Dual Crosslinked Methacrylated Alginate Hydrogel Micron Fibers and Tissue Constructs for Cell Biology
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Alg-MA and Dual Crosslinked Alg-MA Hydrogel Microfibers
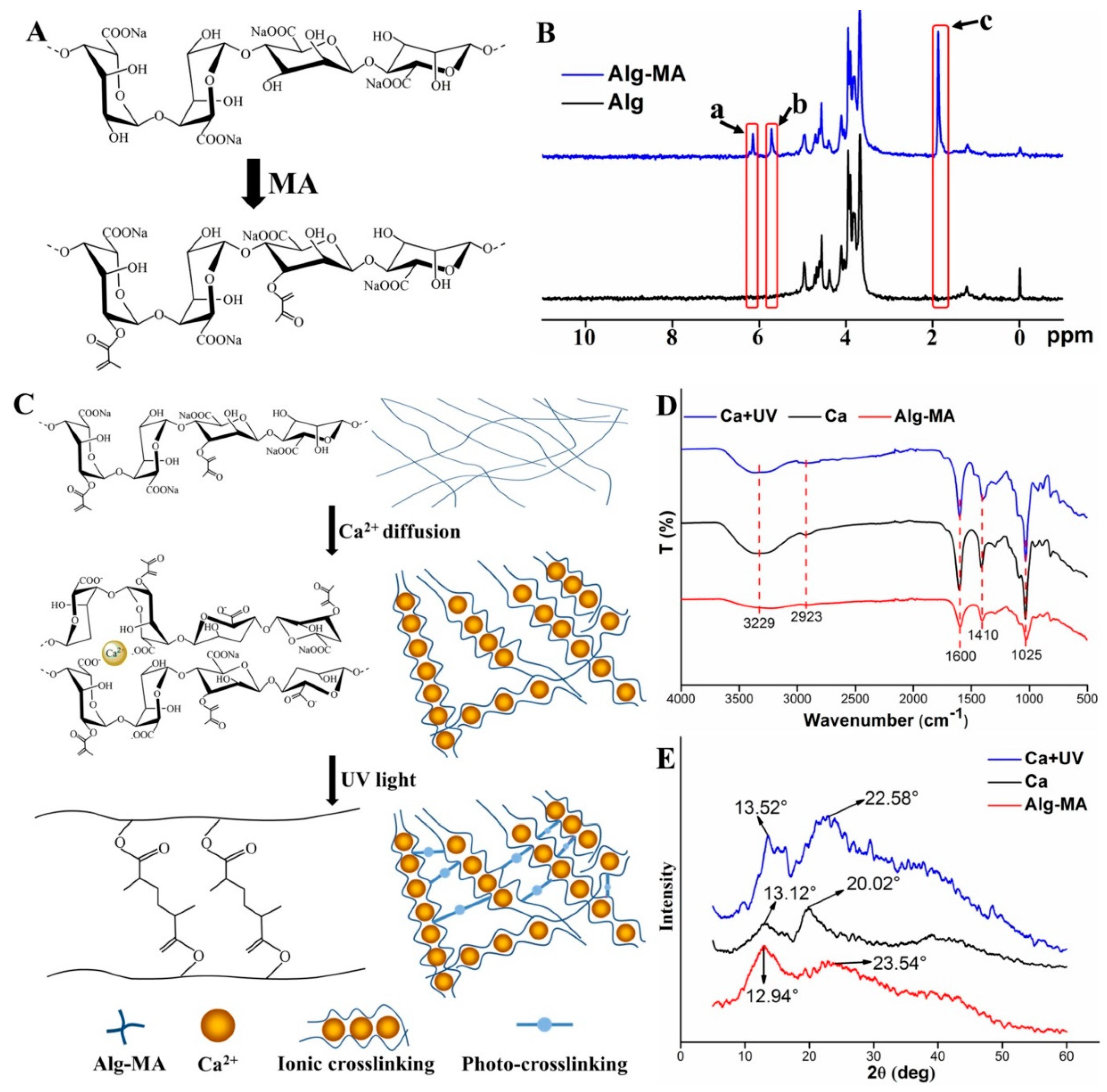
2.1.1. Synthesis of Alg-MA and Characterization
2.1.2. Effects of Crosslinking on the Alg-MA Macromolecules Structure
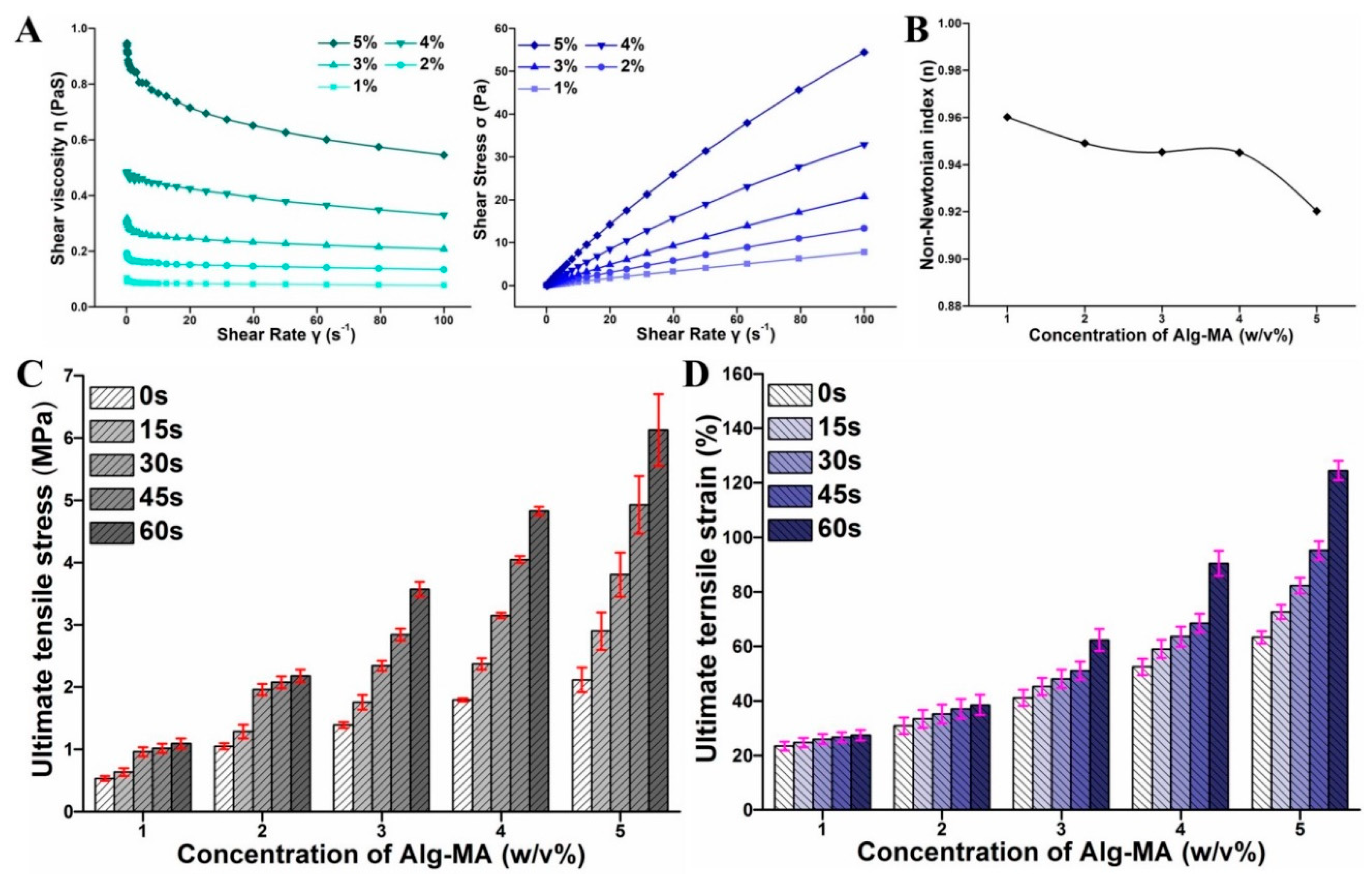
2.1.3. Rheological Behaviors of Alg-MA Solution
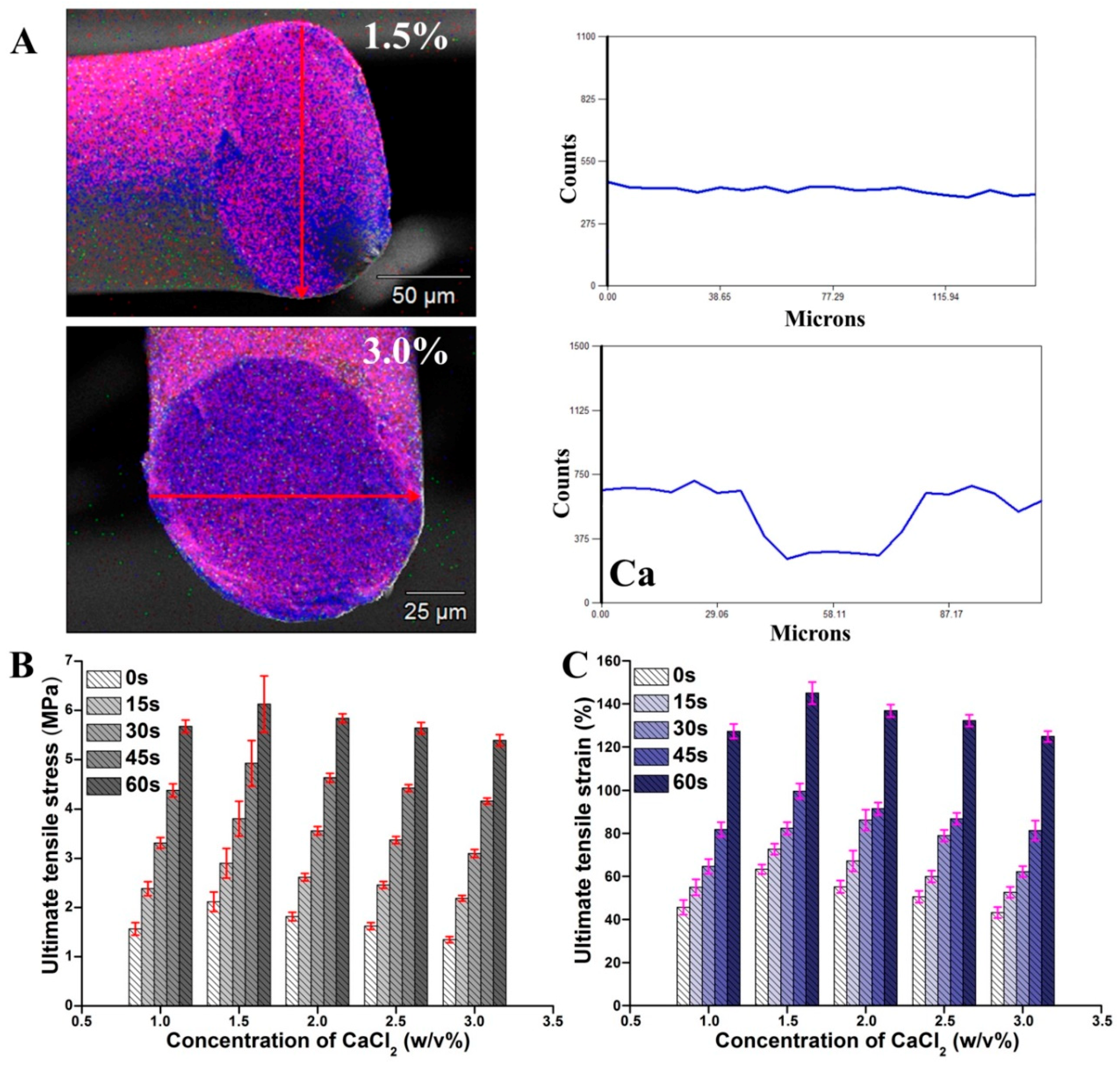
2.2. Tensile Mechanical Properties
2.3. Swelling Ratio Characteristics
2.4. Weight Loss Characteristics
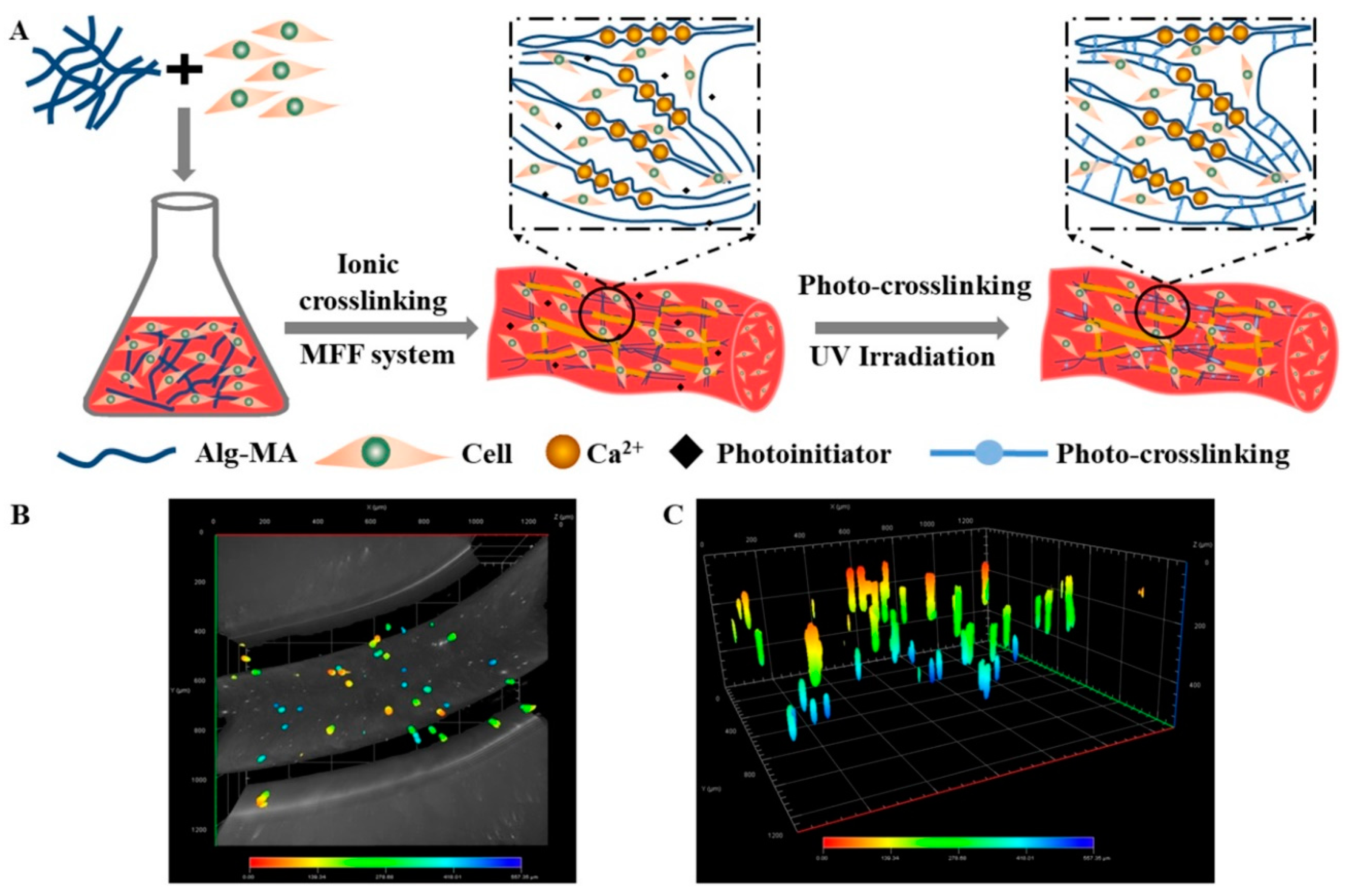
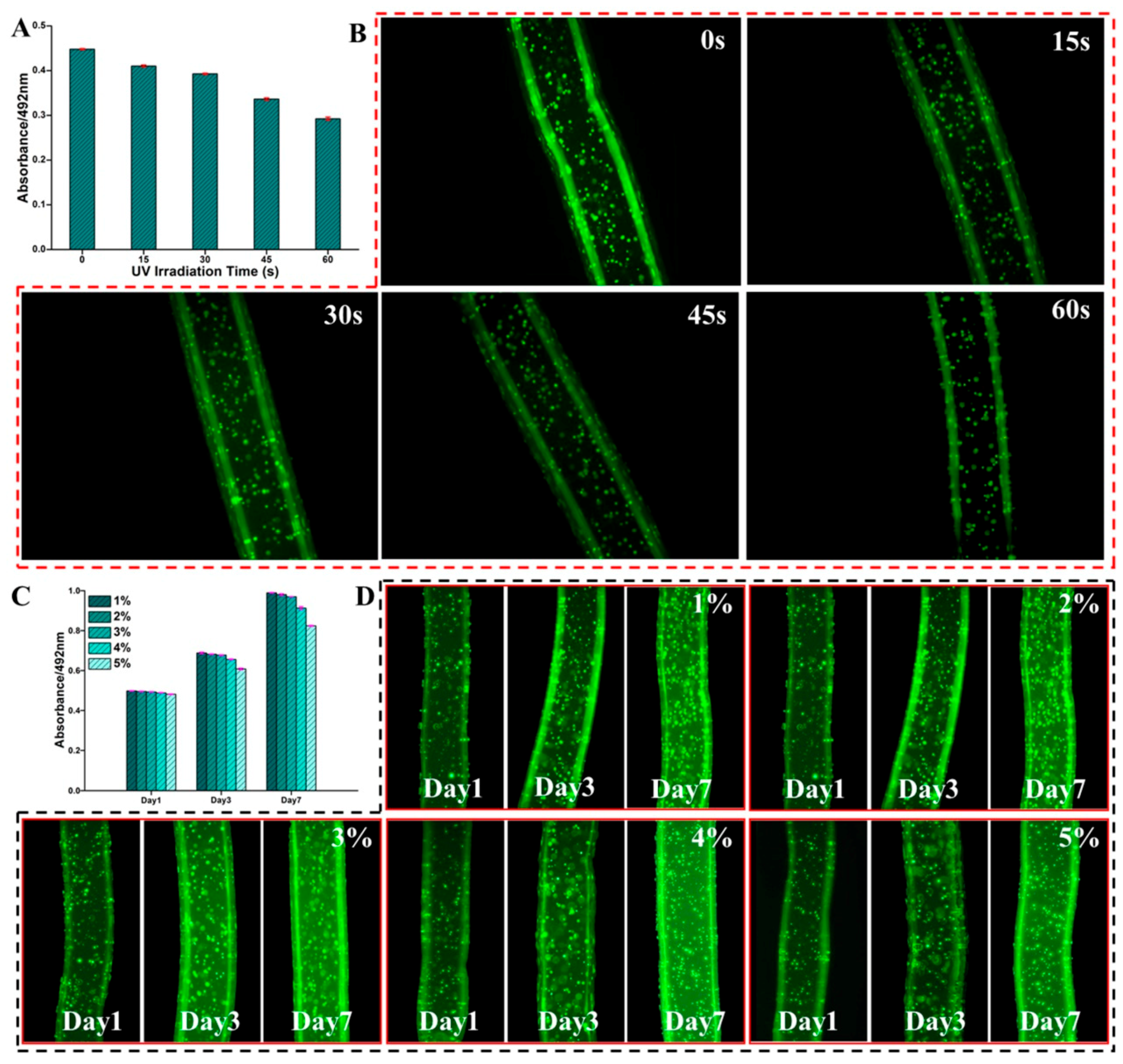
2.5. Cell Distribution in the Dual Crosslinked Hydrogel Microfibers
2.6. Cell Proliferation in the Dual Crosslinked Hydrogel Microfibers
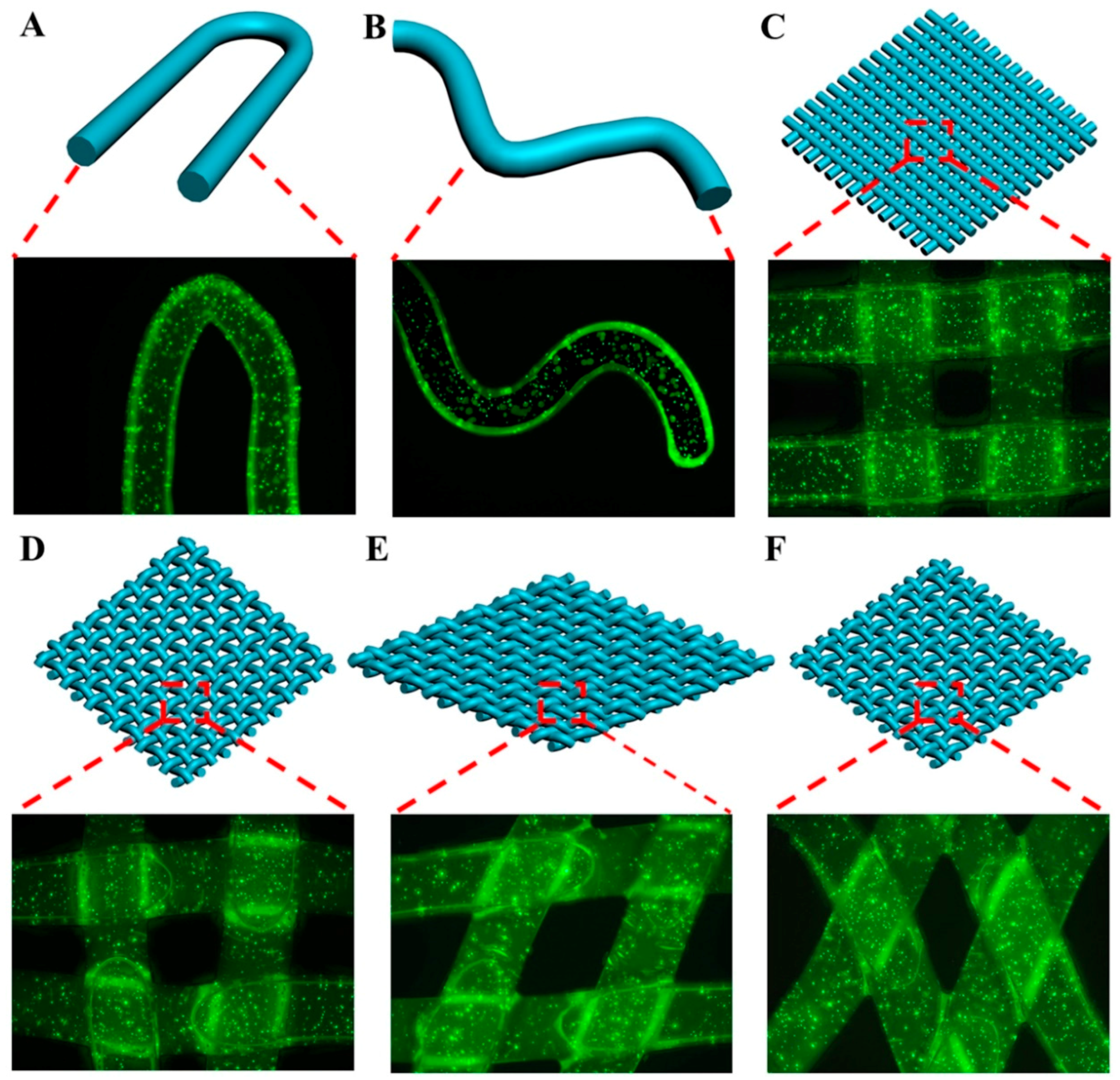
2.7. Assemblies of the Cell-encapsulated Dual Crosslinked Hydrogel Microfibers
3. Materials and Methods
3.1. Materials
3.2. Alg-MA Synthesis and Characterization
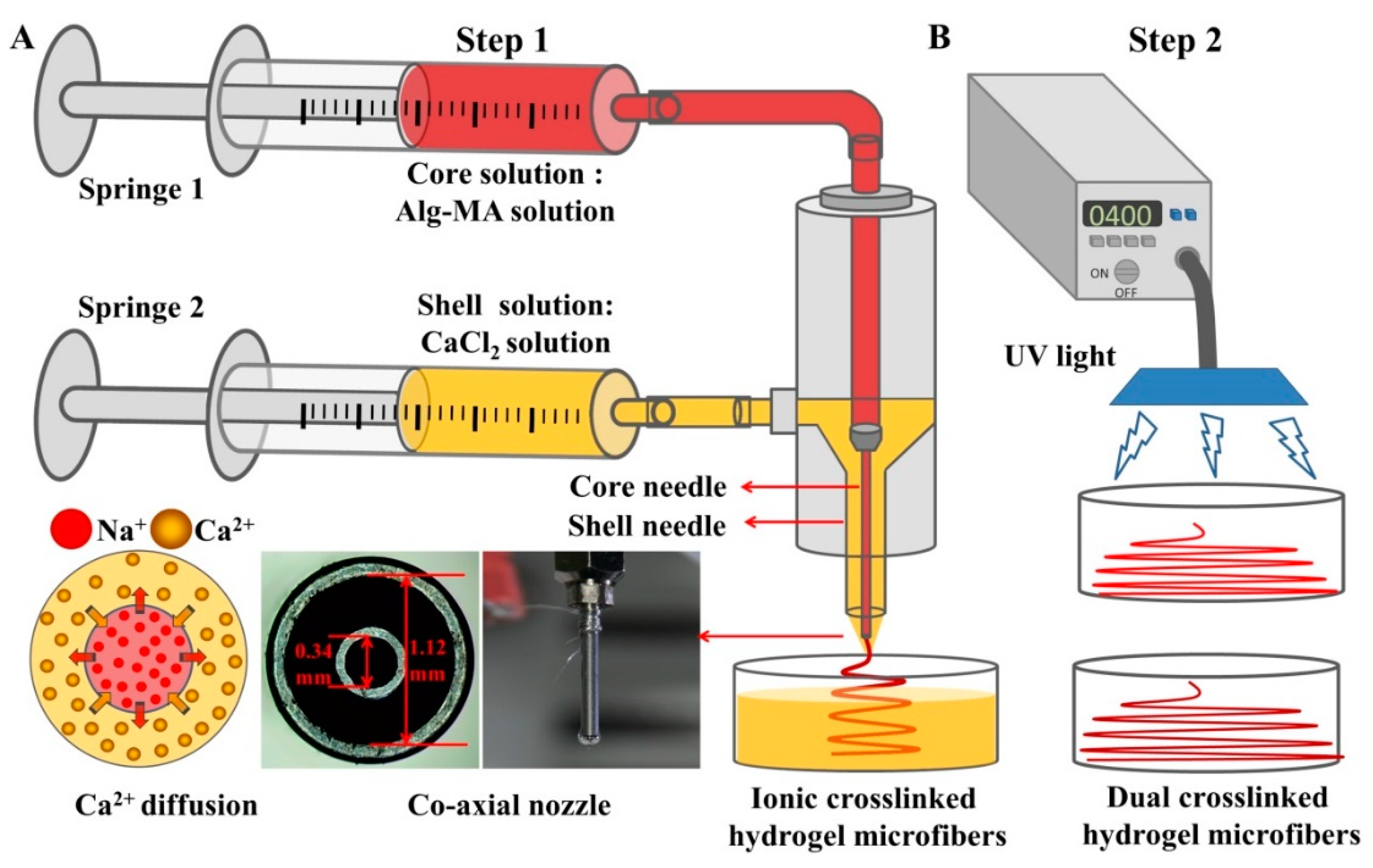
3.3. Preparation of the Dual Crosslinked Alg-MA Hydrogel Microfibers
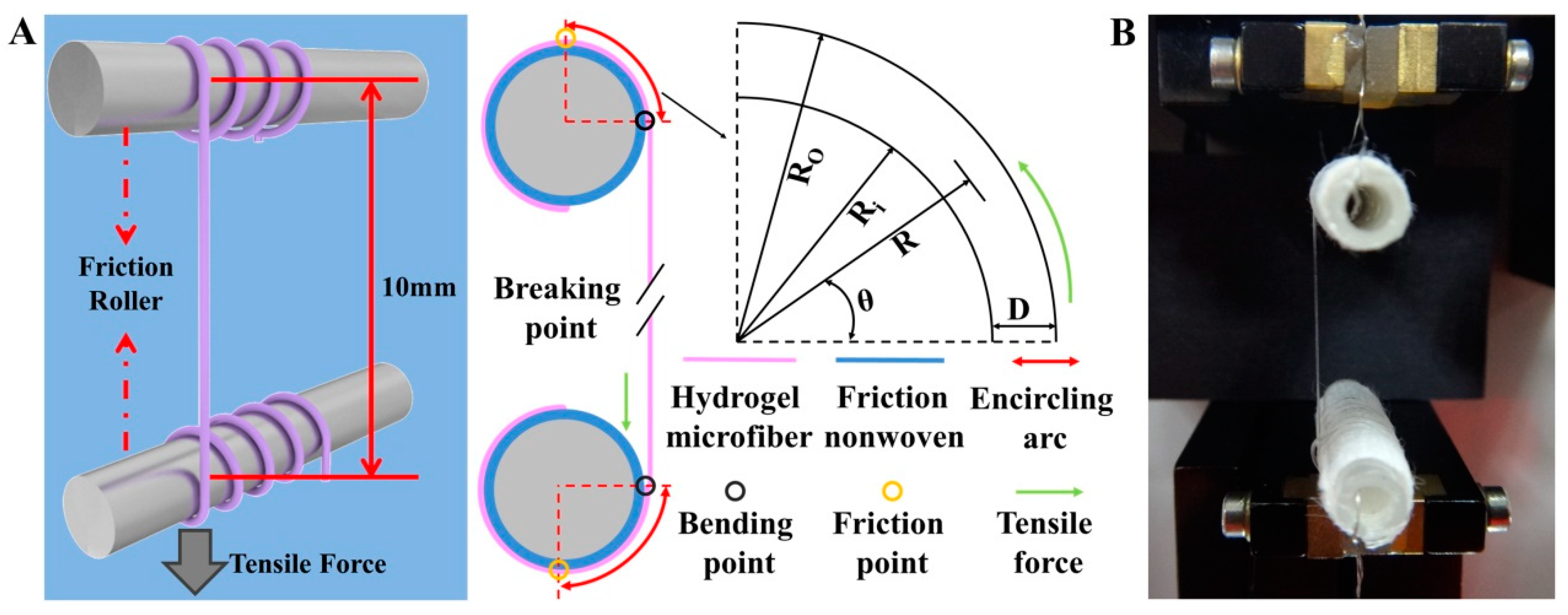
3.4. Tensile Mechanical Testing
3.5. Swelling Testing
3.6. Weight Loss Testing
3.7. Cell Culture and Preparation of Cell-Encapsulated Dual Crosslinked Hydrogel Microfibers
3.8. Cell Viability and Proliferation in Cell-encapsulated Dual Crosslinked Hydrogel Microfibers
