Lipoxygenase Pathways in Diatoms: Occurrence and Correlation with Grazer Toxicity in Four Benthic Species
Abstract
1. Introduction
2. Results
2.1. LC-MS Analysis of LOFAs
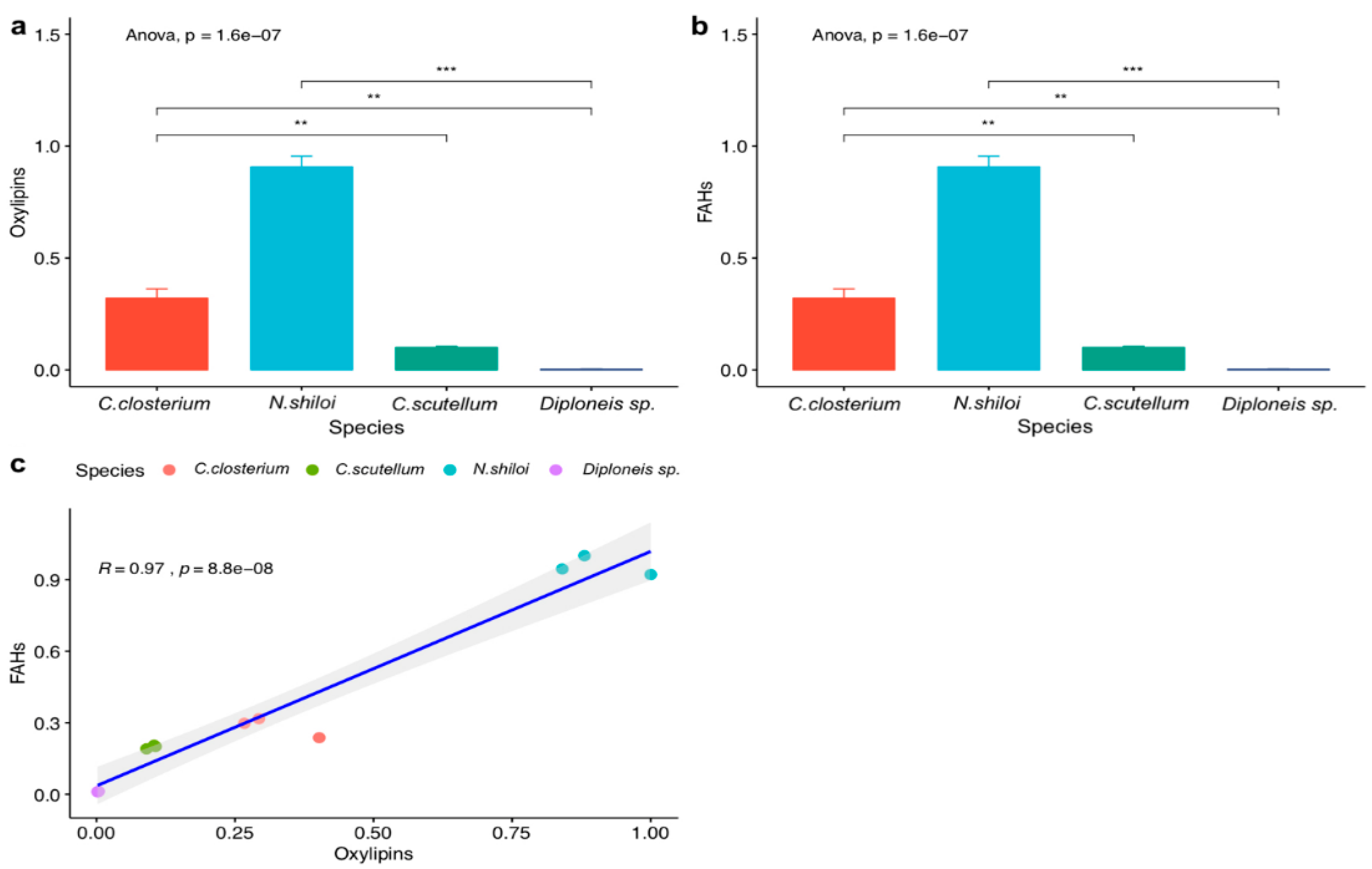
2.2. FOX2 Assay
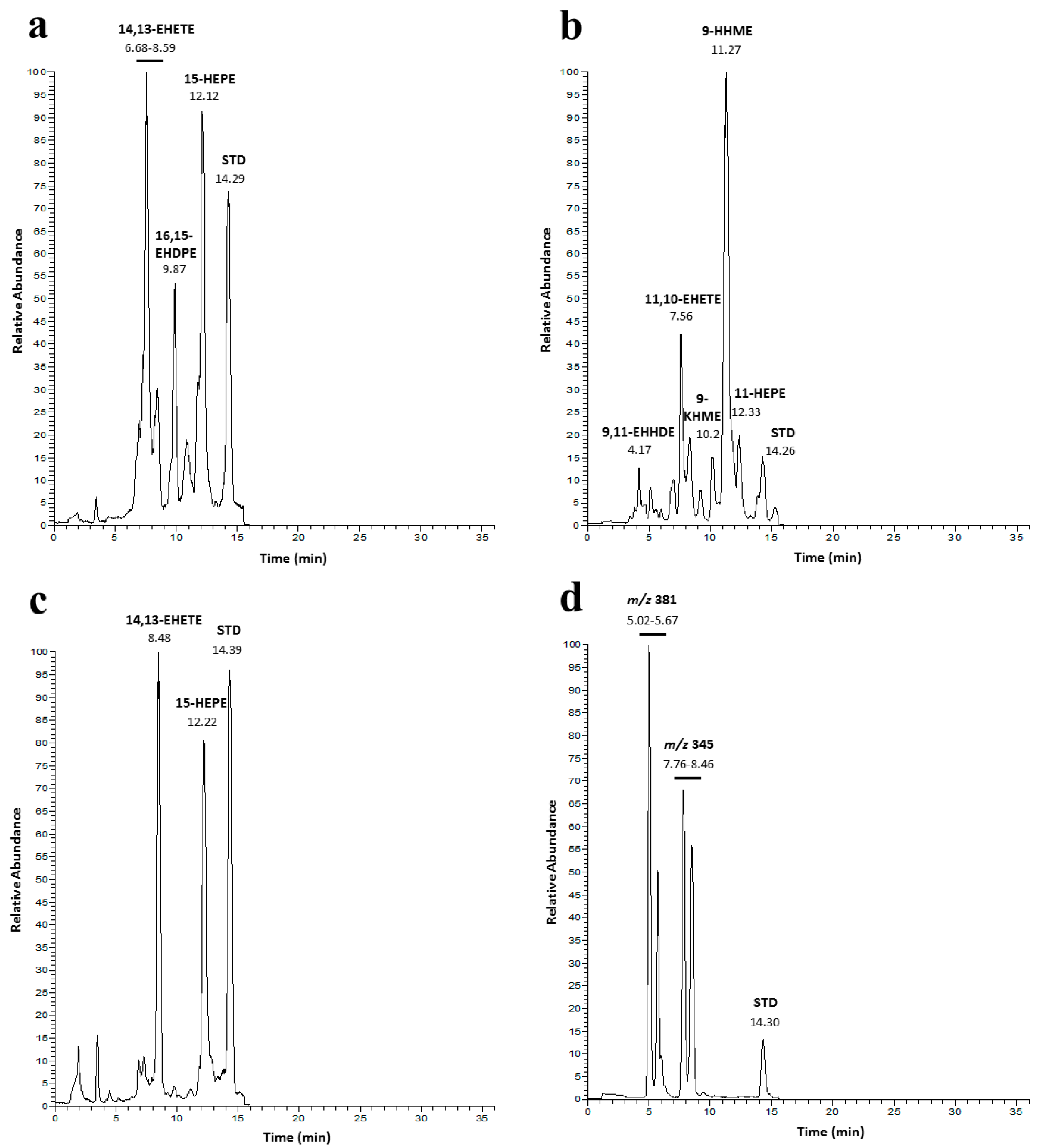
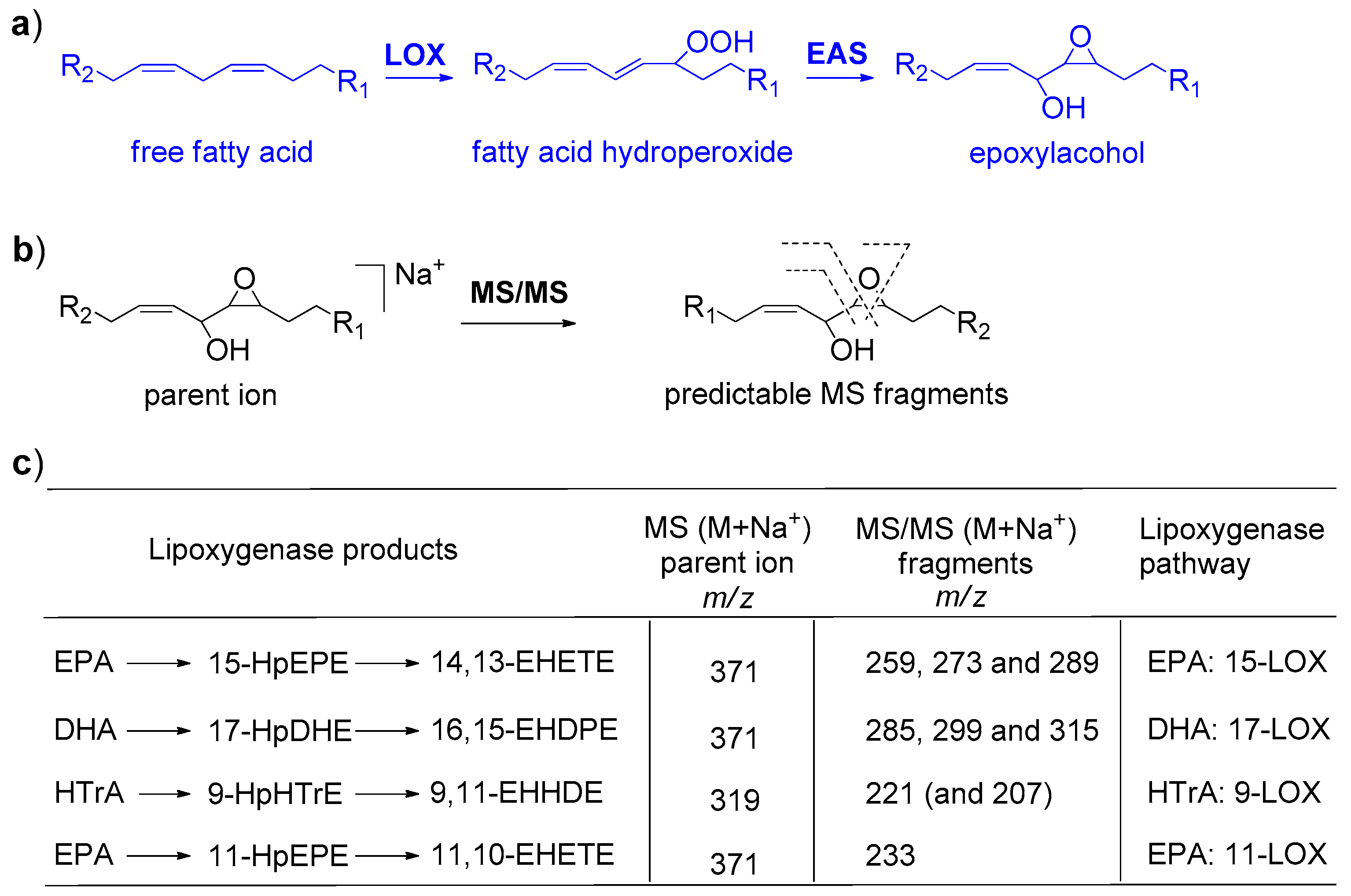
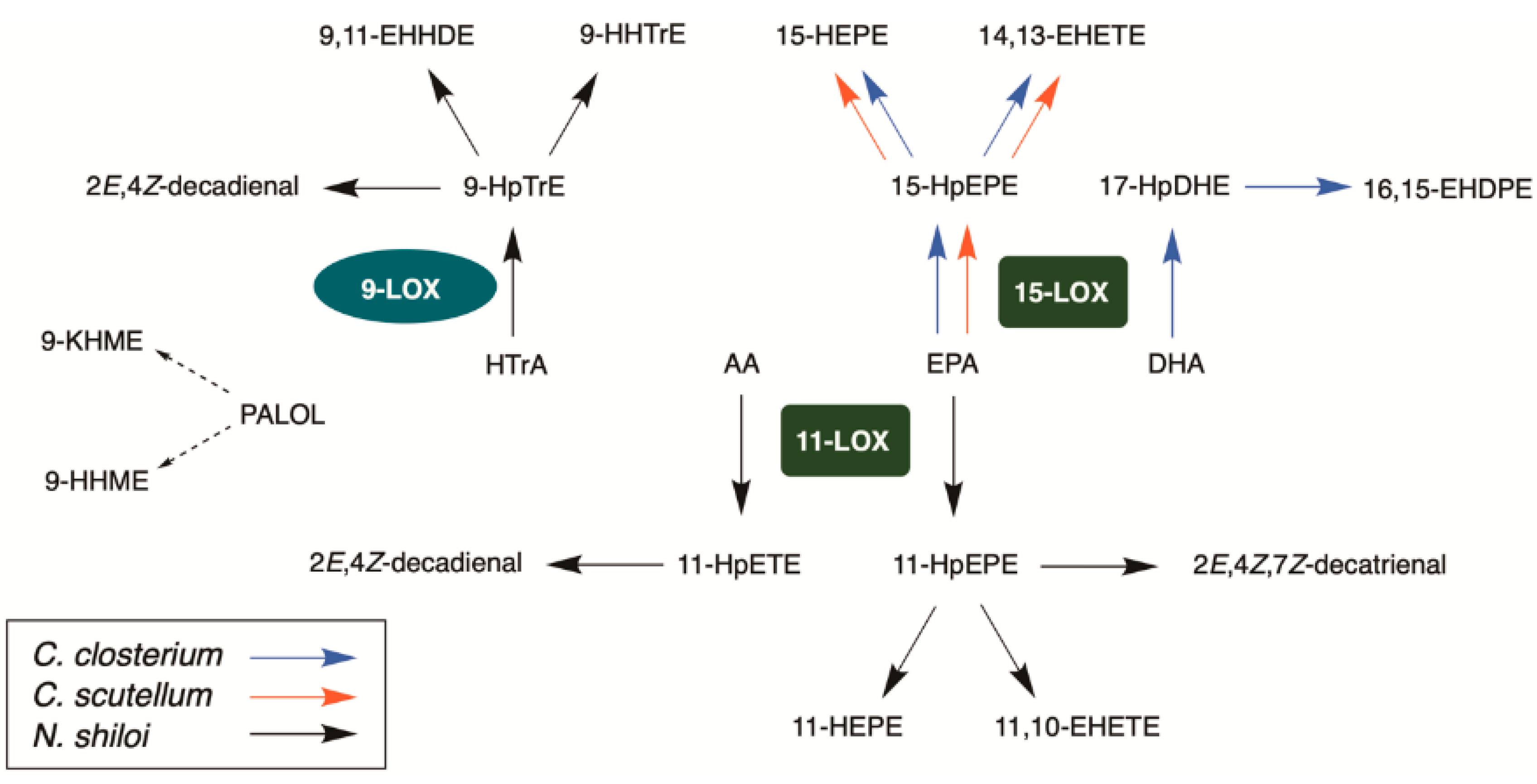
2.3. Identification of Lipoxygenase Pathways of C. closterium
2.4. Identification of Lipoxygenase Pathway of C. scutellum
2.5. Identification of Lipoxygenase Pathway of N. shiloi
2.6. GC-MS Analysis of PUAs
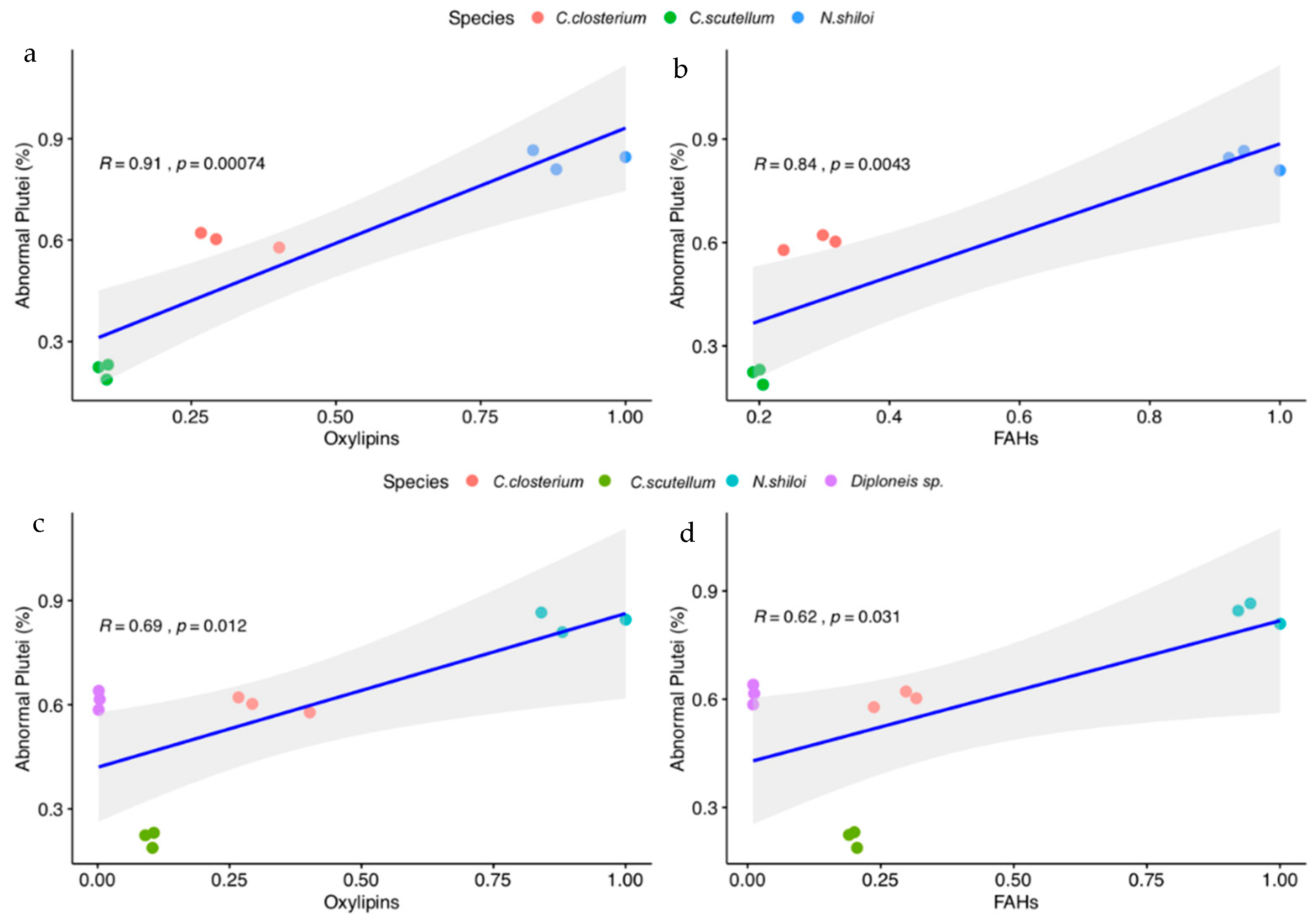
2.7. Effect of LOX Products on Sea Urchin Development
3. Discussion
4. Materials and Methods
4.1. General
4.2. Cell Culturing and Collection
4.3. Elemental Analysis by CHNS Analyzer
4.4. GC-MS Analysis of Total Fatty Acids
4.5. LOX Activation and Oxylipin Extraction
4.6. Analysis of Polyunsaturated Aldehydes (PUAs) and Linear Oxygenated Fatty Acids (LOFAs)
4.7. Assessment of LOX Activity
4.8. Feeding Experiments on the Sea Urchin Paracentrotus lividus
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 11,10-EHETE | 11,12-epoxy-10-hydroxy-eicosa-5Z,8Z,14Z,17Z-tetraenoic acid |
| 11-HEPE | 11-hydroxy-eicosa-5Z,8Z,12E,14Z,17Z-pentaenoic acid |
| 11-HpEPE | 11-hydroperoxy-eicosa-5Z,8Z,12E,14Z,17Z-pentaenoic acid |
| 11-HpETE | 11-hydroperoxy-eicosa-5Z,8Z,12E,14Z -tetraenoic acid |
| 14,13-EHETE | 14,15-epoxy-13R-hydroxy-eicosa-5Z,8Z,11Z,17Z-tetraenoic acid |
| 15-HEPE | 15-hydroxy-eicosa-5Z,8Z,11Z,13E,17Z-pentaenoic acid |
| 15-HpEPE | 15-hydroperoxy-eicosa-5Z,8Z,11Z,13E,17Z-pentaenoic acid |
| 16,15-EHDPE | 16,17-epoxy-15-hydroxy-docosa-4Z,7Z,10Z,13Z,19Z-pentaenoic acid |
| 17-HpDHE | 17-hydroperoxy-docosa-4Z,7Z,10Z,13Z,16Z,19Z-esenoic acid |
| 5-HEPE | hydroxy-eicosa-6E,8Z,11Z,14Z,17Z-pentaenoic acid |
| 9,11-EHHDE | 9,10-epoxy-11-hydroxy-hexadecadienoic acid |
| 9-HHME | 9-hydroxyhexadec-7E-enoic acid |
| 9-HpHTrE | 9-hydroperoxy-6Z,10E,13Z-hexadecatrienoic acid |
