Ciguatera in Mexico (1984–2013)
Abstract
1. Introduction
1.1. Historical Records
1.2. Recent Cases
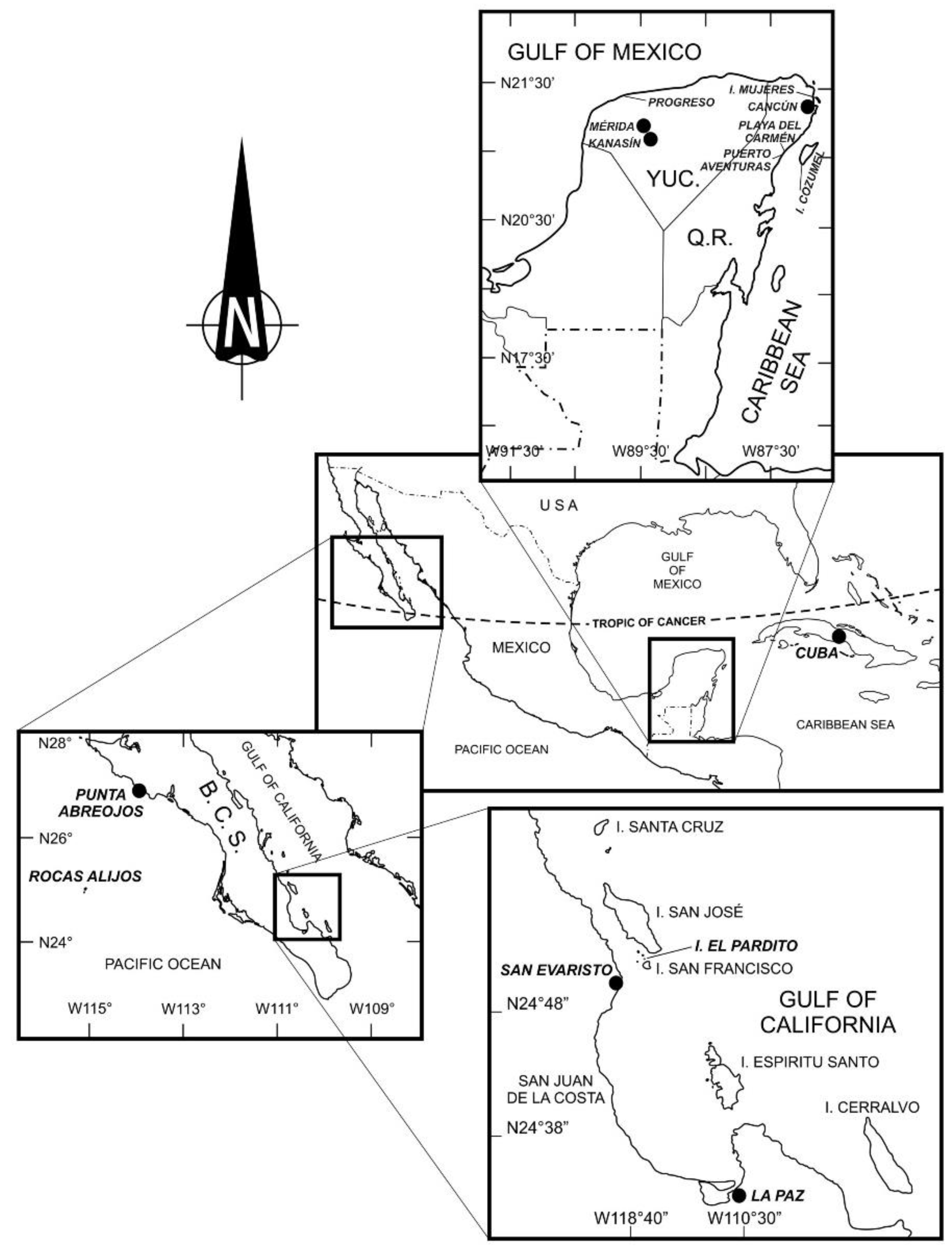
1.2.1. Ciguatera in the Mexican Pacific
1.2.2. Ciguatera in the Mexican Atlantic (Gulf of Mexico and Caribbean)
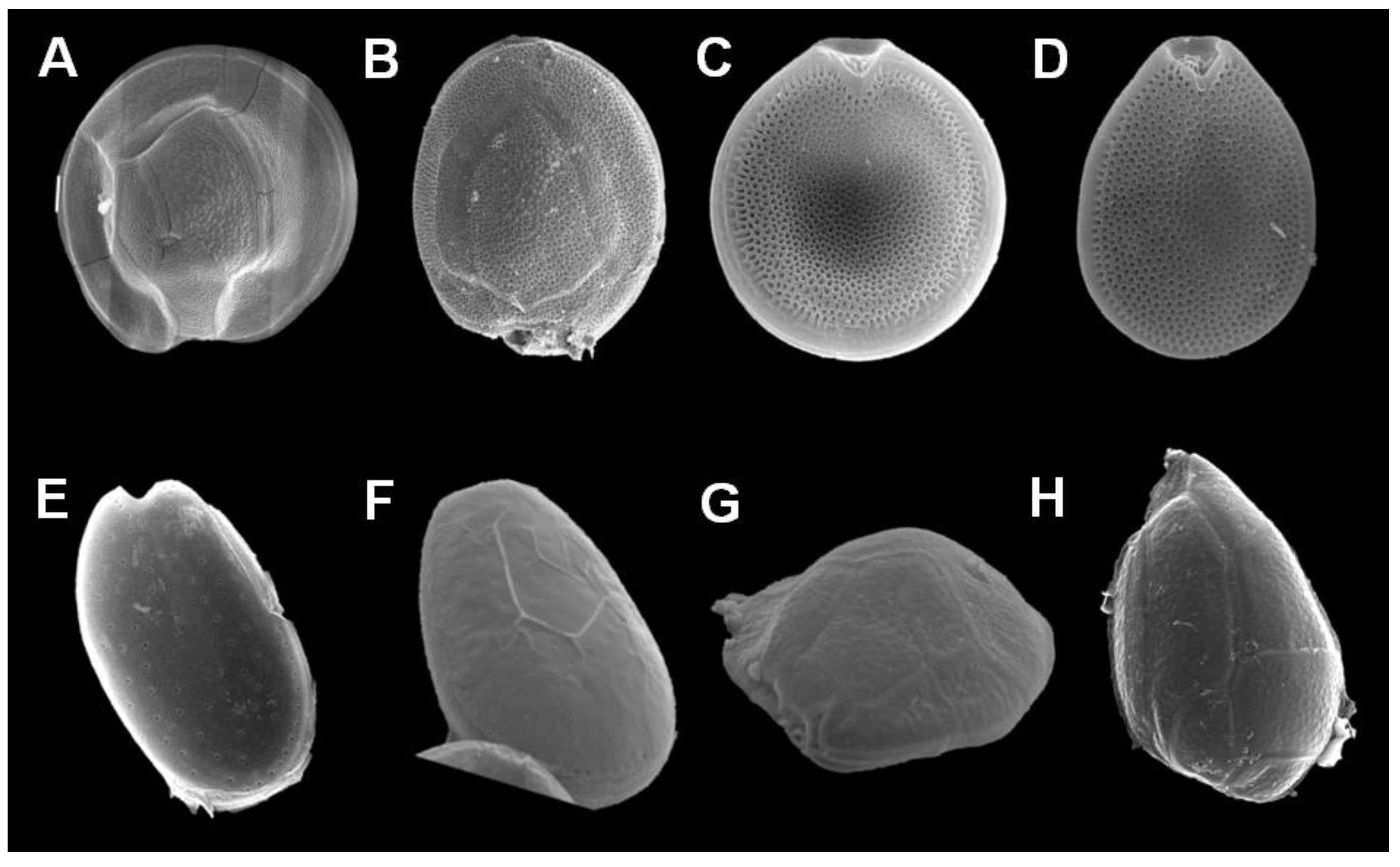
1.3. Dinoflagellate Species Associated with CFP
1.4. Fish Species Involved in CFP
1.5. Marine Toxins
1.5.1. Dinoflagellate Toxins
1.5.2. Fish Toxins
1.6. Ecological Risk
1.7. Legislation, Management, and Prevention
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yasumoto, T.; Satake, M. Chemistry, etiology and determination methods of Ciguatera toxins. J. Toxicol.-Toxin Rev. 1996, 15, 91–107. [Google Scholar] [CrossRef]
- Yasumoto, T. The Chemistry and Biological function of natural marine toxins. Chem. Rec. 2001, 1, 228–242. [Google Scholar] [CrossRef]
- Fleming, L.E.; Baden, D.G.; Bean, J.A.; Weisman, R.; Blythe, D.G. Seafood toxin diseases: Issues in epidemiology and community outreach. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta Galicia and Intergovernmental Oceanographic Commission of UNESCO: Vigo, Spain, 1998; pp. 245–248. [Google Scholar]
- Lehane, L.; Lewis, R. Ciguatera, recent advances but the risk remains. Int. J. Food Microbiol. 2000, 36, 1515–1518. [Google Scholar] [CrossRef]
- FAO. Marine Biotoxins; FAO Food and Nutrition paper 80; Organization of the United Nations: Rome, Italy, 2005. [Google Scholar]
- Friedman, M.A.; Fleming, L.E.; Fernández, M.; Bienfang, P.; Schrak, K.; Dickey, R.; Bottien, M.-Y.; Backer, L.; Ayyar, R.; Weisman, R.; et al. Ciguatera Fish Poisoning: Treatment, Prevention and Management. Mar. Drugs 2008, 6, 456–479. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.W. Ciguatera Fish Poisoning. Am. Fam. Phys. 1994, 50, 579–584. [Google Scholar]
- Legrand, M. Ciguatera toxins: Origin, transfer through the food chain and toxicity humans. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta Galicia and Intergovernmental Oceanographic Commission of UNESCO: Vigo, Spain, 1998; pp. 39–43. [Google Scholar]
- Holmes, M.J.; Lewis, R. The Origin of Ciguatera. Mem. Qld. Mus. Brisb. 1994, 34, 497–504. [Google Scholar]
- Hokama, Y.; Yoshikawa-Ebesu, J.S.M. Ciguatera Fish Poisoning: A Foodborne Disease. J. Toxicol.-Toxin Rev. 2001, 20, 85–139. [Google Scholar] [CrossRef]
- Adachi, R.; Fukuyo, Y. The thecal plate structure of a marine toxic dinoflagellate Gambierdiscus toxicus gen. et sp. nov. collected in a ciguatera endemic area. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 67–71. [Google Scholar] [CrossRef]
- Faust, M. Observation of sand-dwelling toxic dinoflagellates (Dinophyceae) from widely differing sites, including two new species. J. Phycol. 1995, 31, 996–1003. [Google Scholar] [CrossRef]
