Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes Simplex Virus Type 1
Abstract
1. Introduction
2. Results and Discussion
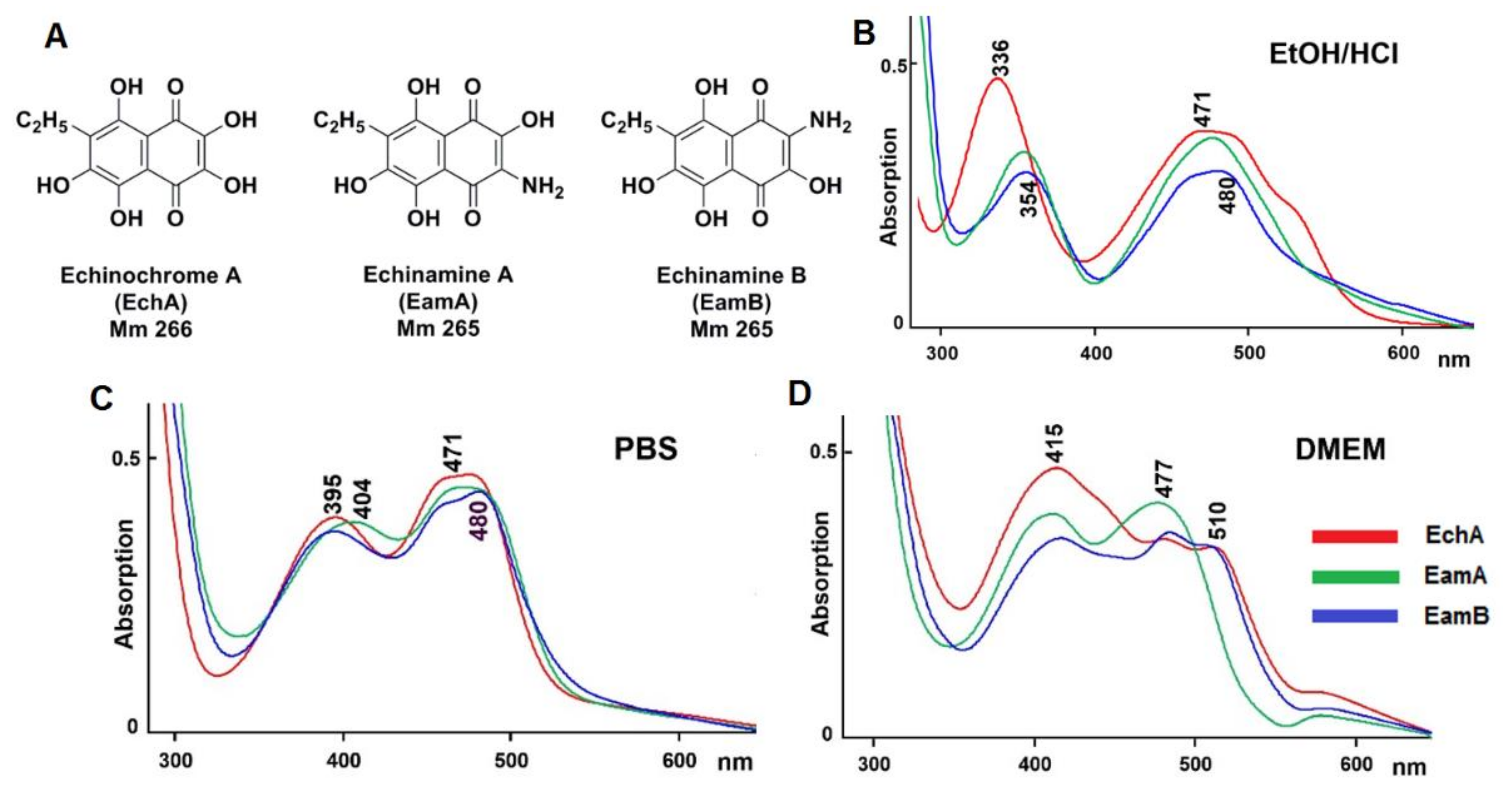
2.1. Physico-Chemical Properties of Aminated Spinochromes
2.2. Cytotoxicity and Anti-HSV-1 Activity of the Tested Compounds
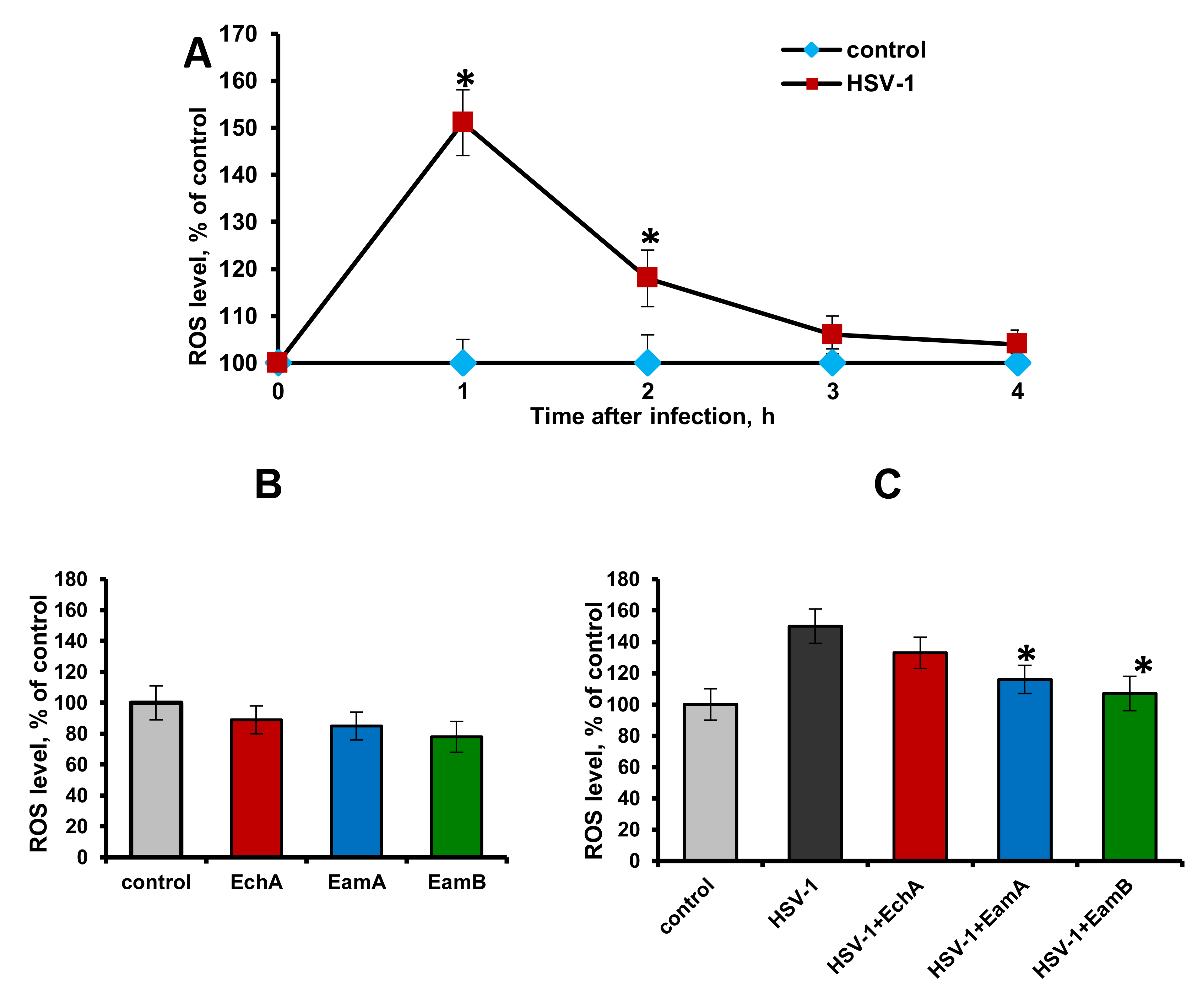
2.3. Effect of Spinochromes on HSV-1-Induced Intracellular ROS Production
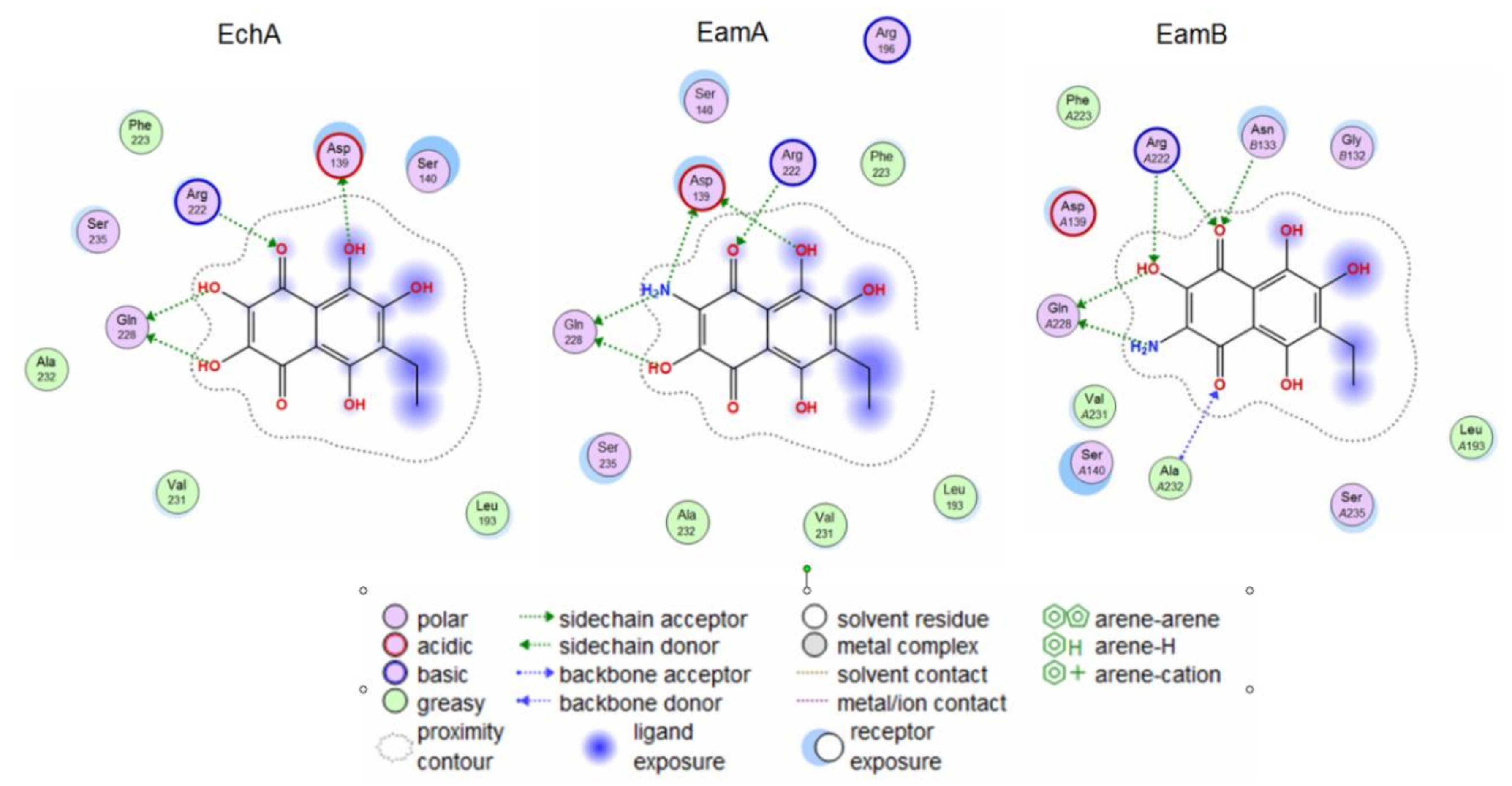
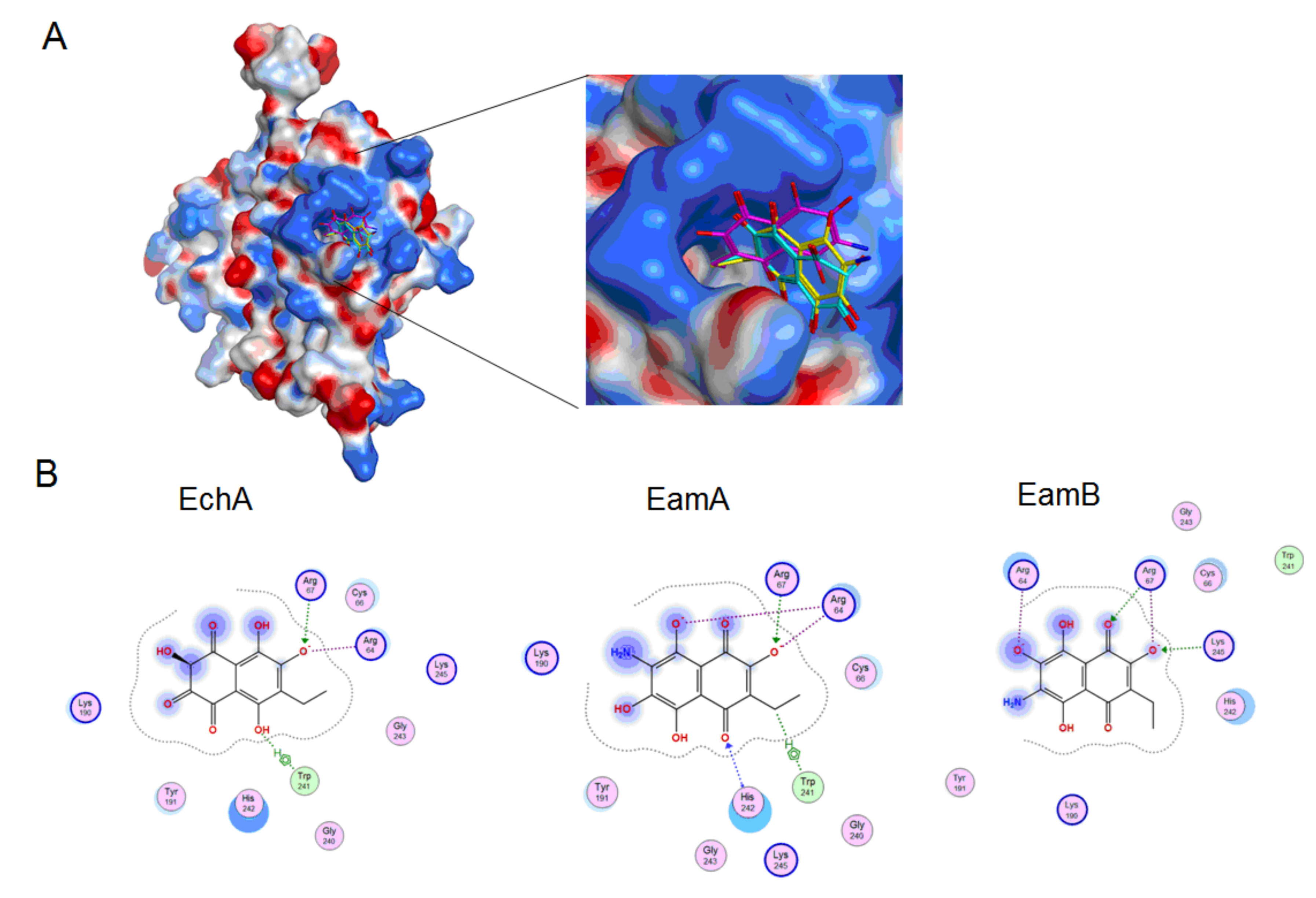
2.4. Molecular Docking
3. Materials and Methods