3.9. Assembly of Cell-encapsulated Dual Crosslinked Hydrogel Microfibers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogel for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Tan, W.H.; Takeuchi, S.J. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater. 2007, 19, 2696–2701. [Google Scholar] [CrossRef]
- Mazumder, M.A.J.; Burke, N.A.D.; Shen, F.; Potter, M.A.; Stover, H.D.H. Core-cross-linked alginate microcapsules for cell encapsulation. Biomacromolecules 2009, 10, 1365–1373. [Google Scholar] [CrossRef]
- Mu, C.J.; Sakai, S.J.; Ijima, H.; Kawakami, K. Preparation of cell-enclosing microcapsules through photopolymerization of methacrylated alginate solution triggered by irradiation with visible light. J. Biosci. Bioeng. 2010, 109, 618–621. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Cha, C.; Kim, S.Y.; Cao, L.; Kong, H. Decoupled control of stiffness and permeability with a cell-encapsulating poly (ethylene glycol) dimethacrylate hydrogel. Biomaterials 2010, 31, 4864–4871. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, M.; Kim, T.H.; Kim, J.H.; Lee, J.H.; Park, J.H.; Knowles, J.C.; Kim, H.W. Utilizing core–shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng. Part A 2014, 20, 103–114. [Google Scholar] [CrossRef]
- Onoe, H.; Okitsu, T.; Itou, A.; Negishi, M.K.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Shigetomi, K.K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef]
- Daniele, M.A.; Boyd, D.A.; Adams, A.A.; Ligler, F.S. Microfluidic strategies for design and assembly of microfibers and nanofibers with tissue engineering and regenerative medicine applications. Adv. Healthcare Mater. 2015, 4, 11–28. [Google Scholar] [CrossRef]