| 9-HHTrE | 9-hydroxy-hexadeca-6Z,10E,12Z-trienoic acid |
| 9-KHME | 9-ketohexadec-7E-enoic acid |
| AA | arachidonic acid |
| DHA | docosa-4Z,7Z,10Z,13Z,16Z,19Z-esenoic acid |
| EAS | epoxyalcohol synthase |
| EPA | eicosa-5Z,8Z,11Z,14Z,17Z-pentaenoic acid |
| ESI | electrospray ionization |
| FAH | fatty acid hydroperoxide |
| GC-MS | gas chromatography-mass spectrometry |
| HPL | hydroperoxide lyase |
| HTrA | 6Z,9Z,12Z-hexadecatrienoic acid |
| LC-MS | liquid chromatography-mass spectrometry |
| LOFAs | linear oxygenated fatty acids |
| LOX | Lipoxygenase |
| PALOL | palmitoleic acid |
| PUA | polyunsaturated aldehyde |
| PUFA | polyunsaturated fatty acid |
References
- Nelson, D.M.; Trrguer, P.; Brzezinski, M.A. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycle 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Raven, J.A. Photosynthesis and primary production in nature. In Aquatic Photosynthesis, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Ban, S.; Burns, C.; Castel, J.; Chaudron, Y.; Christou, E.; Escribano, R.; Umani, S.F.; Gasparini, S.; Ruiz, F.G.; Hoffmeyer, M.; et al. The paradox of diatom-copepod interactions. Mar. Ecol. Prog. Ser. 1997, 157, 287–293. [Google Scholar] [CrossRef]
- Poulet, S.A.; Ianora, A.; Miralto, A.; Meijer, L. Do diatoms arrest embryonic development in copepods? Mar. Ecol. Prog. Ser. 1994, 111, 79–86. [Google Scholar] [CrossRef]
- Ianora, A.; Poulet, S.A.; Miralto, A.; Grottoli, R. The diatom Thalassiosira rotula affects reproductive success in the copepod Acartia clausi. Mar. Biol. 1996, 125, 279–286. [Google Scholar] [CrossRef]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Romano, G.; Caruso, T.; Spinella, A.; Cimino, G.; Fontana, A. Production of octadienal in the marine diatom Skeletonema costatum. Org. Lett. 2003, 5, 885–887. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Iadicicco, O.; Romano, G.; Fontana, A. Detection of short-chain aldehydes in marine organisms: The diatom Thalassiosira rotula. Tetrahedron Lett. 2002, 43, 6137–6140. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Romano, G.; Iadicicco, O.; Miralto, A.; Ianora, A.; Cimino, G.; Fontana, A. New birth-control aldehydes from the marine diatom Skeletonema costatum: Characterization and biogenesis. Tetrahedron Lett. 2002, 43, 6133–6136. [Google Scholar] [CrossRef]
