The Potential of Neoagaro-Oligosaccharides as a Treatment of Type II Diabetes in Mice
Abstract
1. Introduction
2. Results
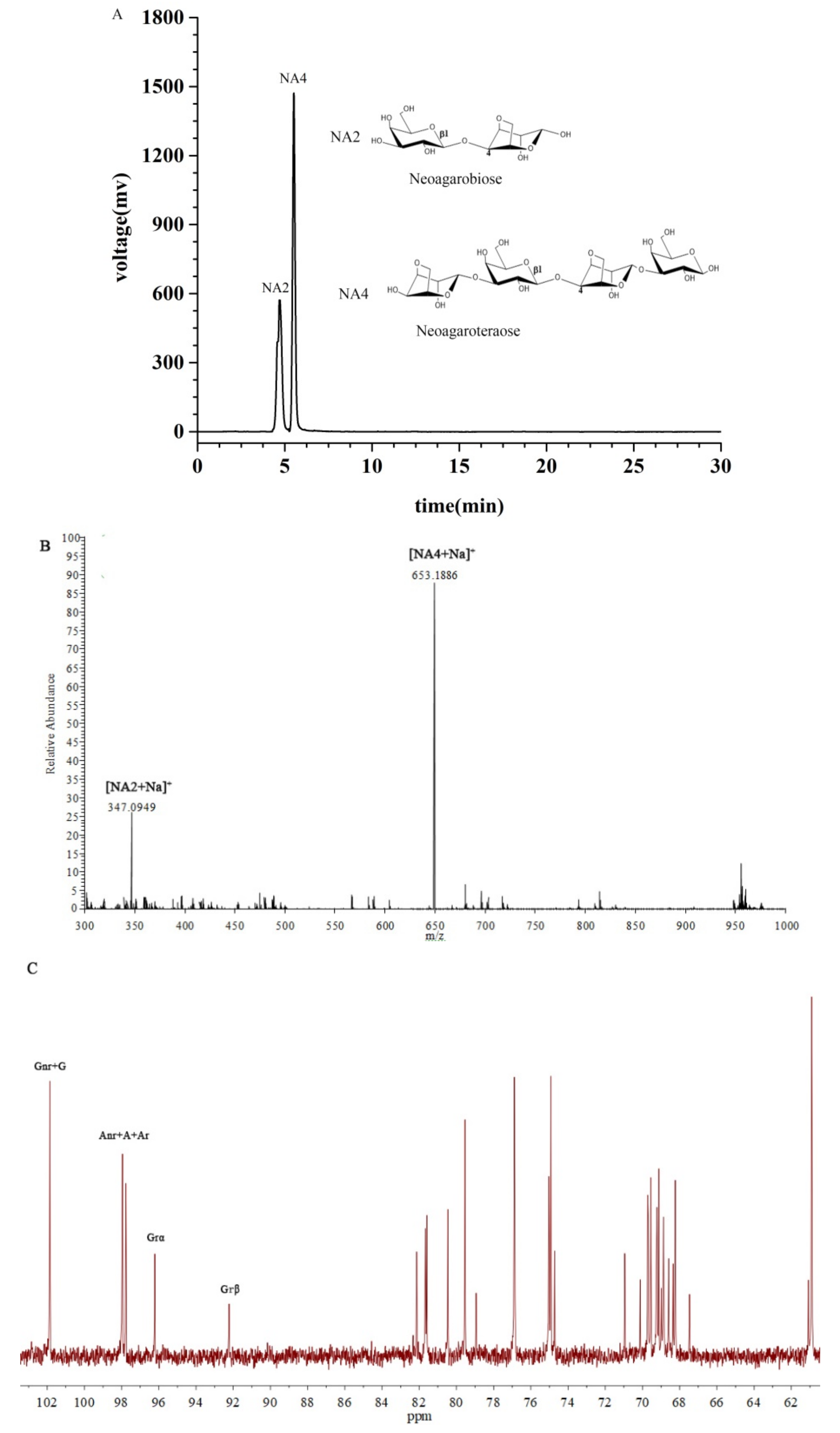
2.1. Characterization of the NAOs
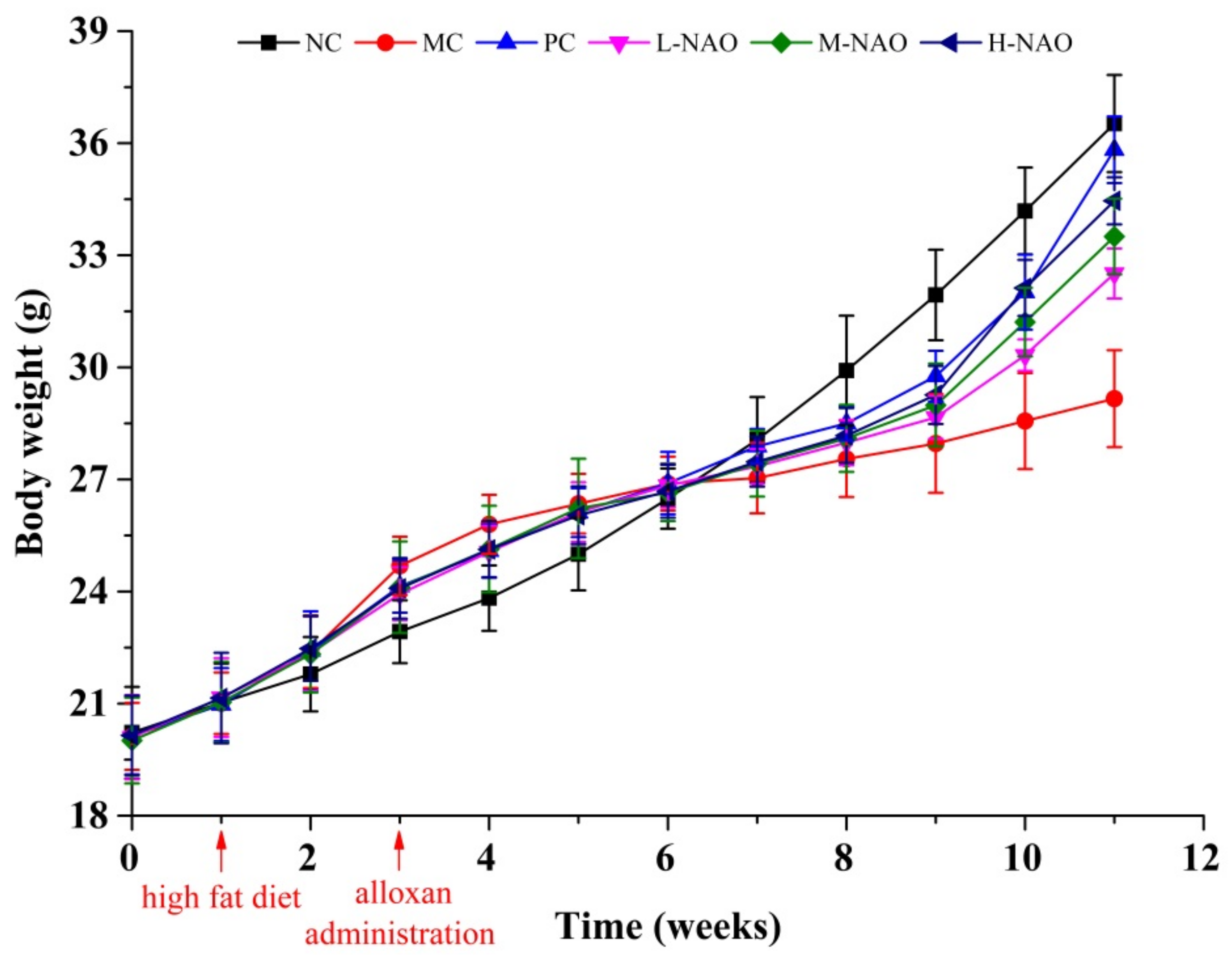
2.2. Effects of NAOs on Body Mass and Food Intake
2.3. Fasting Blood Glucose (FBG) Determination and Oral Glucose Tolerance Test (OGTT)
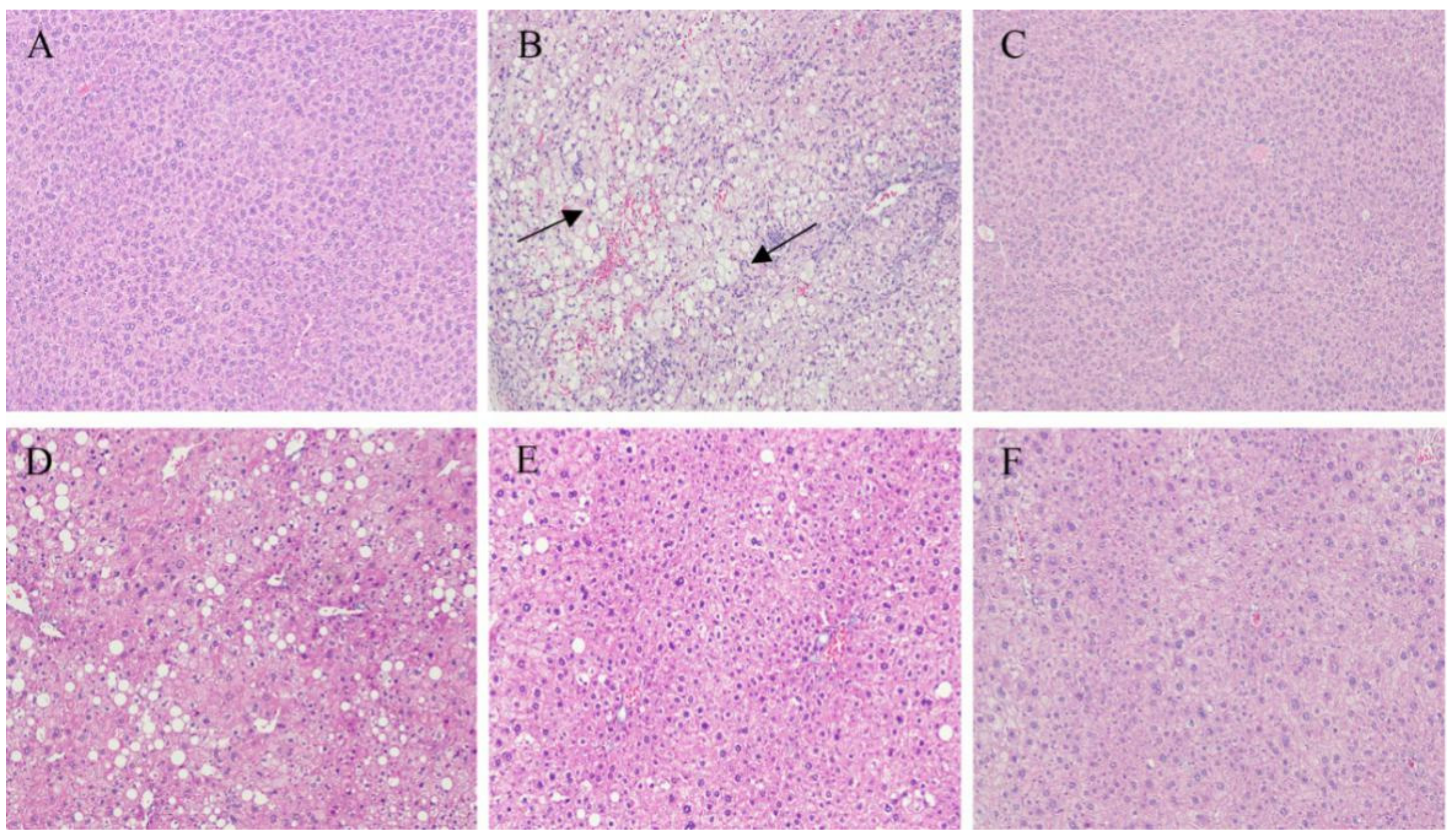
2.4. Immunohistochemistry of the Liver and Pancreatic Islets
2.5. Effects of NAOs on Serum Lipids
2.6. Effects of NAOs on the Hepatic Antioxidation System
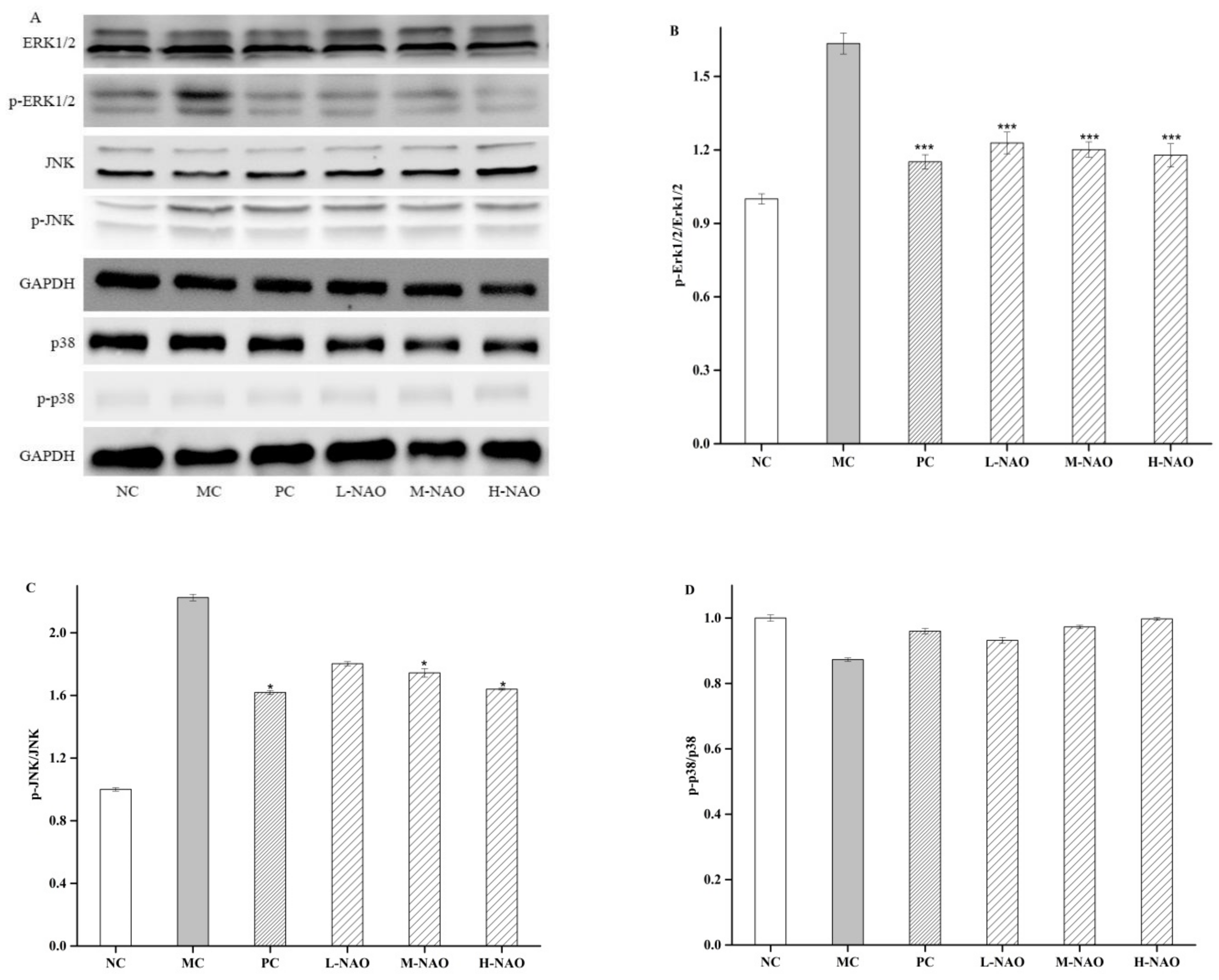
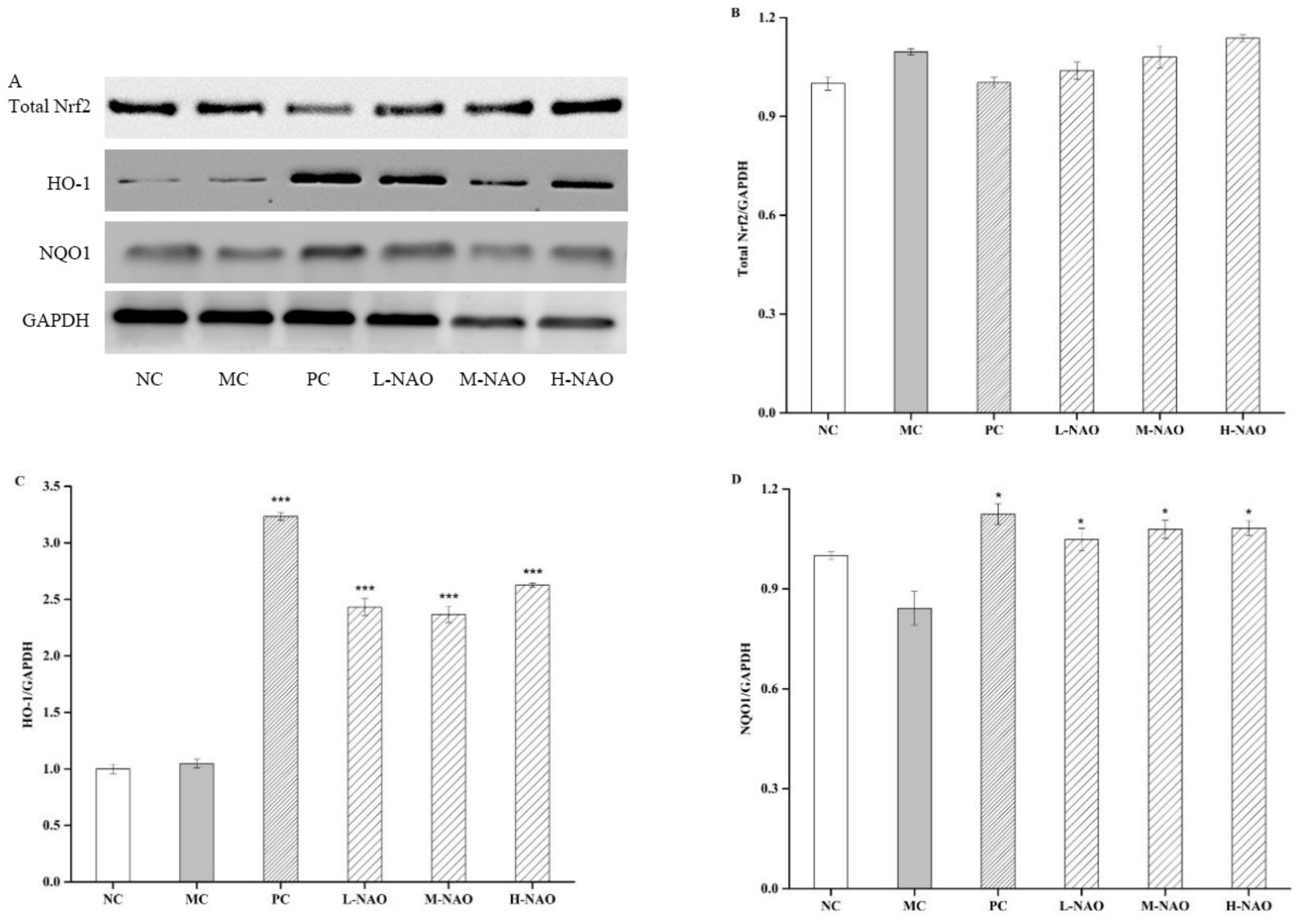
2.7. NAOs Regulated the MAPK-Nrf2 Pathway
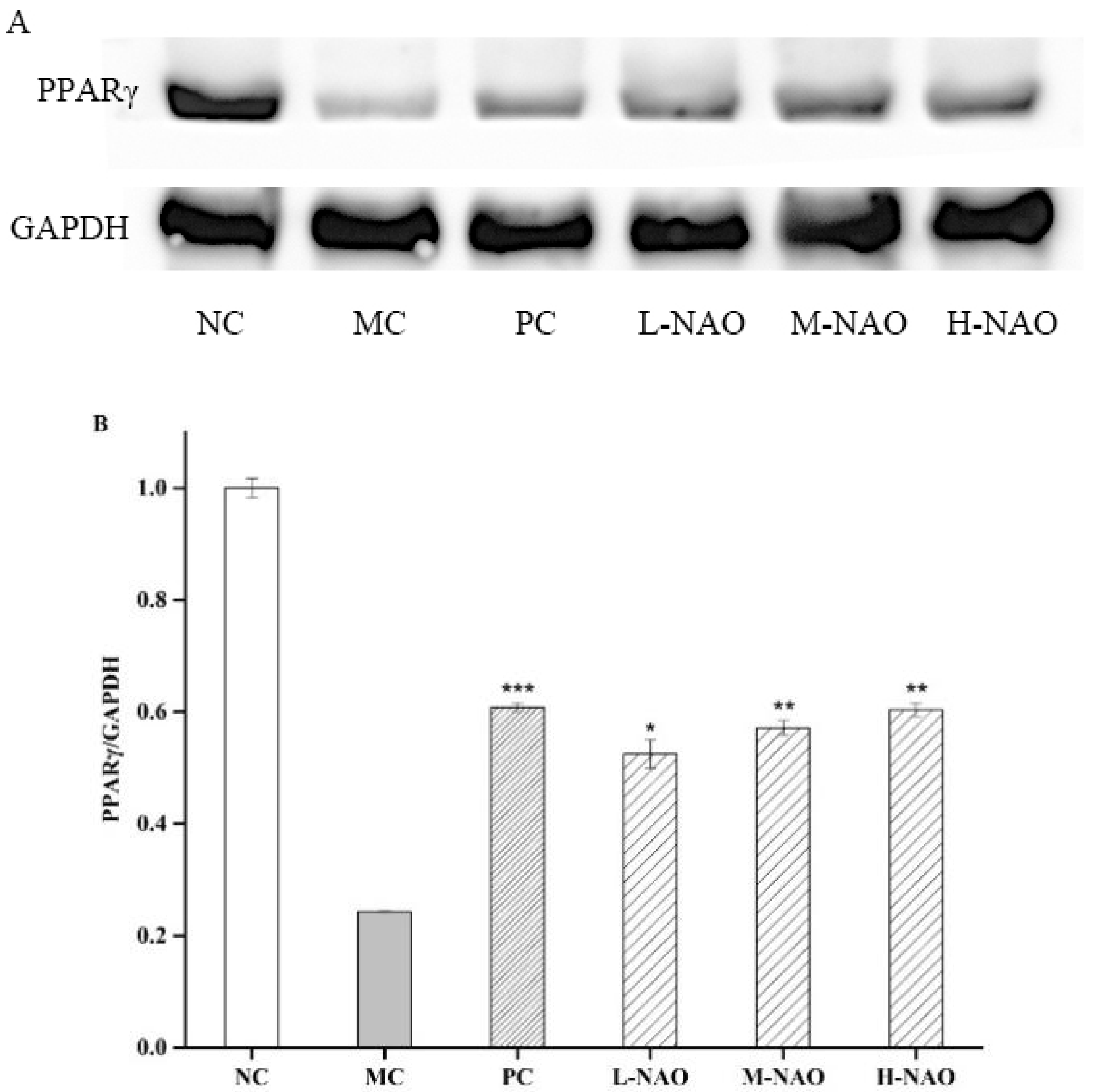
2.8. NAOs Regulated the PPARγ Pathway
3. Discussion
4. Materials and Methods
4.1. Materials and Animals
4.2. Preparation of NAOs
4.3. Experimental Design
4.4. FBG and OGTT
4.5. Biochemical Analysis
4.6. Determination of GSH-Px, SOD, and MDA
4.7. Histologic Assessment of the Liver and Pancreas
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abreviations
References
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kakkar, P. Modulation of liver function, antioxidant responses, insulin resistance and glucose transport by Oroxylum indicum stem bark in STZ induced diabetic rats. Food Chem. Toxicol. 2013, 62, 722–731. [Google Scholar] [CrossRef]
- Sun, W.; Sang, Y.; Zhang, B.; Yu, X.; Xu, Q.; Xiu, Z.; Dong, Y. Synergistic effects of acarbose and an Oroxylum indicum seed extract in streptozotocin and high-Fat-Diet induced prediabetic mice. Biomed. Pharmacother. 