- Yu, Y.R.; Fu, F.F.; Shang, L.R.; Cheng, Y.; Gu, Z.Z.; Zhao, Y.J. Bioinspired helical microfibers from microfluidics. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Asthana, A.; Lee, K.H.; Shin, S.J.; Perumal, J.; Butler, L.; Lee, S.H.; Kim, D.P. Bromo-oxidation reaction in enzyme-entrapped alginate hollow microfibers. Biomicrofluidics 2011, 5, 024117. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.R.; Fu, F.F.; Wang, J.; Shang, L.R.; Gu, Z.Z.; Zhao, Y.J. Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS Appl. Mater. Interfaces 2016, 8, 1080–1086. [Google Scholar] [CrossRef]
- Attalla, R.; Ling, C.; Selvaganapathy, P. Fabrication and characterization of gels with integrated channels using 3d printing with microfluidic nozzle for tissue engineering applications. Biomed. Microdevices 2016, 18, 17. [Google Scholar] [CrossRef]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef]
- Ghorbanian, S.; Qasaimeh, M.A.; Akbari, M.; Tamayol, A.; Juncker, D. Microfluidic direct writer with integrated declogging mechanism for fabricating cell-laden hydrogel constructs. Biomed. Microdevices 2014, 16, 387–395. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, K.Y.; Won, S.W.; Cha, C.Y.; Gaharwar, A.K.; Selimović, Š.; Bae, H.J.; Lee, K.H.; Lee, D.H.; Lee, S.H.; et al. Microfluidic fabrication of cell adhesive chitosan microtubes. Biomed. Microdevices 2013, 15, 465–472. [Google Scholar] [CrossRef]
