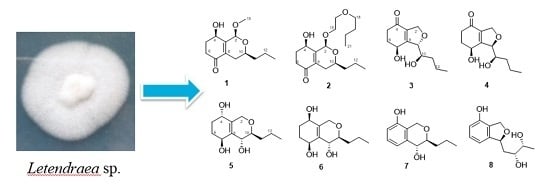
New Polyketides from the Marine-Derived Fungus Letendraea Sp. 5XNZ4-2
Abstract
:1. Introduction
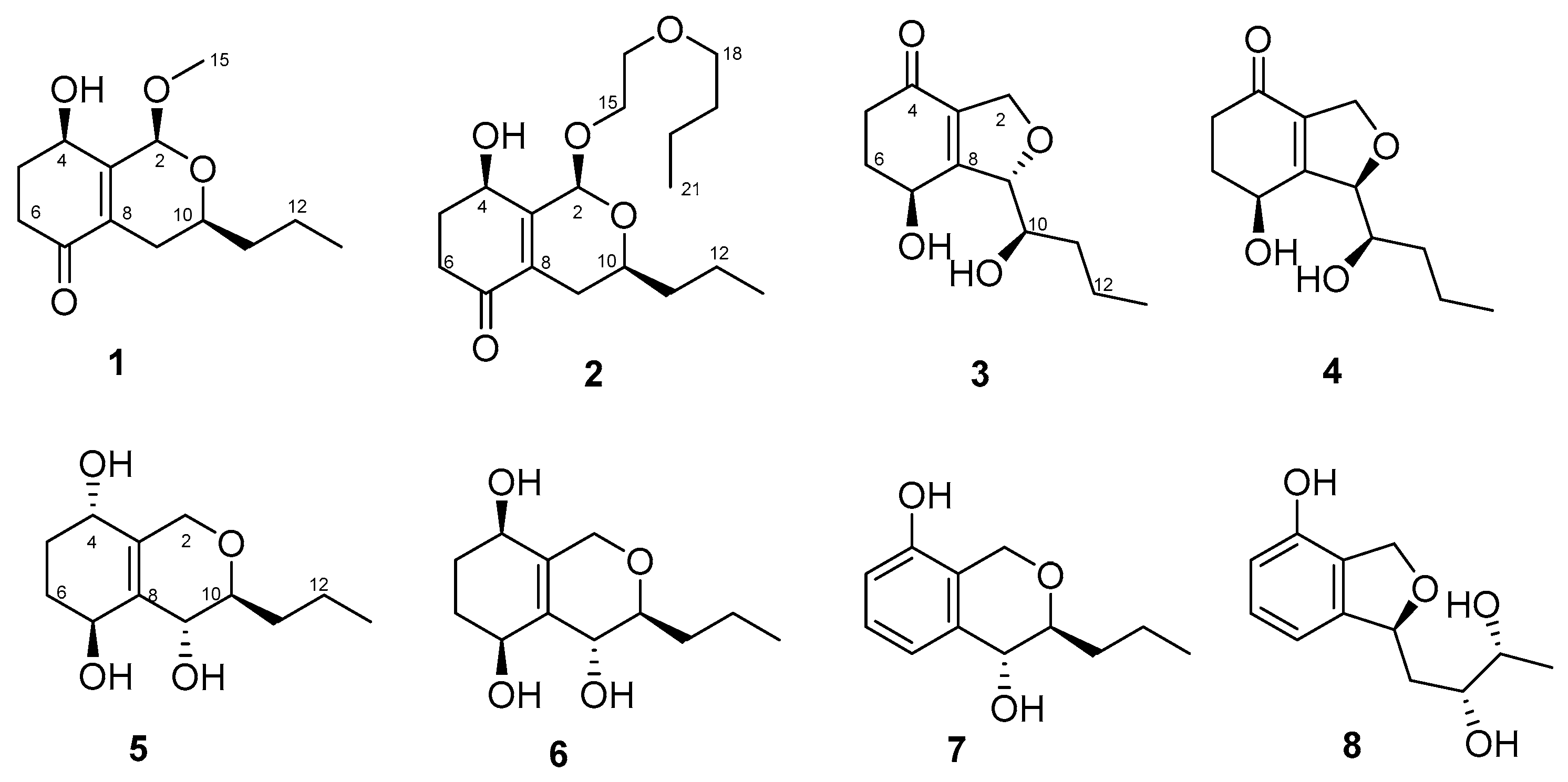
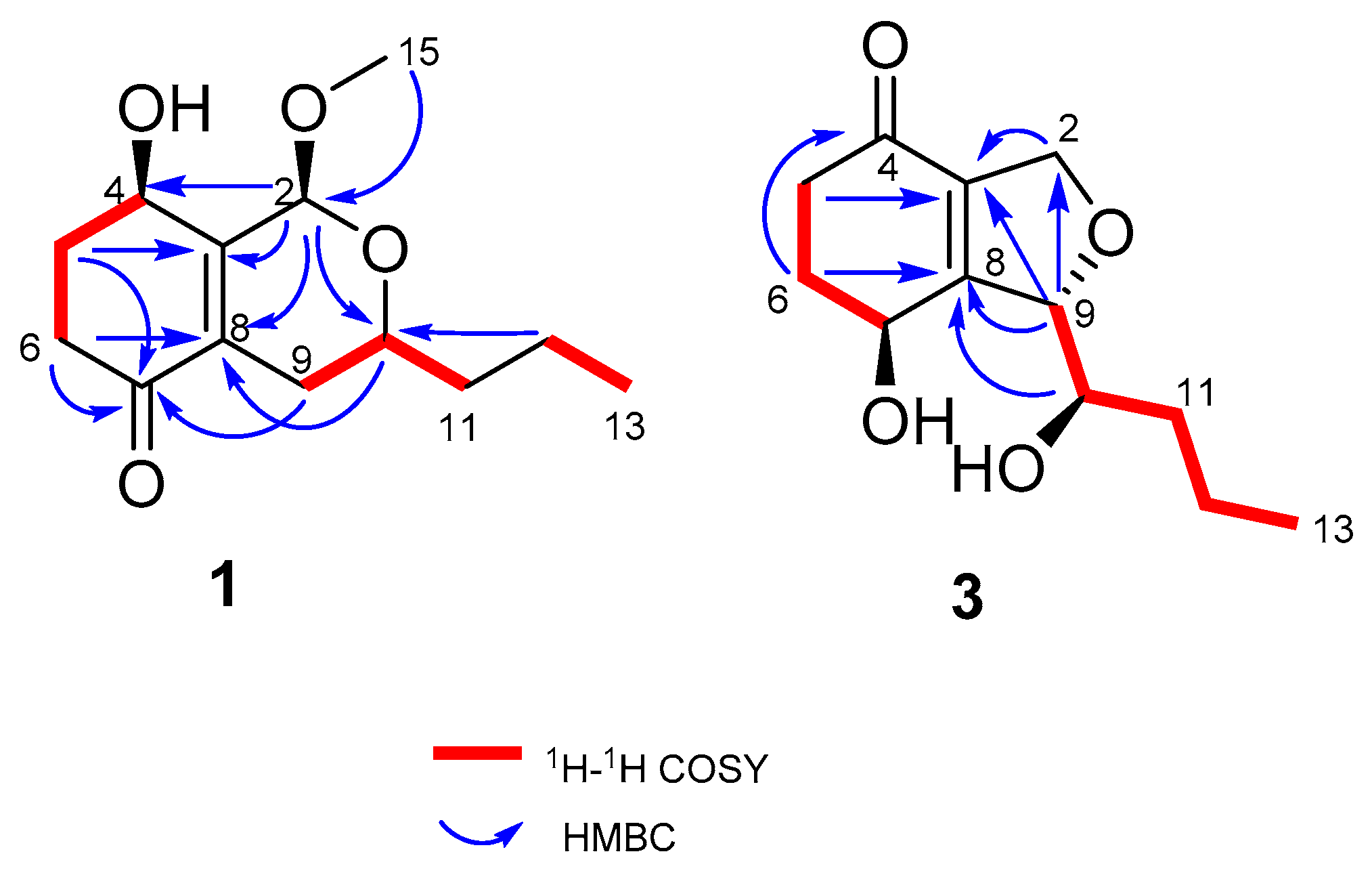
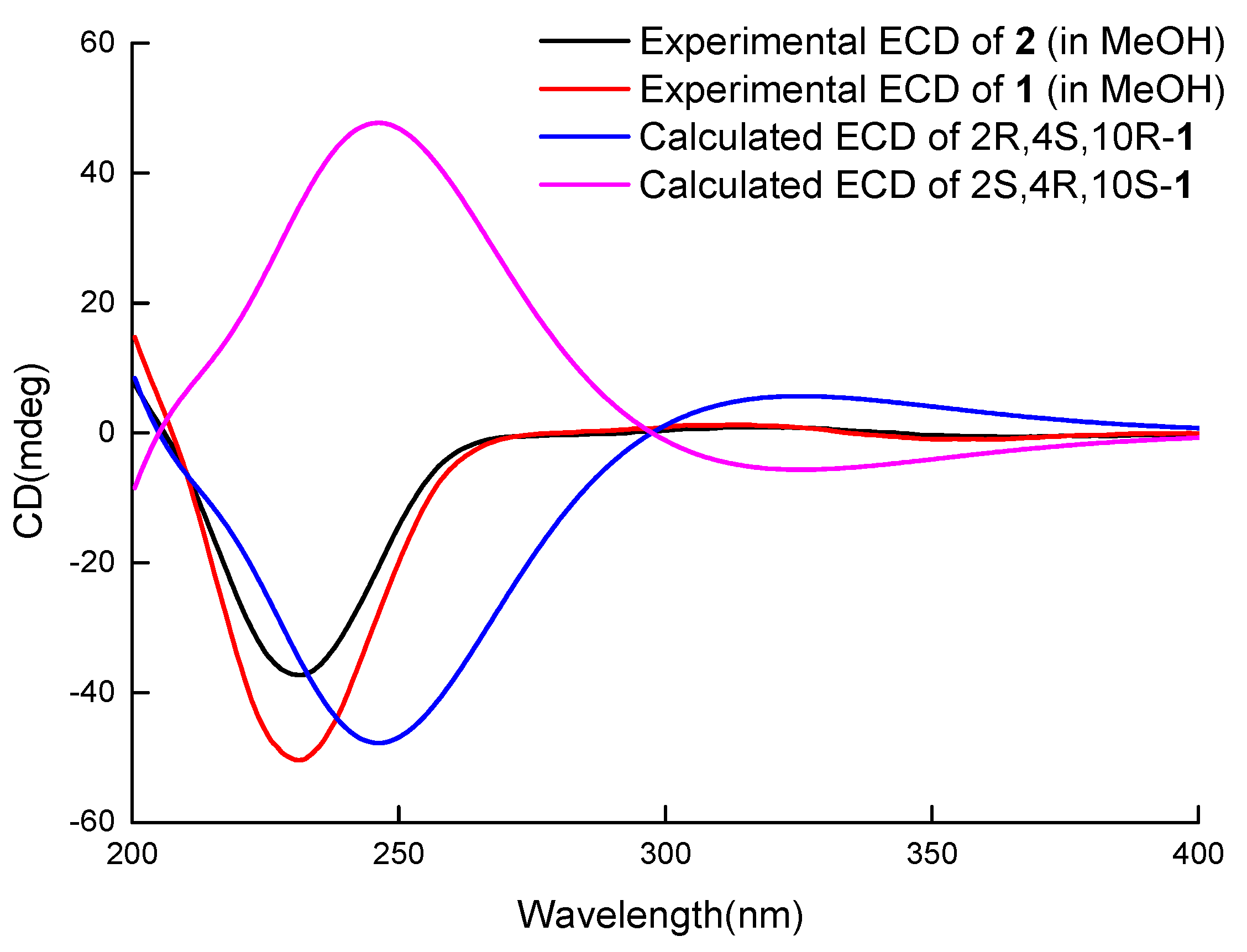
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
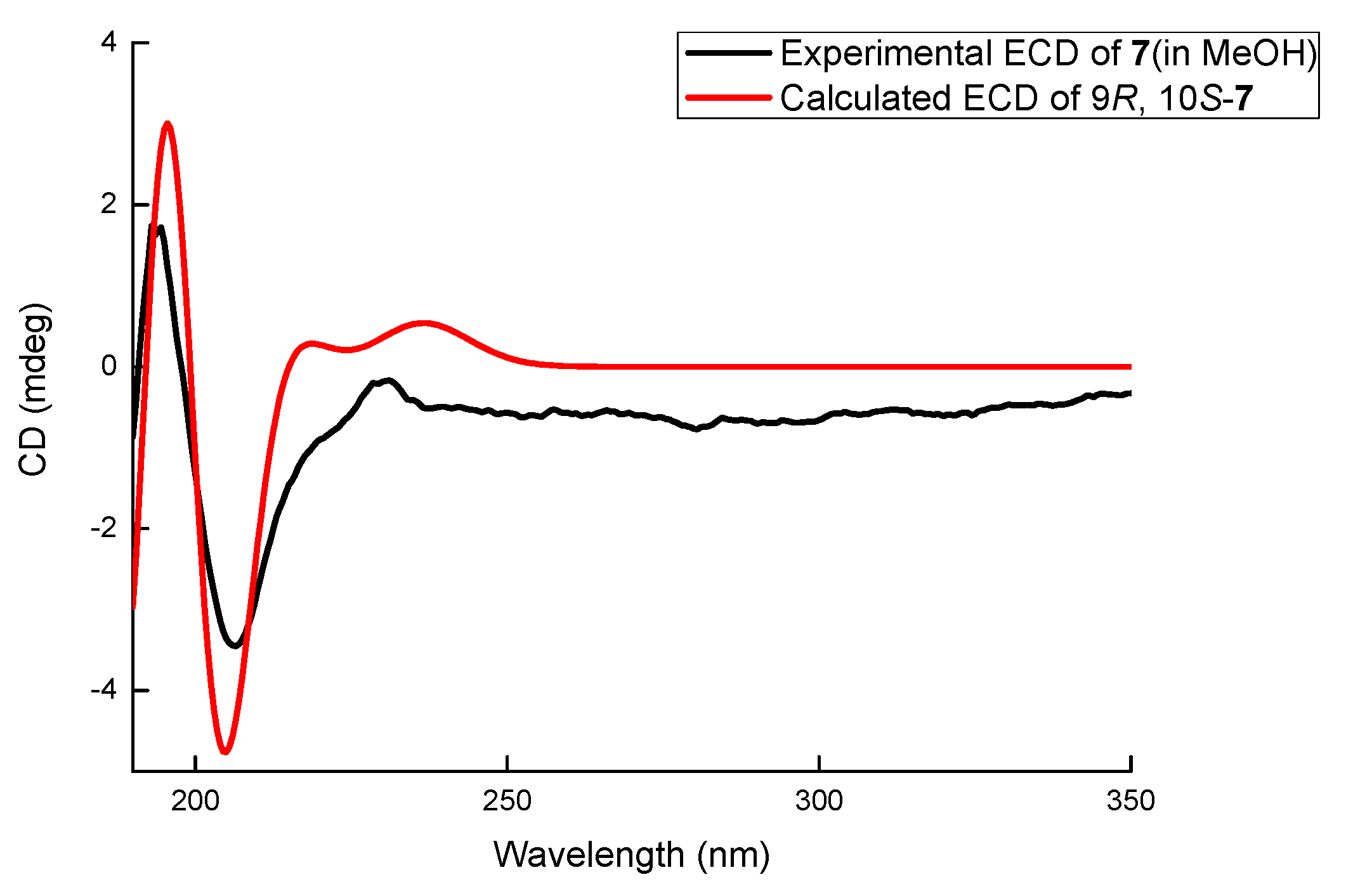
3.4. ECD Calculation of Compound 1
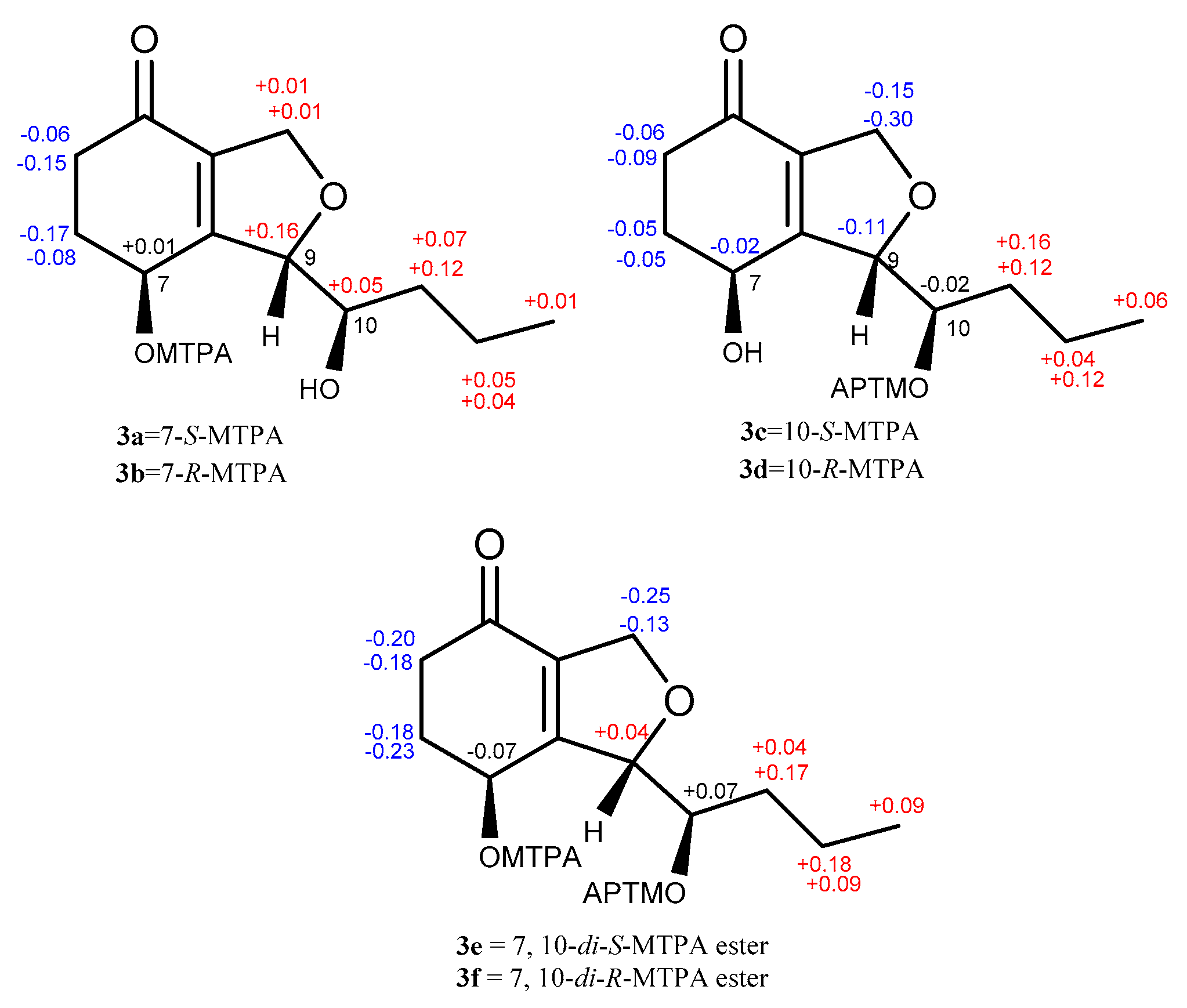
3.5. Preparation of MTPA Esters of Compound 3
3.6. Preparation of MTPA Esters of Compounds 4–6
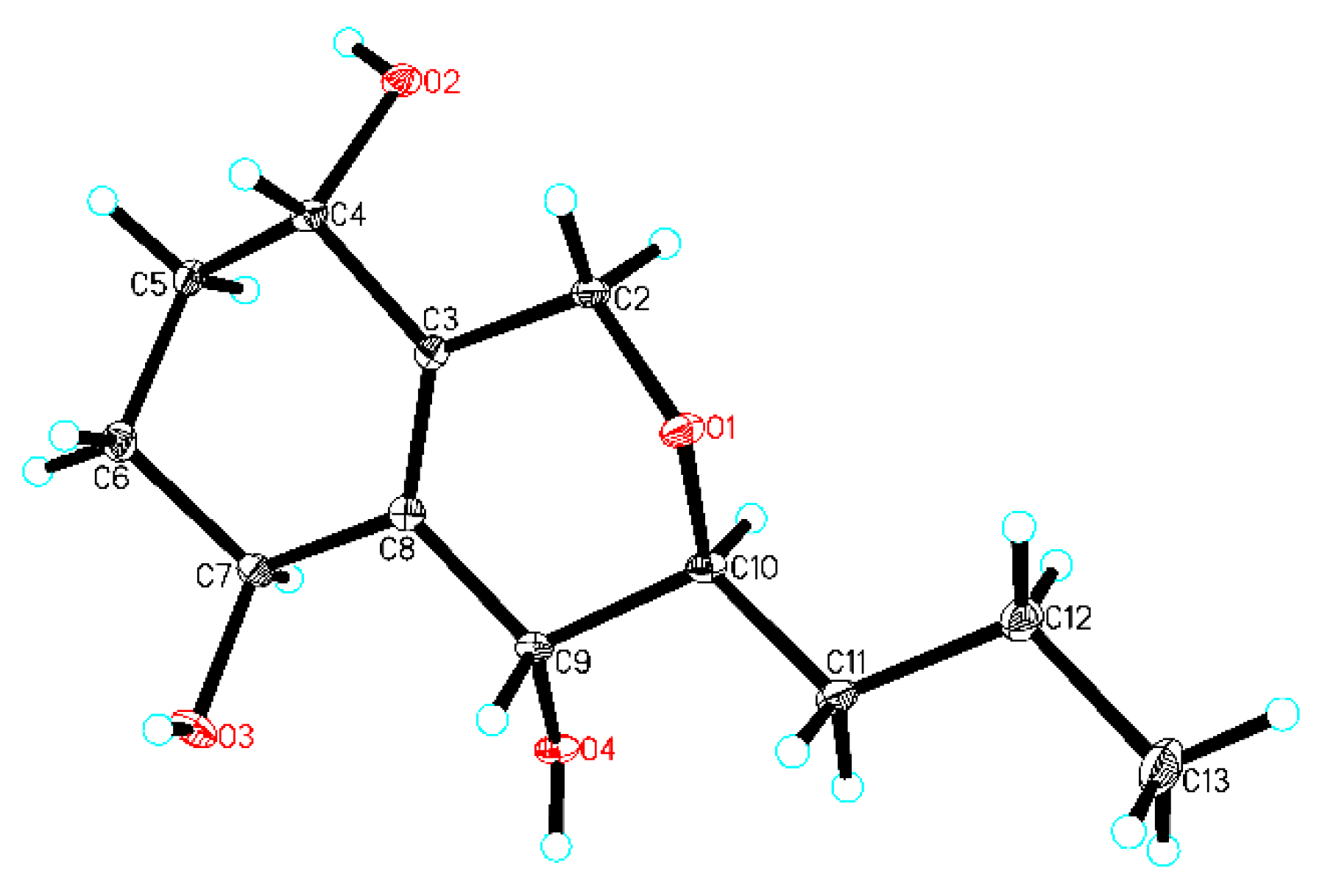
3.7. X-Ray Crystallographic Analysis
3.8. NO Inhibition Assay
3.9. Cytotoxic Assay
3.10. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gudina, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactants Produced by Marine Microorganisms with Therapeutic Applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortorella, E.; Tedesco, P.; Palma Esposito, F.; January, G.G.; Fani, R.; Jaspars, M.; de Pascale, D. Antibiotics from Deep-Sea Microorganisms: Current Discoveries and Perspectives. Mar. Drugs 2018, 16, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro MH, G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [Green Version]