- Holmes, M.J. Gambierdiscus yasumotoi, sp. nov. (Dinophyceae), a toxic benthic dinoflagellate from Southeastern Asia. J. Phycol. 1998, 34, 661–668. [Google Scholar] [CrossRef]
- Chinain, M.; Faust, M.A.; Pauillac, S. Morphology and molecular analysis of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol. 1999, 35, 1282–1296. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Chinain, M.; Holmes, M.J.; Holland, W.C.; Tester, P. Taxonomy of Gambierdiscus: Including four new species, Gambierdiscus caribaeus sp. nov., Gambierdiscus carolinianus sp. nov., Gambierdiscus carpentei sp. nov. and Gambierdiscus ruetzleri sp. nov. (Gonyaulacales Dinophyceae). Phycologia 2009, 48, 344–390. [Google Scholar] [CrossRef]
- Fraga, S.; Rodríguez, F.; Caillau, A.; Diogène, J.; Raho, N.; Zapata, M. Gambierdiscus excentricus sp. nov (Dinpophyceae), a benthic toxic dinoflagellate from Canary Islands (NE Atlantic Ocean). Harmful Algae 2010, 11, 10–22. [Google Scholar] [CrossRef]
- Fraga, S.; Rodríguez, F. Genus Gambierdiscus in the Canary Islands (NE Atlantic Ocean) with description of Gambierdiscus silvae sp. nov., a new potentially toxic epiphytic benthic dinoflagellate. Protist 2014, 125, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Sato, S.; Tawong, W.; Sakanari, H.; Yamaguchi, H.; Adachi, M. Morphology of Gambierdiscus scabrosus sp. nov. (Gonyaulacales): A new epiphytic dinoflagellate from coastal areas of Japan. J. Phycol. 2014, 50, 506–514. [Google Scholar] [CrossRef]
- Fraga, S.; Rodríguez, F.; Riobó, P.; Bravo, I. Gambierdiscus balechii sp. nov (Dinophyceae), a new benthic toxic dinoflagellate from the Celebes Sea (SW Pacific Ocean). Harmful Algae 2016, 58, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.F.; Rhodes, L.; Verma, A.; Curley, B.G.; Harwood, D.; Kohli, G.S.; Solomona, D.; Rongo, T.; Munday, R.; Murray, S.A. A new Gambierdiscus species (Dinophyceae) from Rarotonga, Cook Islands: Gambierdiscus cheloniae sp. nov. Harmful Algae 2016, 60, 45–56. [Google Scholar] [CrossRef]
- Kretzschmar, A.L.; Verma, A.; Harwood, T.; Hoppenrath, M.; Murray, S. Characterization of Gambierdiscus lapillus sp. nov. (Gonyaulacales, Dinophyceae): A new toxic dinoflagellate from the Great Barrier Reef (Australia). J. Phycol. 2017, 53, 283–297. [Google Scholar] [CrossRef]
- Tosteson, T.R. The diversity and origins of toxins in ciguatera fish poisoning. PRHSJ 1995, 14, 117–129. [Google Scholar]
- Lewis, R.; Holmes, M. Origin and transfer of toxins involved in Ciguatera. Comp. Biochem. Physiol. 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Halstead, B.W. Poisonous and Venomous Marine Animals of the World, Vol. II; U.S. Government Printing Office: Washington, DC, USA, 1967; 1070p.