- Chung, B.G.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip 2012, 12, 45–59. [Google Scholar] [CrossRef]
- Shi, X.T.; Ostrovidov, S.; Zhao, Y.H.; Liang, X.B.; Kasuya, M.; Kurihara, K.; Nakajima, K.; Bae, H.; Wu, H.K.; Khademhosseini, A. Microfluidic spinning of cell-responsive grooved microfibers. Adv. Funct. Mater. 2015, 25, 2250–2259. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 06–126. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, G.; Stokke, B.T. Evidence for egg-box-compatible interactions in calcium-alginate gels from fiber x-ray diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Braccini, I.; Perez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Tellechea, A.; Silva, E.A.; Min, J.H.; Leal, E.C.; Auster, M.E.; Nabzdyk, L.P.; Shih, W.; Mooney, D.J.; Aristidis, V. Alginate and DNA gels are suitable delivery systems for diabetic wound healing. Int. J. Lower Extrem. Wounds 2015, 14, 146–153. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Rödel, P.; Anamur, C.; Seeliger, C.; Imhoff, A.B.; Herbst, E.; Vogt, S.; Griensven, M.; Winter, G.; Engert, J. Calcium alginate gels as stem cell matrix–making paracrine stem cell activity available for enhanced healing after surgery. PLoS ONE 2015, 10, e0118937. [Google Scholar] [CrossRef] [PubMed]
- Barralet, J.E.; Wang, L.; Lawson, M.; Triffitt, J.T.; Cooper, P.R.; Shelton, R.M. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J. Mater. Sci. Mater. Med. 2005, 16, 515–519. [Google Scholar] [CrossRef]