- Imhoff, J.F. Natural Products from Marine Fungi—Still an Underrepresented Resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Ye, B.; Ding, W.; Wang, P.; Xu, J. Letenketals A and B, two novel spirocyclic polyketides from a marine crab-derived Letendraea sp. fungus. Phytochem. Lett. 2019, 30, 165–168. [Google Scholar] [CrossRef]
- Koizumi, F.; Takahashi, Y.; Ishiguro, H.; Tanaka, R.; Ohtaki, S.; Yoshida, M.; Nakanishi, S.; Ikeda, S.I. Structure elucidation of EI-1941-1 and -2, novel interleukin-1β converting enzyme inhibitors produced by Farrowia sp. E-1941. Tetrahedron Lett. 2004, 45, 7419–7422. [Google Scholar] [CrossRef]
- Petrovic, A.G.; Navarro-Vazquez, A.; Lorenzo Alonso-Gomez, J. From Relative to Absolute Configuration of Complex Natural Products: Interplay between NMR, ECD, VCD, and ORD Assisted by ab initio Calculations. Curr. Org. Chem. 2010, 14, 1612–1628. [Google Scholar] [CrossRef]
- Cerra, B.; Carotti, A.; Passer, D.; Sardella, R.; Moroni, G.; Di Michele, A.; Macchiarulo, A.; Pellicciar, R.; Gioielle, A. Exploiting Chemical Toolboxes for the Expedited Generation of Tetracyclic Quinolines as a Novel Class of PXR Agonists. ACS Med. Chem. Lett. 2019, 10, 677–681. [Google Scholar] [CrossRef]
- Tang, J.W.; Wang, W.G.; Li, A.; Yan, B.C.; Chen, R.; Li, X.N.; Du, X.; Sun, H.D.; Pu, J.X. Polyketides from the endophytic fungus Phomopsis sp. sh917 by using the one strain/many compounds strategy. Tetrahedron 2017, 73, 3577–3584. [Google Scholar] [CrossRef]
- Fischer, T.C.; Cerra, B.; Fink, M.J.; Rudroff, F.; Horkel, E.; Mihovilovic, M.D. First Total Synthesis of Piperenol B and Configuration Revision of the Enantiomers Piperenol B and Uvarirufol A. Eur. J. Org. Chem. 2015, 1464–1471. [Google Scholar] [CrossRef]
- Seco, J.M.; Quinoa, E.; Riguera, R. Assignment of the Absolute Configuration of Polyfunctional Compounds by NMR Using Chiral Derivatizing Agents. Chem. Rev. 2012, 112, 4603–4641. [Google Scholar] [CrossRef] [PubMed]
- Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Bioactive polyketides from the sea fan-derived fungus Penicillium citrinum PSU-F51. Tetrahedron 2012, 68, 8245–8250. [Google Scholar] [CrossRef]
- Mullapudi, V.; Ramana, C.V. The Total Synthesis and Structural Assignment of Hexaketide Xylarinol B and its C1’-Epimer. Asian J. Org. Chem. 2016, 5, 417–422. [Google Scholar] [CrossRef]
- Han, J.J.; Chen, Y.H.; Bao, L.; Yang, X.L.; Liu, D.; Li, S.J.; Zhao, F.; Liu, H. Anti-inflammatory and cytotoxic cyathane diterpenoids from the medicinal fungus Cyathus africanus. Fitoterapia 2013, 84, 22–31. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Ding, W.; Ye, B.; Wang, P.; Xu, J. Aspochalazine A, a Novel Polycyclic Aspochalasin from the Fungus Aspergillus sp. Z4. Tetrahedron Lett. 2017, 58, 2405–2408. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, R.; Yi, W.; Chai, W.; Zhang, Z.; Lian, X.Y. Peniciphenalenins A–F from the culture of a marine-associated fungus Penicillium sp. ZZ901. Phytochemistry 2018, 152, 53–60. [Google Scholar] [CrossRef]