- Núñez-Vázquez, E.J. Biotoxinas Marinas en peces comestibles de Baja California Sur: Origen, Identificación y Cuantificación. Bachelor’s Thesis, Universidad Autónoma de Baja California Sur, La Paz, Mexico, 2005. [Google Scholar]
- Núñez-Vázquez, E.J.; Almazán-Becerril, A.; Heredia-Tapia, A.; Hernández-Becerril, D.U.; Troccoli-Ghinaglia, L.; Arredondo-Vega, B.O.; Herrera-Silveira, J.A.; Vázquez-Castellanos, J.L.; Ochoa, J.L. Incidencia del envenenamiento por Ciguatera en Mexico. In 4ª Reunión de expertos en envenenamientos por animales ponzoñosos; Instituto de Biotecnología-Universidad Nacional Autónoma de Mexico, International Society Toxinology-Panamerican Section, Inst. Bioclon, Lab. Silanes: Cuernavaca, Mexico, 2000; pp. 56–57. [Google Scholar]
- Núñez-Vázquez, E.J.; Ochoa, J.L.; Band-Schmidt, C.J.; Gárate-Lizárraga, I.; Heredia-Tapia, A.; López-Cortés, A.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J. Ciguatera in Mexico. In Proceedings of the Abstracts of the 13th International Conference on Harmful Algae, Hong Kong, China, 3–7 November 2008; p. 98. [Google Scholar]
- Núñez-Vázquez, E.J.; Bustillos-Guzmán, J.; Heredia-Tapia, A.; Yasumoto, T.; Cruz-Villacorta, A.; Band-Schmidt, C.J.; Gárate-Lizárraga, I.; López-Cortés, D.; Hernández-Sandoval, F.E.; Ochoa, J.L. Múltiples toxinas marinas en el hígado de Mycteroperca prionura, M. rosacea y Lutjanus colorado asociados a la ciguatera en la isla El Pardito, B.C.S., México. In Resúmenes del III Taller sobre Florecimientos Algales Nocivos: Integración del conocimiento sobre eventos de FAN en México; Laboratorio Estatal de Salud Pública Dr. Galo Soberón y Parra, Secretaría de Salud México: Acapulco, Mexico, 2009; p. 52. [Google Scholar]
- Parrilla-Cerrillo, M.C.; Vázquez-Castellanos, J.L.; Sáldate-Castañeda, E.O.; Nava-Fernández, L.M. Brotes de toxiinfecciones alimentarias de origen microbiano y parasitario. Salud Pública Mex. 1993, 35, 456–463. [Google Scholar] [PubMed]
- Barton, E.D.; Tanner, P.; Turchen, S.G.; Tungen, C.L.; Manoguerra, A.; Clarck, R.F. Ciguatera fish poisoning: A Southern California epidemic. West J. Med. 1995, 163, 31–35. [Google Scholar] [PubMed]
- Lechuga-Devéze, C.; Sierra-Beltrán, A. Documented case of ciguatera on the Mexican Pacific Coast. Nat. Toxins 1995, 3, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Vázquez, E.; Sierra-Beltrán, A.; Cruz-Villacorta, A.; Ochoa, J.L. Ciguatoxins on Serranidae and Lutjanidae fish of Baja California Sur, Mexico. Toxicon 1998, 36, 1224. [Google Scholar]
- Arcila-Herrera, H.; Castello-Navarrete, A.; Mendoza-Ayora, J.; Montero-Cervantes, L.; González-Franco, M.; Brito-Villanueva, O.W. Diez casos de ciguatera en Yucatán. Rev. Investig. Clín. 1998, 50, 149–152. [Google Scholar]
- De Haro, L.; Hayek-Lanthois, M.; Joosen, F.; Pes, P.; Castanier, L.; Jouglard, J. Medical management in the Marseilles Poison Centre o a ciguatera poisoning collective case after a meal of one barracuda in Mexico. Toxicon 1997, 35, 810. [Google Scholar] [CrossRef]
- De Haro, L.; Hayek-Lanthois, M.; Joossen, F.; Affaton, M.-F.; Jouglard, J. Intoxication collective ciguaterique apres ingestion d´ un barracuda au Mexique: Deductions pron ostique et therapeutique. Med. Trop. 1997, 57, 55–58. [Google Scholar]
- Chávez-Peón, F. Reporte de 21 casos de Ciguatera en Yucatán. CENIDS-Secretaria de Salubridad y Asistencia; Dirección General de Epidemiología: Ciudad de México, México, 1997; pp. 1–2. [Google Scholar]
- Quiñones-Vega, C.M. Intoxicación por ciguatera en Yucatán. Boletín epidemiológico; Hospital General “Agustín O’Horán” Urgencias Pediátricas: Mérida, México, 2000; pp. 3–6. [Google Scholar]
- Farstad, D.J.; Chow, T. A brief case report and review of ciguatera poisoning. Wild. Environ. Med. 2001, 12, 263–269. [Google Scholar] [CrossRef]
- Keynan, Y.; Pottesman, I. Neurological symptoms in a traveler returning from Central America. J. Intern. Med. 2004, 256, 174–175. [Google Scholar] [CrossRef]
- Slobbe, L.; van Genderen, P.J.J.; Wismans, P.J. Two patients with ciguatera toxicity: A seafood poisoning in travellers to (sub) tropical areas. Neth. J. Med. 2008, 66, 389–391. [Google Scholar]
- Montesano-Castellanos, R.; Galindo-Rodríguez, C. Flores-Dinoris. Intoxicación por ciguatera: Informe de un brote en turistas mexicanos y revisión bibliográfica. Arch. Neurocien. 1996, 1, 124–129. [Google Scholar]
- Gatti, C.; Oelher, E.; Legrand, A.M. Severe seafood poisoning in French Polynesia: A retrospective analysis of 129 medical files. Toxicon 2008, 51, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Boucaud-Maitre, D.; Vernoux, J.P.; Pelczar, S.; Daudens-Vaysee, E.; Aubert, L.; Boa, S.; Ferraci, S.; Garnier, R. Incidence and clinical characteristics of ciguatera fish poisoning in Guadeloupe (French West Indies) between 2013 and 2016: A retrospective cases-series. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Gordon, C.J.; Ramsdell, J.S. Effects of marine algal toxins on thermoregulation in mice. Neurotoxicol. Teratol. 2005, 27, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, P.R.; Jollow, D.J.; Scheuer, P.J.; York, R.; McMillan, J.P.; Withers, N.W.; Fudenberg, H.H.; Higerd, T.B. Effect of Ciguatera-Associated Toxins on Body Temperature in Mice. In Seafood Toxins; Ragelis, E.P., Ed.; American Chemical Society: Washington, DC, USA, 1984; Volume 262, pp. 321–329. [Google Scholar] [CrossRef]
