Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus flocculosus
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
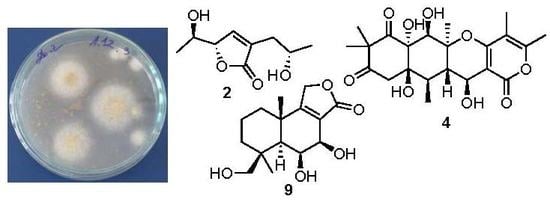
3.2. Fungal Strain
3.3. Cultivation of the Fungus
3.4. Extraction and Isolation
3.5. Preparation of (S)-MTPA and (R)-MTPA Esters of Aspilactonol F (1)
3.6. Preparation of (S)-MTPA and (R)-MTPA Esters of Aspilactonol G (2)
3.7. Cell Culture
3.8. Cytotoxicity Assay
3.9. Colony Formation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Tóth, B.; Rigó, K.; Téren, J.; Hoekstra, R.F.; Kozakiewicz, Z. Phylogenetic analysis of aspergillus section circumdati based on sequences of the internal transcribed spacer regions and the 5.8 S rRNA gene. Fungal Genet. Biol. 2000, 30, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Fuchser, J.; Zeeck, A. Secondary metabolites by chemical screening, 34—Aspinolides and Aspinonene/Aspyrone co-metabolites, new pentaketides produced by aspergillus ochraceus. Liebigs Ann. 1997, 1997, 87–95. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.M.; Meng, L.H.; Wang, B.G. Polyketides from the marine mangrove-derived fungus aspergillus ochraceus ma-15 and their activity against aquatic pathogenic bacteria. Phytochem. Lett. 2015, 12, 232–236. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, S.; Zhan, Y.; Zhang, N.; Wu, A.A.; Gui, F.; Guo, K.; Yang, Y.; Cao, S.; Hu, Z.; et al. Aspertetranones a-d, putative meroterpenoids from the marine algal-associated fungus aspergillus sp. ZL0-1b14. J. Nat. Prod. 2015, 78, 2405–2410. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Qin, X.; Tian, X.; Liao, L.; Li, K.; Zhou, X.; Yang, X.; Wang, F.; Zhang, T.; et al. Antiviral merosesquiterpenoids produced by the antarctic fungus aspergillus ochraceopetaliformis SCSIO 05702. J. Nat. Prod. 2016, 79, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Choi, B.K.; Trinh, P.T.H.; Lee, H.S.; Kang, J.S.; Van, T.T.T.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Lee, J. Suppression of rankl-induced osteoclastogenesis by the metabolites from the marine fungus aspergillus flocculosus isolated from a sponge stylissa sp. Mar. Drugs 2018, 16, 14. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Jensen, P.R.; Renner, M.K.; Fenical, W. New cytotoxic sesquiterpenoid nitrobenzoyl esters from a marine isolate of the fungus aspergillus versicolor. Tetrahedron 1998, 54, 1715–1724. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, B.; Lin, X.; Luo, X.; Pang, X.; Tang, L.; Liu, Y.; Li, X.; Zhou, X. Nitrobenzoyl sesquiterpenoids with cytotoxic activities from a marine-derived aspergillus ochraceus fungus. J. Nat. Prod. 2018, 81, 92–97. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Wang, J.; Liu, P.; Li, J.; Zhu, W. Antimicrobial ergosteroids and pyrrole derivatives from halotolerant aspergillus flocculosus PT05-1 cultured in a hypersaline medium. Extrem. Life Under Extrem. Cond. 2013, 17, 963–971. [Google Scholar] [CrossRef]
- Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z.; et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus aspergillus ochraceus Jcma1F17. MedChemComm 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Trinh, P.T.H.; Ivanets, E.V.; Smetanina, O.F.; Yurchenko, A.N. Neuroprotective activity of some marine fungal metabolites in the 6-hydroxydopamin- and paraquat-induced parkinson’s disease models. Mar. Drugs 2018, 16, 457. [Google Scholar] [CrossRef] [PubMed]
