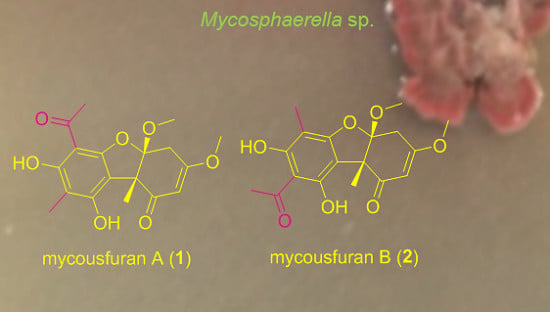
Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment
Abstract
1. Introduction
2. Results
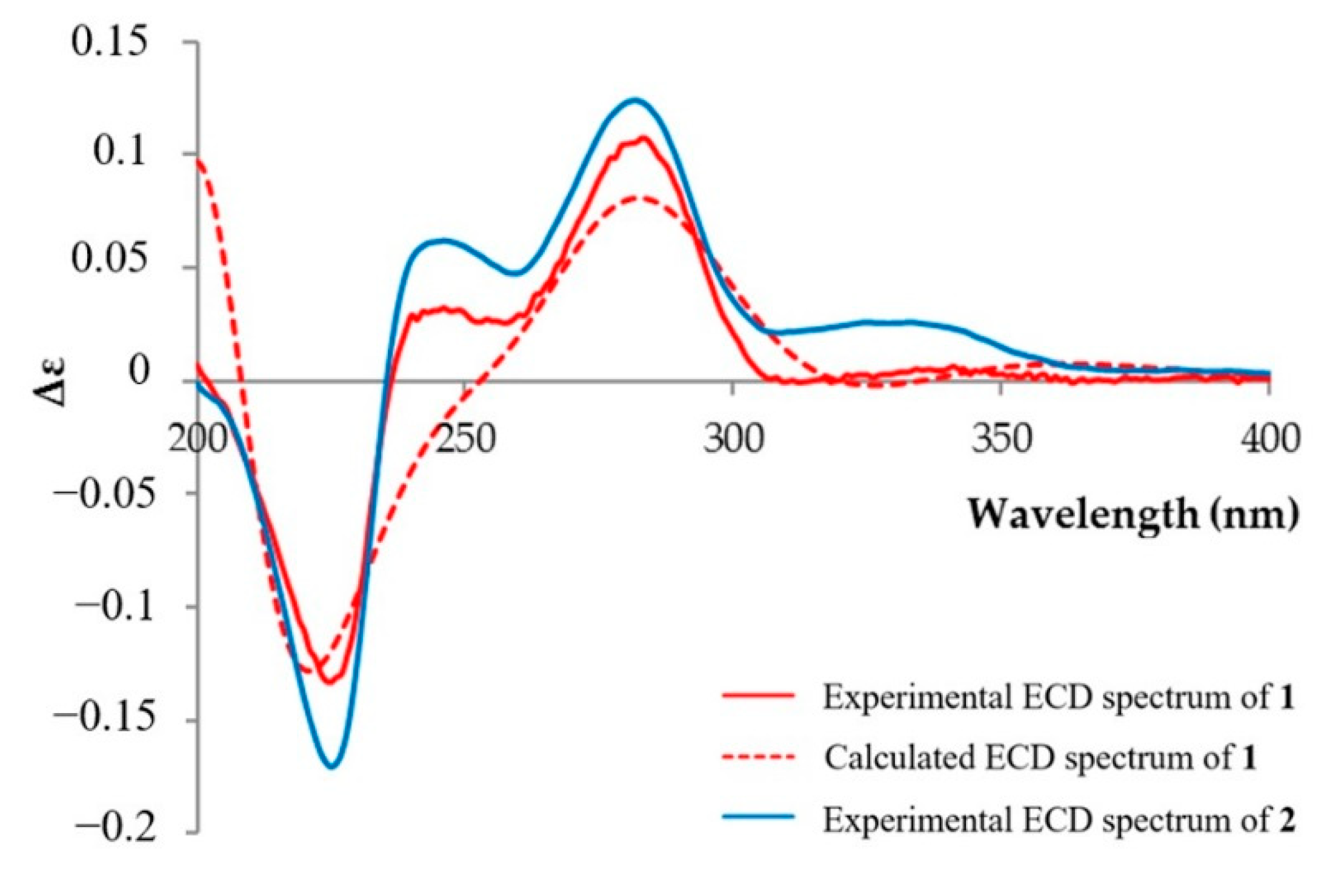
2.1. Isolation and Structure Elucidation
2.2. Bioactivity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Purifircation
3.4. Computer-Assisted Conformational Analyses and ECD Calculations
3.5. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Millot, M.; Dieua, A.; Tomasib, S. Dibenzofurans and derivatives from lichens and ascomycetes. Nat. Prod. Rep. 2016, 33, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsdóttir, K. Molecules of interest usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Usnic Aacid and its derivatives for pharmaceutical use: A patent review (2000-2017). Expert Opin. Ther. Pat. 2018, 28, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Cocchietto, M.; Skert, N.; Nimis, P.L.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwissenschaften 2002, 89, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.A. Usnic acid biological activity: History, evaluation and usage. Int. J. Basic Clin. Pharmacol. 2017, 6, 2752–2759. [Google Scholar] [CrossRef]
- Kim, K.-J.; Jeong, M.-H.; Lee, Y.; Hwang, S.-J.; Shin, H.-B.; Hur, J.-S.; Son, Y.-J. Effect of usnic acid on osteoclastogenic activity. J. Clin. Med. 2018, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and tts derivatives: Part 1. Activity against unicellular organisms. Russ. J. Bioorg. Chem. 2016, 42, 115–132. [Google Scholar] [CrossRef]
- Albinati, A.; Bruckner, S.; Camarda, L.; Nasini, G. Rosigenin, an unusual metabolite from Mycosphaerella rosigena. Tetrahedron 1980, 36, 117–121. [Google Scholar] [CrossRef]
- Arnone, A.; Camarda, L.; Nasini, G.; Assante, G. Secondary metabolites. Part 15. Structure elucidation of rubellins A and B, two novel anthraquinone metabolites from Mycosphaerella rubella. J. Chem. Soc. 1986, 1, 255–260. [Google Scholar] [CrossRef]
