Abstract
One of the most widely distributed soft coral species, found especially in shallow waters of the Indo-Pacific region, Red Sea, Mediterranean Sea, and also the Arctic, is genus Sacrophyton. The total number of species belonging to it was estimated to be 40. Sarcophyton species are considered to be a reservoir of bioactive natural metabolites. Secondary metabolites isolated from members belonging to this genus show great chemical diversity. They are rich in terpenoids, in particular, cembranoids diterpenes, tetratepenoids, triterpenoids, and ceramide, in addition to steroids, sesquiterpenes, and fatty acids. They showed a broad range of potent biological activities, such as antitumor, neuroprotective, antimicrobial, antiviral, antidiabetic, antifouling, and anti-inflammatory activity. This review presents all isolated secondary metabolites from species of genera Sacrophyton, as well as their reported biological activities covering a period of about two decades (1998–2019). It deals with 481 metabolites, including 323 diterpenes, 39 biscembranoids, 11 sesquiterpenes, 53 polyoxygenated sterols, and 55 miscellaneous and their pharmacological activities.
Keywords:
Sacrphyton; soft coral; terpenoids; antimicrobial; antitumor; antidiabetic; anti-inflammatory 1. Introduction
Classification of alcyonacean corals, subclass Octocorallia implies the existence of polyps with eight tentacles, which differentiates them from hexacorallian Scleractinia corals. Alcyonaceans are sessile large invertebrate with distinct stalk and a smooth, mushroom-shaped top known as capitulum, and their tissue comprises sclerites, which give support to the colony [1,2]. Traditionally, identification and classification of most soft coral have been carried out by sclerite classification. Sarcophyton covers 35 species, and another six species of Sarcophyton were described [3,4,5,6,7,8]. Later, [9] reported that, within Sarcophyton samples, Sarcophyton glaucum contains six different genetic clades, signifying that this morphologically heterogeneous species was mysterious [10]. Studies revealed that Sarcophyton were mostly seen in shallow water of the Indo-Pacific region [11,12], Red Sea [13], Mediterranean Sea [14], and also the Arctic area [10,15]. However, to our knowledge, nothing was reported from North and South of America (Figure 1). Sarcophyton sp. synonyms include Toadstool Mushroom Leather, Toadstool Leather Coral, Umbrella Coral, Toadstool Mushroom Coral, Mushroom Leather Coral, Sarcophyton Coral, and Mushroom Coral. Sarcophyton sp. were considered a reservoir of bioactive natural metabolites such as diterpenes, steroids, sesquiterpenes, and fatty acids [16,17]. These metabolites, mainly macrocyclic cembranes and their byproducts, represented an important natural bioactive product, with significant biological activities, including anticancer [18,19], antimicrobial [20], anti-inflammatory [21], anti-osteoporotic, antimetastatic, antiangiogenic, and neuroprotective [22]. One metabolite, sarcophytol A 15, isolated from Sarcophyton obtained from Ishigaki Island, Okinawa, Southern Japan, was studied and highlighted because of its important anticancer activity [23]. Some recent articles had partially covered the chemistry and pharmacology of secondary metabolites from Sarcophyton sp. [24,25,26]. This review concentrates on marine bioactive metabolites isolated from Sarcophyton species, their biological properties, and studies of the biosynthesis of marine metabolites. In this review, we reported all metabolites isolated from Sarcophyton species and their reported biological activities stated in the literature over the years from 1998 to 2019. Different online databases were utilized through this review, including Scifinder, Marinlit, and Web of Science. The present review aims to present the progress made in the last two decades regarding the potential application of biomolecules (481 compounds) isolated from Sacrophyton soft corals, to complete the previously published papers (Figure 2 and Figure 3) on the interesting subject of Sacrophyton. It deals with the chemistry, as well as the biological activity of secondary metabolites, including terpenoids, in particular diterpenes, sesquiterpenes, biscembranoids, and polyhydroxysterols, in addition to a number of miscellaneous compounds. The percentage of different chemical classes is represented in (Figure 2), and Figure 3 shows a diagram of isolated classes from each Sarcophyton sp.
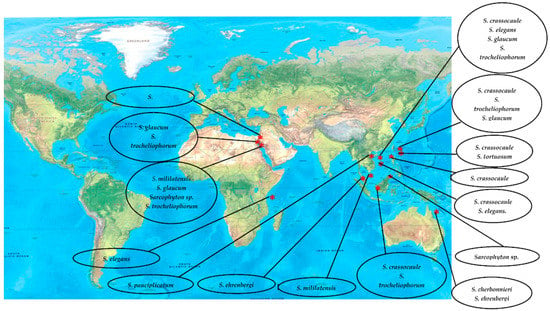
Figure 1.
Worldwide distribution of chemically studied Sarcophyton soft coral.
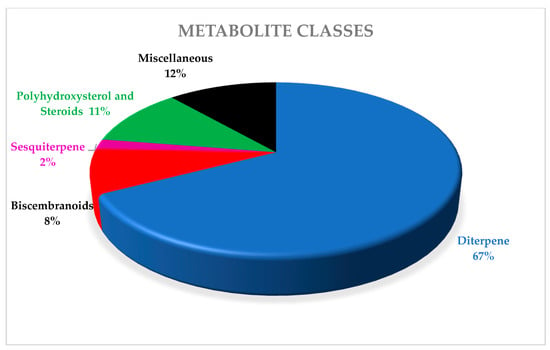
Figure 2.
Pie chart showing the percentage of each class of metabolites identified in Sarcophyton sp.
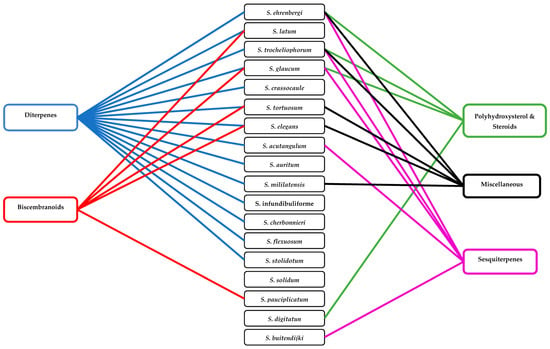
Figure 3.
A diagram of isolated classes from each Sarcophyton sp.
2. Classes of Secondary Metabolites
2.1. Diterpenes
Sarcophyton ehrenbergi dichloromethane extract yielded sarcophytol T 1, (1E,3E,7E,11R*12R*)-15-(acetoxymethyl)cembra-11,12-epoxy-1,3,7-triene 2, and (11S*,12S*)-15-(acetoxymethyl) cembra-3,4:11,12-diepoxy-1,7-diene 3, together with known isoneocembrene A 4, an isomer to neocembrane A 5, and (2S*,11R*,12R*)-isosarcophytoxide 6. Compound 2 was found to possess several structural similarities with the former two isolates in conjugated diene system (C-1 and C-4) and Δ7,8 double bond and 11,12-epoxy functional group [27].
Another three cembrenolide diterpenes identified as crassolide 7, sarcocrassolide A 8, and 13-acetoxysarcocrassolide 9, alongside known cembrenolide denticulatolide 10, were reported from S. crassocaule [28].
From S. trocheliophorum, the isolation of 7,8-epoxy-1(E),3(E),11(E)cembratrien-15-ol 11, 7,8-epoxy1(E),3(E),11(E)-cembratriene 12, and sarcophin 13 was reported, and the absolute configuration of sarcophin 13 was investigated through modified Mosher’s assay [29].
Using chromatographic techniques, cembrane alcohol identified as acutanol 14 beside sarcophytol A 15 and sarcophytol A acetate 16 were isolated from S. acutangulum extract. The absolute configuration of sarcophytol A 15 was assessed with the use of many chiral anisotropic reagents, as 1-naphthylmethoxyacetic acid [30].
Four cembranes, (1S,2E,4R,6E,8S,11S,12S)-11,12-Epoxy-2,6-cembrane-4,8-diol 17, (1S,2E,4R,6E,8R,11S,12S)-11,12-Epoxy-2,6-cembrane-4,8-diol 18, (1S,2E,4R,7S)-11,12-Epoxy-2,8(19)-cembradiene-4,7-diol 19, and (1S,2E,4R,7R)-11,12-Epoxy-2,8(19)-cembradiene-4,7-diol 20, were isolated from Sarcophyton sp. It is worth noticing that these metabolites were not previously found in nature. Their absolute configurations were validated with X-ray analysis [31].
The hydroperoxide cembrane diterpenoid, sarcophycrassolide A 21, together with sacophyocrassolide B 22 and compound 8, was reported from S. crassocaule. Identification of compound 21 was resolved by using X-ray diffraction and spectral analysis [32].
Three furano-cembranoids and two seco-cembranoid acetates, which were identified as 13-dehydroxysarcoglaucol 23, 13-dehydroxysarcoglaucol-16-one 24 and sarcoglaucol-16-one 25, (3E)-7-hydroxy-4,8,15,15-tetramethyl-1-[(E)-12-methyl-10-oxo-12-pentenyl]-3,8-decadienyl acetate 26, (3E)-7-hydroxy-4,8,15,15-tetramethyl-1-[(Z)-12-methyl-10-oxo-12-pentenyl]-3,8-decadienyl acetate 27 beside sarcoglaucol 28, and decaryiol 29, were isolated form S. cherbonnieri. Spectral data showed that compound 25 was a 16-keto derivative of compound 28 and the 13-hydroxy derivative of compound 24 [33]. The absolute configuration of compound 25 was investigated similarly to compound 13 by using the modified Mosher’s method [34]. Another two bicyclic cembranolides metabolites, with infrequent structures in marine literature, having a 12Z double bond, identified as (4Z,8S,9S,12Z,14E)-9-Hydroxy-1-isopropyl-8,12-dimethyl-oxabicyclo [9.3.2]-hexadeca-4,12,14-trien-18-one 30, and (4Z,12Z,14E)-sarcophytolide 31, in addition to sarcophytolide 32, (4Z,8S,9R,12E,14E)-9-Hydroxy-1-isopropyl-8,12-dimethyloxabicyclo[9.3.2]-hexadeca-4,12,14-trien-18-one 33 and (4Z,8S,9R,12E,14E)-1-Isopropyl-8,12-dimethyl-18-oxo-oxabicyclo[9.3.2]-hexadeca-4,12,14-trien-2-yl acetate 34, were reported from Sarcophyton new sp. Additionally, the authors presented biosynthetic pathways for all isolated compounds which resulted from the common acyclic precursor (all-E)-geranylgeranyl pyrophosphate (GGPP), by converting geranylgeranyl-PP [GGPP] into geranylneryl-PP [GNPP], using diterpene synthase, followed by cyclization to cembranoid ring with a 12Z double bond [35]. Three diterpenes, sarcophytolol 35, sarcophytolide B 36, and sarcophytolide C 37, were reported from S. glaucum [34].
Sarcophytonolides A–D 38–41, four cembranolides were isolated from S. tortuosum. Sarcophytonolide B 39 was found to be the 12-(methoxycarbonyl) derivative of compound 38, in which it exhibited αβ-unsaturated methyl ester instead of the methyl group. Sarcophytonolide D 41 was similar in structure to compound 40, while, compound 41 possessed an extra trisubstituted C=C and acetoxy group [36]. Four more sarcophytonolides E-H 42–45 from S. latum were isolated. All isolated compounds were related in structure to compound 40, with an α, β-unsaturated butanolide group. Sarcophytonolide G 44 was found to be the epimer of Sarcophytonolide F 43 at C-6, while sarcophytonolide H 45 was 14-acetoxy methoxycarbonyl derivative of compound 43. The absolute configuration was investigated by using the modified Mosher’s assay as they all possessed secondary alcohol at C-6. It is worth noting that the structural configuration supporting all cembrane diterpenes stated, in the order alcyonacea, with the identified absolute configuration at C-1, belonged to α-series [37]. Moreover, sarcophytonolides I–L 46–49 were isolated from S. latum. All compounds were related in structure to the previously isolated compounds 38–45; all possessed α, β-unsaturated butenolactone group. The absolute configuration of compounds 38–45 still need further determination. Considering the fact that these compounds were structurally related to previously isolated sarcophytonolide, the structure of sarcophytonolide I 46 differs from sarcophytonolide D 41, in the olefinic C7=C8 bond and absence of C=O group at C6 [38]. Another five cembranolide, sarcophytonolides N–R 50–54, ketoemblide 55, and (E,E,E)-1-isopropenyl-4,8,12-trimethylcyclotetradeca-3,7,11-tiene 56 were isolated from S. trocheliophorum Marenzeller. A detailed spectroscopic analysis was done, in which sarcophytonolides N–R 50–54 were found to be either mono- or bicyclic cembranoids possessing oxidized methyl groups and three/four double bonds [39]. The absolute configuration of another six metabolites isolated from S. trocheliophorum, sarcophytonolides S–U 57–59 and sartrolides H–J; α,β-unsaturated ε-lactone 60–62, along with seven known analogues, were carried out through different techniques [40]. Chemical determination of S. trocheliophorum yielded seven cembranolides, sartrolides A–G 63–69 and bissartrolide dimer 70; a third member of this scarce class of cembrane dimers [41]. Yalongenes A and B 71 and 72 another two cembranoids, isolated from S. trocheliophorum [42], and another two cembranoids, trochelioids A and B 73 and 74, and 16-oxosarcophytonin E 75 were isolated [43].