- Kito, K.; Ookura, R.; Yoshida, S.; Namikoshi, M.; Ooi, T.; Kusumi, T. Pentaketides relating to aspinonene and dihydroaspyrone from a marine-derived fungus, aspergillus ostianus. J. Nat. Prod. 2007, 70, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Li, C.W.; Cui, C.B.; Hua, W.; Zhu, T.J.; Gu, Q.Q. Nine new and five known polyketides derived from a deep sea-sourced aspergillus sp. 16-02-1. Mar. Drugs 2014, 12, 3116–3137. [Google Scholar] [CrossRef] [PubMed]
- Rahbaek, L.; Christophersen, C.; Frisvad, J.; Bengaard, H.S.; Larsen, S.; Rassing, B.R. Insulicolide a: A new nitrobenzoyloxy-substituted sesquiterpene from the marine fungus aspergillus insulicola. J. Nat. Prod. 1997, 60, 811–813. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Anbuchezhian, R.; Sun, W.; Shao, C.L.; Zhang, F.L.; Yin, Y.; Yu, Z.S.; Li, Z.Y.; Wang, C.Y. Cytotoxic nitrobenzoyloxy-substituted sesquiterpenes from spongederived endozoic fungus aspergillus insulicola md10-2. Curr. Pharm. Biotechnol. 2016, 17, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Kalinovsky, A.I.; Khudyakova, Y.V.; Dyshlovoy, S.A.; Von Amsberg, G.; Yurchenko, E.A.; Afiyatullov, S.S. Unique prostate cancer-toxic polyketides from marine sediment-derived fungus isaria felina. J. Antibiot. 2017, 70, 856–858. [Google Scholar] [CrossRef]
- Yurchenko, A.; Smetanina, O.; Ivanets, E.; Kalinovsky, A.; Khudyakova, Y.; Kirichuk, N.; Popov, R.; Bokemeyer, C.; von Amsberg, G.; Chingizova, E.; et al. Pretrichodermamides d–f from a marine algicolous fungus penicillium sp. KMM 4672. Mar. Drugs 2016, 14, 122. [Google Scholar] [CrossRef]
