. Biol. 2002, 12, R197–R199. [Google Scholar] [CrossRef]
- Schultz-Johansen, M.; Cueff, M.; Hardouin, K.; Jam, M.; Larocque, R.; Glaring, M.A.; Hervé, C.; Czjzek, M.; Stougaard, P. Discovery and screening of novel metagenome-derived GH 107 enzymes targeting sulfated fucans from brown algae. FEBS J 2018, 285, 4281–4295. [Google Scholar] [CrossRef]
- Nelson, S.S.; Bollampalli, S.; McBride, M.J. SprB Is a Cell Surface Component of the Flavobacterium johnsoniae Gliding Motility Machinery. JB 2008, 190, 2851–2857. [Google Scholar] [CrossRef]
- Cao, H.; Mikkelsen, M.; Lezyk, M.; Bui, L.; Tran, V.; Silchenko, A.; Kusaykin, M.; Pham, T.; Truong, B.; Holck, J.; et al. Novel Enzyme Actions for Sulphated Galactofucan Depolymerisation and a New Engineering Strategy for Molecular Stabilisation of Fucoidan Degrading Enzymes. Mar. Drugs 2018, 16, 422. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Nazarenko, E.L.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural analysis of a fucoidan from the brown alga Fucus evanescens by MALDI-TOF and tandem ESI mass spectrometry. Carbohydr. Res. 2009, 344, 779–787. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.-M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.; Kusaykin, M.; Kurilenko, V.; Zakharenko, A.; Isakov, V.; Zaporozhets, T.; Gazha, A.; Zvyagintseva, T. Hydrolysis of Fucoidan by Fucoidanase Isolated from the Marine Bacterium, Formosa algae. Mar. Drugs 2013, 11, 2413–2430. [Google Scholar] [CrossRef] [PubMed]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Popivnich, I.B.; Isakov, V.V.; Scobun, A.S.; Sundukova, E.V.; Elyakova, L.A. A new procedure for the separation of water-soluble polysaccharides from brown seaweeds. Carbohydr. Res. 1999, 322, 32–39. [Google Scholar] [CrossRef]
- Kusaykin, M.I.; Chizhov, A.O.; Grachev, A.A.; Alekseeva, S.A.; Bakunina, I.Y.; Nedashkovskaya, O.I.; Sova, V.V.; Zvyagintseva, T.N. A comparative study of specificity of fucoidanases from marine microorganisms and invertebrates. J. Appl. Phycol. 2006, 18, 369–373. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Rasin, A.B.; Zueva, A.O.; Kusaykin, M.I.; Zvyagintseva, T.N.; Kalinovsky, A.I.; Kurilenko, V.V.; Ermakova, S.P. Fucoidan Sulfatases from Marine Bacterium Wenyingzhuangia fucanilytica CZ1127T. Biomolecules 2018, 8, 98. [Google Scholar] [CrossRef]


| Enzyme | Organism | Accession Number | Linkage Specificity | Homology with Fhf1 (D1 Domain) | |
|---|---|---|---|---|---|
| % Identity | % Similarity | ||||
| MfFcnA | Mariniflexile fuconivorans | CAI47003.1 | α-(1,4) | 69 | 79 |
| FFA2 | Formosa algae | WP_057784219.1 | α-(1,4) | 64 | 77 |
| SVI_0379 | Shewanella violacea | BAJ00350.1 | n.d | 25 | 42 |
| Fda1 | Alteromonas sp. | AAO00508.1 | α-(1,3) | 24 | 39 |
| Fda2 | Alteromonas sp. | AAO00509.1 | α-(1,3) | 24 | 38 |
 |
 |
| Residue | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 |
|---|---|---|---|---|---|---|
| A1 | 5.27/100.3 | 4.58/74.6 | 4.17/73.5 | 4.11/70.0 | 4.41/68.6 | 1.24/16.5 |
| B1 | 5.34/95.3 | 4.47/76.6 | 4.15/68.6 | 3.99/83.7 | 4.51/68.9 | 1.38/16.8 |
| C1 | 5.34/95.0 | 4.45/76.5 | 4.10/68.6 | 3.89/73.3 | 4.51/67.9 | 1.22/16.4 |
| D1 | 5.48/91.7 | 4.51/74.6 | 4.05/73.9 | 4.07/69.8 | 4.22/67.1 | 1.24/16.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuillemin, M.; Silchenko, A.S.; Cao, H.T.T.; Kokoulin, M.S.; Trang, V.T.D.; Holck, J.; Ermakova, S.P.; Meyer, A.S.; Mikkelsen, M.D. Functional Characterization of a New GH107 Endo-α-(1,4)-Fucoidanase from the Marine Bacterium Formosa haliotis. Mar. Drugs 2020, 18, 562. https://doi.org/10.3390/md18110562
Vuillemin M, Silchenko AS, Cao HTT, Kokoulin MS, Trang VTD, Holck J, Ermakova SP, Meyer AS, Mikkelsen MD. Functional Characterization of a New GH107 Endo-α-(1,4)-Fucoidanase from the Marine Bacterium Formosa haliotis. Marine Drugs. 2020; 18(11):562. https://doi.org/10.3390/md18110562
Chicago/Turabian StyleVuillemin, Marlene, Artem S. Silchenko, Hang Thi Thuy Cao, Maxim S. Kokoulin, Vo Thi Dieu Trang, Jesper Holck, Svetlana P. Ermakova, Anne S. Meyer, and Maria Dalgaard Mikkelsen. 2020. "Functional Characterization of a New GH107 Endo-α-(1,4)-Fucoidanase from the Marine Bacterium Formosa haliotis" Marine Drugs 18, no. 11: 562. https://doi.org/10.3390/md18110562
APA StyleVuillemin, M., Silchenko, A. S., Cao, H. T. T., Kokoulin, M. S., Trang, V. T. D., Holck, J., Ermakova, S. P., Meyer, A. S., & Mikkelsen, M. D. (2020). Functional Characterization of a New GH107 Endo-α-(1,4)-Fucoidanase from the Marine Bacterium Formosa haliotis. Marine Drugs, 18(11), 562. https://doi.org/10.3390/md18110562