3.1. Reagents
3.2. Viruses and Cell Cultures
3.3. Spinochromes Isolation
3.4. Cytotoxicity of the Tested Compounds
3.5. Anti-HSV-1 Activity of the Tested Compounds
- Virucidal assay (the pretreatment of the HSV-1 with compounds). HSV-1 suspension was pre-incubated with an equal volume of DMEM or various concentrations of tested compounds for 1 h at 37 °C, then the mixture was used to infect cellular monolayers. After viral adsorption for 1 h at 37 °C, the plates were washed, covered with the maintenance medium (DMEM) containing 1% CMC for 72 h at 37 °C (5% CO2) until plaques formed.
- Time-of-addition assay. The tested compounds, at various concentrations, were added to cells at 1 h before viral infection (pretreatment of cells), at the same time with infection (simultaneous treatment) or 1 h after infection (post-treatment). To study a preventive effect, the cells were pretreated with compounds for 1 h, then infected with HSV-1 for 1 h after removal of compounds by washing and overlaid with DMEM with 1% CMC. To study the effect on virus adsorption, cells were treated with compounds and simultaneously infected with HSV-1, then overlaid with DMEM with 1% CMC after removal of the compounds and unbound virus by washing at 1 h after adsorption. To study the effect on the early stage of virus replication, the cells were infected with the HSV-1 for 1 h, and then overlaid with DMEM with 1% CMC containing different concentrations of the studied compounds after removal of virus by washing. Within all procedures, the cells were incubated for 72 h at 37 °C (5% CO2) until plaques formed.