- Barofsky, A.; Pohnert, G. Biosynthesis of polyunsaturated short chain aldehydes in the diatom Thalassiosira rotula. Org. Lett. 2007, 9, 1017–1020. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar] [CrossRef]
- Paffenhöfer, G.A.; Ianora, A.; Miralto, A.; Turner, J.T.; Kleppel, G.S.; Ribera D’Alcalà, M.; Casotti, R.; Caldwell, G.S.; Pohnert, G.; Fontana, A.; et al. Colloquium on diatom-copepod interactions. Mar. Ecol. Prog. Ser. 2005, 286, 293–305. [Google Scholar] [CrossRef][Green Version]
- Ianora, A.; Poulet, S.A.; Miralto, A. The effects of diatoms on copepod reproduction: A review. Phycologia 2003, 42, 351–363. [Google Scholar] [CrossRef]
- Pohnert, G. Diatom/copepod interactions in plankton: The indirect chemical defense of unicellular algae. ChemBioChem 2005, 6, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Blée, E. Phytooxylipins and plant defense reactions. Prog. Lipid Res. 1998, 37, 33–72. [Google Scholar] [CrossRef]
- Blée, E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002, 7, 315–321. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Tucci, S.; Cutignano, A.; Romano, G.; Cimino, G.; Miralto, A.; Fontana, A. The role of complex lipids in the synthesis of bioactive aldehydes of the marine diatom Skeletonema costatum. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2004, 1686, 100–107. [Google Scholar] [CrossRef]
- Cutignano, A.; D’Ippolito, G.; Romano, G.; Lamari, N.; Cimino, G.; Febbraio, F.; Nucci, R.; Fontana, A. Chloroplastic glycolipids fuel aldehyde biosynthesis in the marine diatom Thalassiosira rotula. ChemBioChem 2006, 7, 450–456. [Google Scholar] [CrossRef]