- Van der Sluis, P.; Hezemans, A.M.F.; Kroon, J. Crystallization of low-molecular-weight organic compounds for X-ray crystallography. J. Appl. Crystallogr. 1989, 22, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Spingler, B.; Schnidrig, S.; Todorova, T.; Wild, F. Some thoughts about the single crystal growth of small molecules. CrystEngComm 2012, 14, 751–757. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Kratzert, D.; Holstein, J.J.; Krossing, I. DSR: Enhanced modelling and refinement of disordered structures with SHELXL. J. Appl. Crystallogr. 2015, 48, 933–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| No. | 1 a | 2 a | 3 b | 4 c | 5 b | 6 b | 7 b |
|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 2 | 5.29, s | 5.48, s | 4.69, ddd (12.5, 3.1, 2.2) 4.74, ddd (12.0, 5.5, 3.0) | 4.76, m 4.97, m | 4.02, overlapped 4.30, dt (16.5, 2.3) | 4.00, overlapped 4.37, overlapped | 4.60, d (15.6) 4.82, d (15.6) |
| 4 | 4.59, br s | 4.63, d (9.2) | 4.06, m | 3.95, dd (4.5, 5.6) | |||
| 5 | 2.06, m 2.28, m | 2.01, m 2.30, m | 2.48, m 2.52, m | 2.47, m 2.53, dt (16.0, 4.7) | 1.54, m 2.02, m | 1.74, m 1.79, m | 6.63, d (8.0) |