2017, 87, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, B.; Yu, X.; Zhuang, C.; Li, X.; Sun, J.; Xing, Y.; Xiu, Z.; Dong, Y. Oroxin A from Oroxylum indicum prevents the progression from prediabetes to diabetes in streptozotocin and high-Fat diet induced mice. Phytomedicine 2018, 38, 24–34. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhang, H.H.; Yu, C.H.; Fang, J.; Ying, H.Z. Ethanol extract of Atractylodis macrocephalae Rhizoma ameliorates insulin resistance and gut microbiota in type 2 diabetic db/db mice. J. Funct. Foods 2017, 39, 139–151. [Google Scholar] [CrossRef]
- Li, J.; Luo, M.; Luo, Z.; Guo, A.Y.; Yang, X.; Hu, M.; Zhang, Q.; Zhu, Y. Transcriptome profiling reveals the anti-Diabetic molecular mechanism of Cyclocarya paliurus polysaccharides. J. Funct. Foods 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Parillo, M.; Riccardi, G. Diet composition and the risk of type 2 diabetes: Epidemiological and clinical evidence. Br. J. Nutr. 2004, 92, 7–19. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Wang, J.; Yang, H.; Li, S.; Jiang, W.; Liu, Y.; Li, J. Long-Chain bases from sea cucumber inhibits renal fibrosis and apoptosis in type 2 diabetic mice. J. Funct. Foods 2018, 40, 760–768. [Google Scholar] [CrossRef]
- Kannel, W.B. Lipids, diabetes, and coronary heart disease: Insights from the Framingham Study. Am. Heart J. 1985, 110, 1100–1107. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, X.Y.; Li, J.; Xu, Z.G.; Chen, L. The characterization of high-Fat diet and multiple low-Dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 2008, 1–9. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Antidiabetic effect of Lactobacillus casei CCFM0412 on mice with type 2 diabetes induced by a high-Fat diet and streptozotocin. Nutrition 2014, 30, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Yan, X.J. Antioxidant activities of agaro-Oligosaccharides with different degrees of polymerization in cell-Based system. Biochim. Biophys. Acta 2005, 1722, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, X.; Zhu, P.; Lin, J. Antioxidant activity and hepatoprotective potential of agaro-oligosaccharides in vitro and in vivo. Nutr. J. 2006, 5, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Wen, T.N.; Pan, C.L. Algal-Oligosaccharide-Lysates prepared by two bacterial agarases stepwise hydrolyzed and their anti-Oxidative properties. Fish. Sci. 2010, 71, 1149–1159. [Google Scholar] [CrossRef]
- Kang, O.L.; Ghani, M.; Hassan, O.; Rahmati, S.; Ramli, N. Novel agaro-Oligosaccharide production through enzymatic hydrolysis: Physicochemical properties and antioxidant activities. Food Hydrocoll. 2014, 42, 304–308. [Google Scholar] [CrossRef]
- Enoki, T.; Tanabe, M.; Shimomura, M.; Ohnogi, H. Induction mechanism of heme oxygenase-1 and anti-inflammatory activity by agaro-oligosaccharides. Nippon Shokuhin Kogyo Gakkaishi 2010, 57, 157–162. [Google Scholar] [CrossRef][Green Version]
- Enoki, T.; Tominaga, T.; Takashima, F.; Ohnogi, H.; Sagawa, H.; Kato, I. Anti-Tumor-Promoting activities of agaro-Oligosaccharides on two-Stage mouse skin carcinogenesis. Biol. Pharm. Bull. 2012, 35, 1145–1149. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Tanimura, Y.; Mizushima, K.; Harusato, A.; Fukui, A.; Yoriki, H.; Handa, O.; Ohnogi, H.; et al. Preventive effect of agaro-Oligosaccharides on non-Steroidal anti-Inflammatory drug-Induced small intestinal injury in mice. J. Gastroenterol. Hepatol. 2014, 29, 310–317. [Google Scholar] [CrossRef]