- Adelfi, M.G.; Vitale, R.M.; D’Ippolito, G.; Nuzzo, G.; Gallo, C.; Amodeo, P.; Manzo, E.; Pagano, D.; Landi, S.; Picariello, G.; et al. Patatin-like lipolytic acyl hydrolases and galactolipid metabolism in marine diatoms of the genus Pseudo-nitzschia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G. Phospholipase A2 activity triggers the wound-activated chemical defense in the diatom Thalassiosira rotula. Plant Physiol. 2002, 129, 103–111. [Google Scholar] [CrossRef]
- Wichard, T.; Poulet, S.A.; Pohnert, G. Determination and quantification of α,β,g,δ-unsaturated aldehydes as pentafluorobenzyl-oxime derivates in diatom cultures and natural phytoplankton populations: Application in marine field studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 814, 155–161. [Google Scholar] [CrossRef]
- Wichard, T.; Poulet, S.A.; Halsband-Lenk, C.; Albaina, A.; Harris, R.; Liu, D.; Pohnert, G. Survey of the chemical defence potential of diatoms: Screening of fifty species for α,β,γ,δ-unsaturated aldehydes. J. Chem. Ecol. 2005, 31, 949–958. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Lamari, N.; Montresor, M.; Romano, G.; Cutignano, A.; Gerecht, A.; Cimino, G.; Fontana, A. 15S-Lipoxygenase metabolism in the marine diatom Pseudo-nitzschia delicatissima. New Phytol. 2009, 183, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Cutignano, A.; Tucci, S.; Romano, G.; Cimino, G.; Fontana, A. Biosynthetic intermediates and stereochemical aspects of aldehyde biosynthesis in the marine diatom Thalassiosira rotula. Phytochemistry 2006, 67, 314–322. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Cutignano, A.; Briante, R.; Febbraio, F.; Cimino, G.; Fontana, A. New C16 fatty-acid-based oxylipin pathway in the marine diatom Thalassiosira rotula. Org. Biomol. Chem. 2005, 3, 4065–4070. [Google Scholar] [CrossRef]
- Cutignano, A.; Lamari, N.; D’Ippolito, G.; Manzo, E.; Cimino, G.; Fontana, A. Lipoxygenase products in marine diatoms: A concise analytical method to explore the functional potential of oxylipins. J. Phycol. 2011, 47, 233–243. [Google Scholar] [CrossRef]