| 6 | 2.40, m 2.70, ddd (17.0, 7.0, 5.0) | 2.40, m 2.65, dt (17.0, 4.0) | 2.01, m 2.34, ddd (12.6, 9.4, 4.7) | 2.01, m 2.31, ddd (12.8, 9.4, 4.8) | 1.56, m 2.07, m | 1.72, m 1.82, m | 7.07, t (8.0) |
| 7 | 4.67, m | 4.64, m | 4.43, m | 4.35, overlapped | 7.01, d (8.0) | ||
| 9 | 1.88, m 2.36, m | 1.84, m 2.36, m | 5.10, m | 4.94, m | 3.99, overlapped | 3.98, overlapped | 4.30, d (8.5) |
| 10 | 3.74, m | 3.70, m | 3.80, dt (8.0, 4.0) | 3.83, dt (9.5, 3.2) | 3.33, overlapped | 3.30, overlapped | 3.37, m |
| 11 | 1.55, m 1.61, m | 1.54, m 1.59, m | 1.52, m 1.54, m | 1.39, m 1.44, m | 1.46, m 1.75, m | 1.48, m 1.72, m | 1.50, m 1.89, m |
| 12 | 1.43, m 1.52, m | 1.43, m 1.50, m | 1.58, m 1.42, m | 1.53, m 1.33, m | 1.41, m 1.59, m | 1.40, m 1.56, m | 1.48, m 1.65, m |
| 13 | 0.95, t (7.0) | 0.94, t (7.0) | 0.96, t (7.0) | 0.96, t (7.0) | 0.96, t (7.2) | 0.96, t (7.3) | 0.99, t (7.2) |
| 15 | 3.50, s | 3.86, ddd (12.1,8.8,2.8) 3.97, dt (12.0, 3.0) | |||||
| 16 | 3.60, dt (11.0, 3.0) 3.66, m | ||||||
| 18 | 3.53, m 3.53, m | ||||||
| 19 | 1.61, m 1.63, m | ||||||