- Kobayashi, R.; Takisada, M.; Suzuki, T.; Kirimuraab, K.; Usamiab, S. Neoagarobiose as a novel moisturizer with whitening effect. Biosci. Biotechnol. Biochem. 1997, 61, 162–163. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Yu, S.; Kim, K.H.; Kang, N.J. Different Levels of Skin Whitening Activity among 3,6-Anhydro-l-Galactose, Agarooligosaccharides, and Neoagarooligosaccharides. Mar. Drugs 2017, 15, 321. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, J.H.; Kim, E.J.; Yang, H.J.; Park, J.S.; Hong, S.K. Anti-Obesity and Anti-Diabetic Effect of Neoagarooligosaccharides on High-Fat Diet-Induced Obesity in Mice. Mar. Drugs 2017, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Cho, S.S.; Kim, K.M.; Kim, J.Y.; Kim, E.J.; Park, E.Y.; Lee, J.H.; Ki, S.H. Neoagarooligosaccharides enhance the level and efficiency of LDL receptor and improve cholesterol homeostasis. J. Funct. Foods 2017, 38, 529–539. [Google Scholar] [CrossRef]
- Wang, W.; Liu, P.; Hao, C.; Wu, L.; Wan, W.; Mao, X. Neoagaro-Oligosaccharide monomers inhibit inflammation in LPS-Stimulated macrophages through suppression of MAPK and NF-Kappab pathways. Sci. Rep. 2017, 7, 44252. [Google Scholar] [CrossRef]
- Kang, D.R.; Yoon, G.Y.; Cho, J.; Lee, S.J.; Lee, S.J.; Park, H.J.; Kang, T.H.; Han, H.D.; Park, W.S.; Yoon, Y.K.; et al. Neoagarooligosaccharides prevent septic shock by modulating A20-and cyclooxygenase-2-Mediated interleukin-10 secretion in a septic-Shock mouse model. Biochem. Biophys. Res. Commun. 2017, 486, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, X.; Xin, J.; Wu, Y.; Li, H. Coumarins improved type 2 diabetes induced by high-Fat diet and streptozotocin in mice via antioxidation. Can. J. Physiol. Pharmacol. 