- Jüttner, F.; Messina, P.; Patalano, C.; Zupo, V. Odour compounds of the diatom Cocconeis scutellum: Effects on benthic herbivores living on Posidonia oceanica. Mar. Ecol. Prog. Ser. 2010, 400, 63–73. [Google Scholar] [CrossRef]
- Fink, P.; Von Elert, E.; Jüttner, F. Oxylipins from freshwater diatoms act as attractants for a benthic herbivore. Arch. Hydrobiol. 2006, 167, 561–574. [Google Scholar] [CrossRef]
- Pezzolesi, L.; Pichierri, S.; Samorì, C.; Totti, C.; Pistocchi, R. PUFAs and PUAs production in three benthic diatoms from the northern Adriatic Sea. Phytochemistry 2017, 142, 85–91. [Google Scholar] [CrossRef]
- Maibam, C.; Fink, P.; Romano, G.; Buia, M.C.; Gambi, M.C.; Scipione, M.B.; Patti, F.P.; Lorenti, M.; Butera, E.; Zupo, V. Relevance of wound-activated compounds produced by diatoms as toxins and infochemicals for benthic invertebrates. Mar. Biol. 2014, 161, 1639–1652. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Zupo, V.; Lauritano, C.; Caramiello, D.; Ianora, A.; Budillon, A.; Romano, G.; Nuzzo, G.; D’Ippolito, G.; et al. Toxigenic effects of two benthic diatoms upon grazing activity of the sea urchin: Morphological, metabolomic and de novo transcriptomic analysis. Sci. Rep. 2018, 8, 5622. [Google Scholar] [CrossRef]
- Ruocco, N.; Cavaccini, V.; Caramiello, D.; Ianora, A.; Fontana, A. Noxious effects of the benthic diatoms Cocconeis scutellum and Diploneis sp. on sea urchin development: Morphological and de novo transcriptomic analysis. Harmful Algae 2019, 86, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Orefice, I.; Gerecht, A.; D’Ippolito, G.; Fontana, A.; Ianora, A.; Romano, G. Determination of lipid hydroperoxides in marine diatoms by the FOX2 Assay. Mar. Drugs 2015, 13, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Nuzzo, G.; Sardo, A.; Manzo, E.; Gallo, C.; Fontana, A. Lipoxygenases and lipoxygenase products in marine diatoms. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 69–100. [Google Scholar]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Miralto, A.; Ianora, A.; Romano, G.; Cimino, G. Chemistry of oxylipin pathways in marine diatoms. Pure Appl. Chem. 2007, 79, 481–490. [Google Scholar] [CrossRef]