- Gawronski, J.K.; Van Oeveren, A.; Van Der Deen, H.; Leung, C.W.; Feringa, B.L. Simple circular dichroic method for the determination of absolute configuration of 5-substituted 2(5H)-furanones. J. Org. Chem. 1996, 61, 1513–1515. [Google Scholar] [CrossRef]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef]
- Beekman, A.C.; Woerdenbag, H.J.; Van Uden, W.; Pras, N.; Konings, A.W.T.; Wikström, H.V.; Schmidt, T.J. Structure-cytotoxicity relationships of some helenanolide-type sesquiterpene lactones. J. Nat. Prod. 1997, 60, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Menchinskaya, E.S.; Venz, S.; Rast, S.; Amann, K.; Hauschild, J.; Otte, K.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; et al. The marine triterpene glycoside frondoside a exhibits activity in vitro and in vivo in prostate cancer. Int. J. Cancer 2016, 138, 2450–2465. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Venz, S.; Shubina, L.K.; Fedorov, S.N.; Walther, R.; Jacobsen, C.; Stonik, V.A.; Bokemeyer, C.; Balabanov, S.; Honecker, F. Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer. J. Proteom. 2014, 96, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Hauschild, J.; Amann, K.; Tabakmakher, K.M.; Venz, S.; Walther, R.; Guzii, A.G.; Makarieva, T.N.; Shubina, L.K.; Fedorov, S.N.; et al. Marine alkaloid monanchocidin a overcomes drug resistance by induction of autophagy and lysosomal membrane permeabilization. Oncotarget 2015, 6, 17328–17341. [Google Scholar] [CrossRef] [PubMed]
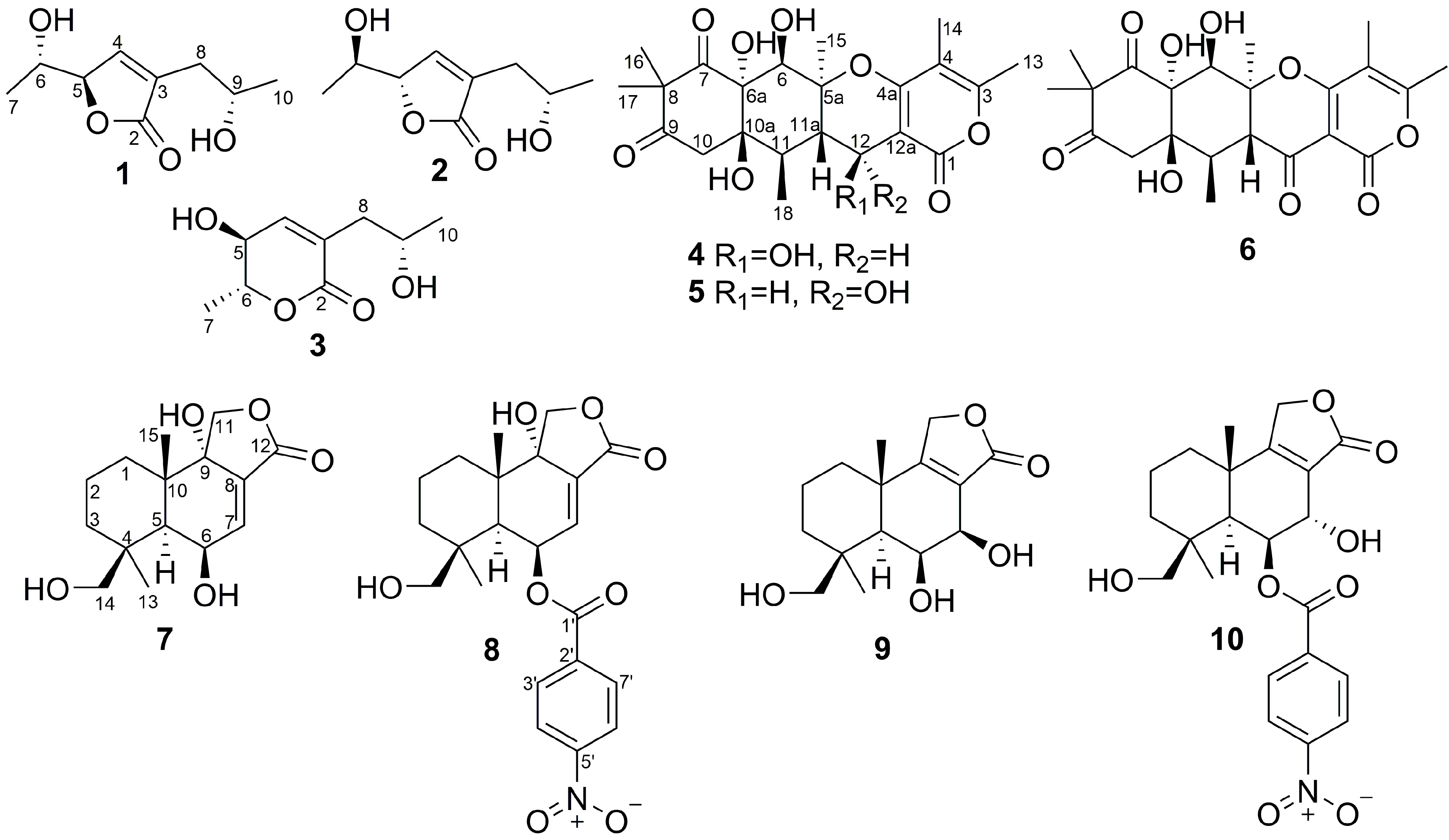