2018, 96, 1–24. [Google Scholar] [CrossRef]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. Jama 1986, 256, 2835–2838. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V. Obesity and cardiovascular disease: The hippocrates paradox? J. Am. Coll. Cardiol. 2003, 42, 677–679. [Google Scholar] [CrossRef]
- Carr, M.C.; Brunzell, J.D. Abdominal obesity and dyslipidemia in the metabolic syndrome: Importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J. Clin. Endocrinol. Metab. 2004, 89, 2601–2607. [Google Scholar] [CrossRef]
- Ansell, B.J.; Watson, K.E.; Fogelman, A.M.; Navab, M.; Fonarow, G.C. High-Density lipoprotein function recent advances. J. Am. Coll. Cardiol. 2005, 46, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P. Chronic Oxidative Stress as a Central Mechanism for Glucose Toxicity in Pancreatic Islet Beta Cells in Diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.L.; Rasouli, N.; Sinha, A.K.; Molavi, B. Oxidative stress in diabetes: A mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int. J. Biochem. Cell Biol. 2006, 38, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Iqbal, M.; Karam, J.; Salifu, M.; McFarlane, S.I. Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: Pathophysiological insights. Antioxid. Redox Signal. 2007, 9, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.Q.; Wei, W.; Chen, L.M.; Liu, S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J. Ethnopharmacol. 2006, 108, 109–115. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, X.; Shao, N.; Ye, R.; Zhang, N.; Zhang, Y. Protective and antiangiopathy effects of ginsenoside Re against diabetes mellitus via the activation of p38 MAPK, ERK1/2 and JNK signaling. Mol. Med. Rep. 2016, 14, 4849–4856. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-Induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, X.; Pan, Z.; Shen, G.; Kim, J.H.; Yu, S.; Khor, T.O.; Li, W.; Ma, J.; Kong, A.N. Mechanism of action of isothiocyanates: The induction of ARE-Regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther. 2006, 5, 1918–1926. [Google Scholar] [CrossRef]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-Derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar]
- Barroso, I.; Gurnell, M.; Crowley, V.E.F.; Agostini, M.; Schwabe, J.W.; Soos, M.A.; Maslen, G.L.; Williams, T.D.M.; Lewis, H.; Schafer, A.J.; et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999, 402, 880–883. [Google Scholar] [CrossRef]
- Dubois, S.G.; Heilbronn, L.K.; Smith, S.R.; Albu, J.B.; Kelley, D.E.; Ravussin, E.; Look, A.A.R.G. Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity 2006, 14, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Han, J.P.; Huang, Y.Y.; Ye, J.; Xiao, M.T. Screening and identification of a bacterium capable of converting agar to neoagaro oligosaccharides. Acta Microbiol. Sin. 2015, 55, 1126–1132. [Google Scholar]
- Lin, F.; Ye, J.; Huang, Y.; Yang, Y.; Xiao, M. Simple Preparation of Diverse Neoagaro-Oligosaccharides. Processes 2019, 7, 267. [Google Scholar] [CrossRef]



| Group | Body Weight (g) | Food Intake (g/day) | |||
|---|---|---|---|---|---|
| 0 Week | 1 Week (HFD) | 3 Weeks (Alloxan Administration) | 11 Weeks | ||
| NC | 20.23 ± 1.23 | 21.03 ± 1.05 | 22.93 ± 0.84 | 36.53 ± 1.30 | 5.16 ± 0.28 |
| MC | 20.13 ± 0.90 | 21.01 ± 0.82 | 24.69 ± 0.78 ## | 29.16 ± 1.30 ## | 4.60 ± 0.29 |