- Shin, S.J.; Park, J.Y.; Lee, J.Y.; Park, H.; Park, Y.D.; Lee, K.B.; Whang, C.M.; Lee, S.H. “On the fly” continuous generation of alginate fibers using a microfluidic device. Langmuir 2007, 23, 9104–9108. [Google Scholar] [CrossRef] [PubMed]
- Mørch, Y.A.; Donati, I.; Strand, B.L.; Skjak-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Guo, B.L.; Ma, P.X. Multifunctional interpenetrating polymer network hydrogels based on methacrylated alginate for the delivery of small molecule drugs and sustained release of protein. Biomacromolecules 2014, 15, 3246–3252. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Berglund, C.M.; Lee, J.Y.; Polacheck, W.J.; Tsui, Y.; Bonassar, L.J.; Kirby, B.J. Methods for photocrosslinking alginate hydrogel scaffolds with high cell viability. Tissue Eng. Part C 2011, 17, 173–179. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef]
- Coates, E.E.; Riggin, C.N.; Fisher, J.P. Photocrosslinked alginate with hyaluronic acid hydrogels as vehicles for mesenchymal stem cell encapsulation and chondrogenesis. J. Biomed. Mater. Res. A 2012, 101, 1962–1970. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Jeon, O.; Powell, C.; Ahmed, S.M.; Alsberg, E. Biodegradable, photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng. Part A 2010, 16, 2915–2925. [Google Scholar] [CrossRef] [PubMed]
- Boddupalli, A.; Bratlie, K.M. Collagen organization deposited by fibroblasts encapsulated in ph responsive methacrylated alginate hydrogels. J. Biomed. Mater. Res. Part A 2018, 106, 2934–2943. [Google Scholar] [CrossRef]