- Lamari, N.; Ruggiero, M.V.; D’Ippolito, G.; Kooistra, W.H.C.F.; Fontana, A.; Montresor, M. Specificity of lipoxygenase pathways supports species delineation in the marine diatom genus Pseudo-nitzschia. PLoS ONE 2013, 8, e73281. [Google Scholar] [CrossRef] [PubMed]
- Gerecht, A.; Romano, G.; Ianora, A.; D’Ippolito, G.; Cutignano, A.; Fontana, A. Plasticity of oxylipin metabolism among clones of the marine diatom Skeletonema marinoi (Bacillariophyceae). J. Phycol. 2011, 47, 1050–1056. [Google Scholar] [CrossRef]
- Nanjappa, D.; D’Ippolito, G.; Gallo, C.; Zingone, A.; Fontana, A. Oxylipin diversity in the diatom family leptocylindraceae reveals DHA derivatives in marine diatoms. Mar. Drugs 2014, 12, 368–384. [Google Scholar] [CrossRef]
- Fontana, A.; Cutignano, A.; Romano, G.; Lamari, N.; Gallucci, M.; Cimino, G.; Miralto, A.; Ianora, A. LOX-induced lipid peroxidation mechanism responsible for the detrimental effect of marine diatoms on zooplankton grazers. ChemBioChem 2007, 8, 1810–1818. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat. Prod. Rep. 2002, 19, 108–122. [Google Scholar]
- Adolph, S.; Poulet, S.A.; Pohnert, G. Synthesis and biological activity of α,β,γ,δ-unsaturated aldehydes from diatoms. Tetrahedron 2003, 59, 3003–3008. [Google Scholar] [CrossRef]
- Ianora, A.; Romano, G.; Carotenuto, Y.; Esposito, F.; Roncalli, V.; Buttino, I.; Miralto, A. Impact of the diatom oxylipin 15S-HEPE on the reproductive success of the copepod Temora stylifera. Hydrobiologia 2011, 666, 265–275. [Google Scholar] [CrossRef]
- Gerecht, A.; Carotenuto, Y.; Ianora, A.; Romano, G.; Fontana, A.; D’Ippolito, G.; Jakobsen, H.H.; Nejstgaard, J.C. Oxylipin production during a mesocosm bloom of Skeletonema marinoi. J. Exp. Mar. Biol. Ecol. 2013, 446, 159–165. [Google Scholar] [CrossRef]