Five diterpenes cembrane type, sarcrassins A–E 76–80, beside emblide 81 isolated from S. crassocaule were identified based on 1D and 2D NMR. Sarcrassins B and C 77 and 78, cyclic diterpenes, derivatives of sarcrassin A 76 in which the double bond in sarcrassin A 76 was replaced by an epoxy ring in sarcrassin B 77. However, in sarcrassin C 78 the epoxy ring in sarcrassin A 76 was replaced by a hydroxyl and methoxy group. As for sarcrassin D 79, its bicyclic diterpene structure was confirmed through spectral data [44], and its absolute configuration, as well as that of emblide 81, was determined by X-ray analysis [41,45].
Investigation of ethyl acetate extract of S. crassocaule yielded six polyoxygenated cembrane-diterpenoids with a trans-fused α-methylene-γ-lactone, identified as crassocolides A–F 82–87 alongside lobophytolide 88. Absolute configuration for crassocolide A 82 was resolved by using modified Mosher’s method [46]. Another seven polyoxygenated cembranoids with α-methylene-γ-lactone group identified as crassocolides G–M 89–95, were reported. The structures of all compounds were determined through a full spectral data analysis, and the absolute configuration of crassocolide G 89 was investigated by modified reaction of Mosher’s assay [47]. Other crassocolides N–P 96–98 were isolated from S. crassocaule [48]. The CHCl3/MeOH extract of S. flexuosum yielded three cembranes, identified through spectral data as flexusines A, B, and epimukulol 99–101 [49].
From ethyl acetate extract of S. stolidotum, seven cembranes, sarcostolides A–G 102–108, alongside isosarcophin 109, were reported, and their structures were elucidated through spectral data. The authors also proposed a reasonable biogenetic pathway for all isolates, in which cyclization of GPP with lactonization and oxidation may lead to the production of sarcostolide C 104. Sarcostolides A and B 102 and 103 and D–G 105–108 were converted from sacostolide C 104 through migration and isomerization of double bonds [50].
Sarcophyton mililatensis methanol extract yielded cembranoid diterpenes identified as (−)-7β-hydroxy-8α-methoxy-deepoxy-sarcophytoxide 110, (−)-7β,8β-dihydroxy-deepoxy-sarcophytoxide 111, (−)-17-hydroxysarcophytonin A 112, sarcophytol V 113, and sarcophytoxide 114 [51].
Two cembrane diterpenes known as 17-hydroxysarcophytoxide 115 and 7β-acetoxy-8α-hydroxydeepoxysarcophine 116, along with 7β,8α, dihydroxydeepoxysarcophine 117, sarcophytonin A 118, and (−)-β-elemene 119 reported from Sarcophyton sp., were isolated from S. glaucum [52]. Investigation of S. glaucum extract led to the isolation of two cembranoids, (7R,8S)-dihydroxydeepoxy-ent-sarcophine 120 and secosarcophinolide 121, in addition to, ent-sarcophin 122. Structural elucidation of the isolates was established by their spectral data and chemical correlation, as (7R,8S)-dihydroxydeepoxy-ent-sarcophine 120 was found to be the enantiomer of (7S,8R)-dihydroxydeepoxysarcophine 123 and compound 121 has a unique butyl ester group at C-16 [53].
Seven cembranoids were isolated from Sarcophyton sp., 5-epi-sinuleptolide 124, lobohedleolide 125, (7Z)-lobohedleolide 126, and two uncommon cembranoids, sarcofuranocembrenolide A 127; with a unique carbon skeleton of 8,19-bisnorfuranocembrenolide, and sarcofuranocembrenolide B 128; a furanocembrenolide [54]. Sarcophytonins F and G 129 and 130, another two dihydrofuranocembranoids, were reported from Sarcophyton sp. [55]. Nineteen compounds from Sarcophyton sp., of which five cembrane diterpenoids were isolated and identified as 7-acetyl-8-epi- sinumaximol G 131, 8-epi- sinumaximol G 132, 12-acetyl-7,12-epi- sinumaximol G 133, 12-hydroxysarcoph-10-ene 134, and 8-hydroxy-epi-sarcophinone 135, together with sinumaximol G 136, were reported [56].
Five isolated cembranoids, sarcocrassocolides A–E 137–141, together with sinularolide 142, were isolated from S. crassocaule. Structural elucidation of the compounds was determined through spectral analysis, and the absolute configuration of sarcocrassocolide A 137 was investigated by modified Mosher’s method. It is worth mentioning that sarcocrassocolides A–D 137–140 possessed a tetrahydrofuran group with a seldomly found 4,7-ether bond, which was discovered previously in Eunicea mammosa soft coral [57,58]. Another seven cembranoids with α-methylene-γ-lactonic group and rare trans 6,7-disubstituted double bond, uncovered earlier only in soft coral Eunicea pinta, identified as sarcocrassocolides F–L 143–149, were isolated from S. crassocaule [59]. Besides the abovementioned sarcocrassocolides, another three sarcocrassocolides, M–O 150–152, from S. crassocaule, were identified. Through structural analysis, sarcocrassocolide N 151 was found to have the same relative configuration of sarcocrassocolide M 150, while sarcocrassocolide O 152 was found to be the 13-deacetoxy derivative of sarcocrassocolide M 150 [60]. Three more cembranoids, sarcocrassocolides P–R 153–155, were identified, and their structures were investigated by an extensive spectral study [61].
Investigation of n-hexane fraction for S. ehrenbergi led to the isolation of (+)-7,8-epoxy-7,8-dihydrocembrene C 156, in which its optical rotation indicated that it was (+)- (7S,8S)-7,8-epoxy-7,8-dihydrocembrene C 156, not (−)-7,8-Epoxy-7,8-dihydrocembrene C, which was reported previously from S. crassocaule [62].
Six cembranoids, (+)-12-carboxy-11Z-sarcophytoxide 157, (+)-12-methoxycarbonyl-11Z-sarcophine 158, ehrenberoxides A–C 159–161 and lobophynin C 162 were isolated from S. ehrenbergi. Compound 157 has a 2,5-dihydrofuran ring attached to a 14 membered ring at carbon-1 and carbon-2, a carboxylic acid at carbon-12 and an epoxide moiety at carbon-7 and carbon-8. Moreover, the authors mentioned that both ehrenberoxides B and C 160–161 raised from the exact precursor with a 7,8-epoxide through a transannular cleavage of the 7,8-epoxide by both ends of an 11,12-diol, while compound 160 has a unique oxepane ring, which was not detected previously in cembranoid [63] and from S. infundibuliforme diterpenoids cembrene C 163, sarcophytol B 164, sarcophytol E 165, and sarcophytol H 166, (−)-marasol 167 were reported [64].
A cembrane diterpene identified as 2R,7R,8R-dihydroxydeepoxysarcophine 168 was isolated from S. glaucum [65], and three compounds were reported from its ethyl acetate fraction, of which two were peroxide diterpenes identified as 11(S)-hydroperoxylsarcoph-12(20)-ene 169, 12(S)-hydroperoxylsarcoph-10-ene 170, and 8-epi-sarcophinone 171. All structures were investigated by spectral data, and their relative configuration was assigned by X-ray diffraction [66].
Methyl sarcotroates A and B 172 and 173 two diterpenes, along with sarcophytonolide M 174, a precursor for the former two compounds, were isolated from S. trocheliophorum, and their biogenetic pathways were proposed, in which isomaration, cycloaddition followed by oxidation of compound 174 led to the formation of both compounds 172 and 173. The authors also studied the absolute configuration of methyl sarcotroate B 173 through TDDFT ECD calculations, helping in determining the absolute configurations for methyl sarcotroate A 172 and sarcophytonolide M 174 by a biogenetic relationship and ECD comparison, respectively [67].
Cembranoid diterpene, identified as (1S,2E,4R,6E,8S,11R,12S)-8,11-epoxy-4,12-epoxy- 2,6-cembradiene 175, (1S,2E,4R,6E,8R,11S,12R)-8,12-epoxy-2,6-cembradiene-4,11-diol 176, and (1S,4R,13S)-cembra-2E,7E,11E-trien-4,13-diol 177, were reported from nature for the first time, from S. glaucum [68].
From an acetone extract of S. ehrenbergi, three cembranoids were isolated. Through full NMR data, the existence of α, β unsaturated ethyl ester and α, β unsaturated methyl ester of both (+)-12-ethoxycarbonyl-11Z-sarcophine; ehrenbergol A and B 178–180 were confirmed. Ehrenbergol B 179 showed a trisubstituted epoxide and two trisubstituted olefins. [69].
Fifteen cembrane-type diterpenoids were isolated from S. elegans, sarcophyolides B–E 181–184, along with sarcophytol L 185, 13α-hydroxysarcophytol L 186, sarcophyolide A 187, sarcophinone 188, 7α-hydroxy-Δ8(19)-deepoxysarcophine 189, 4β-hydroxy-Δ2(3)-sarcophine 190, 1,15β-epoxy-2-epi-16-deoxysarcophine 191, sarcophytol Q 192, and lobocrasol 193. A detailed structural elucidation was determined by spectral data and reported data. The absolute configurations of sarcophyolides B–E 181–184 were approved by single-crystal X-ray diffraction assay, using Flack’s assay [22], and the structure of lobocrasol 193 was further studied [70].
From the ethyl acetate extract of S. ehrenbergi two diterpenes were isolated, acetyl ehrenberoxide B 194 and ehrenbergol C 195. Ehrenbergol C 195 shared a structure similar to lobocrasol 193, isolated from Lobophytum crassum [71]. Yet, relative stereochemistry of carbon-7 and carbon-8 in ehrenbergol C 195 differed from lobocrasol 193 in hydroxy group and a conjugated enone evidenced by the IR spectrum at 3444 and 1696 cm−1, respectively [72].
An oxygenated cembranoid diterpene, sarcophytol W 196, together with (2E,7E)-4,1l-dihydroxy-1,12-oxidocembra-2,7-dien 197, were isolated before from S. infundibuliforme and S. glaucum, (+)-11,12-epoxy-11,12-dihydrocembrene-C 198, (+)-11,12-epoxysarcophytol A 199 and sarcolactone A 200, previously known, were reported from Sarcophyton sp. Structures were determined through spectral data and comparing the reported data. The absolute configuration of sarcophytol W 196 was elucidated based on the modified Mosher’s assay [73].
Two diterpenes were isolated from S. tortuosum, identified as tortuosenes A and B 201 and 202. Structural elucidation of compounds 201 and 202 were investigated by spectral data. The absolute configuration of tortuosene A 201 was investigated using TDDFT ECD method. Moreover, the authors proposed a biosynthetic pathway for tortuosenes A and B 201 and 202 from the assumed cembranoidal precursor; (1Z, 3Z, 7E, 11E)-4-isopropyl-1,7,11-trimethylcyclotetradeca-1,3,7,11-tertaene, by oxidation of carbon-20 and the carbon-7/carbon-8 double bond was epoxidize, forming aldehydocembrane, a structure related to emblide 81. The resulting aldehydocembrane additionally formed a cycle from carbon-2 to carbon-20 by acid-catalyzed affecting the carbon-1/carbon-2 double bond of the carbonyl moiety [74].
2-epi-sarcophine 203 and (1R,2E,4S,6E,8R,11R,12R)-2,6-cembradiene-4,8,11,12-tetrol 204, two diterpenes were isolated from S. auritum [75]. An extensive chemical investigation of Sarcophyton sp. extract yielded four cembranoids, sarcophytons A–D 205–208, along with cembranoids, 2-[(E,E,E)-7′,8′-epoxy-4′,8′,12′-trimethylcyclotetradeca-1′,3′,11-trienyl]propan-2-ol 209, (1E,3E,7R*,8R*,11E)-1-(2-methoxy-propan-2-yl)-4,8,12-trimethyloxabicyclo[12.1.0]-pentadeca-1,3,11-triene 210, crassumol C 211, and laevigatol A 212. [76]. Two unique pyrane-based cembranoids, sarcotrocheliol acetate and sarcotrocheliol 213 and 214 were isolated from S. trocheliophorum [77]. Investigation of S. glaucum organic extract resulted in the isolation of sarcophinediol 215, previously processed semi-syntheticaly [78].
Cembranoid diterpenes, 7-keto-8α-hydroxy-deepoxysarcophine 216 similar to compound 13, in which the carbon at carbon-3 and carbon-11 were presumed to be in E configuration established on compound 13 derivatives; this was established through spectral data. 7β-chloro-8α-hydroxy-12acetoxy-deepoxysarcophine 217 was close to 7-keto-8α-hydroxy-deepoxysarcophine 216 except for the disappearance of ketone signal at C-7 which co-exists with the presence of an up fielded signal at δ62.9 (C-7), a downfield of C-20 and the presence of carbonyl and methyl group at 170 and 22.2, respectively, were isolated from S. ehrenbergi. [79].