- Sun, J.; Wei, D.; Yang, K.; Yang, Y.; Liu, X.L.; Fan, H.S.; Zhang, X.D. The development of cell-initiated degradable hydrogel based on methacrylated alginate applicable to multiple microfabrication technologies. J. Mater. Chem. B 2017, 5, 8060–8069. [Google Scholar] [CrossRef]
- Spencer, L.F.; Tian, X.M.; Ryan, M.S.; Rachael, A.O. Dual-cross-linked methacrylated alginate sub-microspheres for intracellular chemotherapeutic delivery. ACS Appl. Mater. Interfaces 2016, 8, 17775–17783. [Google Scholar]
- Etter, N.J.; Karasinski, M.; Ware, J.; Oldinski, A.R. Dual-crosslinked homogeneous alginate microspheres for mesenchymal stem cell encapsulation. J. Mater. Sci-Mater. Med. 2018, 29, 143. [Google Scholar] [CrossRef] [PubMed]
- Sami, I.S.; Kelly, L.; Chin, Y.Y.; Marcella, K.V.; Veronica, I.; Alyssa, A.A.; Banu, A.; Ming, H.C.; Eric, M.B. Synthesis and evaluation of dual crosslinked alginate microbeads. Acta Biomater. 2018, 65, 33–65. [Google Scholar]
- Xu, X.J.; Li, F.X.; Yu, J.Y.; Cao, A.M. Effects of molecular weight and rheological properties on spinnability.of biodegradable PBST copolyesters. Int. J. Nonlin. Sci. Num. 2008, 9, 31–36. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Q.H.; Chen, L.Y.; Zhang, Y.Y.; Guan, F.C. Rheological behavior of sodium alginate and its fiber properties. Synth. Fiber Ind. 2013, 36, 28–31. [Google Scholar]
- Matyash, M.; Despang, F.; Ikonomidou, C.; Gelinsky, M. Swelling and mechanical properties of alginate hydrogels with respect to promotion of neural growth. Tissue Eng. Part C 2014, 20, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.E.; Stabler, C.L.; Simpson, C.P.; Sambanis, A.; Constantinidis, I. The role of the CaCl2-guluronic acid interaction on alginate encapsulated βTC3 cell. Biomaterials 2004, 25, 2603–2610. [Google Scholar] [CrossRef]