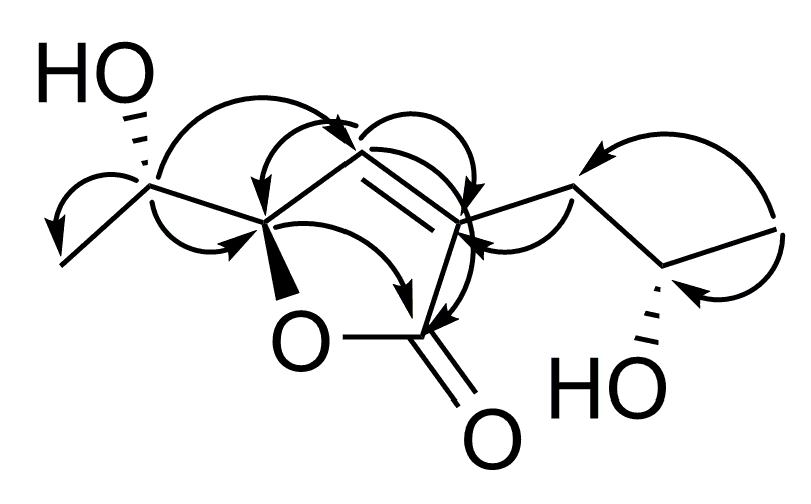
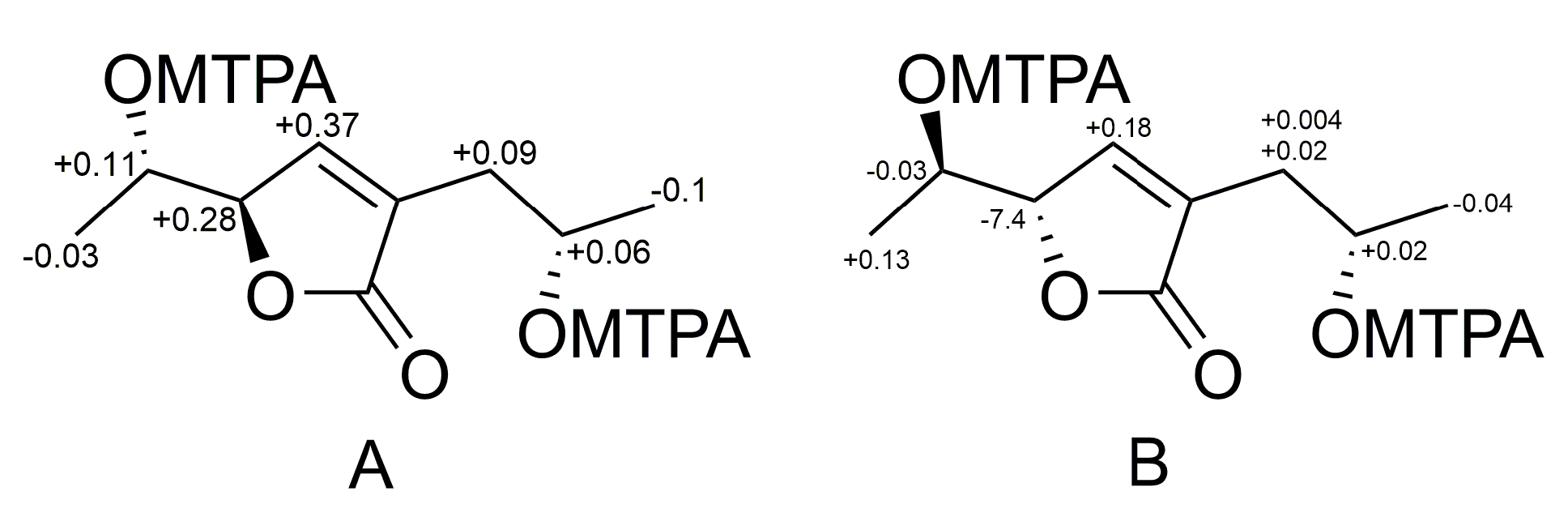
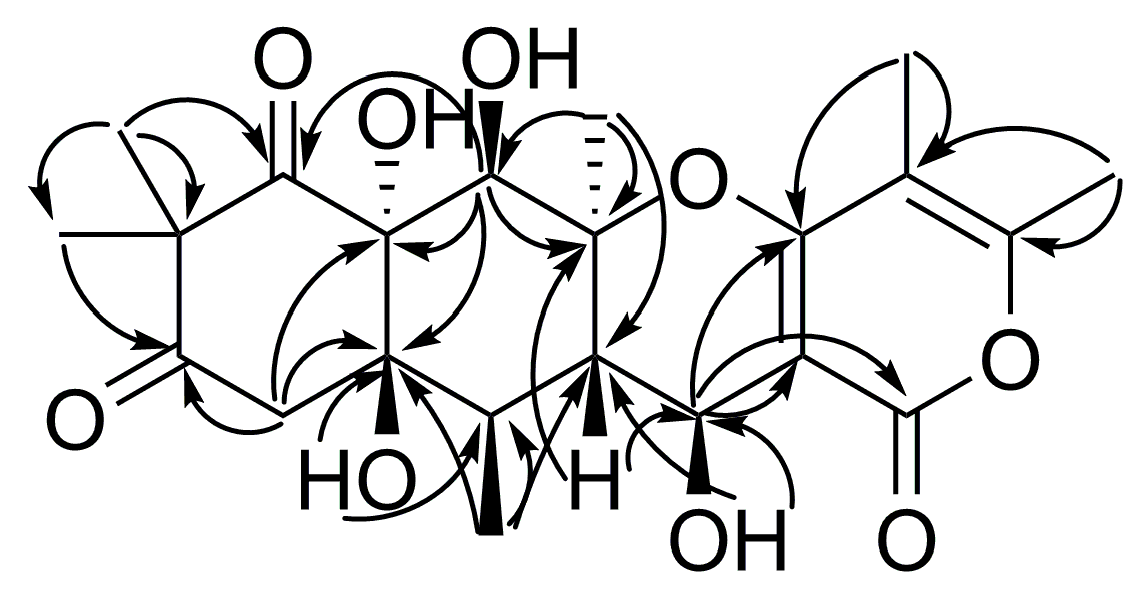
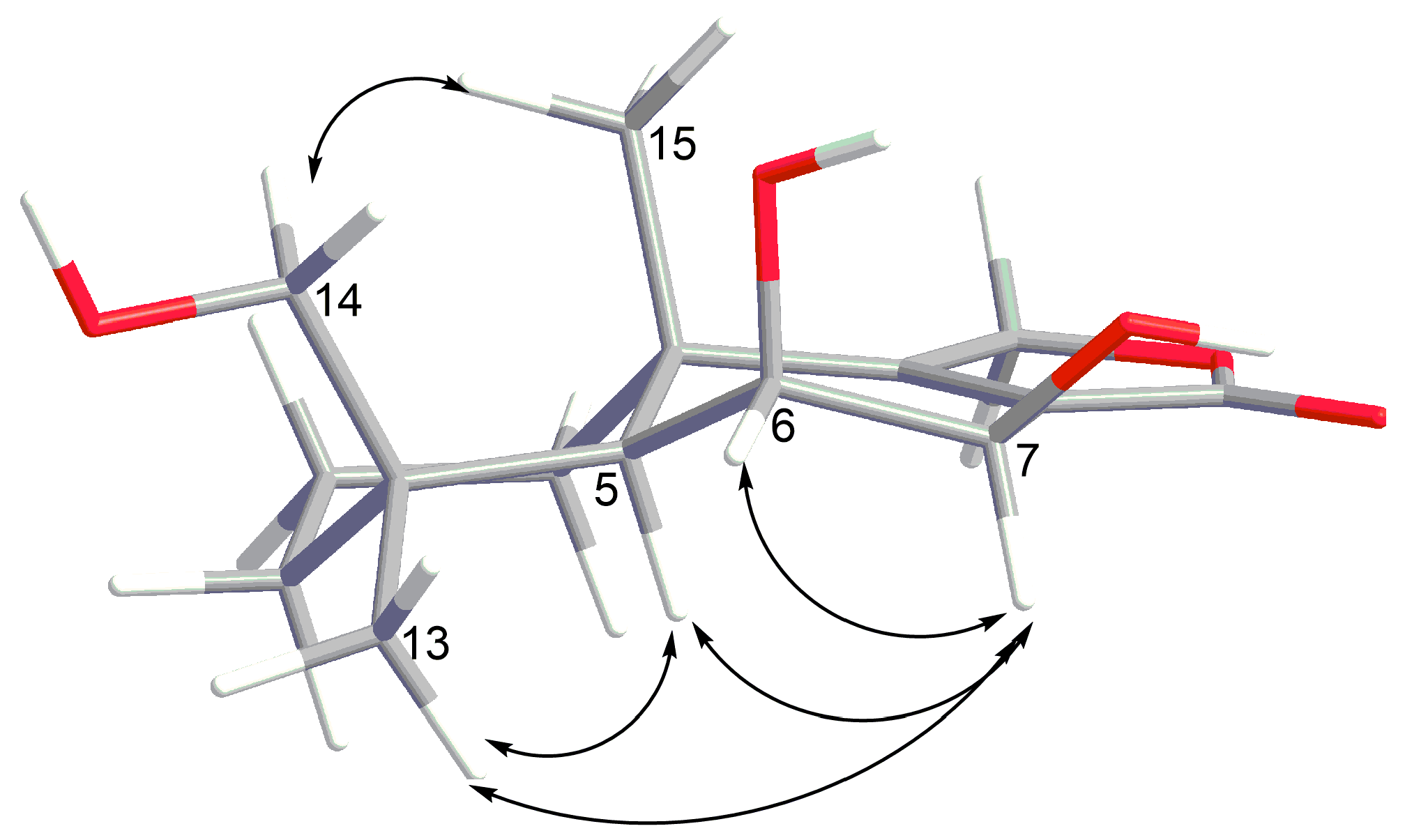
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, mult | δH (J in Hz) | δC, mult | δH (J in Hz) | |
| 2 | 174.2, C | 174.1, C | ||
| 3 | 132.8, C | 132.9, C | ||
| 4 | 147.4, CH | 7.27, d (1.4) | 147.3, CH | 7.25, d (1.2) |
| 5 | 84.9, CH | 4.85, dd (4.4, 1.4) | 84.8, CH | 4.86, dd (4.2, 1.4) |
| 6 | 67.8, CH | 4.05, qd (6.4, 4.4) | 67.6, CH | 4.08, qd (6.6, 4.2) |
| 7 | 18.8, CH3 | 1.31, d (6.4) | 18.8, CH3 | 1.31, d (6.6) |
| 8 | 34.9, CH2 | 2.52, ddt (15.0, 3.8, 1.4) 2.45, ddt (15.0, 7.8, 1.4) | 35.2, CH2 | 2.55, ddt (14.6, 3.6, 1.4) 2.40, dd (14.6, 8.5) |
| 9 | 66.2, CH | 4.08, m | 65.8, CH | 4.04, m |
| 10 | 23.3, CH3 | 1.25, d (6.3) | 23.2, CH3 | 1.25, d (6.2) |
| Position | δC, Mult | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 164.4, C | ||
| 3 | 157.9, C | ||
| 4 | 107.3, C | ||
| 4a | 162.5, C | ||
| 5a | 83.0, C | ||
| 6 | 75.15, CH | 4.36, s | 5a, 6a, 7, 10a, 11a, 15 |
| 6a | 76.5, C | ||
| 7 | 211.4, C | ||
| 8 | 55.5, C | ||
| 9 | 209.1, C | ||
| 10 | 45.6, CH2 | 2.86, d (17.7) 2.76, dd (17.7, 2.7) | 6a, 9, 10a 9, 10a |
| 10a | 75.07, C | ||
| 11 | 39.5, CH | 2.00, dd (12.0, 6.8) | 5a, 10a, 11a, 18 |
| 11a | 39.3, CH | 2.32, dd (12.0, 9.4) | 5a, 6, 10a, 11, 12, 18 |
| 12 | 63.5, CH | 4.63, d (9.4) | 1, 4a, 11, 11a, 12a |
| 12a | 102.2, C | ||
| 13 | 17.3, CH3 | 2.24, s | 3, 4, 4a |
| 14 | 9.5, CH3 | 1.89, s | 3, 4, 4a |
| 15 | 18.5, CH3 | 1.43, s | 5a, 6, 11a |
| 16 | 25.1, CH3 | 1.39, s | 7, 8, 9, 17 |
| 17 | 24.0, CH3 | 1.41, s | 7, 8, 9, 16 |