- Babinchak, J.A.; Moeller, P.D.R.; Van Dolah, F.M.; Eyo, P.B.; Ramsdell, J.S. Productions of ciguatoxins in cultured Gambierdiscus toxicus. Mem. Qld. Mus. 1994, 34, 447–453. [Google Scholar]
- Tindall, D.; Tindall, P. Tropical Dinoflagellates. Living Collections. Southern Illinois University, Carbondale. 1997. Available online: http//www.siu.edu/~dinos/collect.htm (accessed on 10 December 1997).
- Troccoli-Ghinaglia, L.; Herrera-Silviera, J.A. Fitoplancton e hidrografía en una zona costera con descargas de agua subterránea. In Resúmenes de la X Reunión Nacional de la Sociedad Mexicana de Planctología, A. C. y III Reunión Internacional de Planctología; Mazatlán, Sinaloa, México, 1999; SOMPAC: Mazatlán, México, 1999; p. 26. [Google Scholar]
- Almazán-Becerril, A. Estudio taxonómico de algunos dinoflagelados potencialmente tóxicos en el Caribe Mexicano. Master’s Thesis, Universidad Nacional Autónoma de Mexico, Mexico City, Mexico, 2000. [Google Scholar]
- Hernández-Becerril, D.U.; Almazán-Becerril, A. Especies de dinoflagelados del género Gambierdiscus (Dinophyceae) del Mar Caribe Mexicano. Rev. Biol. Trop. 2004, 52 (Suppl. 1), 77–87. [Google Scholar] [PubMed]
- Okolodkov, Y.B.; Campos-Bautista, G.; Gárate-Lizárraga, I.; González-González, J.A.G.; Hoppenrath, M.; Arenas, V. Seasonal changes of benthic and epiphytic dinoflagellates in the Veracruz reef zone, Gulf of Mexico. Aquat. Microb. Ecol. 2007, 47, 223–237. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Merino-Virgilio, F.C.; Herrera-Silveira, J.A.; Espinosa-Matías, S.; Parsons, M.R. Gambierdiscus toxicus in the southeastern Gulf of Mexico. Harmful Algal News 2009, 40, 12–14. [Google Scholar]
- Mier y Terán-Suárez, J.M.; Castro, G.V.; Mayor-Nucamendi, H.F.; Brito-López, J.A. Florecimientos Algales en Tabasco. Salud en Tabasco 2006, 12, 414–422. [Google Scholar]
- Poot-Delgado, C.A.; Rosado-García, P. Fitoplancton marino potencialmente nocivo en las aguas costeras de Champotón, Campeche. In Proceedings of the Memorias del XX Congreso Nacional de Ciencia y Tecnología del Mar, Los Cabos, México, 1–5 October 2013; Dirección General de Ciencia y Tecnología del Mar, Secretaría de Educación Pública: Ciudad de México, México, 2013; pp. 1–11. [Google Scholar]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibbler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of Ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef]
- Martinez-Cruz, M.E.; Okolodkov, Y.B.; Aguilar-Trujillo, C.; Herrera-Silveira, J.A. Epiphytic dinoflagellates on the seagrass Thalassia testudinum at Dzilam, southeastern Gulf of Mexico. Cymbella 2015, 1, 2–9. [Google Scholar]
- Almazán-Becerril, A.; Escobar-Morales, S.; Rosiles-González, G.; Valadez, F. Benthic epiphytic dinoflagellates from the northen portion of the Mesoamerican Reff System. Bot. Mar. 2015, 58, 115–128. [Google Scholar] [CrossRef]
- Almazán-Becerril, A.; Irola-Sansores, E.D.; Escobar-Morales, S. El género Gambierdiscus de Quintana Roo, Mexico. In Florecimientos Algales Nocivos en Mexico; García-Mendoza, E., Quijano-Scheggia, S.I., Olivos-Ortíz, A., Núñez-Vázquez, E.J., Eds.; CICESE: Ensenada, Mexico, 2016; pp. 366–377. ISBN 978-607-95688-5-6. [Google Scholar]
- Holland, W.C.; Litaker, R.W.; Tomas, C.R.; Kibler, S.R.; Place, A.R.; Davenport, E.D.; Tester, P.A. Differences in the toxicity of six Gambierdiscus (Dinophyceae) species measured using an in vitro human erythrocyte lysis assay. Toxicon 2013, 65, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, F.; Sibat, M.; Herrenknecht, C.; Lhaute, K.; Gaiani, G.; Ferron, P.-J.; Fessard, V.; Fraga, S.; Nascimento, S.M.; Litaker, W.; et al. Maitotoxin-4, a novel MTX analog produced by Gambierdiscus excentricus. Mar. Drugs 2017, 15, 220. [Google Scholar] [CrossRef]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef] [PubMed]
- Sierra, B.A.; Cruz, A.; Núñez-Vázquez, E.J.; Del Villar, L.M.; Cerecero, J.; Ochoa, J.L. An overview of the marine food poisoning in Mexico. Toxicon 1998, 36, 1493–1502. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Gárate-Lizárraga, I. An annoted checklist of dinoflagellates (Dinophyceae) from the Mexican Pacific. Acta Bot. Mex. 2006, 72, 1–154. [Google Scholar] [CrossRef]
- Gárate-Lizárraga, I.; Band-Schmidt, C.J.; Verdugo-Díaz, G.; Muñetón-Gómez, M.J.; Félix-Pico, E. Dinoflagelados (Dinophyceae) del sistema lagunar Magdalena-Almejas. In Estudios Ecológicos en Bahía Magdalena; Funes-Rodríguez, R., Gómez-Gutiérrez, J., Palomares-García, R., Eds.; Gobierno del Estado de B. C. S., Secretaria de Turismo de B. C. S., Fondo para la Protección de los Recursos Marinos de B. C. S., Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas: La Paz, México, 2007; pp. 145–174. [Google Scholar]
- Cortés-Altamirano, R. Two new localities for Gambierdiscus toxicus in the Mexican Pacific. Harmful Algae News 2012, 45, 10–11. [Google Scholar]