- Potter, K.; Balcom, B.J.; Carpenter, T.A.; Hall, L.D. The gelation of sodium alginate with calcium ions studied by magnetic resonance imaging (MRI). Carbohyd. Res. 1994, 257, 117–126. [Google Scholar] [CrossRef]
- Cuadros, T.R.; Skurtys, O.; Aguilera, J.M. Mechanical properties of calcium alginate fibers produced with a microfluidic device. Carbohyd. Polym. 2012, 89, 1198–1206. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. A quantitative analysis of alginate swelling. Carbohyd. Polym. 2010, 79, 1020–1027. [Google Scholar] [CrossRef]
- Mirabedini, A.; Foroughi, J.; Romeo, T.; Wallace, G.G. Development and characterization of novel hybrid hydrogel fibers. Macromol. Mater. Eng. 2015, 12, 1217–1225. [Google Scholar] [CrossRef]
- Yuta, K.; Akishige, H.; Tomoaki, T.; Yasuhiko, T.; Hisashi, K. Regeneration of anterior cruciate ligament by biodegradable scaffold combined with local controlled release of basic fibroblast growth factor and collagen wrapping. Tissue Eng. Part C 2008, 14, 47–57. [Google Scholar]
- Xiao, W.Z.; Gyeong, J.K.; Min, G.K.; Jung, K.L.; Jeong, W.S.; Jeong, T.D.; Kwonho, H.; Jae, M.C.; Su, R.S.; Hojae, B. Marine biomaterial-based bioinks for generating 3D printed tissue constructs. Mar. Drugs 2018, 16, 484–496. [Google Scholar]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef]