| PC | 20.15 ± 1.05 | 20.98 ± 0.98 | 24.14 ± 0.70 | 35.82 ± 0.89 ** | 4.61 ± 0.27 |
| L-NAO | 20.10 ± 1.13 | 21.16 ± 1.05 | 23.94 ± 0.71 | 32.51 ± 0.67 ** | 4.42 ± 0.24 |
| M-NAO | 20.01 ± 1.15 | 21.04 ± 1.08 | 24.11 ± 1.23 | 33.50 ± 1.01 ** | 4.43 ± 0.22 |
| H-NAO | 20.15 ± 1.06 | 21.15 ± 1.21 | 24.09 ± 0.81 | 34.46 ± 0.63 ** | 4.39 ± 0.17 |
| Group | 0 Week | 1 Week (HFD) | 3 Weeks (Alloxan Administration) | 7 Weeks | 11 Weeks |
|---|---|---|---|---|---|
| NC | 4.44 ± 0.49 | 4.59 ± 0.59 | 4.44 ± 0.49 | 4.10 ± 0.43 | 4.83 ± 0.69 |
| MC | 4.18 ± 0.56 | 4.53 ± 0.49 | 22.90 ± 6.16 ## | 22.96 ± 4.80 ## | 22.24 ± 5.76 ## |
| PC | 4.33 ± 0.52 | 4.44 ± 0.45 | 22.60 ± 5.69 | 17.79 ± 8.63 | 9.95 ± 2.59 ** |
| L-NAO | 4.68 ± 0.44 | 4.59 ± 0.51 | 22.63 ± 5.68 | 17.94 ± 10.62 | 17.85 ± 7.90 |
| M-NAO | 4.33 ± 0.50 | 4.55 ± 0.49 | 22.46 ± 5.55 | 18.14 ± 8.42 | 15.95 ± 4.72 * |
| H-NAO | 4.33 ± 0.46 | 4.73 ± 0.32 | 22.44 ± 5.34 | 14.89 ±6.46 * | 12.06 ± 3.77 ** |
| Group | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) |
|---|---|---|---|---|
| NC | 2.60 ± 0.24 | 0.91 ± 0.08 | 2.76 ± 0.34 | 1.67 ± 0.20 |
| MC | 5.59 ± 0.22 ## | 2.01 ± 0.06 ## | 1.91 ± 0.27 ## | 3.60 ± 0.23 ## |
| PC | 2.83 ± 0.07 ** | 1.21 ± 0.05 ** | 2.87 ± 0.19 ** | 1.79 ± 0.22 ** |
| L-NAO | 5.39 ± 0.13 | 1.94 ± 0.09 | 2.01 ± 0.07 | 3.52 ± 0.45 |
| M-NAO | 5.04 ± 0.14 * | 1.87 ± 0.06 * | 2.03 ± 0.41 | 3.08 ± 0.32 * |
| H-NAO | 4.60 ± 0.28 ** | 1.35 ± 0.05 ** | 2.33 ± 0.18 * | 2.91 ± 0.32 * |
| Groups | MDA (nmol/g Protein) | GSH-Px (U/mg Protein) | SOD (U/mg Protein) |
|---|---|---|---|
| NC | 71.37 ± 0.96 | 13.02 ± 0.47 | 82.48 ± 1.85 |
| MC | 92.01 ± 5.68 ## | 9.65 ± 0.26 ## | 52.37 ± 2.85 ## |
| PC | 75.54 ± 3.82 ** | 12.34 ± 0.43 ** | 75.84 ± 1.41 ** |