| 18 | 10.8, CH3 | 1.31, d (6.8) | 10a, 11, 11a |
| 6-OH | 3.57, brs | ||
| 6a-OH | 3.12, brs | ||
| 10a-OH | 4.01, d (2.7) | 10, 10a | |
| 12-OH | 4.43, brs | 11a, 12 |
| Position | 7 a | 9 b | ||||
|---|---|---|---|---|---|---|
| δC, mult | δH (J in Hz) | HMBC | δC, mult | δH (J in Hz) | HMBC | |
| 1 | 32.6, CH2 | 1.24, m 2.13, td (12.7, 5.7) | 2, 3, 5, 9, 10, 15 | 37.8, CH2 | 1.59, m 1.54, m | 2, 3, 5, 15 |
| 2 | 17.6, CH2 | 1.50, m | 1, 3, 4 | 18.0, CH2 | 1.71, m 1.45, m | 1, 3 |
| 3 | 42.0, CH2 | 1.38, td (12.9, 5.3) 1.63, m | 2, 4, 13, 14 1, 2, 4, 5, 14 | 37.8, CH2 | 1.32, td (13.0, 3.8) 1.10, td (13.6, 4.3) | 1, 2, 13, 14 |
| 4 | 38.3, C | 38.3, C | ||||
| 5 | 47.1, CH | 2.00, d (4.0) | 4, 6, 9, 13, 14, 15 | 48.6, CH | 1.57, brs | 1, 6, 9, 10, 14, 15 |
| 6 | 63.5, CH | 4.62, t (4.2) | 7, 8, 10 | 70.0, CH | 3.99, brs | 5, 7, 8, 9, 10 |
| 7 | 139.1, CH | 6.96, d (4.0) | 5, 9, 12 | 64.1, CH | 4.00, d (2.1) | 5, 6, 12 |
| 8 | 130.1, C | 122.1, C | ||||
| 9 | 77.5, C | 173.1, C | ||||
| 10 | 39.0, C | 36.3, C | ||||
| 11 | 75.0, CH2 | 4.24, d (9.8) 4.44, d (9.8) | 8, 9, 12 | 68.1, CH2 | 4.94, dd (17.6, 1.7) 4.79, brd (17.6) | 7, 8, 9 |
| 12 | 169.6, C | 173.4, C | ||||
| 13 | 26.8, CH3 | 1.15, s | 3, 4, 5, 14 | 27.9, CH3 | 0.97, s | 3, 4, 5, 14 |
| 14 | 68.4, CH2 | 3.42, d (11.4) 4.41, d (11.4) | 3, 4, 5, 13 | 65.6, CH2 | 3.94, dd (11.3, 3.8) 3.26, dd (11.3, 6.0) | 3, 4, 5, 13 |
| 15 | 20.8, CH3 | 1.23, s | 1, 5, 9, 10 | 21.6, CH3 | 1.40, s | 1, 5, 9, 10 |
| Compounds | Cytotoxicity IC50, µM | Colony Formation, % | ||
|---|---|---|---|---|
| Neuro-2a | 22Rv1 | MCF-7 | 22Rv1 | |
| 1 | >100 | >100 | nt | - |