From S. trocheliophorum, sarsolenane diterpenes and capnosane diterpenes were obtained. Sarsolenane diterpenes are uncommon in nature, symbolized only by sarsolenone isolated from S. solidum. Two sarsolenane diterpenes, dihydrosarsolenone 218, methyl dihydrosarsolenoneate 219, and two capnosane diterpenes, sarsolilides B and C 220 and 221, together with sarsolilide A 222 were isolated. Dihydrosarsolenone 218 resulting from sarsolenone 223 by terminal double bond Δ15 reduction followed by the oxidation of C-18 gave methyl dihydrosarsolenoneate 219. Capnosane diterpenes were first isolated from S. solidum and S. trocheliphorum. The only example reported with α, β-unsaturated ε-lactone subunit was sarsolilide A 222, from S. solidum, in which, the hydration of the exomethylene group provided carbon-10 epimers, sarsolilides B and C 220 and 221 [80]
Ethyl acetate extract of S. trocheliophorum yielded twenty-three isolates, of which nineteen were cembranoids with unique capnosane skeleton identified as trocheliophols A–S 224–242 and two analogues, 4-epi-sarcophytol L 243 and sarcophyolide C 182. The structures were investigated by a full spectral data, and their absolute configurations were established through modified Mosher’s assay, CD and X-ray diffraction. Trocheliophols C 226, E 228, F 229, and M 236 all possessed a structure similar to sarcophytolide C 176, while, trocheliophol Q 240 was identified as the C-8 methoxylated model of trocheliophol F 229. However, trocheliophol R 241 possessed a similar structure to trocheliophol F 229 but it differed in the presence of the methoxy group [81].
Chemical determination of S. elegans CH2Cl2/MeOH extract resulted in isolation of four cembranoids identified as sarcophelegans A–D 244–247. Sarcophelegan A 244 was found to be the 11,12-epoxy derivative of sarcophelegan C 246. Through X-ray crystallographic examination using anomalous scattering of Cu Kα radiation, sarcophelegan A 244 structure was verified. Moreover, sarcophelegan C 246 was found to be the 7-hydrogenated derivative of sarcophelegan B 245 [18].
Five polyoxygenated cembranoids were identified as polyoxygenated cembranoids, (+)-1,15-epoxy-2-methoxy-12methoxycarbonyl-11E-sarcophytoxide 248, (+)-2-epi-12-methoxycarbonyl-11E-sarcophine 249, 3,4-epoxyehrenberoxide A 250, ehrenbergol D 251 and ehrenbergol E 252 in S. ehrenbergi. The authors proposed that (+)-1,15-epoxy-2-methoxy-12methoxycarbonyl-11E-sarcophytoxide 248 was the 1,15 epoxy-2-methoxylated equivalent of lobophynin C 162. Through investigating the spectral data and X-ray crystallization of (+)-2-epi-12-methoxycarbonyl-11E-sarcophine 249 it was found that it differed in the alignment of the α,β-unsaturated γ-lactone ring attached to C-2 of the 14-membered ring [63]. 3,4-epoxyehrenberoxide A 250; an analogue to ehrenberoxide A 159 where the epoxide in ehrenberoxide A 159 was substituted by a double bond at C3 and C4 [82].
Eight metabolites were isolated from S. solidum, three sarsolenanes, 7-deacetyl-sarsolenone 253, sarsolenone 223, and methyl dihydro-sarsolenoneate 219 together with, sarsolilide B 220. All 7-deacetyl-sarsolenone 253, sarsolenone 223, sarsolilide B 220, could be used as a chemotaxonomic marker for this species [83].
Three isolates; trocheliane 254, tetracyclic biscembrane and two cembranoid diterpenes, sarcotrocheldiols A and B 255 and 256, were isolated from S. trocheliophorum. Their relative configuration and structure of the isolates were investigated by spectral data [84].
From Sarcophyton sp., one cembrane diterpene, 16-hydroxycembra-1,3,7,11-tetraene 257, besides, 15-hydroxycembra-1,3,7,11-tetraene 258 were reported. Structures were investigated by spectral data [85].
Three cembranoids from S. trocheliophorum, sarcophytrols D–F 259–261 highly oxidative compounds, besides, 11,12-epoxy-1(E),3(E), 7(E)-cembratrien-15-ol 262 and sinugibberol 263 were isolated. All structures were investigated by a full spectral data and by comparing with previous stated data [86]. Another six cembranoids, sarcophytrols G–L 264–269 together with crassumol A 270, were isolated from S. trocheliophorum [87]. Additionally, another nine cembranoids, sarcophytrols M–U 271–279, were also reported. Their structures were interpreted with extensive spectral analysis and chemical conversion and the absolute configuration for sarcophytrols M–S 271–277 were investigated by the modified Mosher’s assay. Sarcophytrols R and S 276 and 277 revealed a unique decaryiol skeleton with an uncommon C12/C15 cyclization [88]. Another cembranoid, trocheliolide B 280 from S. trocheliophorum was isolated [89]. Chemical determination of S. trocheliophorum organic extract, yielded pyrane-based diterpene, 9-Hydroxy-10,11-dehydro-sarcotrocheliol 281 [90].
From S. ehrenbergi eight cembranoids, sarcophytonoxides A–E 282–286 were identified. Sarcophytonoxide A 282, a cembrane diterpene with epoxide, dihydrofuran, acetyl group and three olefin bonds were confirmed by spectral data analysis while sarcophytonoxide D 285 was the deacetylated form of sarcophytonoxide C 284 which has a structure similar to sarcophytonoxide A 282. However, sarcophytonoxide C 283 differed in the chemical shift of C-19, C-6, C-7, and C-9 because of the 7,8-double bond configuration or chiral center of C-6. However, sarcophytonoxide E 286 differed in the position of acetyl group and the exocyclic double bond. [91]. From S. trocheliophorum a sarsolenane diterpene, secodihydrosarsolenone 287 was identified [92].
The chemical investigation of both diethyl ether and dichloromethane extracts of S. stellatum yielded the isolation of three cembranoid diterpenes and enantiomer, (+)-(1E,3E,11E)-7,8-epoxycembra-1,3,11,15-tetraene 288, (+)-(7R,8R,14S,1Z,3E,11E)-14-acetoxy-7,8-epoxycembra-1,3,11-triene 289 [93].
Five isoprenoids from S. glaucum, 3,4,8,16-tetra-epi-lobocrasol, 1,15β-epoxy-deoxysarcophine, 3,4-dihydro-4α,7β,8α-trihydroxy-∆2-sarcophine, ent-sarcophyolide E 290–293, together with, 3,4-dihydro-4α-hydroxy-∆2-sarcophine, 3,4-dihydro-4β-hydroxy-∆2-sarcophine 294 and 295 and klyflaccicembranol F 296 were reported and their structures were elucidated by spectral data. [70]. Moreover, five cembranoids, sarelengans C–G 297–301 from S. elegans were also stated. Isolates structures were established by spectral data, and absolute configuration of sarelengans D–F 298–300 were investigated through single crystal X-ray diffraction [94].
Isolation of seven diterpenes were reported from S. ehrenbergi and identified as sarcoehrenbergilids A–C 302–304 together with sinulolides A and B 305 and 306. The absolute configuration of sarcoehrenbergilid A 301 was investigated by scattering of CaKα radiation with the flack parameter [95]. Moreover, sarcoehrenbergilid D–F 307–309, diterpenes isolated from S. ehrenbergi were isolated and their absolute configurations were investigated by experimental and TDDFT-simulated ECD spectra. Sarcoehrenbergilid D 307 was found to differ from compound 301 only in stereochemistry [96]. Furthermore, five cembranes diterpenes, Sarcoehrenolides A–E 310–314 were isolated from S. ehrenbergi. Their chemical structures were determined through extensive spectral data. All isolates were related to ehrenbergol D 251 in structure, having an α,β-unsaturated-γlactone group at carbon-6 to carbon-19, however, they differ in migration of double bonds and/or oxidative configurations. Additionally, the absolute configuration of sarcoehrenolide A 310 was investigated by a single-crystal X-ray diffraction assay by Cu Kα radiation, and the absolute configurations of sarcoehrenolides B 311 and D 313 by TDDFT/ECD calculations [97].
From S. infundibuliforme two nitrogenous diterpenoids with unusual tricycle [6.3.1.01,5] dodecane skeleton named, sarinfacetamides A and B 315 and 316 and a known compound; nanolobatin B 317 were reported. Their structures were clarified by a thorough spectral data, TDDFT-ECD calculation and the absolute configuration of sarinfacetamide A 315 was investigated. The authors proposed a probable biosynthetic pathway for sarinfacetamides A and B 315 and 316, in which, the development of the carbon-12−carbon-4 bond together with epoxide ring opening of nanolobatin B 317 created an intermediary carbon cation molecule which reacted with the nitrogen lone pair electrons attacking carbon-9 followed by the opening of carbon-1/carbon-9 bond and generation of carbon-1/carbon-8 bond offering sarinfacetamides skeleton, of which acetylation of carbon -4/carbon -8 or carbon -4/carbon -8/carbon -16 yielded sarinfacetamides B 316 and A 315, respectively [98]. From genus sarcophyton, (1S,2E,4R,6E,8S,11S,12S)-11,12-epoxy-8-hydroperoxy-4-hydroxy-2,6-cembradiene 318 was reported. Its structure was fully determined through a complete spectroscopic analysis [99].
Sarcomililatols A, B and sarcomililate A 319–321, which possessed tricyclo [11.3.0.02,16] hexadecane skeleton, along with diterpenoids sarcophytol M 322, were isolated from S. mililatensis. Absolute configuration for sarcomililatol A 319 and sarcomililate A 321 were elucidated by combination of residual dipolar coupling-based NMR analysis, Snatzke’s assay and TDDFT-ECD calculation and anomalous X-ray diffraction with sarcomililatol A 319. The authors also proposed a biogenetic pathway relationship for sarcomililatols A, B and sarcomililate A 319–321. Based on structural resemblance between the three compounds, acetylation of sarcomililatol B 320 gave sarcomililatol A 319, and with dehydration under acid, isomerization and intramolecular [4 + 2] cycloaddition, sarcomililate A 321 was formed [100]. A pyrane-cembranoid diterpenes, 9-hydroxy-7,8dehydro-sarcotrocheliol and 8,9-expoy-sarcotrocheliol acetate 323 and 324 were isolated from S. trocheliophorum [101]. Figure 4 summarizes diterpenes isolated from Sacrophyton sp.
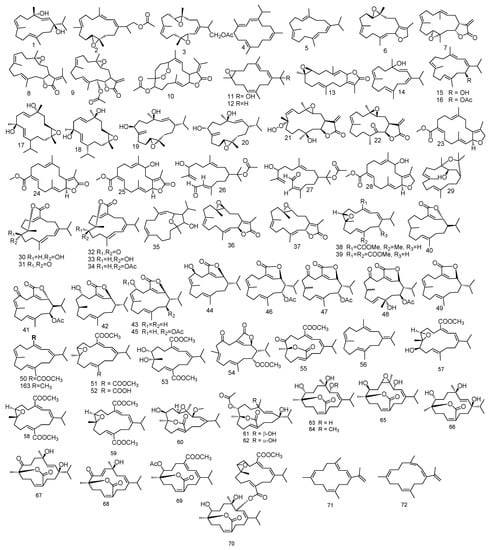
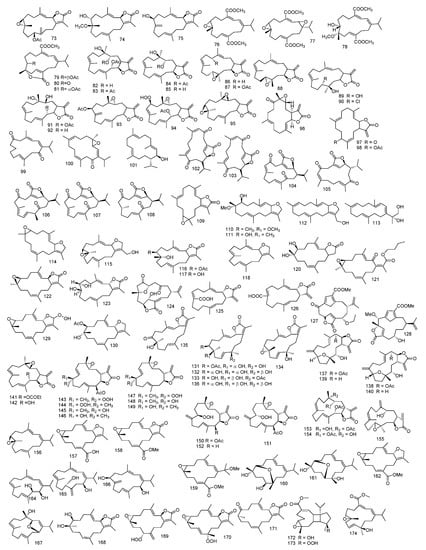
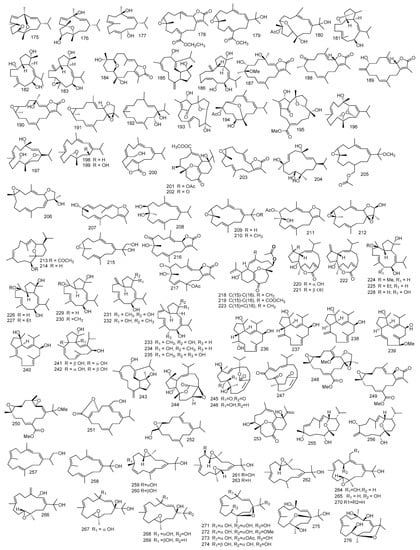
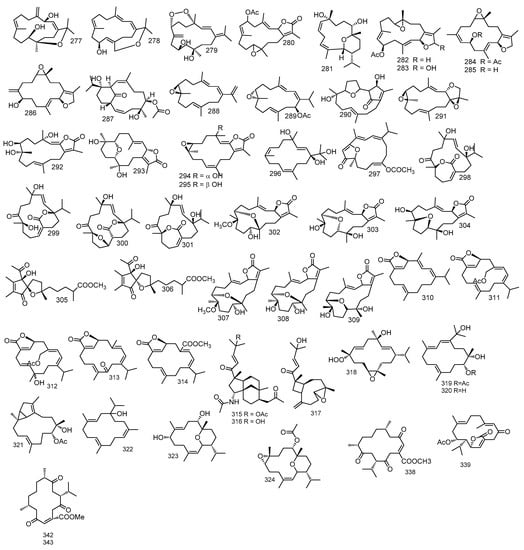
Figure 4.