| L-NAO | 89.83 ± 3.82 | 10.18 ± 0.42 * | 55.02 ± 2.53 |
| M-NAO | 85.41 ± 4.67 * | 10.82 ± 0.31 ** | 60.62 ± 1.34 ** |
| H-NAO | 79.61 ± 2.32 ** | 11.41 ± 0.58 ** | 65.54 ± 2.88 ** |
| Constituents | gm% | kcal% |
|---|---|---|
| Protein | 24 | 20 |
| Carbohydrate | 41 | 35 |
| Fat | 24 | 45 |
| Ingredients | gm | kcal |
| Casein, 30 Mesh | 200 | 800 |
| L-Cystine | 3 | 12 |
| Corn Starch | 72.8 | 271 |
| Maltodextrin 10 | 100 | 400 |
| Sucrose | 172.8 | 691 |
| Cellulose, BW200 | 50 | 0 |
| Soybean Oil | 25 | 225 |
| Lard | 177.5 | 1598 |
| Mineral Mix S10026 | 10 | 0 |
| DiCalcium Phosphate | 13 | 0 |
| Calcium Carbonate | 5.5 | 0 |
| Potassium Citrate, 1 H2O | 16.5 | 0 |
| Vitamin Mix V10001 | 10 | 40 |
| Choline Bitartrate | 2 | 0 |
| FD&C Red Dye #40 | 0.05 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.; Yang, D.; Huang, Y.; Zhao, Y.; Ye, J.; Xiao, M. The Potential of Neoagaro-Oligosaccharides as a Treatment of Type II Diabetes in Mice. Mar. Drugs 2019, 17, 541. https://doi.org/10.3390/md17100541
Lin F, Yang D, Huang Y, Zhao Y, Ye J, Xiao M. The Potential of Neoagaro-Oligosaccharides as a Treatment of Type II Diabetes in Mice. Marine Drugs. 2019; 17(10):541. https://doi.org/10.3390/md17100541
Chicago/Turabian StyleLin, Fudi, Dongda Yang, Yayan Huang, Yan Zhao, Jing Ye, and Meitian Xiao. 2019. "The Potential of Neoagaro-Oligosaccharides as a Treatment of Type II Diabetes in Mice" Marine Drugs 17, no. 10: 541. https://doi.org/10.3390/md17100541
APA StyleLin, F., Yang, D., Huang, Y., Zhao, Y., Ye, J., & Xiao, M. (2019). The Potential of Neoagaro-Oligosaccharides as a Treatment of Type II Diabetes in Mice. Marine Drugs, 17(10), 541. https://doi.org/10.3390/md17100541