| 20 | 1.36, m 1.39, m | ||||||
| 21 | 0.94, t (7.0) |
| No. | 1 a | 2 a | 3 b | 4 c | 5 b | 6 b | 7 b |
|---|---|---|---|---|---|---|---|
| δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | |
| 2 | 95.2, CH | 95.6, CH | 73.8, CH2 | 76.0, CH2 | 67.1, CH2 | 65.6, CH2 | 65.3, CH2 |
| 3 | 151.4, C | 154.4, C | 136.0, C | 134.6, C | 137.1, C | 137.0, C | 123.3, C |
| 4 | 65.3, CH | 66.5, CH | 197.1, C | 197.1, C | 67.2, CH | 66.1, CH | 153.5, C |
| 5 | 31.3, CH2 | 30.9, CH2 | 37.1, CH2 | 37.6, CH2 | 29.6, CH2 | 29.1, CH2 | 113.8, CH |
| 6 | 34.4, CH2 | 36.1, CH2 | 34.5, CH2 | 34.1, CH2 | 29.8, CH2 | 29.2, CH2 | 128.3, CH |
| 7 | 198.2, C | 197.9, C | 66.0, CH | 65.7, CH | 64.2, CH | 64.4, CH | 118.9, CH |
| 8 | 132.5, C | 131.5, C | 163.6, C | 167.0, C | 135.5, C | 135.3, C | 140.0, C |
| 9 | 27.4, CH2 | 27.2, CH2 | 91.3, CH | 90.0, CH | 67.0, CH | 67.0, CH | 70.1, CH |
| 10 | 66.7, CH | 67.4, CH | 74.4, CH | 74.0, CH | 80.4, CH | 80.2, CH | 80.4, CH |
| 11 | 37.4, CH2 | 37.2, CH2 | 35.6, CH2 | 34.1, CH2 | 35.2, CH2 | 35.0, CH2 | 35.5, CH2 |