- The attachment assay. Pre-chilled at 4 °C for 1 h Vero cells were infected with the virus (100 PFU/mL), and incubated for 3 h at 4 °C with different concentrations of the studied compounds. Then, the compounds and unbound viruses were washed away with cold PBS. The cells were supplied with DMEM containing 1% CMC and incubated for 72 h at 37 °C (5% CO2) until plaques formed.
- The penetration assay. Pre-chilled at 4 °C for 1 h Vero cells were infected with the virus (100 PFU/mL), and incubated for 3 h at 4 °C. The unbound viruses were removed with cold PBS, the cells were treated with medium containing different concentrations of the compounds, and then incubated for 1 h at 37 °C. Viruses that did not enter cells were inactivated with citrate buffer (pH 3.0). Then, the cells were washed with PBS, supplied with DMEM containing 1% CMC, and incubated for 72 h at 37 °C (5% CO2) until plaques formed.
3.6. Measurement of the ROS Level
- ROS production in HSV-1-infected cells: Cells were treated with HSV-1 (100 PFU/mL) and cultured at 37 °C. After 1, 2, 3, and 4 h, the cells were washed with PBS and the ROS level was measured in infected (HSV-1) and uninfected cells (control).
- ROS production in control cells with the presence of spinochromes: The monolayer of cells treated with the tested spinochromes (5 μg/mL, 100 μL/well) and incubated for 2 h at 37 °C. After washing with PBS, the ROS level was measured. Untreated cells were used as the control.
- ROS production in control and HSV-1-infected cells with the presence of spinochromes: HSV-1 (100 PFU/mL) was mixed with the tested spinochromes (5 μg/mL) in a 1:1 (v/v) ratio and incubated for 1 h at 37 °C. Then, the mixture and HSV-1 (100 PFU/mL) were applied to a monolayer of Vero cells. After 1 h of incubation at 37 °C, the cells were washed with PBS and the ROS level was measured. Uninfected and HSV-1-infected cells were used as the control.
3.7. Molecular Docking
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Herpes Simplex Virus. News Bulletin. 2016. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 22 October 2020).
- Banerjee, A.; Kulkarni, S.; Mukherjee, A. Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home. Front. Microbiol. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef]
- Turner, L.D.; Beckingsale, P. Acyclovir-resistant herpetic keratitis in a solid-organ transplant recipient on systemic immunosuppression. Clin. Ophthalmol. 2013, 7, 229–232. [Google Scholar] [CrossRef]
- Pan, D.; Kaye, S.B.; Hopkins, M.; Kirwan, R.; Hart, I.J.; Coen, D.M. Common and new acyclovir resistant herpes simplex virus-1 mutants causing bilateral recurrent herpetic keratitis in an immunocompetent patient. J. Infect. Dis. 2014, 209, 345–349. [Google Scholar] [CrossRef]
- Santana, S.; Sastre, I.; Recuero, M.; Bullido, M.J.; Aldudo, J. Oxidative stress enhances neurodegeneration markers induced by herpes simplex virus type 1 infection in human neuroblastoma cells. PLoS ONE 2013, 8, e75842. [Google Scholar] [CrossRef]
- Georgieva, A.; Vilhelmova, N.; Muckova, L.; Tzvetanova, E.; Alexandrova, A.; Milevaet, M. Alterations in Oxidative Stress Parameters in MDBK Cells, Infected by Herpes Simplex Virus-1. Compt. Rend. Acad. Bulg. Sci. 2017, 70, 731–738. [Google Scholar]
- Marino-Merlo, F.; Papaianni, E.; Frezza, C.; Pedatella, S.; De Nisco, M.; Macchi, B.; Grelli, S.; Mastino, A. NF-κB-Dependent Production of ROS and Restriction of HSV-1 Infection in U937 Monocytic Cells. Viruses 2019, 11, 428. [Google Scholar] [CrossRef]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant therapy: Current status and future prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Kandasamy, D.; Nithyanand, P. A review on antioxidant activity of marine organisms. Int. J. Chem. Tech. Res. 2014, 6, 3431–3436. [Google Scholar]