| 2 | >100 | >100 | nt | - |
| 3 | >100 | >100 | nt | - |
| 4 | >100 | >100 | nt | 41 |
| 5 | >100 | >100 | nt | - |
| 6 | >100 | >100 | nt | - |
| 7 | 24.1 | 31.5 | >100 | - |
| 8 | 4.9 | 3.0 | 59.6 | - |
| 9 | >100 | >100 | >100 | 36 |
| 10 | >100 | >100 | >100 | - |
| Docetaxel | nt | 0.02 | nt | nt |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchenko, A.N.; Trinh, P.T.H.; Girich, E.V.; Smetanina, O.F.; Rasin, A.B.; Popov, R.S.; Dyshlovoy, S.A.; von Amsberg, G.; Menchinskaya, E.S.; Thanh Van, T.T.; et al. Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus flocculosus. Mar. Drugs 2019, 17, 579. https://doi.org/10.3390/md17100579
Yurchenko AN, Trinh PTH, Girich EV, Smetanina OF, Rasin AB, Popov RS, Dyshlovoy SA, von Amsberg G, Menchinskaya ES, Thanh Van TT, et al. Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus flocculosus. Marine Drugs. 2019; 17(10):579. https://doi.org/10.3390/md17100579
Chicago/Turabian StyleYurchenko, Anton N., Phan Thi Hoai Trinh, Elena V. Girich (Ivanets), Olga F. Smetanina, Anton B. Rasin, Roman S. Popov, Sergey A. Dyshlovoy, Gunhild von Amsberg, Ekaterina S. Menchinskaya, Tran Thi Thanh Van, and et al. 2019. "Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus flocculosus" Marine Drugs 17, no. 10: 579. https://doi.org/10.3390/md17100579
APA StyleYurchenko, A. N., Trinh, P. T. H., Girich, E. V., Smetanina, O. F., Rasin, A. B., Popov, R. S., Dyshlovoy, S. A., von Amsberg, G., Menchinskaya, E. S., Thanh Van, T. T., & Afiyatullov, S. S. (2019). Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus flocculosus. Marine Drugs, 17(10), 579. https://doi.org/10.3390/md17100579