| 12 | 18.9, CH2 | 18.8, CH2 | 20.0, CH2 | 20.1, CH2 | 20.0, CH2 | 19.9, CH2 | 19.8, CH2 |
| 13 | 14.1, CH3 | 14.0, CH3 | 14.4, CH3 | 14.4, CH3 | 14.4, CH3 | 14.4, CH3 | 14.4, CH3 |
| 15 | 55.8, CH3 | 68.4, CH2 | |||||
| 16 | 69.9, CH2 | ||||||
| 18 | 71.5, CH2 | ||||||
| 19 | 31.5, CH2 | ||||||
| 20 | 19.3, CH2 | ||||||
| 21 | 14.0, CH3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Huang, R.; Liu, H.; Yan, T.; Ding, W.; Jiang, Y.; Wang, P.; Zheng, D.; Xu, J. New Polyketides from the Marine-Derived Fungus Letendraea Sp. 5XNZ4-2. Mar. Drugs 2020, 18, 18. https://doi.org/10.3390/md18010018
Xu Y, Huang R, Liu H, Yan T, Ding W, Jiang Y, Wang P, Zheng D, Xu J. New Polyketides from the Marine-Derived Fungus Letendraea Sp. 5XNZ4-2. Marine Drugs. 2020; 18(1):18. https://doi.org/10.3390/md18010018
Chicago/Turabian StyleXu, Yan, Ruibao Huang, Hongwei Liu, Tingting Yan, Wanjing Ding, Yongjun Jiang, Pinmei Wang, Daoqiong Zheng, and Jinzhong Xu. 2020. "New Polyketides from the Marine-Derived Fungus Letendraea Sp. 5XNZ4-2" Marine Drugs 18, no. 1: 18. https://doi.org/10.3390/md18010018