- Sassa, T.; Igarashi, M. Structures of (−)-mycousnine, (+)-isomycousnine and (+)-oxymycousnine. Agric. Biol. Chem. 1990, 54, 2231–2237. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Pereira, C.B.; Mendes, G.; Junker, J.; Kolloff, M.; Rosa, L.H.; Rosa, C.A.; Alves, T.M.A.; Zani, C.L.; Johann, S.; et al. Two new usnic acid derivatives from the endophytic fungus Mycosphaerella sp. Naturforsch C. 2018, 73, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Webber, F.C. Observations on the structure, life history and biology of Mycosphaerella ascophylli. Trans. Br. Mycol. Soc. 1967, 50, 583–601. [Google Scholar] [CrossRef]
- Kohlmeyer, J. Geography of marine fungi. Aust. J. Bot. Suppl. Ser. 1983, 10, 67–76. [Google Scholar] [CrossRef]
- Fries, N. Physiological characteristics of Mycosphaerella ascophylli, a fungal endophyte of the marine brown alga Ascophyllum nodosum. Physiol. Plant. 1979, 45, 117–121. [Google Scholar] [CrossRef]
- Lyons, J.I.; Alber, M.; Hollibaugh, J.T. Ascomycete fungal communities associated with early decaying leaves of Spartina spp. from central California estuaries. Oecologia 2010, 162, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Newell, S.Y.; Blum, L.K.; Crawford, R.E.; Dai, T.; Dionne, M. Autumnal biomass and potential productivity of salt marsh fungi from 29° to 43° north latitude along the United States Atlantic coast. Appl. Environ. Microbiol. 2000, 66, 180–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Connolly, J.D.; Freer, A.A.; Huneck, S. Crystal Structure of (-)-placodiolic acid, a dibenzofuran derivative from the lichen Rhizoplaca chrysoleuca. Phytochem. 1984, 23, 702. [Google Scholar] [CrossRef]
- Seo, C.; Sohn, J.H.; Park, S.M.; Yim, J.H.; Lee, H.K.; Oh, H. Usimines A-C, bioactive usnic acid derivatives from the Antarctic lichen Stereocaulon alpinum. J. Nat. Prod. 2008, 71, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]


| Position | 1 | HMBC | 2 | ||
|---|---|---|---|---|---|
| δC, Type | δH, mult. (J in Hz) | δC, Type | δH, mult. (J in Hz) | ||
| 1 | 200.5, C | 201.0, C | |||
| 2 | 100.6, CH | 5.55, s | 100.2, CH | 5.55, s | |
| 3 | 175.4, C | 175.9, C | |||
| 4α 4β | 34.3, CH2 | 3.15, d (17.5), 2.96, d (17.5) | 2, 4a 2, 4a | 34.2, CH2 | 3.20, d (17.5) 2.94, d (17.5) |
| 4a | 111.6, C | 110.6, C | |||