Diterpenes reported from Sarcophyton sp.
2.2. Biscembranes
Four biscembranes, bisglaucumlides A–D 325–328 were isolated from S. glaucum. Spectral data showed that bisglaucumlide A 325 possessed a biscembranoid skeleton. Bisglaucumlide B 326 was confirmed to be 32-acetylbisglaucumlide A by the positive Cotton effect in the CD spectrum. As for bisglaucumlide C 327 it was found to be the geometrical isomer of bisglaucumlide B 326 while considering the geometry of the C-4 olefin. Bisglaucumlide D 328 was an isomer to bisglaucumlide C 327, its absolute configuration indicated an anticlockwise relation among the enone chromopores revealing a negative Cotton effect CD spectrum [102]. Moreover, chemical investigation of S. glaucum extract yielded two biscembranes with an uncommon α, β-unsaturated ε-lactone, Glaucumolides A and B 329–330 [103].
Ximaolides A–G 331–337, seven biscembranoid, together with methyl tortuosoate A 338 where isolated from S. tortuosum. Their structures were elucidated through spectral analysis and Ximaolide A 331 and E 335 relative stereochemistry were investigated using X-ray diffraction method. The authors demonstrated that methyl tortuosoate A 338 could be the biogenetic precursor for all isolated metabolites since their upper parts were closely related to compound 338 [104].
A cembranolide diterpene identified as isosarcophytonolide D 339, an isomer to the previously isolated compound 41 from S. tortuosum, along with two biscembranes, bislatumlides A and B 340–341, were isolated from S. latum. A detailed spectral analysis revealed that the structure of bislatumlide B 341 matched that of bislatumlide A 340. However, 13C NMR data revealed a significant difference from compound 340 in the chemical shifts of carbon-19 and carbon-10 demonstrating the Z nature of Δ11 olefin in compound 340. Thus, compound 340 was found to be the 11Z isomer of bislatumlide B 341. Interestingly the authors have proposed a biosynthetic pathway for bislatumlides A and B 340–341 in which isosarcophytonolide D 339 was found to be one of the precursors for bislatumlide A 340. Moreover, the authors investigated the effect of long-term storage in CDCl3, where it showed isomerization of bislatumlide A 340 to bislatumlide B 341 at ∆11 [105].
Methyl tetrahydrosarcoate and methyl tetrahydroisosarcoate 342 and 343, two cembranoids isolated from S. elegans, along with four biscembranoids, nyalolide, desacetylnyalolide, diepoxynyalolide, and dioxanyalolide 344–347. The authors proposed that diepoxynyalolide 346 could be a precursor for both compound nyalolide 344 and dioxanyalolide 347 [106].
Investigation on S. elegans extract led to the isolation of six biscembranoids identified as sarcophytolides G–L 348–353, together with biscembranoids, lobophytones H, Q, K, W, U 354–358. Isolates structure were determined by spectroscopic analysis. Absolute configuration of the compound sarcophytolide G 348 was determined using Mosher reaction [22,107]. From the methanol extract of S. pauciplicatum, sarcophytolides M and N 359 and 360, along with lobophytone O 361, were isolated [108].
Two biscembranoids, sarelengans A and B 362 and 363, were reported from S. elegans. Their chemical structures were investigated by spectral and chemical methods, and the absolute configuration of sarelengans A determined by single crystal X-ray diffraction. Sarelengans A and B 362 and 363 possessed a conjuncted trans-fused A/B-ring between two cembranoid entities. The authors mentioned that this structure feature led to an uncommon biosynthetic pathway including a cembranoid-∆8 instead of cembranoid-∆1 unit in endo-Diels-Alder cycloaddition [94]. Figure 5 summarizes biscembranes isolated from Sacrophyton sp.
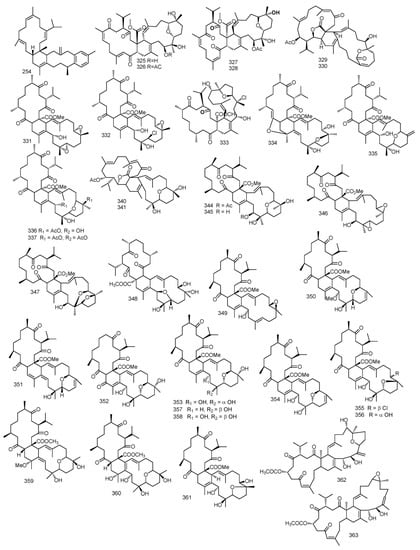
Figure 5.
Biscembranes reported from Sarcophyton sp.
2.3. Sesquiterpenes
Investigation of the methylene chloride extract of S. acutangulum yielded tetracyclic terpenoid hydrocarbon (+)-alloaromadendrene 364 which showed similar spectral data as that of (−)-alloaromadendrene but with different optical rotation [R]D +25.8° (−)-alloaromadendrene and cyclosinularane 365 [109].
Two guaiane sesquiterpenes 4α-ethoxy-10α-hydroxyguai-6-ene and 10α-hydroxy-4α-methoxyguai-6-ene 366 and 367 were isolated from S. buitendijki and their structures were elucidated through 1 and 2D NMR [110]. One 1,2-dioxolane sesquiterpene alcohol named, dioxosarcoguaiacol 368, was isolated from S. glaucum [111].
Trocheliophorin 369 was isolated from S. trocheliophorum ethyl acetate extract. Through spectral data, its structure was elucidated, revealing that it could be the result of aromatization with dehydration of ring B of sarcophytin which co-exist in the extract, and removal of ring C and the ring junction methyl and breakage of ring A [112]. In addition, aromadendrene sesquiterpenoid, palustrol 370 from S. trocheliophorum was reported [77]. Moreover, sesquiterpene guajacophine 371 and 1,4-peroxymuurol-5-ene 372 from S. ehrenbergi were stated. [62]. Continuing the abovementioned isolation from S. glaucum sesquiterpenoid, 6-oxo-germacra-4(15),8,11-triene 373 was also reported [78]. Figure 6 summarizes sesquiterpenes isolated from Sacrophyton sp.
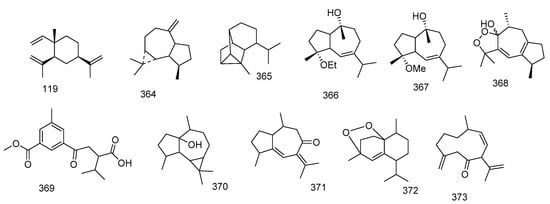
Figure 6.
Sesquiterpenes reported from Sarcophyton sp.
2.4. Polyhydroxysterol and Steroids
One polyhydroxysetrol, 23,24-dimethylcholest-16(17)-E-en-3β,5α,6β,20(S)-tetraol 374, along with 24-methylcholestane-3β,5α,6β,25-tetraol-25-monoacetate 375 and gorosten-5(E)-3β-ol 376, were reported from S. trocheliophorum. Interpretation using 1 and 2D NMR analysis pointed out the existence of 23,24-dimethyl cholesterol derivatives which were further approved by the mass fragmentation pattern [29]. The isolation of (24S)-24methylcholestane-3β,5α,6β-triol 377 from S. crassocaule were also reported [28].
Sardisterol 378 was isolated from S. digitatun Moser. The carbon NMR matched that of (22R)-methylcholest-5-en-3β, 22,25,28-tetraol-3,22,28-triacetate 379 indicating that sardisterol 378 has the same steroidal nucleus as (22R) -methylcholest-5-en-3β, 22,25,28-tetraol-3,22,28-triacetate 378 but the OH groups in carbon 22 and 28 were replaced by acetoxy groups [113].
(24S)-24-methylcholestane-3β,5α,6β,25γ,26-pentol-25,26-diacetate 380 and (24S)-24-methylcholestane-3β,5α,6β,25γ,26-pentol-26-n-decanoate 381, was isolated from S. trocheliophorum, while, (24S)-24-methylcholestane-3β,5α,6β,25γ-tetrol 382 and (24S)-24- methylcholestane-3β,5α,6β,25γ pentol-25-monoacetate 383 were reported from S. glaucum [114].
Fourteen polyoxygenated steroids with 3β,5α,6β-hydroxy group, showing ergostane, cholestane, gorgostane and 23,24-dimethyl cholestane carbon skeletons were reported from Sarcophyton sp., 11α-acetoxy-cholesta-24-en-3β,5α,6β-triol 384, (22E,24S)-11α-acetoxy-ergostane-22,25-dien-3β,5α,6β-triol 385, (24S)-ergostane-1α,3β,5α,6β,11α-pentaol 386, (24S)-23,24-dimethylcholesta-22-en-3β,5α,6β,11α-tetraol 387, (23R,24R)-23,24-dimethylcholesta-17(20)-en-3β,5α,6β-triol 388, 11α-acetoxy-gorgostane-3β,5α,6β,12α-tetraol 389 and 12α-acetoxy-gorgostane-3β,5α,6β,11α-tetraol 390, sarcoaldosterol A 391, (24S)-ergostane-3β,5α,6β-triol 392, (24S)-ergostane-3β,5α,6β,11α-tetraol 393, (24S)-ergostane-7-en-3β,5α,6β-triol 394, 11α-acetoxy-gorgostane-3β,5α,6β-triol 395, sarcoaldosterol B 396 and gorgostane-1α,3β,5α,6β,11α-pentaol 397. Structural elucidation for all isolates were done based on spectral analysis and comparing with reported literature [115].
Six polyhydroxy steroids, (24S)-ergostan-3β,5α,6β,25-tetraol-25-monoacetate 398, (24S)-24-methylcholestan-3β,6β,25-triol-25-O-acetate 399, (24S)-methylcholestan-3β,5α,6β,25-tetraol-3,25-diacetate 400, (24S)-24-methylcholestan-1β,3β,5α,6β,25-pentaol-25-monoacetate 401 and (24S)-methylcholestan-3β,5α,6β,12β,25-pentaol-25-O-acetate 402, were reported from Sarcophyton sp., one was reported as 18-oxygenated polyhydroxy steroid, (24S)-ergostan-3β,5α,6β,18,25-pentaol 18,25-diacetate 403. The structure of this compound was determined through spectroscopic data, and its absolute configuration was elucidated by the modified Mosher’s assay [116].
Chemical investigation of the polar fraction of S. trocheliophorum, yielded two poly-hydroxy steroids, identified through extensive spectral analysis as zahramycins A and B 404 and 405. Zahramycin A 404 was characterized by the existence of oxirane ring at carbon-5 and carbon-6, while zahramycin B 405 possessed a keto-hydroxy sterol structure [117].
Ten polyhydroxylated steroids were isolated from Sarcophyton sp., (23R,24R,17Z)-11α-acetoxy-16β-methoxy-23,24-dimethylcholest-17(20)-en-3β,5α,6β-triol 406, (24R)-gorgost-25-en-3β,5α,6β,11α-tetraol 407 and 11α-acetoxycholest-24-en-1α,3β,5α,6β-tetraol 408, (24R)-methylcholest-7-en-3β,5α,6β-triol 409, 11α-acetoxy-cholest-24-en-3β,5α,6β-triol 410, (22E,24S)-11α-acetoxy-ergost-22,25-dien-3β,5α,6β-triol 411, (24S)-11α-acetoxy-ergost-3β,5α,6β-triol 412, (24R)-11α-acetoxy-gorgost-3β,5α,6β-triol 413, (24S)-ergost-3β,5α,6β,11α-tetraol 414, and (24S)-23,24-dimethylcholest-22-en-3β,5α,6β,11α-tetraol 415. Their structural elucidation was based on spectral data, and it was found that all isolated compounds have a distinguishable 3β,5α,6β-trihydroxy group; however, they differ in side chains and substitutions. These steroids could be alienated structurally into four categories including, cholesterol, ergosterol, gorgosterol and 23,24-dimethyl cholesterol. (23R,24R,17Z)-11α-acetoxy-16β-methoxy-23,24-dimethylcholest-17(20)-en-3β,5α,6β-triol 406 has a distinctive 17(20)-en-23,24-dimethyl side chain, while (24R)-gorgost-25-en-3β,5α,6β,11α-tetraol 407 was a gorgosterol having a 25-ene side chain [118].
Ethanol-soluble fraction of the acetone extract of S. trocheliophorum yielded 9,11-secosteroid named, 25(26)-dehydrosarcomilasterol 416 and three polyhydroxylated steroids, 7α- hydrocrassorosterol A 417, 11α-acetoxy-7α-Hydrocrassorosterol A 418, sarcomilasterol 419, 3β,6α,11-trihydroxy-9,11-seco-5α-cholest-7-ene-9-one 420 and 3β,6α,11-trihydroxy-24-methylene-9,11-seco-5α-cholest-7-ene-9-one 421. The 9,11-secostroids nucleus can be described as the chemotaxonomic indicators for genus Sarcophyton [119].
Beside the abovementioned isoprenoids obtained from S. glaucum, 16-deacetylhalicrasterol B 422, together with sarcoaldesterol B 396, sarglaucsterol 423 were isolated too and their structures were elucidated by spectral data [70]. Furthermore, from S. ehrenbergi the isolation of two formerly isolated hippurine 424 and 425 [120] alongside pregnenolone 426 were reported [121]. Figure 7 summarizes polyhydroxylated sterols isolated from Sacrophyton sp.