- Garate-Lizarraga, I.; Okolodkov, Y.B.; Cortés-Altamirano, R. Microalgas formadoras de florecimientos algales en el Golfo de California. In Florecimientos Algales Nocivos en Mexico; García-Mendoza, E., Quijano-Scheggia, S.I., Olivos-Ortíz, A., Núñez-Vázquez, E.J., Eds.; CICESE: Ensenada, México, 2016; pp. 130–145. ISBN 978-607-95688-5-6. [Google Scholar]
- Osorio-Tafall, B.F. El Mar de Cortés y la productividad fitoplanctónica de sus aguas. Anales de la Escuela Nacional de Ciencias Biológicas 1942, 3, 73–118. [Google Scholar]
- Gilmartin, M.; Revelante, N. The phytoplankton characteristics of the barrier island lagoons of the Gulf of California. Estuar. Coast. Mar. Sci. 1978, 7, 29–47. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U. Especies del fitoplancton tropical del Pacífico mexicano. II. Dinoflagelados y cianobacterias. Rev. Latinam. Microbiol. 1987, 30, 187–196. [Google Scholar]
- Hernández-Becerril, D.U.; Vázquez-Martínez, A. Fitoplancton en aguas costeras de Quinta Roo: Composición y Distribución. Memorias del V Congreso Latinoamericano de Ciencias del Mar, La Paz, México, 27 September–1 October 1993; COLACMAR: La Paz, México, 1993; p. 96. [Google Scholar]
- Licea, S.; Moreno, J.L.; Santoyo, H.; Figueroa, G. Dinoflageladas del Golfo de California; UABCS-FOMES-SEP-PROMARCO: La Paz, Mexico, 1996. [Google Scholar]
- Gárate-Lizárraga, I.; Martínez-López, A. Primer registro de una marea roja de Prorocentrum mexicanum (Prorocentraceae) en el Golfo de California. Rev. Biol. Trop. 1997, 45, 1263. [Google Scholar]
- Almazán-Becerril, A.; Hernández-Becerril, D.U. Toxic and potentially toxic dinoflagellates from the Mexican Caribbean Sea. In Proceedings of the Abstracts of the ninth Intenational Conference on Harmful Algal Blooms, Hobart, Tasmania, Australia, 7–11 February 2000; p. 111. [Google Scholar]
- Popowsky, G.; Alvarez-Cadena, J.N.; Delgado, G.; Sánchez, M. Inventario de la microflora fitoplantónica de la laguna Bojorquez, Caribe Mexicano. Rev. Investig. Mar. 2002, 23, 173–178. [Google Scholar]
- Heredia-Tapia, A.; Arredondo-Vega, B.O.; Núñez-Vázquez, E.J.; Yasumoto, T.; Yasuda, M.; Ochoa, J.L. Isolation of Prorocentrum lima (Syn. Exuviaella lima) and diarrhetic shellfish poisoning (DSP) risk assessment in the Gulf of California, Mexico. Toxicon 2002, 40, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Altamirano, R.; Sierra-Beltrán, A.P. Morphology and taxonomy of Prorocentrum mexicanum and reinstatement of Prorocentrum rhathymum (Dinophyceae). J. Phycol. 2003, 39, 221–225. [Google Scholar] [CrossRef]
- Cortés-Lara, M.C.; Cortés-Altamirano, R.; Sierra-Beltrán, A.; Reyes-Juárez, A. Ostreopsis siamensis (Dinophyceae) a new tychoplanktonic record from Isabel Island National Park, Pacific Mexico. Harmful Algal News 2006, 28, 4–5. [Google Scholar]
- Zepeda-Esquivel, M.A.; Resendíz-Flores, M.I.; Morales-Zamorano, L.A. Identificación de Ostreopsis sp en la columna de agua de Bahía Azufre en la Isla Clarión. In Resúmenes del II Taller sobre Florecimientos Algales Nocivos; CICESE: Ensenada, México, 2007; p. 11. [Google Scholar]
- Okolodkov, Y.B.; Merino-Virgilio, F.C.; Aké-Castillo, J.A.; Aguilar-Trujillo, A.C.; Espinosa-Matias, S.; Herrera-Silveira, J.A. Seasonal changes in epiphytic dinoflagellates assemnlages near the northern coast of the Yucatan Peninsula, Gulf of Mexico. Acta Bot. Mex. 2014, 107, 121–151. [Google Scholar] [CrossRef]
- Aguilar-Trujillo, A.C.; Okolodkov, Y.B.; Herrera-Silveira, J.A.; Merino-Virgilio, F.C.; Galicia-García, C. Taxocoenosis of epibenthic dinoflagellates in coastal waters of the northern Yucatan Peninsula before and after the harmful algal bloom event in 2011–2012. Mar. Pollut. Bull. 2017, 119, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Becerril, A.; Escobar-Morales, S.; Irola-Sansores, E.D.; Delgado-Pech, B. Morfología y taxonomía de los géneros Ostreopsis y Coolia en el Caribe Mexicano. In Florecimientos Algales Nocivos en Mexico; García-Mendoza, E., Quijano-Scheggia, S.I., Olivos-Ortíz, A., Núñez-Vázquez, E.J., Eds.; CICESE: Ensenada, Mexico, 2016; pp. 378–393. ISBN 978-607-95688-5-6. [Google Scholar]
- Gárate-Lizárraga, I.; González-Armas, R.; Okolodkov, Y.B. Ocurrence of Ostreopsis lenticualris (Dinophyceae:Gonyaulacales) from the Archipiélago de Revillagigedo, Mexican Pacific. Mar. Pollut. Bull. 2018, 128, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Babinchak, J.A.; Doucette, G.J.; Ball, R.M. Partial characterization of the LSU rRNA gene from the ciguatoxic dinoflagellate. Gambierdiscus toxicus. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; IOC-UNESCO: Vigo, Spain, 1996; pp. 459–462. [Google Scholar]
- Richlen, M.L.; Morton, S.L.; Barber, P.H.; Lobel, P.S. Phylogeography, morphological variation and taxonomy of the toxic dinoflagellate Gambierdiscus toxicus (Dinophyceae). Harmful Algae 2008, 7, 614–629. [Google Scholar] [CrossRef]
- Irola-Sansores, E.D. Dinámica poblacional de los dinoflagelados bentónicos em dos géneros de macroalgas: Dictyota y Amphiroa em dos sistemas arrecifales de norte de Quintana Roo. Master’s Thesis, Centro de Investigación Científica de Yucatán, Cancún, México, 2016. [Google Scholar]
- Colección de Dinoflagelados Marinos (CODIMAR). Available online: https://www.cibnor.gob.mx/investigacion/colecciones-biologicas/codimar (accessed on 12 October 2018).