- Torky, Z.A.; Hossain, M.M. Pharmacological evaluation of the hibiscus herbal extract against herpes simplex virus-type 1 as an antiviral drug in vitro. Int. J. Virol. 2017, 13, 68–79. [Google Scholar] [CrossRef]
- Thomson, R.H. Naturally Occurring Quinones, 2nd ed.; Academic Press: London, UK; New York, NY, USA, 1971; 734p. [Google Scholar]
- Hou, Y.; Vasileva, E.A.; Carne, A.; McConnell, M.; Bekhit, A.E.D.A.; Mishchenko, N.P. Naphthoquinones of the spinochrome class: Occurrence, isolation, biosynthesis and biomedical applications. RSC Adv. 2018, 8, 32637–32650. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Krishtopina, A.S.; Makarov, V.G. Naphthoquinone pigments from sea urchins: Chemistry and pharmacology. Phytochem. Rev. 2018, 17, 509–534. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and antioxidant properties of echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef]
- Krylova, N.V.; Leneva, I.A.; Fedoreev, S.A.; Ebralidze, L.K.; Mishchenko, N.P.; Vasileva, E.A.; Falynskova, I.N.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A. Activity of compounds containing echinochrome A against herpes simplex virus type 2 in vitro and in vivo. J. Microbiol. Epidemiol. Immunobiol. 2019, 6, 56–64. [Google Scholar] [CrossRef]
- Rubilar Panasiuk, C.T.; Barbieri, E.S.; Gázquez, A.; Avaro, M.; Vera Piombo, M.; Gittardi Calderón, A.A.; Seiler, E.N.; Fernandez, J.P.; Sepúlveda, L.R.; Chaar, F. In Silico Analysis of Sea Urchin Pigments as Potential Therapeutic Agents Against SARS-CoV-2: Main Protease (Mpro) as a Target. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Barbieri, E.S.; Rubilar Panasiuk, C.T.; Gázquez, A.; Avaro, M.; Seiler, E.N.; Vera Piombo, M.; Gittardi Calderón, A.A.; Chaar, F.; Fernandez, J.P.; Sepúlveda, L.R. Sea Urchin Pigments as Potential Therapeutic Agents Against the Spike Protein of SARS-CoV-2 Based on in Silico Analysis. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Fedoreyev, S.A.; Pokhilo, N.D.; Anufriev, V.P.; Denisenko, V.A.; Glazunov, V.P. Echinamines A and B, first aminated hydroxynaphthazarins from the sea urchin Scaphechinus mirabilis. J. Nat. Prod. 2005, 68, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, E.A.; Mishchenko, N.P.; Zadorozhny, P.A.; Fedoreyev, S.A. New aminonaphthoquinone from the sea urchins Strongylocentrotus pallidus and Mesocentrotus nudus. Nat. Prod. Commun. 2016, 11, 821–824. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Qin, L.; Zhu, B.W.; Wang, X.D.; Tan, H.; Yang, J.F.; Li, D.M.; Dong, X.P.; Wu, H.T.; Sun, L.M. Extraction and antioxidant property of polyhydroxylated naphthoquinone pigments from spines of purple sea urchin. Food Chem. 2011, 129, 1591–1597. [Google Scholar] [CrossRef]
- Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A. Diversity of polyhydroxynaphthoquinone pigments in North Pacific sea urchins. Chem. Biodivers. 2017, 14, e1700182. [Google Scholar] [CrossRef]
- Hou, Y.; Vasileva, E.A.; Mishchenko, N.P.; Carne, A.; McConnell, M.; Bekhit, A.E.A. Extraction, structural characterization and stability of polyhydroxylated naphthoquinones from shell and spine of New Zealand sea urchin (Evechinus chloroticus). Food Chem. 2019, 272, 379–387. [Google Scholar] [CrossRef]
- Powell, C.; Hughes, A.D.; Kelly, M.S.; Conner, S.; McDougall, G.J. Extraction and identification of antioxidant polyhydroxynaphthoquinone pigments from the sea urchin, Psammechinus miliaris. LWT-Food Sci. Technol. 2014, 59, 455–460. [Google Scholar] [CrossRef]
- Soleimani, S.; Yousefzadi, M.; Moe, S. Antibacterial and antioxidant characteristics of pigments and coelomic fluid of sea urchin, Echinodermata Mathaei species, from the Persian Gulf. J. Kerman Univ. Med. Sci. 2015, 22, 614–628. [Google Scholar]