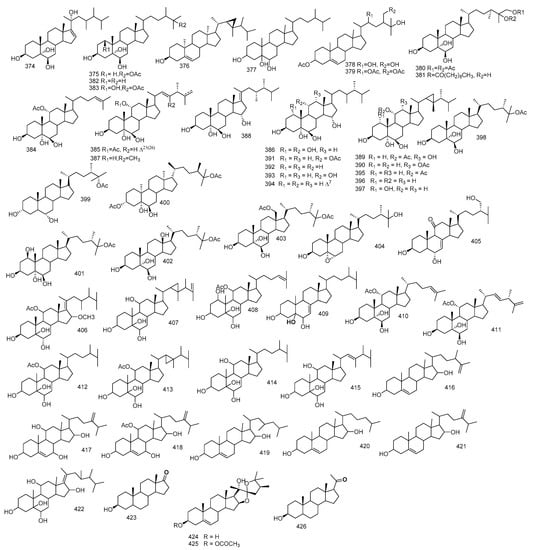
Figure 7.
Polyhydroxylated sterols reported from Sarcophyton sp.
2.5. Miscellaneous
From S. trocheliophorum, tetradecyl octadecenoate 427, 2,3-dihydroxypropyloctadecyl ether 428 and tetradecyl-9-Z-octadecenoate 429 were identified [29]. In addition, purification of the total lipid extract of S. trocheliophorum provided four butenolides 430–433 with different chain substitutions and saturation together with three fatty acids, arachidonic acid, eicosapentaenoic and docosahexaenoic methyl esters 434–436 and prostaglandin PGB2 437 [122].
An infrequent prostaglandin was isolated from S. crassocaule, (5Z)-9,15-dioxoprosta-5,8(12)-dien-1-oate 438 based on spectral analysis. This was the first time to report a prostaglandin with a C-15 keto group from natural origin [123]. Furthermore, from the ethyl acetate and n-butanol fractions of S. crassocaule, two isolated metabolites identified as sarcophytonone 439 a tetra-substituted quinone, and sarcophytonamine 440 a quaternary amine were reported. It might be valuable to know that these quinone derivatives are scarce in marine organisms and only sarcophytonone 439 was identified in S. mayi [124].
Five compounds were isolated from S. infundibuliforme, three were reported O-glycosylglycerol known as sarcoglycosides A–C 441–443 and chimyl alcohol and hexadecanol 444 and 445. Sarcoglycoside A 441 was the first glycoglycerolipid to be isolated from soft coral, while sarcoglycosides B and C 442 and 443 were rare marine isolates, composed of a lyxose residue and chimyl alcohol moiety [125]. Moreover, one α-tocopheryl quinone derivative, 3,5,6-trimethyl-2-14S-3,11,14-trihydroxy-3,7,11,15-tetramethylhexadecylcyclohexa-2,5-diene-1,4-dione 446, was isolated [64].
Purification of ethyl acetate extract of S. ehrenbergi yielded ten prostaglandins, sarcoehrendin A−J 447–456 together with five correlated compounds 457–461. Sarcoehrendin A 447 was found to be the acetylated derivative of arachidonic acid ethyl; previously isolated from Lobophyton depressum [126,127]. Another six prostaglandins 462–467, were reported from S. ehrenbergi, three were reported to be of marine origin [121]. From S. ehrenbergi extract, 2-methyl-1-octanol ester of (E)-3-(4methoxyphenyl) propenoic acid 468 was reported. The authors mentioned that stereochemical structure of 2-methyl-1-octanol ester of (E)-3-(4methoxyphenyl) propenoic acid 468 was ensured via synthesis of two possible isomers (S)-1 and (R)-1 which was recognized by an asymmetric synthesis using 4-benzyl-2-oxazolidinone chiral auxiliaries from octanoic acid [128]. From S. ehrenbergi ceramide 469 was reported alongside two cerebrosides, sarcoehrenosides A and B 470 and 471. A detailed spectral analysis revealed the occurrence of an amide linkage, a long chain, and a sugar, dependable with the C-9 methyl cerebroside nature of sarcoehrenoside A 470 [129].
Three carotenoids, peridinin, peridininol and peridininol-5,8-furanoxide 472–474 were reported for the first time from S. elegans. Chemical structures were interpreted by using spectral data and reported data [130]. Additionally, from Sarcophyton sp. another carotenoid, all-trans-(9′Z,11′Z)-(3R,3′S,5′R,6′R)-pyrrhoxanthin 475 was isolated [76].
Methyl tortuoate A and methyl tortuoate B 476 and 477, two tetracyclic tetraterpenoids, together with methyl sartortuoate 478 and methyl isosartortuoate 479, were reported from S. tortuosum. Methyl tortuoate A 476 was similar to methyl sartortuoate 478 in structure, except for the presence of secondary hydroxyl group in methyl tortuoate A 476 and absence of one tertiary hydroxyl functional group and conjugated diene. As for, methyl tortuoate B 477, it was found to be similar to methyl isosartortuoate 479 in structure, but with no hydroxyl group at C-27 [131]. Tetraterpenoid, methyl tortuoate C 480 after further investigation of the same ethanolic extract of S. tortuosum was isolated and a full spectral data was done to investigate its structure [132]. Another tetracyclic tetraterpenoid; methyl tortuoate D 481, was also reported from S. tortuosum and was identified using direct infusion electrospray ionization mass spectrometry [133]. Figure 8 summarizes miscellaneous isolated from Sacrophyton sp.
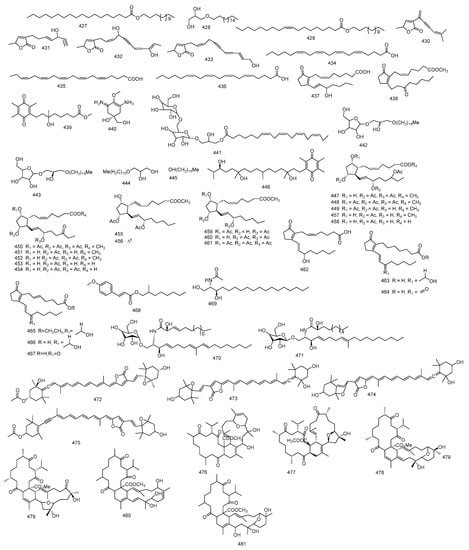
Figure 8.
Miscellaneous isolated from Sacrophyton sp.
3. Biological Activities
3.1. Cytotoxic Activity
The capability of 13- Acetoxysarcocrassolide 9 was investigated, as a cytotoxic agent against gastric carcinoma using MTT method, colony formation method, cell morphology assessments, and wound-healing method. It suppressed the development and migration of gastric cancer cells in a dose-dependent manner and initiated both early and late cell death examined by flow cytometer assay [134]. The authors mentioned that there was a relationship between the structure of sarcrassin A, B, D, and E 76, 77, 79, and 80, and emblide 81, and its activity, showing that loss of acetoxy group as in crassocolide C 84 led to loss of activity against all tested cell lines. While, acetylation at 4-OH position in crassocolide B 83 resulted in a decrease in activity cytotoxicity. However, the existence of two hydroxy moiety present at carbon-3 and carbon-4 and no oxidation at carbon-13 as in crassocolide D 85 showed potent activity against MCF-7 and A549 cell lines. While, crassocolide A 82 and F 87 exhibited potent activity toward Hep G2, MCF-7, MDA-MB-231 and A549, because of the 5-O-acetyl group [46]. Furthermore, crassocolide H and L 90 and 94, from S. crassocaule, showed strong activity toward KB, Hela, and Daoy cell lines owing to the presence of Cl atom at C-11 instead of OH group in crassocolide H 90 [47].
Sarcocrassocolides A–D 137–140, showed potent activity toward MCF-7, WiDr, HEp-2 and Daoy cell lines [58]. The authors maintained that the existence of acetoxy group at C-13 was important for activity. Sarcocrassocolides F–I 143–146, showed cytotoxicity toward all or part cell lines. However, sarcocrassocolide I 146 was most potent toward Daoy, HEp-2, MCF-7 and WiDr cell lines while sarcocrassocolide J and L 147 and 149 13-deacetoxy derivatives, were least potent against all tested cell lines with ED50 = >20 μM. Furthermore, hydroxy moiety at carbon-8 improve the cytotoxic activity in contrast with carbon-8 hydroperoxy-bearing correspondents sarcocrassocolide F and H 143 and 145 were most potent toward MCF-7 [59].
Owing to the α,β-unsaturated ε-lactone ring in glaucumolides A and B 329 and 330 both exhibited strong cytotoxicity toward HL-60 and CCRF-CEM cell [103]. The authors specified that the less degrees of oxidation the more immunosuppressive activity, yalongene A 71 was the most potent even better than the positive control Cyclosporin A [100]. (24S)-24-methylcholestane-3β,5α,6β,25-tetrol-25-monoacetate 375 exhibited potent activity toward P-388, A549, and HT-29 cell lines [114]. The authors reported that there was a structure activity relationship in which the existence of an extra free hydroxyl group at C-20 position in 23,24-dimethylcholest-16(17)-E-ene-3β,5α,6β,20(S)-tetraol 374, and acetyl group at C-25 position in 24-methylcholestane-3β,5α,6β,25-tetraol 25-monoacetate 375 led to strong cytotoxicity toward human M14, HL60, and MCF7 cells with a dose-dependent manner [29]. The occurrence of OAc moiety at carbon-11 was important for cytotoxic activity, as in (23R,24R,17Z)-11α-acetoxy-16β-methoxy-23,24-dimethylcholest-17(20)-en-3β,5α,6β-triol 406, (22E,24S)-11α-acetoxy-ergost-22, 25-dien-3β,5α,6β-triol 411 and (24R)-11α-acetoxy-gorgost-3β,5α,6β-triol 413 showed a strong cytotoxicity toward K562, HL-60, HeLa cell lines, while, 11α-acetoxycholest-24-en-1α,3β,5α,6β-tetraol 408, 11α-acetoxy-cholest-24-en-3β,5α,6β-triol 410 and (24S)-11α-acetoxy-ergost-3β,5α,6β-triol 412 exhibited a potent activity toward K562 and HL-60 [118].
3.2. Anti-Inflammatory Activity
Sarcocrassocolide M 150 could be a leading anti-inflammatory. Sarcocrassocolides M–O 150–152 might be beneficial anti-inflammatory agents because of the structure relationship and the existence of β-hydroperoxy moiety at carbon-7 [60]. Sarcocrassocolides F–L 137–143 activity was attributed to the ring-opening of the α,β-unsaturated-β-ether ketone group leading to an increase in the enzyme inhibitory activity [58]. Sarcoehrenolide A, B, and D 310, 311, and 313 and ehrenbergol D 251 showed significant TNF-α inhibition in which sarcoehrenolide B 311 was most active due to the existence of acetoxy at carbon-18. A structure activity relationship was demonstrated in which the keto moiety at carbon-13 and hydroxyl group at carbon-18 could be responsible for the slight increase in activity. However, the presence of carbomethoxy moiety at carbon-18 led to a reduction in activity [97].
3.3. Antidiabetic Activity
Methyl sarcotroate B 173 has strong inhibitory activity toward PTP1B because of the hydroperoxide group which binds to the active site of the Cys residue [67]. Potency of sarcophytonolide N 50 and sarcrassin E 80 may be because of the existence of methyl ester moiety at carbon-18, which significantly increases the enzyme inhibitory activity toward human PTP1B enzyme [39].
3.4. Antimicrobial Activity
Sarcophytolide 32 showed a strong antibacterial activity toward methicillin-sensitive S. aureus Newman strain because of the diene at C-1/C-3 [41]. The crude extract exhibited antimicrobial activity toward most of the examined bacteria, yeasts, and fungi. [77]. Trocheliophols H, I, L, N, O, and R 231, 232, 235, 237, 238, and 241, 4-epi-sarcophytol L 243 showed antibacterial activity toward Xanthomonas vesicatoria, Agrobacterium tumefaciens, Pseudomonas lachrymans, Bacillus subtilis, and Staphylococcus aureus. The authors mentioned that the structure activity relationship and the existence of exomethylene group at C-8 add to the antibacterial activity, while H-3β orientation, which was present only in compound trocheliophol S 242, gave the most potent activity against the selected bacteria [81]. The toxicity of the novel γ-lactones compounds butenolides 430–433 were evaluated by using shrimp bioassay, and bioactivity was shown. Additionally, they showed activity against Gram-positive bacteria only [122].
Because of the structure activity relationship, 11α-acetoxy-cholesta-24-en-3β,5α,6β-triol 384, (22E,24S)-11α-acetoxy-ergostane-22,25-dien-3β,5α,6β-triol 385, 11α-acetoxy-gorgostane-3β,5α,6β,12α-tetraol 389, 12α-acetoxy-gorgostane-3β,5α,6β,11α-tetraol 390, and sarcoaldosterol A 391 were more potent toward antibacterial activity toward Escherichia coli and Bacillus megaterium, and antifungal activity toward Microbotryum violaceum and Septoria tritici fungi, because of the 11α-acetoxy group, cyclopropane side chain and terminal-double bond [115].