- Diario Oficial de la Federación. Carta Nacional Pesquera; Secretaria de Agricultura, Ganaderia, Desarrollo Rural, Pesca y Alimentación (SAGARPA): Ciudad de México, México, 2006. [Google Scholar]
- Castro-González, M.I.; Ojeda, V.A.; Montaño, B.S.; Ledesma, C.E.; Pérez-Gil, R.F. Evaluación de los ácidos grasos n-3 de 18 especies de pescados marinos mexicanos como alimentos funcionales. Archiv. Latinoam. Nutrición 2007, 57, 1–10. [Google Scholar]
- Pottier, I.; Vernoux, J.P.; Lewis, R. Ciguatera Fish Poisoning in the Caribbean islands and Western Atlantic. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 2001; Volume 168, pp. 99–141. ISBN 978-1-4613-0143-1. [Google Scholar]
- Lewis, R.L. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar] [CrossRef]
- Núñez-Vázquez, E.J.; Heredia-Tapia, A.; Pérez-Urbiola, J.C.; Alonso-Rodríguez, R.; Arellano-Blanco, J.; Cordero-Tapia, A.; Pérez-Linares, J.; Ochoa, J.L. Evaluation of dinoflagellate toxicity implicated in recent HAB events in the Gulf of California, Mexico. In Proceedings of the HABTech 2003, APEC. A Workshop on Technologies for Monitoring of Harmful Algal Blooms and Marine Biotoxins, Cawtron Report No. 906, Nelson, New Zealand, 26–30 November 2003; p. 64. [Google Scholar]
- Gamboa, P.M.; Parck, D.L.; Fremy, J.M. Extraction and purification of toxic fractions from barracuda (Sphyraena barracuda) implicated in ciguatera poisoning. In Proceedings of the Third International Conference on Ciguatera Fish Poisoning, Puerto Rico; Tosteson, T.R., Ed.; Polyscience Publications: Laval, QC, Canada, 1992; pp. 13–24. [Google Scholar]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Lewis, R.L.; Sellin, M.; Poli, M.A.; Norton, R.S.; Mac Leod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- Lewis, R.J.; Norton, R.S.; Brereton, I.M.; Eccles, C.D. Ciguatoxin-2 is a diastereomer of ciguatoxin-3. Toxicon 1993, 31, 637–643. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. The structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdiscus toxicus. Tetrahedron Lett. 1993, 34, 1975–1978. [Google Scholar] [CrossRef]
- Lewis, R.; Vernoux, J.-P.; Brereton, I.M. Structure of Caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc. 1998, 120, 5914–5920. [Google Scholar] [CrossRef]
- Satake, M.; Ishibashi, Y.; Legrand, A.M.; Yasumoto, T. Isolation and structure of ciguatoxin-4A, a new ciguatoxin precursor, from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem. 1997, 60, 2103–2105. [Google Scholar] [CrossRef]
- Alcala, A.C.; Alcala, L.C.; Garth, J.S.; Yasumura, D.; Yasumoto, T. Human fatality due to ingestion of the crab Demania reynaudii that contained a palytoxin-like toxin. Toxicon 1988, 26, 105–107. [Google Scholar] [CrossRef]
- Kodama, A.M.; Hokama, Y.; Yasumoto, T.; Fukui, M.; Manea, S.J.; Sutherland, N. Clinical and laboratory findings implicating palytoxin as cause of ciguatera poisoing due to Decapterus macrosoma (mackerel). Toxicon 1989, 27, 1051–1053. [Google Scholar] [CrossRef]
- Ito, E.; Ohkusu, M.; Yasumoto, T. Intestinal injuries caused by experimental palytoxicosis in mice. Toxicon 1996, 34, 643–652. [Google Scholar] [CrossRef]
- Ito, E.; Ohkusu, M.; Terao, K.; Yasumoto, T. Effects of repeated injections of palytoxin on lymphoid tissues in mice. Toxicon 1997, 35, 679–688. [Google Scholar] [CrossRef]
- Onuma, Y.; Satake, M.; Ukena, T.; Roux, J.; Chanteau, S.; Rasolofonirina, N.; Ratsimaloto, M.; Naoki, H.; Yasumoto, T. Identification of putative palytoxin as the cause clupeotoxism. Toxicon 1999, 37, 55–65. [Google Scholar] [CrossRef]
- Wiles, J.S.; Vick, J.A.; Christensen, M.K. Toxicological evaluation of palytoxin in several animal species. Toxicon 1974, 12, 427–433. [Google Scholar] [CrossRef]
- Fukui, M.; Murata, M.; Inou, A.; Gawel, M.; Yasumoto, T. Occurrence of palytoxin in a trigger fish Melichtys vidua. Toxicon 1987, 25, 1121–1124. [Google Scholar] [CrossRef]
- Moore, A.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climatic variability and future climate change on harmful algal blooms ans human health. Environ. Health 2008, 7 (Suppl. 2), 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ruff, T.A. Ciguatera in the Pacific: A link with military activities. Lancet 1989, 8631, 201–205. [Google Scholar] [CrossRef]
- Kholer, S.T.; Kholer, C.C. Dead bleached coral provides new surfaces dinoflagellates implicated in ciguatera fish poisonings. Environ. Biol. Fish. 1992, 35, 413–416. [Google Scholar] [CrossRef]
- Hales, S.; Weinstein, P.; Woodward, A. Ciguatera (fish poisoning), El Niño, and Pacific Sea surface temperature. Ecosyst. Health 1999, 5, 20–25. [Google Scholar] [CrossRef]
- Hales, S.; Kowats, S.; Woodward, A. What El Niño can tell us about human health and global climate change. Glob. Chance Hum. Health 2000, 1, 66–77. [Google Scholar] [CrossRef]
- Lehane, L. Ciguatera update. Med. J. Aust. 2000, 172, 176–179. [Google Scholar] [PubMed]
- Villarreal, T.; Hanson, S.; Qualia, S.; Jester, E.L.E.; Granade, H.R.; Dickey, R.W. Petroleum production platforms as sites for the expansion of ciguatera in northwestern Gulf of Mexico. Harmful Algae 2007, 6, 253–259. [Google Scholar] [CrossRef]
- Herrera-Silveira, J.A.; Morales-Ojeda, S.M. Evaluation of the health status of a coastal ecosystem in southeast Mexico: Assessment of water quality, phytoplankton and submerged aquatic vegetation. Mar. Poll. Bull. 2009, 59, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadistica, Geografia e Informatica (INEGI), 2005–2007. Available online: http://www.inegi.org.mx/est/contenidos/espanol/sistemas/conteo2005/default.asp?c=6790 (accessed on 13 December 2018).