- Jia, W.T.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.J.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Cristina, C.; Su, R.S.; Vijayan, M.; Solange, M.; Marco, C.; Andrea, B.; Mehmet, R.D.; Mariella, D.; Ali, K. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low viscosity bioink. Adv. Mater. 2015, 28, 677–684. [Google Scholar]
- Ehrbar, M.; Sala, A.; Lienemann, P.; Ranga, A.; Mosiewicz, K.; Birrermann, A.; Rizzi, S.C.; Weber, F.E.; Lutolf, M.P. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys. J. 2011, 100, 284–293. [Google Scholar] [CrossRef]
- Huang, Z.M. The mechanical properties of composites reinforced with woven and braided fabrics. Compos. Sci. Technol. 2000, 60, 479–498. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Jin, X. Dual Crosslinked Methacrylated Alginate Hydrogel Micron Fibers and Tissue Constructs for Cell Biology. Mar. Drugs 2019, 17, 557. https://doi.org/10.3390/md17100557
Gao Y, Jin X. Dual Crosslinked Methacrylated Alginate Hydrogel Micron Fibers and Tissue Constructs for Cell Biology. Marine Drugs. 2019; 17(10):557. https://doi.org/10.3390/md17100557
Chicago/Turabian StyleGao, Yingjun, and Xiangyu Jin. 2019. "Dual Crosslinked Methacrylated Alginate Hydrogel Micron Fibers and Tissue Constructs for Cell Biology" Marine Drugs 17, no. 10: 557. https://doi.org/10.3390/md17100557
APA StyleGao, Y., & Jin, X. (2019). Dual Crosslinked Methacrylated Alginate Hydrogel Micron Fibers and Tissue Constructs for Cell Biology. Marine Drugs, 17(10), 557. https://doi.org/10.3390/md17100557