- Vidoudez, C.; Pohnert, G. Growth phase-specific release of polyunsaturated aldehydes by the diatom Skeletonema marinoi. J. Plankton Res. 2008, 30, 1305–1313. [Google Scholar] [CrossRef]
- Gallina, A.A.; Brunet, C.; Palumbo, A.; Casotti, R. The effect of polyunsaturated aldehydes on Skeletonema marinoi (Bacillariophyceae): The involvement of reactive oxygen species and nitric oxide. Mar. Drugs 2014, 12, 4165–4187. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, A.; Carotenuto, Y.; Lamari, N.; Esposito, F.; D’Ippolito, G.; Fontana, A.; Romano, G.; Ianora, A.; Miralto, A.; Guisande, C. Diatom induction of reproductive failure in copepods: The effect of PUAs versus non volatile oxylipins. J. Exp. Mar. Bio. Ecol. 2011, 401, 13–19. [Google Scholar] [CrossRef]
- Caldwell, G.S. The Influence of bioactive oxylipins from marine diatoms on invertebrate reproduction and development. Mar. Drugs 2009, 367–400. [Google Scholar] [CrossRef]
- Poulet, S.A.; Wichard, T.; Ledoux, J.B.; Lebreton, B.; Marchetti, J.; Dancie, C.; Bonnet, D.; Cueff, A.; Morin, P.; Pohnert, G. Influence of diatoms on copepod reproduction. I. Field and laboratory observations related to Calanus helgolandicus egg production. Mar. Ecol. Prog. Ser. 2006, 308, 129–142. [Google Scholar] [CrossRef]
- Adolph, S.; Bach, S.; Blondel, M.; Cueff, A.; Moreau, M.; Pohnert, G.; Poulet, S.A.; Wichard, T.; Zuccaro, A. Cytotoxicity of diatom-derived oxylipins in organisms belonging to different phyla. J. Exp. Biol. 2004, 207, 2935–2946. [Google Scholar] [CrossRef]
- Ianora, A.; Bastianini, M.; Carotenuto, Y.; Casotti, R.; Roncalli, V.; Miralto, A.; Romano, G.; Gerecht, A.; Fontana, A.; Turner, J.T. Non-volatile oxylipins can render some diatom blooms more toxic for copepod reproduction. Harmful Algae 2015, 44, 1–7. [Google Scholar] [CrossRef]
- Wang, R.; Shimizu, Y.; Steiner, J.R.; Clardy, J. The absolute configuration of bacillariolides I and II, a new type of cyclopentane icosanoids from a marine diatom. J. Chem. Soc. Chem. Commun. 1993, 379–381. [Google Scholar] [CrossRef]