- Consejo Nacional de Población. Situación sociodemografica de las zonas costeras. In La situación demográfica de Mexico, 1999; CONAPO: Mexico City, Mexico, 1999; pp. 73–89. ISBN 970-628-397-8. [Google Scholar]
- Jordan-Dahlgren, E. Los Arrecifes coralinos del Golfo de Mexico: Caracterización y Diagnóstico. In Diagnóstico Ambiental del Golfo de Mexico; Caso, M., Pisanty, I., Escurra, E., Eds.; Instituto Nacional de Ecologia, Secretaria de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2004; pp. 555–572. [Google Scholar]
- Villanueva, G.E. El ciclón Liza: Historia de los huracanes en B.C.S; Universidad Autónoma de Baja California Sur: La Paz, Mexico, 2004; p. 227. ISBN 9688961418. [Google Scholar]
- Martínez-Gutiérrez, G.; Mayer, L. Huracanes en Baja California, Mexico y sus implicaciones en la sedimentación en el Golfo de California. GEOS 2004, 24, 57–64. [Google Scholar]
- Bagnis, R. Natural versus anthropogenic disturbance to coral reefs: Comparison in epidemiological patterns of ciguatera. Mem. Qld. Mus. 1994, 34, 455–460. [Google Scholar]
- Yasumoto, T.; Fujimoto, K.; Oshima, Y.; Inoue, A.; Ochi, T.; Fukuyo, Y. Environmental studies on a toxic dinoflagellate responsable for ciguatera. Bull. Jpn. Soc. Sci. Fish 1980, 46, 1397–1404. [Google Scholar] [CrossRef]
- Bomber, J.W.; Rubio, M.G.; Norris, D.R. Epiphytism of dinoflagellates associated with the disease ciguatera: Substrate specificity and nutrition. Phycologia 1989, 28, 360–368. [Google Scholar] [CrossRef]
- Parsons, M.L.; Preskitt, L.B. A survey of epiphytic dinoflagellates from the coastal water of the island of Hawaii. Harmful Algae 2007, 6, 658–669. [Google Scholar] [CrossRef]
- Herrera-Silveira, J.A.; Comin, F.A.; Aranda-Cirero, N.; Troccoli, L.; Capurro, L. Coastal water quality assessment in the Yucatan Peninsula: Management implications. Ocean Coast. Manag. 2004, 47, 625–639. [Google Scholar] [CrossRef]
- Tapia-Gonzalez, F.U.; Herrera-Silveira, J.A.; Aguirre-Macedo, M.L. Water quality variability and eutrophic trends in karstic tropical coastal lagoons of Yucatán Peninsula. Estuar. Coast. Shelf Sci. 2008, 76, 418–430. [Google Scholar] [CrossRef]
- Smayda, T.J. Eutrophication and phytoplankton. In Drainage Basin Nutrient Inputs and Eutrophication: An Integral Approach; Wassmann, P., Olli, K., Eds.; University of Tromsø: Tromsø, Norway, 2005; pp. 89–98. [Google Scholar]
- Convention on Wetlands of International Importance especially as Waterfowl Habitat. Ramsar (Iran). Mexico reaches 86 wetlands of International Importance. 2009. Available online: ramsar.conanp.gob.mx/sitios_ramsar.html (accessed on 13 December 2018).