| 5a | 157.1, C | 160.7, C | |||
| 6 | 102.0, C | 100.4, C | |||
| 7 | 163.3, C | 165.5, C | |||
| 8 | 107.5, C | 107.1, C | |||
| 9 | 159.6, C | 156.4, C | |||
| 9a | 106.5, C | 106.1, C | |||
| 9b | 57.9, C | 58.9, C | |||
| 10 | 16.6, CH3 | 1.62, s | 1, 4a, 9a, 9b | 16.2, CH3 | 1.66, s |
| 11 | 7.4, CH3 | 2.04, s | 8 | 7.5, CH3 | 2.02, s |
| 12 | 201.2, C | 203.8, C | |||
| 13 | 31.3, CH3 | 2.61, s | 6, 12 | 32.9, CH3 | 2.73, s |
| 14 | 50.9, CH3 | 3.49, s | 4a | 50.3, CH3 | 3.47, s |
| 15 | 56.9, CH3 | 3.81, s | 2, 3 | 56.8, CH3 | 3.84, s |
| 7-OH | 13.34, s | 6, 7, 8 | 14.32, s | ||
| 9-OH | 9.34, s | 8, 9a | 9.61, s | ||
| Compound | Gram (+) Bacteria | Gram (−) Bacteria | ||||
|---|---|---|---|---|---|---|
| B. subtilis ATCC 6633 | K. rhizophila ATCC 9341 | S. aureus ATCC 6538 | E. coli ATCC 11775 | S. typhimurium ATCC 14208 | K. pneumonia ATCC 4352 | |
| 1 | >128 | 8 | 32 | >128 | >128 | >128 |
| 2 | >128 | 16 | 32 | >128 | >128 | >128 |
| 3 | 4 | 8 | 4 | >128 | >128 | >128 |
| 4 | 4 | 8 | 4 | >128 | >128 | >128 |
| 5 | 2 | 8 | 16 | >128 | >128 | >128 |
| Vancomycin | 0.25 | 0.25 | 0.5 | >128 | >128 | >128 |
| Ampicillin | 0.5 | 0.25 | 2 | 16 | 8 | >128 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, J.; Kim, G.J.; Yang, I.; Wang, W.; Nam, J.-W.; Choi, H.; Nam, S.-J.; Kang, H. Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment. Mar. Drugs 2019, 17, 422. https://doi.org/10.3390/md17070422
Lee J, Lee J, Kim GJ, Yang I, Wang W, Nam J-W, Choi H, Nam S-J, Kang H. Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment. Marine Drugs. 2019; 17(7):422. https://doi.org/10.3390/md17070422
Chicago/Turabian StyleLee, Jihye, Jusung Lee, Geum Jin Kim, Inho Yang, Weihong Wang, Joo-Won Nam, Hyukjae Choi, Sang-Jip Nam, and Heonjoong Kang. 2019. "Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment" Marine Drugs 17, no. 7: 422. https://doi.org/10.3390/md17070422
APA StyleLee, J., Lee, J., Kim, G. J., Yang, I., Wang, W., Nam, J.-W., Choi, H., Nam, S.-J., & Kang, H. (2019). Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment. Marine Drugs, 17(7), 422. https://doi.org/10.3390/md17070422