- Kuwahara, R.; Hatate, H.; Yuki, T.; Murata, H.; Tanaka, R.; Hama, Y. Antioxidant property of polyhydroxylated naphthoquinone pigments from shells of purple sea urchin Anthocidaris crassispina. LWT-Food Sci. Technol. 2009, 42, 1296–1300. [Google Scholar] [CrossRef]
- Brasseur, L.; Hennebert, E.; Fievez, L.; Caulier, G.; Bureau, F.; Tafforeau, L.; Flammang, P.; Gerbaux, P.; Eeckhaut, I. The roles of spinochromes in four shallow water tropical sea urchins and their potential as bioactive pharmacological agents. Mar. Drugs 2017, 15, 179. [Google Scholar] [CrossRef]
- Pokhilo, N.D.; Shuvalova, M.I.; Lebedko, M.V.; Sopelnyak, G.I.; Yakubovskaya, A.Y.; Mischenko, N.P.; Fedoreyev, S.A.; Anufriev, V.P. Synthesis of Echinamines A and B, the first aminated hydroxynaphthazarins produced by the sea urchin Scaphechinus mirabilis and Its Analogues. J. Nat. Prod. 2006, 69, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Mel’man, G.I.; Mishchenko, N.P.; Denisenko, V.A.; Berdyshev, D.V.; Glazunov, V.P.; Anufriev, V.F. Amination of 2-hydroxy-and 2, 3-dihydroxynaphthazarins. Synthesis of echinamines A and B, metabolites produced by the sand dollar Scaphechinus mirabilis. Russ. J. Org. Chem. 2009, 45, 37–43. [Google Scholar] [CrossRef]
- Pokhilo, N.D.; Yakubovskaya, A.Y.; Denisenko, V.A.; Anufriev, V.P. Regiospecificity in the reaction of 2,3-dichloronaphthazarins with azide anions. Synthesis of echinamine A—A metabolite produced by the sea urchin Scaphechinus mirabilis. Tetrahedron Lett. 2006, 47, 1385–1387. [Google Scholar] [CrossRef]
- Polonik, S.G.; Polonik, N.S.; Denisenko, V.A.; Moiseenko, O.P.; Anufriev, V.F. Synthesis and transformation of 2-amino-3-hydroxynaphthazarin. Russ. J. Org. Chem. 2009, 45, 1410–1411. [Google Scholar] [CrossRef]
- Polonik, N.S.; Anufriev, V.P.; Polonik, S.G. Short and Regiospecific Synthesis of Echinamine A—The Pigment of Sea Urchin Scaphechinus mirabilis. Nat. Prod. Commun. 2011, 6, 217–222. [Google Scholar]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aided Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pKa prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Petukh, M.; Stefl, S.; Alexov, E. The role of protonation states in ligand-receptor recognition and binding. Curr. Pharm. Des. 2013, 19, 4182–4190. [Google Scholar] [CrossRef]
- Dai, W.; Wu, Y.; Bi, J.; Wang, S.; Li, F.; Kong, W.; Barbier, J.; Cintrat, J.C.; Gao, F.; Gillet, D.; et al. Antiviral Effects of ABMA against Herpes Simplex Virus Type 2 In Vitro and In Vivo. Viruses 2018, 10, 119. [Google Scholar] [CrossRef]
- Klysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses—A Review. Curr. Med. Chem. 2020, 27, 4118–4137. [Google Scholar] [CrossRef]
- Tai, C.J.; Li, C.L.; Tai, C.J.; Wang, C.K.; Lin, L.T. Early Viral Entry Assays for the Identification and Evaluation of Antiviral Compounds. J. Vis. Exp. 2015, 104, e53124. [Google Scholar] [CrossRef]
- Wang, G.; Hernandez, R.; Weninger, K.; Brown, D.T. Infection of cells by Sindbis virus at low temperature. Virology 2007, 362, 461–467. [Google Scholar] [CrossRef][Green Version]
- Akhtar, J.; Shukla, D. Viral entry mechanisms: Cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009, 276, 7228–7236. [Google Scholar] [CrossRef]
- Luo, Z.; Kuang, X.P.; Zhou, Q.Q.; Yan, C.Y.; Li, W.; Gong, H.B.; Kurihara, H.; Li, W.X.; Li, Y.F.; He, R.R. Inhibitory effects of baicalein against herpes simplex virus type 1. Acta Pharm. Sin. B 2020, in press. [Google Scholar] [CrossRef]
- Arii, J.; Kawaguchi, Y. The Role of HSV Glycoproteins in Mediating Cell Entry. In Human Herpesviruses. Advances in Experimental Medicine and Biology; Kawaguchi, Y., Mori, Y., Kimura, H., Eds.; Springer: Singapore, 2018; Volume 1045, pp. 3–21. [Google Scholar]