3.5. Miscellaneous
Anticonvulsant activity of ceramide 469, measured in vivo by the pentylenetetrazole (PTZ)-induced seizure assay, has successfully opposed the lethality of pentylenetetrazole in mice. It showed also a significant anxiolytic activity when used in the light–dark transition box. This could be caused possibly by GABA and serotonin receptors modulation [135]. Table 1 summarizes the main biological activities of secondary metabolites from genus Sacrophyton.

Table 1.
The main biological activities of secondary metabolites isolated from genus Sacrophyton.
4. Conclusions
Based on reviewing the available current literature, a huge library of metabolites was isolated, and it possessed unique structures. Up to 481 compounds with different structures belonging to different chemical classes were reported from the Sarcophyton species. The chemical structures were classified as terpenoids (majority), biscembranes, polyhydroxylated sterols, sesquiterpenes (minority), and miscellaneous compounds. S. trocheliophorum gave the highest number of compounds. Members of genus Sarcophyton possessed valuable and interesting biological activities, such as antibacterial, cytotoxicity, antifungal, and antidiabetic.
Supplementary Files
Supplementary File 1Author Contributions
Resources, Y.A.E. and M.M.B.; data curation, A.M.E. and O.M.S.; writing—original draft preparation, Y.A.E.; writing—review and editing, A.M.E., M.S.E., O.M.S., and M.M.B.; visualization, N.M.M. and E.M.K.; supervision, O.M.S. and A.N.B.S.; project administration, O.M.S.; funding acquisition, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank M. El-Kalay, head of Department of English Postgraduate Studies (EPS) and International Exams (CELTA, OET, ILETS), Future University in Egypt, for her kind and valuable help with English language correction.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lewis, J.C.; Wallis, E.V. The function of surface sclerites in gorgonians (Coelenterata, Octocorallia). Biol. Bull. 1991, 181, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, S.; Raghunathan, C.; Chandra, K. New record of Sarcophyton cornispiculatum Verseveldt, 1971 (Octocorallia: Alcyonacea: Alcyoniidae) in India, from the Andaman Islands. Eur. Zool. J. 2017, 84, 167–171. [Google Scholar] [CrossRef]
- Verseveldt, J. A revision of the genus Sarcophyton Lesson (Octocorallia, Alcyonacea). Zool. Verhandel. 1982, 192, 1–91. [Google Scholar]
- Alderslade, P. A redescription of Alcyonium agaricum Stimpson with a generic placement in Sarcophyton (Coelenterata: Octocorallia). Precious Corals Octocorals Res. 1993, 1, 20–29. [Google Scholar]
- Alderslade, P.; Shirwaiker, P. New species of soft corals (Coelenterata: Octocorallia) from the Laccadive Archipelago. Beagle 1991, 8, 189–233. [Google Scholar]
- Benayahu, Y.; Perkol-Finkel, S. Soft corals (Octocorallia: Alcyonacea) from southern Taiwan. I. Sarcophyton nanwanensis sp. nov. (Octocorallia: Alcyonacea). Zool. Stud. 2004, 43, 537–543. [Google Scholar]
- Benayahu, Y.; van Ofwegen, L.P. New species of Sarcophyton and Lobophytum (Octocorallia: Alcyonacea) from Hong Kong. Zool. Meded. 2009, 83, 863–876. [Google Scholar]
- Li, C. Studies on the Alcyonacea of the South China Sea II. Genera Lobophytum and Sarcophyton from the Xisha Islands, Guangdong Province. J. Chin. Chem. Soc. 1984, 6, 103–119. [Google Scholar]
- McFadden, C.S.; Alderslade, P.; Ofwegen, L.P.; Johnsen, H.; Rusmevichientong, A. Phylogenetic relationships within the tropical soft coral genera Sarcophyton and Lobophytum (Anthozoa, Octocorallia). Invertebr. Biol. 2006, 125, 288–305. [Google Scholar] [CrossRef]
- Aratake, S.; Tomura, T.; Saitoh, S.; Yokokura, R.; Kawanishi, Y.; Shinjo, R.; Reimer, J.D.; Tanaka, J.; Maekawa, H. Soft coral Sarcophyton (Cnidaria: Anthozoa: Octocorallia) species diversity and chemotypes. PLoS ONE 2012, 7, e30410. [Google Scholar] [CrossRef]
- Dineson, Z. Patterns in the distribution of soft corals across the Central Great Barrier Reef. Coral Reefs 1983, 1, 229–236. [Google Scholar] [CrossRef]
- Mignk, C.A.; Davoult, D. Quantitative distribution of benthic macrofauna of the Dover Strait pebble community (English Channel, France). Oceanol. Acta 1997, 20, 453–460. [Google Scholar]
- Benayahu, Y.; Loya, Y. Competition for space among coral reef sessile organisms at Eilat, Red Sea. Bull. Mar. Sci. 1981, 31, 514–522. [Google Scholar]
- Ros, J.; Romero, J.; Ballesteros, E.; Gili, J. Diving in blue water. The benthos. In The Western Mediterranean; Pergamon Press: Oxford, UK, 1985; pp. 233–295. [Google Scholar]
- Slattery, M.; McClintock, J.B. Population structure and feeding deterrence in three shallow-water antarctic soft corals. Mar. Biol. 1995, 122, 461–470. [Google Scholar] [CrossRef]
- Faulkner, D.J. Interesting aspects of marine natural products chemistry. Tetrahedron 1977, 33, 1421–1443. [Google Scholar] [CrossRef]
- Kobayashi, J.; Ohizumi, Y.; Nakamura, H.; Yamakado, T.; Matsuzaki, T.; Hirata, Y. Ca-antagonistic substance from soft coral of the genus Sarcophyton. Experientia 1983, 39, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Liu, Q.; Tang, G.; Wang, H.; Fan, C.; Yin, S. Bioactive cembranoids from the South China Sea soft coral Sarcophyton elegans. Molecules 2015, 20, 13324–13335. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Sylvester, P.W.; Avery, M.A.; Desai, P.; Youssef, D.T.A.; El Sayed, K.A. Bioactive Rearranged and Halogenated Semisynthetic Derivatives of the Marine Natural Product Sarcophine. J. Nat. Prod. 2004, 67, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Mohamed, S.Z.; Abu El-Regal, M.; Farhat, A.Z. Antibacterial activity of some Red Sea soft corals, Egypt. Blue Biotechnol. J. 2013, 1, 119–138. [Google Scholar]
- Lai, K.; You, W.; Lin, C.; El-Shazly, M.; Liao, Z.; Su, J. Anti-Inflammatory Dembranoids from the Soft Coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; Ofwegen, L.; Proksch, P.; Lin, W. Sarcophyolides B–E, New Cembranoids from the Soft Coral Sarcophyton elegans. Mar. Drugs 2013, 11, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Suguri, H.; Yoshizawa, S.; Takagi, K.; Kobayashi, M. Sarcophytols A and B inhibit tumor promotion by teleocidin in two-stage carcinogenesis in mouse skin. J. Cancer Res. Clin. Oncol. 1989, 115, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Guo, Y.W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodivers. 2013, 10, 2161–2196. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef]
- Zubaira, M.S.; Al-Footy, K.O.; Ayyada, S.N.; Al-Lihaibib, S.S.; Alarifb, W.M. A review of steroids from Sarcophyton species. Nat. Prod. Res. 2015, 30, 869–879. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D. New Cembranoid Diterpenes from the Soft Coral Sarcophyton ehrenbergi. J. Nat. Prod. 1998, 61, 494–496. [Google Scholar] [CrossRef]
- Duh, C.; Wang, S.; Chung, S.; Chou, G.; Dai, C. Cytotoxic Cembrenolides and Steroids from the Formosan Soft Coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 1634–1637. [Google Scholar] [CrossRef]
- Dong, H.; Gou, Y.; Kini, R.M.; Xu, H.; Chen, S.; Teo, S.L.M.; But, P.P. A New Cytotoxic Polyhydroxysterol from Soft Coral Sarcophyton trocheliophorum. Chem. Pharm. Bull. 2000, 48, 1087–1089. [Google Scholar] [CrossRef]
- Mada, K.; Ooi, T.; Kusumi, T. NMR study of acutanol, a new cembrene alcohol, and sarcophytol A isolated from the soft coral Sarcophyton acutangulum. J. Spectrosc. 2001, 15, 177–182. [Google Scholar] [CrossRef][Green Version]
- Pham, N.; Butler, M.; Quinn, R.J. Naturally Occurring Cembranes from an Australian Sarcophyton Species. J. Nat. prod. 2002, 65, 1147–1150. [Google Scholar] [CrossRef]
- Xu, X.; Kong, C.; Lin, C.; Wang, X.; Zhu, Y.; Yang, H. A Novel Diterpenoid from the Soft Coral Sarcophyton crassocaule. Chin. J. Chem. 2003, 21, 1506–1509. [Google Scholar] [CrossRef]
- Gross, H.; Kehraus, S.; Nett, M.; König, G.M.; Beil, W.; Wright, A.D. New cytotoxic cembrane based diterpenes from the soft corals Sarcophyton cherbonnieri and Nephthea sp. Org. Biomol. Chem. 2003, 1, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Al-Lihaibi, S.; Alarif, W.; Abdel-Lateff, A.; Ayyad, S.; Abdel-Naim, A.; El-Senduny, F.; Badria, F. Three New Cembranoid-Type Diterpenes from Red Sea Soft Coral Sarcophyton glaucum: Isolation and Antiproliferative Activity against HepG2 Cells. Eur. J. Med. Chem. 2014, 81, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Wright, A.D.; Beil, W.; König, G.M. Two new bicyclic cembranolides from a new Sarcophyton species and determination of the absolute configuration of sarcoglaucol-16-one. Org. Biomol. Chem. 2004, 2, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Guo, Y.; Mollo, E.; Cimino, G. Sarcophytonolides AD, Four New Cembranolides from the Hainan Soft Coral Sarcophyton sp. Helv. Chim. Acta 2005, 88, 1028–1033. [Google Scholar] [CrossRef]
- Jia, R.; Guo, Y.; Mollo, E.; Gavagnin, M.; Cimino, G. Sarcophytonolides E−H, Cembranolides from the Hainan Soft Coral Sarcophyton latum. J. Nat. prod. 2006, 69, 819–822. [Google Scholar] [CrossRef]
- Yan, X.; Li, Z.; Guo, Y. Further New Cembranoid Diterpenes from the Hainan Soft Coral Sarcophyton latum. Helv. Chim. Acta 2007, 38, 1574–1580. [Google Scholar] [CrossRef]
- Liang, L.; Gao, L.; Li, J.; Taglialatela-Scafati, O.; Guo, Y. Cembrane diterpenoids from the soft coral Sarcophyton trocheliophorum Marenzeller as a new class of PTP1B inhibitors. Bioorg. Med. Chem. 2013, 21, 5076–5080. [Google Scholar] [CrossRef]
- Liang, L.; Kurtán, T.; Mándi, A.; Yao, L.; Li, J.; Lan, L.; Guo, Y. Structural, stereochemical, and bioactive studies of cembranoids from Chinese soft coral Sarcophyton trocheliophorum. Tetrahedron 2018, 74, 1933–1941. [Google Scholar] [CrossRef]
- Liang, L.; Lan, L.; Taglialatela-Scafati, O.; Guo, Y. Sartrolides A–G and bissartrolide, new cembranolides from the South China Sea soft coral Sarcophyton trocheliophorum Marenzeller. Tetrahedron 2013, 69, 7381–7386. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, H.; Liang, L.; Guo, X.; Mao, S.; Guo, Y. Yalongenes A and B, Two New Cembranoids with Cytoprotective Effects from the Hainan Soft Coral Sarcophyton trocheliophorum Marenzeller. Helv. Chim. Acta 2012, 95, 235–239. [Google Scholar] [CrossRef]
- Hegazy, M.E.; Mohamed, T.; Abdel-Latif, F.; Alsaid, M.; Shahat, A.; Pare, P. Trochelioid A and B, new cembranoid diterpenes from the Red Sea soft coral Sarcophyton trocheliophorum. Phytochem. Lett. 2013, 6, 383–386. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Su, J.; Liang, Y.; Yang, X.; Zheng, K.; Zeng, L. Cytotoxic diterpenoids from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.A.; Burreson, B.J.; Scheuer, P.J.; Finer-Moore, J.; Clardy, J. Emblide, a new polyfunctional cembranolide from the soft coral Sarcophyton glaucum. Tetrahedron 1980, 36, 1307–1309. [Google Scholar] [CrossRef]