- Zheng, N.; Shimizu, Y. The isolation and structure of bacillariolide III, an extracellular metabolite of the diatom, Pseudo-nitzschia multiseries. Chem. Commun. 1997, 399–400. [Google Scholar] [CrossRef]
- Fadeeva, V.P.; Tikhova, V.D.; Nikulicheva, O.N. Elemental analysis of organic compounds with the use of automated CHNS analyzers. J. Anal. Chem. 2008, 63, 1094–1106. [Google Scholar] [CrossRef]
- Raniello, R.; Iannicelli, M.M.; Nappo, M.; Avila, C.; Zupo, V. Production of Cocconeis neothumensis (Bacillariophyceae) biomass in batch cultures and bioreactors for biotechnological applications: Light and nutrient requirements. J. Appl. Phycol. 2007, 19, 383–391. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruocco, N.; Nuzzo, G.; d’Ippolito, G.; Manzo, E.; Sardo, A.; Ianora, A.; Romano, G.; Iuliano, A.; Zupo, V.; Costantini, M.; et al. Lipoxygenase Pathways in Diatoms: Occurrence and Correlation with Grazer Toxicity in Four Benthic Species. Mar. Drugs 2020, 18, 66. https://doi.org/10.3390/md18010066
Ruocco N, Nuzzo G, d’Ippolito G, Manzo E, Sardo A, Ianora A, Romano G, Iuliano A, Zupo V, Costantini M, et al. Lipoxygenase Pathways in Diatoms: Occurrence and Correlation with Grazer Toxicity in Four Benthic Species. Marine Drugs. 2020; 18(1):66. https://doi.org/10.3390/md18010066
Chicago/Turabian StyleRuocco, Nadia, Genoveffa Nuzzo, Giuliana d’Ippolito, Emiliano Manzo, Angela Sardo, Adrianna Ianora, Giovanna Romano, Antonella Iuliano, Valerio Zupo, Maria Costantini, and et al. 2020. "Lipoxygenase Pathways in Diatoms: Occurrence and Correlation with Grazer Toxicity in Four Benthic Species" Marine Drugs 18, no. 1: 66. https://doi.org/10.3390/md18010066
APA StyleRuocco, N., Nuzzo, G., d’Ippolito, G., Manzo, E., Sardo, A., Ianora, A., Romano, G., Iuliano, A., Zupo, V., Costantini, M., & Fontana, A. (2020). Lipoxygenase Pathways in Diatoms: Occurrence and Correlation with Grazer Toxicity in Four Benthic Species. Marine Drugs, 18(1), 66. https://doi.org/10.3390/md18010066