- O’Donnell, C.D.; Kovacs, M.; Akhtar, J.; Valyi-Nagy, T.; Shukla, D. Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology 2010, 397, 389–398. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Hao, C.; Zhang, Y.; Wang, Z.; Wang, S.; Wang, W. Inhibition of herpes simplex virus by myricetin through targeting viral gD protein and cellular EGFR/PI3K/Akt pathway. Antivir. Res. 2020, 177, 104714. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Weislow, O.S.; Kiser, R.; Fine, D.L.; Bader, J.; Shoemaker, R.H.; Boyd, M.R. New soluble-formazan assay for HIV-1 cytopathic effects: Application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 1989, 81, 577–586. [Google Scholar] [CrossRef]
- Killington, R.A.; Powell, K.L. Growth, assay, and purification of herpesviruses. In Virology: A Practical Approach; Mahy, B.W., Ed.; IRL Press: Oxford, UK, 1991; pp. 207–236. [Google Scholar]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The Amphibian Antimicrobial Peptide Temporin B Inhibits In Vitro Herpes Simplex Virus 1 Infection. Antimicrob. Agents Chemother. 2018, 62, e02367. [Google Scholar] [CrossRef]
- Cardozo, F.T.; Camelini, C.M.; Mascarello, A.; Rossi, M.J.; Nunes, R.J.; Barardi, C.R.; de Mendonca, M.M.; Simoes, C.M. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antivir. Res. 2011, 92, 108–114. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Gerasimenko, A.V.; Fedoreev, S.A.; Mishchenko, N.P. Molecular and Crystal Structure of the Echinochrome Complex with Dioxane. Krist. Russ. Crystallogr. Rep. 2006, 51, 42–47. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE); Chemical Computing Group ULC: Montreal, QC, Canada, 2019.

| Compounds | CC50 | IC50 (SI) | |||||
|---|---|---|---|---|---|---|---|
| Pretreatment of the Virus | Pretreatment of Cells | Attachment | Penetration | Simultaneous Treatment | Post-Infection Treatment | ||
| EchA | 142 ± 6 | 4.1 ± 0.6 (34.6) | 83 ± 14 (1.7) | 33 ± 6 (4.3) | 59 ± 9 (2.4) | 35 ± 6 (4.1) | 95 ± 18 (1.5) |
| EamA | 146 ± 7 | 2.9 ± 0.4 (50.3) * | 96 ± 16 (1.5) | 25 ± 4 (5.8) | 56 ± 8 (2.6) | 34 ± 6 (4.3) | 113 ± 23 (1.3) |
| EamB | 137 ± 6 | 1.7 ± 0.2 (80.6) * | 92 ± 14 (1.5) | 16 ± 3 (8.5) * | 54 ± 7 (2.5) | 30 ± 5 (4.6) | 80 ± 14 (1.7) |
| ACV | >1000 | NA | NA | NA | NA | 2.1 ± 0.4 (476) | 0.1 ± 0.02 (10,000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishchenko, N.P.; Krylova, N.V.; Iunikhina, O.V.; Vasileva, E.A.; Likhatskaya, G.N.; Pislyagin, E.A.; Tarbeeva, D.V.; Dmitrenok, P.S.; Fedoreyev, S.A. Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes Simplex Virus Type 1. Mar. Drugs 2020, 18, 550. https://doi.org/10.3390/md18110550
Mishchenko NP, Krylova NV, Iunikhina OV, Vasileva EA, Likhatskaya GN, Pislyagin EA, Tarbeeva DV, Dmitrenok PS, Fedoreyev SA. Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes Simplex Virus Type 1. Marine Drugs. 2020; 18(11):550. https://doi.org/10.3390/md18110550
Chicago/Turabian StyleMishchenko, Natalia P., Natalia V. Krylova, Olga V. Iunikhina, Elena A. Vasileva, Galina N. Likhatskaya, Evgeny A. Pislyagin, Darya V. Tarbeeva, Pavel S. Dmitrenok, and Sergey A. Fedoreyev. 2020. "Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes Simplex Virus Type 1" Marine Drugs 18, no. 11: 550. https://doi.org/10.3390/md18110550
APA StyleMishchenko, N. P., Krylova, N. V., Iunikhina, O. V., Vasileva, E. A., Likhatskaya, G. N., Pislyagin, E. A., Tarbeeva, D. V., Dmitrenok, P. S., & Fedoreyev, S. A. (2020). Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes Simplex Virus Type 1. Marine Drugs, 18(11), 550. https://doi.org/10.3390/md18110550