- Huang, H.; Ahmed, A.; Su, J.; Chao, C.; Wu, Y.; Chiang, M.; Sheu, J. Crassocolides A−F, Cembranoids with a trans- Fused Lactone from the Soft Coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1554–1559. [Google Scholar] [CrossRef]
- Huang, H.; Chao, C.; Kuo, Y.; Sheu, J. Crassocolides G-M, Cembranoids from the Formosan Soft Coral Sarcophyton crassocaule. Chem. Biodivers. 2009, 6, 1232–1242. [Google Scholar] [CrossRef]
- Wang, G.; Huang, H.; Su, J.; Huang, C.; Hsu, C.; Kuo, Y.; Sheu, J. Crassocolides N–P, three cembranoids from the Formosan soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. Lett. 2011, 21, 7201–7204. [Google Scholar] [CrossRef]
- Bensemhoun, J.; Rudi, A.; Bombarda, I.; Gaydou, E.M.; Kashman, Y.; Aknin, M. Flexusines A and B and Epimukulol from the Soft Coral Sarcophyton flexuosum. J. Nat. Prod. 2008, 71, 1262–1264. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, Y.; Kuo, Y.; Khalil, A.T. Cembrane Diterpenoids from the Taiwanese Soft Coral Sarcophyton stolidotum. J. Nat. Prod. 2008, 71, 1141–1145. [Google Scholar] [CrossRef]
- Coung, N.X.; Tuan, T.A.; Kiem, P.V.; Minh, C.V.; Choi, E.M.; KIM, Y.H. New Cembranoid Diterpenes from the Vietnamese Soft Coral Sarcophyton mililatensis Stimulate Osteoblastic Differentiation in MC3T3-E1 Cells. Chem. Pharm. Bull. 2008, 56, 988–992. [Google Scholar] [CrossRef]
- Grote, D.; Shaker, K.; Soliman, H.; Hegazi, M.; Seifert, K. Cembranoid Diterpenes from the Soft Corals Sarcophyton sp. and Sarcophyton Glaucum. Nat. Prod. Commun. 2008, 3, 1473–1478. [Google Scholar] [CrossRef]
- Yao, L.; Liu, H.; Guo, Y.; Mollo, E. New Cembranoids from the Hainan Soft Coral Sarcophyton glaucum. Helv. Chim. Acta 2009, 92, 1085–1091. [Google Scholar] [CrossRef]
- Magie, M.K.; Lee, J.; Oda, T.; Nakazawa, T.; Takahashi, O.; Ukai, K.; Mangindaan, R.; Rotinsulu, H.; Defny, S.W.; Sachiko, T.; et al. Two unprecedented cembrene-type terpenes from an Indonesian soft coral Sarcophyton sp. Tetrahedron 2010, 66, 641–645. [Google Scholar]
- Chen, S.; Chen, B.; Dai, C.; Sung, P.; Wu, Y.; Sheu, J. Sarcophytonins F and G, New Dihydrofuranocembranoids from a Dongsha Atoll Soft Coral Sarcophyton sp. Bull. Chem. Soc. Jpn. 2012, 85, 920–922. [Google Scholar] [CrossRef]
- Hassan, M.H.; Rateb, E.M.; Hassan, H.M.; Sayed, M.A.; Shabana, S.; Raslan, M.; Amin, E.; Behery, A.F.; Ahmed, M.O.; Bin Muhsinah, A.; et al. New Antiproliferative Cembrane Diterpenes from the Red Sea Sarcophyton Species. Mar. Drugs 2019, 17, 411. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Soto, J.J.; Piña, I.C. Uprolides D-G, 2. A Rare Family of 4,7-Oxa-bridged Cembranolides from the Caribbean Gorgonian Eunicea mammosa. J. Nat. Prod. 1995, 58, 1209–1216. [Google Scholar] [CrossRef]
- Lin, W.; Su, J.; Lu, Y.; Wen, Z.; Dai, C.; Kuo, Y.; Sheu, J. Cytotoxic and Anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010, 18, 1936–1941. [Google Scholar] [CrossRef]
- Lin, W.; Lu, Y.; Su, J.; Wen, Z.; Dai, C.; Kuo, Y.; Sheu, J. Bioactive Cembranoids from the Dongsha Atoll Soft Coral Sarcophyton crassocaule. Mar. Drugs 2011, 9, 994–1006. [Google Scholar] [CrossRef]
- Lin, W.; Lu, Y.; Chen, B.; Huang, C.A.; Su, J.; Wen, Z.; Dai, C.; Kuo, Y.; Sheu, J. Sarcocrassocolides M–O, Bioactive Cembranoids from the Dongsha Atoll Soft Coral Sarcophyton crassocaule. Mar. Drugs 2012, 10, 617–626. [Google Scholar] [CrossRef]
- Lin, W.; Chen, B.; Huang, C.A.; Wen, Z.; Sung, P.; Su, J.; Dai, C.; Sheu, J. Bioactive Cembranoids, Sarcocrassocolides P–R, from the Dongsha Atoll Soft Coral Sarcophyton crassocaule. Mar. Drugs 2014, 12, 840–850. [Google Scholar] [CrossRef]
- Shaker, K.; Müller, M.; Ghani, M.; Dahse, H.; Seifert, K. Terpenes from the Soft Corals Litophyton arboreum and Sarcophyton ehrenbergi. Chem. Biodivers. 2010, 7, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, S.; Chiou, S.; Hsu, C.; Dai, C.; Chiang, M.Y.; Duh, C.Y. Cembranoids from the Octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2010, 73, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, A.; Shao, C.; Li, L.; Xu, Y.; Qian, P. Chemical constituents of soft coral Sarcophyton infundibuliforme from the South China Sea. Biochem. Syst. Ecol. 2011, 39, 853–856. [Google Scholar] [CrossRef]
- Hegazy, M.; El-Beih, A.; Moustafa, A.; A Hamdy, A.; Alhammady, M.; Selim, R.; Abdel-Rehim, M.; Pare, P. Cytotoxic Cembranoids from the Red Sea Soft Coral Sarcophyton glaucum. Nat. Prod. Commun. 2011, 6, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.; Gamal-Eldeen, A.; Shahat, A.; Abdel-Latif, F.; Mohamed, T.; R Whittlesey, B.; Pare, P. Bioactive Hydroperoxyl Cembranoids from the Red Sea Soft Coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef]
- Liang, L.; Kurtán, T.; Mándi, A.; Yao, L.; Li, J.; Zhang, W.; Guo, Y. Unprecedented Diterpenoids as a PTP1B Inhibitor from the Hainan Soft Coral Sarcophyton trocheliophorum Marenzeller. Org. Lett. 2012, 15, 274–277. [Google Scholar] [CrossRef]
- Ross, S.; Abou El-Ezz, R.F.; Sa, A.; Radwan, M.; Ayoub, N.; Afifi, M.; Khalifa, S. Bioactive cembranoids from the Red Sea soft coral Sarcophyton glaucum. Planta Med. 2012, 78, 989–992. [Google Scholar] [CrossRef]
- Wang, S.; Hsieh, M.; Duh, C. Three new cembranoids from the Taiwanese soft coral Sarcophyton ehrenbergi. Mar. Drugs 2012, 10, 1433–1444. [Google Scholar] [CrossRef]
- Chao, C.H.; Li, W.L.; Huang, C.Y.; Ahmed, A.F.; Dai, C.F.; Wu, Y.C.; Lu, M.C.; Liaw, C.C.; Sheu, J.H. Isoprenoids from the Soft Coral Sarcophyton glaucum. Mar. Drugs 2017, 15, 202. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Cheng, S.; Duh, C. Lobocrasol, a New Diterpenoid from the Soft Coral Lobophytum crassum. Org. Lett. 2009, 11, 3012–3014. [Google Scholar] [CrossRef]
- Wang, S.; Hsieh, M.; Duh, C. New diterpenoids from soft coral Sarcophyton ehrenbergi. Mar. Drugs 2013, 11, 4318–4327. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zhou, J.; Xu, K.; Zhang, M.; Wang, C.Y. New Cembranoid Diterpene from the South China Sea Soft Coral Sarcophyton sp. Nat. Prod. Commun. 2013, 8, 1675–1678. [Google Scholar] [CrossRef]
- Lin, K.; Tseng, Y.; Chen, B.; Hwang, T.; Chen, H.; Dai, C.; Sheu, J. Tortuosenes A and B, New Diterpenoid Metabolites from the Formosan Soft Coral Sarcophyton tortuosum. Org. Lett. 2014, 16, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, N.A.; Ibrahim, A.; Radwan, M.; Elsohly, M.A.; Hassanean, H.A.; Ahmed, S.A. Cytotoxic Cembranoids from the Red Sea Soft Coral, Sarcophyton auritum. Tetrahedron Lett. 2014, 46, 3984–3988. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, Q.; Chen, Y.Y.; Fan, C.; Huang, Z.; Xu, X.; Yin, S. Four new cembranoids from the soft coral Sarcophyton sp. Magn. Reson. Chem. 2014, 52, 515–520. [Google Scholar] [CrossRef]
- Al-Footy, K.; Alarif, W.; Asiri, F.; Aly, M.; Ayyad, S. Rare pyrane-based cembranoids from the Red Sea soft coral Sarcophyton troheliophorum as potential antimicrobial-antitumor agents. Med. Chem. Res. 2014, 24, 505–512. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Alarif, W.; Ayyad, S.; Al-Lihaibi, S.; Basaif, S. New cytotoxic isoprenoid derivatives from the Red Sea soft coral Sarcophyton glaucum. Nat. Prod. Res. 2015, 29, 24–30. [Google Scholar] [CrossRef]
- Elkhateeb, A.; El-Beih, A.; Gamal-Eldeen, A.; Alhammady, M.; Ohta, S.; Pare, P.; Hegazy, M.E. New Terpenes from the Egyptian Soft Coral Sarcophyton ehrenbergi. Mar. Drugs 2014, 12, 1977–1986. [Google Scholar] [CrossRef]
- Liang, T.K.; Mándi, A.; Gao, L.; Jia, L.; Zhang, W.; Guo, Y. Sarsolenane and Capnosane Diterpenes from the Hainan Soft Coral Sarcophyton trocheliophorum Marenzeller as PTP1B Inhibitors. Eur. J. Org. Chem. 2014, 2014, 1841–1847. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, W.; Liu, D.; Ofwegen, L.; Proksch, P.; Lin, W. Capnosane-Type Cembranoids from the Soft Coral Sarcophyton trocheliophorum with Antibacterial Effects. Tetrahedron 2014, 70, 8703–8713. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, S.; Hsieh, M.; Duh, C. Polyoxygenated cembrane diterpenoids from the soft coral Sarcophyton ehrenbergi. Int. J. Mol. Sci. 2015, 16, 6140–6152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, W.; Bao, J.; Zhang, J.; Yin, S.; Tang, G. Diterpenoids from the South China Sea soft coral Sarcophyton solidum. Biochem. Syst. Ecol. 2015, 62, 6–10. [Google Scholar] [CrossRef]
- Zubair, M.; Alarif, W.; Al-Footy, K.; P H, M.; Aly, M.; A Basaif, S.; Al-Lihaibi, S.; Ayyad, S. New antimicrobial biscembrane hydrocarbon and cembranoid diterpenes from the soft coral Sarcophyton trocheliophorum. Turk. J. Chem. 2015, 40, 385–392. [Google Scholar] [CrossRef]
- Kamada, T.; Phan, C.S.; Tin, H.S.; Vairappan, C.S.; Muhammad, T.S.T. 16-Hydroxycembra-1,3,7,11-tetraene, a new Cembrane Diterpene from Malaysian Soft Coral Genus Sarcophyton. Nat. Prod. Commun. 2016, 11, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liang, L.F.; Li, X.; Xiao, W.; Guo, Y.W. Further New Highly Oxidative Cembranoids from the Hainan Soft Coral Sarcophyton trocheliophorum. Nat. Prod. Bioprospect. 2016, 6, 97–102. [Google Scholar] [CrossRef]
- Liang, L.; Chen, W.; Mollo, E.; Yao, L.; Wang, H.; Xiao, W.; Guo, Y. Sarcophytrols G–L, Novel Minor Metabolic Components from South China Sea Soft Coral Sarcophyton trocheliophorum Marenzeller. Chem. Biodivers. 2017, 14, e1700079. [Google Scholar] [CrossRef]
- Liang, L.; Chen, W.; Li, X.; Wang, H.; Guo, Y. New Bicyclic Cembranoids from the South China Sea Soft Coral Sarcophyton trocheliophorum. Sci. Rep. 2017, 7, 46584. [Google Scholar] [CrossRef]
- Liu, K.M.; Lan, Y.H.; Su, C.C.; Sung, P.J. Trocheliolide B, a New Cembranoidal Diterpene from the Octocoral Sarcophyton trocheliophorum. Nat. Prod. Commun. 2016, 11, 21–22. [Google Scholar] [CrossRef]
- Shaaban, M.; Ghani, M.A.; Shaaban, K. Unusual pyranosyl cembranoid diterpene from Sarcophyton trocheliophorum. Zeitschrift Für Naturforschung B 2016, 71, 1211–1217. [Google Scholar] [CrossRef]
- Tang, G.; Sun, Z.; Zou, Y.H.; Yin, S. New Cembrane-Type Diterpenoids from the South China Sea Soft Coral Sarcophyton ehrenbergi. Molecules 2016, 21, 587. [Google Scholar] [CrossRef]
- Liang, L.; Wang, J.; Shi, X.; Zhu, Y.; Li, J.; Zhu, W.; Wang, H.; Guo, Y. A Novel Sarsolenane Diterpene as a PTP1B Inhibitor from Hainan Soft Coral Sarcophyton trocheliophorum Marenzeller. Chin. J. Chem. 2017, 35, 1246–1250. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Lubken, T.; Gruner, M.; Kataeva, O.; Ralambondrahety, R.; Andriamanantoanina, H.; Checinski, M.P.; Bauer, I.; Knolker, H.J. Isolation and structure elucidation of natural products of three soft corals and a sponge from the coast of Madagascar. Org. Biomol. Chem. 2017, 15, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zou, Y.H.; Ge, M.X.; Lou, L.L.; Xu, Y.S.; Ahmed, A.; Chen, Y.Y.; Zhang, J.S.; Tang, G.H.; Yin, S. Biscembranoids and Cembranoids from the Soft Coral Sarcophyton elegans. Mar. Drugs 2017, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.F.; Elshamy, A.I.; Mohamed, T.A.; Hamed, A.R.; Ibrahim, M.A.A.; Ohta, S.; Paré, P.W. Cembrene Diterpenoids with Ether Linkages from Sarcophyton ehrenbergi: An Anti-Proliferation and Molecular-Docking Assessment. Mar. Drugs 2017, 15, 192. [Google Scholar] [CrossRef]
- Hegazy, M.F.; Mohamed, T.A.; Elshamy, A.I.; Hamed, A.R.; Ibrahim, M.A.A.; Ohta, S.; Umeyama, A.; Paré, P.W.; Efferth, T. Sarcoehrenbergilides D–F: Cytotoxic cembrene diterpenoids from the soft coral Sarcophyton ehrenbergi. RSC Adv. 2019, 9, 27183–27189. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Zhang, Q.; Yang, M.; Gu, Y.; Liang, L.; Tang, W.; Guo, Y. Rare Cembranoids from Chinese Soft Coral Sarcophyton ehrenbergi: Structural and Stereochemical Studies. J. Org. Chem. 2019, 84, 5091–5098. [Google Scholar] [CrossRef]
- Ye, F.; Li, J.; Wu, Y.; Zhu, Z.D.; Mollo, E.; Gavagnin, M.; Gu, Y.C.; Zhu, W.L.; Li, X.W.; Guo, Y.W. Sarinfacetamides A and B, Nitrogenous Diterpenoids with Tricyclo[6.3.1.0(1,5)]dodecane Scaffold from the South China Sea Soft Coral Sarcophyton infundibuliforme. Org. Lett. 2018, 20, 2637–2640. [Google Scholar] [CrossRef]
- Kamada, T.; Zanil, I.I.; Phan, C.; Vairappan, C. A new cembrane, from soft coral genus Sarcophyton in Borneo. Nat. Prod. Commun. 2018, 13, 123–124. [Google Scholar] [CrossRef]
- Yang, M.; Li, X.L.; Wang, J.R.; Lei, X.; Tang, W.; Li, X.W.; Sun, H.; Guo, Y.W. Sarcomililate A, an Unusual Diterpenoid with Tricyclo[11.3.0.0(2,16)]hexadecane Carbon Skeleton, and Its Potential Biogenetic Precursors from the Hainan Soft Coral Sarcophyton mililatensis. J. Org. Chem. 2019, 84, 2568–2576. [Google Scholar] [CrossRef]
- Shaaban, M.; Issa, M.Y.; Ghani, M.A.; Hamed, A.; Abdelwahab, A.B. New pyranosyl cembranoid diterpenes from Sarcophyton trocheliophorum. Nat. Prod. Res. 2019, 33, 24–33. [Google Scholar] [CrossRef]
- Iwagawa, T.; Hashimoto, K.; Okamura, H.; Kurawaki, J.; Nakatani, M.; Hou, D.X.; Fujii, M.; Doe, M.; Morimoto, Y.; Takemura, K. Biscembranes from the soft coral Sarcophyton glaucum. J. Nat. Prod. 2006, 69, 1130–1133. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, Biscembranoids with New Structural Type from a Cultured Soft Coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Guo, Y.; Chen, P.; Yang, Y.; Mollo, E.; Gavagnin, M.; Cimino, G. Biscembranoids and Their Probable Biogenetic Precursor from the Hainan Soft Coral Sarcophyton tortuosum. J. Nat. Prod. 2007, 70, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gavagnin, M.; Cimino, G.; Guo, Y. Two new biscembranes with unprecedented carbon skeleton and their probable biogenetic precursor from the Hainan soft coral Sarcophyton latum. Tetrahedron Lett. 2007, 48, 5313–5316. [Google Scholar] [CrossRef]
- Bishara, A.; Rudi, A.; Benayahu, Y.; Kashman, Y. Three Biscembranoids and their Monomeric Counterpart Cembranoid, a Biogenetic Diels–Alder Precursor, from the Soft Coral Sarcophyton elegans. J. Nat. Prod. 2007, 70, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; Ofwegen, L.; Chaidir, C.; Lin, W. Sarcophytolides G–L, New Biscembranoids from the Soft Coral Sarcophyton elegans. Helv. Chim. Acta 2013, 96, 2218–2227. [Google Scholar] [CrossRef]
- Nam, N.; The Tung, P.; Thi Ngoc, N.; Thi Hong Hanh, T.; Thao, N.; Van Thanh, N.; Cuong, N.; Thao, D.; Thu Huong, T.; Cong Thung, D.; et al. Cytotoxic Biscembranoids from the Soft Coral Sarcophyton pauciplicatum. Chem. Pharm. Bull. 2015, 63, 636–640. [Google Scholar] [CrossRef]
- Yasumoto, M.; Mada, K.; Ooi, T.; Kusumi, T. New Terpenoid Components from the Volatile Oils of the Soft Corals Clavularia viridis and Sarcophyton acutangulum. J. Nat. Prod. 2000, 63, 1534–1536. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Gowri, P.M. Two New Guaiane Sesquiterpenoids from the Soft Coral Sarcophyton buitendijki of the Andaman and Nicobar Island of the Indian Ocean. Indian J. Chem. 2001, 32, 773–778. [Google Scholar] [CrossRef]
- Sawant, S.S.; Youssef, D.; Sylvester, P.; Wali, V.; El Sayed, K. Antiproliferative Sesquiterpenes from the Red Sea Soft Coral Sarcophyton glaucum. Nat. Prod. Commun. 2007, 2, 117. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G.; Rao, D.V. Trocheliophorin: A novel rearranged sesquiterpenoid from the Indian Ocean soft coral Sarcophyton trocheliophorum. J. Asian Nat. Prod. Res. 2008, 10, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yang, R.; Zeng, L.; Sardisterol, A. New Polyhydroxylated Sterol from the Soft Coral Sarcophyton digitatum Moser. Chin. J. Chem. 2001, 19, 515–517. [Google Scholar] [CrossRef]
- Wang, G.; Su, J.; Chen, C.; Duh, C.; Dai, C.; Sheu, J. Novel Polyhydroxysteroids from the Formosan Soft Coral Sarcophyton glaucum. J. Chin. Chem. Soc. 2004, 51, 217–222. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, H.; Wang, P.; Gong, W.; Xue, M.; Zhang, H.; Liu, T.; Liu, B.; Yi, Y.; Zhang, W. Bioactive Polyoxygenated Steroids from the South China Sea Soft Coral, Sarcophyton sp. Mar. Drugs 2013, 11, 775–787. [Google Scholar] [CrossRef]
- Sun, L.L.; Fu, X.M.; Li, X.B.; Xing, Q.; Wang, C.Y. New 18-oxygenated polyhydroxy steroid from a South China Sea soft coral Sarcophyton sp. Nat. Prod. Res. 2013, 27, 2006–2011. [Google Scholar] [CrossRef]
- Shaaban, M.; Ghani, M.A.; Shaaban, K. Zahramycins A-B, Two New Steroids from the Coral Sarcophyton trocheliophorum. Zeitschrift Für Naturforschung B 2013, 68, 939–945. [Google Scholar] [CrossRef]
- Gong, K.; Tang, X.; Zhang, G.; Cheng, C.; Zhang, X.; Pinglin, L.; Li, G. Polyhydroxylated Steroids from the South China Sea Soft Coral Sarcophyton sp and Their Cytotoxic and Antiviral Activities. Mar. Drugs 2013, 11, 4788–4798. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Yao, L.; Guo, Y. 9,11-Secosteroids and polyhydroxylated steroids from two South China Sea soft corals Sarcophyton trocheliophorum and Sinularia flexibilis. Steroids 2014, 92, 56–61. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Krishnamurthy, M.V.R.; Rao, G.V. Rare aromadendrane diterpenoids from a new soft coral species of Sinularia genus of the Indian Ocean. Tetrahedron 1997, 53, 9301–9312. [Google Scholar] [CrossRef]
- Sekhar, V.C.; Rao, C.B.; Ramana, H.; Rao, D.V. New Prostaglandins from the Soft Coral Sarcophyton ehrenbergi Marengeller of Andaman and Nicobar Islands of Indian Ocean. Asian J. Chem. 2010, 22, 5353–5358. [Google Scholar]
- Řezanka, T.; Dembitsky, V. γ-Lactones from the soft corals Sarcophyton trocheliophorum and Lithophyton arboreum. Tetrahedron 2001, 57, 8743–8749. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Krishna Murthy, M.V.R.; Gowri, P.M.; Venugopal, M.J.R.V.; Laatsch, H. A Rare Prostaglandin from the Soft Coral Sarcophyton crassocaule of the Indian Ocean. J. Nat. Prod. 2000, 63, 1425–1426. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Shao, C.; Han, L.; Sun, X.; Zhao, J.; Guo, Y.; Huang, H.; Guan, H. Two new metabolites from the Hainan soft coral Sarcophyton crassocaule. J. Asian Nat. Prod. Res. 2009, 11, 851–855. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Shao, C.; Guo, Y.; Li, G.; Sun, X.; Han, L.; Huang, H.; Guan, H. Sarcoglycosides A–C, New O-Glycosylglycerol Derivatives from the South China Sea Soft Coral Sarcophyton infundibuliforme. Helv. Chim. Acta 2009, 92, 1495–1502. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y.; Loya, Y.; Benayahu, Y. New prostaglandin (PGF) derivatives from the soft coral Lobophyton depressum. Tetrahedron Lett. 1980, 21, 875–878. [Google Scholar] [CrossRef]
- Cheng, Z.; Deng, Y.; Fan, C.; Han, Q.; Lin, S.; Tang, G.; Luo, H.; Yin, S. Prostaglandin Derivatives: Nonaromatic Phosphodiesterase-4 Inhibitors from the Soft Coral Sarcophyton ehrenbergi. J. Nat. Prod. 2014, 77, 1928–1936. [Google Scholar] [CrossRef]
- Chitturi, B.R.; Dokuburra, C.B.; Tatipamula, V.B.; Dudem, S.; Kalivendi, S.V.; Tuniki, V.R.; Richard, A.B.; Yenamandra, V. Isolation, structural assignment and synthesis of (S E)-2-methyloctyl 3-(4-methoxyphenyl) propenoate from the marine soft coral Sarcophyton ehrenbergi. Nat. Prod. Res. 2014, 29, 70–76. [Google Scholar]
- Cheng, S.; Wen, Z.; Chiou, S.; Tsai, C.; Wang, S.; Hsu, C.; Dai, C.; Chiang, M.Y.; Wang, W.; Duh, C. Ceramide and Cerebrosides from the Octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009, 72, 465–468. [Google Scholar] [CrossRef]
- Minh, C.V.; Kiem, P.V.; Nam, N.; Cuong, N.; Thao, N.; Dang, N.; Quang, T.; Đạt, N.; Thung, D.C.; Thuy, D. Carotenoids from the soft coral Sarcophyton elegans. Vietnam J. Chem. 2010, 48, 627–631. [Google Scholar]
- Zeng, L.; Lan, W.; Su, J.; Zhang, G.; Feng, X.; Liang, Y.; Yang, X. Two New Cytotoxic Tetracyclic Tetraterpenoids from the Soft Coral Sarcophyton tortuosum. J. Nat. Prod. 2004, 67, 1915–1918. [Google Scholar] [CrossRef]
- Lan, W.J.; Li, H.J.; Yan, S.J.; Su, J.Y.; Zeng, L.M. New tetraterpenoid from the soft coral Sarcophyton tortuosum. J. Asian Nat. Prod. Res. 2007, 9, 267–271. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Li, H. Additional New Tetracyclic Tetraterpenoid: Methyl Tortuoate D from Soft Coral Sarcophyton tortuosum. Nat. Prod. Commun. 2009, 4, 1193–1196. [Google Scholar] [CrossRef]
- Su, C.; Chen, J.Y.; Din, Z.; Su, J.; Yang, Z.; Chen, Y.; Wang, R.Y.; Wu, Y. 13-Acetoxysarcocrassolide Induces Apoptosis on Human Gastric Carcinoma Cells Through Mitochondria-Related Apoptotic Pathways: p38/JNK Activation and PI3K/AKT Suppression. Mar. Drugs 2014, 12, 5295–5315. [Google Scholar] [CrossRef]
- Eltahawy, N.A.; Ibrahim, A.K.; Radwan, M.M.; Zaitone, S.A.; Gomaa, M.; ElSohly, M.A.; Hassanean, H.A.; Ahmed, S.A. Mechanism of action of antiepileptic ceramide from Red Sea soft coral Sarcophyton auritum. Bioorg. Med. Chem. Lett. 2015, 25, 5819–5824. [Google Scholar] [CrossRef]
- Szymanski, P.T.; Ahmed, S.A.; Radwan, M.; Khalifa, S.; Fahmy, H. Evaluation of the Anti-melanoma Activities of Sarcophine, (+)-7 alpha,8 beta-Dihydroxydeepoxysarcophine and Sarcophytolide from the Red Sea Soft Coral Sarcophyton glaucum. Nat. Prod. Commun. 2014, 9, 151–154. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).