- Lavín, M.F.; Beier, E.; Badan, A. Estructura hidrográfica y circulación del Golfo de California: Escalas estacional e interanual. In Contribuciones a la Oceanografía Física en Mexico; Lavín, M.F., Ed.; Unión Geofísica Mexicana Monografía No. 3: Ensenada, Mexico, 1997; pp. 141–171. ISBN 9687829001. [Google Scholar]
- Monreal-Gómez, M.A.; Molina-Cruz, A.; Salas-de León, D.A. Water masses and cyclonic circulation in Bay of La Paz, Gulf of California, during June 1988. J. Mar. Syst. 2001, 30, 305–315. [Google Scholar] [CrossRef]
- Obeso-Nieblas, M.; Shirasago-Germán, B.; Gaviño-Rodríguez, J.H.; Obeso-Huerta, H.; Pérez-Lezama, E.L. Jimenéz-Illescas. Hidrografía en la Boca Norte de la Bahía de La Paz, Baja California Sur, México. Cienc. Mar. 2007, 33, 281–291. [Google Scholar] [CrossRef]
- Cervantes-Duarte, R.; Guerrero-Godínez, R. Variación espacio-temporal de nutrientes de la Ensenada de La Paz, B.C.S. México. An. Inst. Cienc. del Mar y Limnol. UNAM 1987, 15, 129–142. [Google Scholar]
- Tosteson, T.R.; Ballantine, D.L.; Winter, A. Sea surface temperature, benthic dinoflagellate tocxicity and toxin transmission in the Ciguatera food web. In Harmul Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernamental Oceanographic Commission of UNESCO: Vigo, Spain, 1998; pp. 48–49. [Google Scholar]
- Glynn, P.W. Widespread coral mortality and the 1982/83 El Niño warming event. Environ. Conserv. 1984, 11, 133–146. [Google Scholar] [CrossRef]
- Wilkinson, C.; Lindén, O.; Cesar, H.; Hodgson, G.; Rubens, J.; Strong, A.E. Ecological and socioeconomic impacts of 1998 coral mortality in the Indian Ocean: An ENSO impact and a warning of future change? Ambio 1999, 28, 188–196. [Google Scholar]
- Goodlett, R.O.; Van Dolah, F.M.; Babinchak, J.A. Re-occurrence of a ciguatera outbreak in a coral reef microcosm at the Pittsburgh Zoo. In Proceeding of the International Symposium on Ciguatera and Marine Natural Products; Hokama, Y., Scheuer, P.J., Yasumoto, T., Eds.; Asian Pacific Research Foundation: Honolulu, HI, USA, 1994; p. 300. [Google Scholar]
- Iglesias-Prieto, R.; Reyes-Bonilla, H.; Riosmena-Rodríguez, R. Effects of 1997-1998 ENSO on coral reef communities in the Gulf of California, Mexico. Geofis. Intern. 2003, 42, 467–471. [Google Scholar]
- Núñez-Vázquez, E.J.; Hernández-Sandoval, F.E.; Cordero-Tapia, A.; Almazán-Becerril, A.; Band-Schmidt, C.J.; Bustillos-Guzmán, J.; López-Cortés, D.; Salinas-Zavala, C.A.; Morales-Zárate, M.V.; Mejía-Rebollo, A.; et al. Presencia de microalgas bénticas potencialmente nocivas y mortandad de peces asociadas al Parque Marino de Cabo Pulmo, B.C.S. In Proceedings of the II Congreso Nacional de Sociedad Mexicana para el estudio de los Florecimientos Algales Nocivos, Manzanillo, México, 30–31 October 2013; p. 41. [Google Scholar]
- Núñez-Vázquez, E.J.; Pérez-Estrada, C.J.; Cordero-Tapia, A.; Martínez-Gutiérrez, C.A.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.; Latisnere-Barragán, H.; López-Cortés, D.J.; Ley-Martínez, T. Proliferación de Lyngbya majuscula en la playa de Balandra, B.C.S. y detección de cianotoxinas tipo lyngbyatoxinas y paralizantes. In Proceedings of the II Congreso Nacional de Sociedad Mexicana para el estudio de los Florecimientos Algales Nocivos, Manzanillo, México, 30–31 October 2013; p. 67. [Google Scholar]
- Pérez-Estrada, C.J.; Núñez-Vázquez, E.J.; Cordero-Tapia, A.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.; Band-Schmidt, C.J.; López-Cortés, D.J.; Martínez-Gutiérrez, C.A.; Ley-Martínez, T. Detección de cianotoxinas en la proliferación de la cianobacteria Lyngbya majuscula y en el opistobranquio Stylocueilus striatus en la Playa de Balandra, Baja California Sur, Mexico. In Proceedings of the I Taller Nacional de Biotoxinas Emergentes, La Paz, Baja California Sur, México, 9–10 November 2015; REDFAN-CONACYT, CIBNOR, CICIMAR-IPN: La Paz, México, 2015; p. 27. [Google Scholar]
- Petróleos Mexicanos (PEMEX). Anuario Estadístico 2008; Exploración y Producción. PEMEX: Mexico City, Mexico, 2017; pp. 1–119. [Google Scholar]
- Comisión Nacional de Áreas Naturales Protegidas (CONANP). Parque Nacional Arrecife Alacranes. 2004. Available online: https://www.gob.mx/conanp/documentos/region-peninsula-de-yucatan-y-caribe-mexicano?state=published (accessed on 13 December 2018).
- Ortíz-Lozano, L.D. Análisis crítico de las zonas de regulación y planeacion en el parque nacional sistema arrecifal veracruzano. Ph.D. Thesis, Universidad Autónoma de Baja California, Ensenda, Mexico, 2006. [Google Scholar]
- Comisión Nacional de Áreas Naturales Protegidas (CONANP). Parque Nacional Sistema Arrecifal Veracruzano. Available online: https://simec.conanp.gob.mx/ficha.php?anp=135®=5 (accessed on 13 December 2018).
- De Fouw, J.C.; Van Egmond, H.P.; Speijers, G.J.A. Ciguatera Fish Poisoning: A Review; RIVM Report No. 388802021; Ministerie van Volksgezondheid, Welzijn en Sport: Bilthoven, The Netherlands, 2001.
- FDA. Fish and Fishery Products Hazards and Control Guidance, 4th ed.; US Department of Health and Human Services, Food Drug Administration, Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2011; 476p. [Google Scholar]
- COFEPRIS. Instrucción de trabajo para el muestreo de fitoplancton y detección de biotoxinas marinas; Secretaria de Salud: Ciudad de México, México, 2005; pp. 21–147. [Google Scholar]
- Norma Oficial Mexicana. Norma Oficial Mexicana NOM-242-SSA1-2009, Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. Diario Oficial de la Federación, 10 febrero 2011. [Google Scholar]
- González-Zihel, M. Quintana Roo desarrolla acciones de protección contra riesgos sanitarios por la ingesta de pescado contaminado por ciguatoxina. Red Sanitaria Secretaria de Salud 2007, 3, 11. [Google Scholar]
- Aguilar, V.; Kolb, M.; Hernández, D.; Urquiza, T.; Koleff, P. Prioridades de Conservación de la Biodiversidad Marina de Mexico. Biodiversitas 2008, 79, 1–15. [Google Scholar]


| Cases (M:F) | Locality (Cases) | Fish Involved | Analysis | Year | Reference |
|---|---|---|---|---|---|
| 200 (n.d.) | La Paz, B.C.S. | Lutjanus sp. | MBA | 1984 | [29] |
| 25 (17:4) * | Rocas Alijos, B.C.S. | Epinephelus labriformis | MBA and immunoassay | 1992 | [30] |
| 7 (7:0) | Rocas Alijos, B.C.S. | Epinephelus sp. and Mycteroperca sp. | MBA | 1993 | [31] |
| 5 (4:1) | Isla El Pardito (3), Punta San Evaristo (2), B.C.S. | Mycteroperca prionura and Lutjanus colorado | MBA and HPLC | 1993–1997 | [32] |
| 3 (1:3) | Punta Abreojos, B.C.S. | Epinephelus sp. | MD | 2004 | [28] |
| 10 (5:5) | Isla Mujeres, Q. Roo | Sphyraena barracuda | MD | 1994 | [33] |
| 30 (14:16) | Isla Mujeres, Q. Roo | S. barracuda | MD | 1995 | [34,35] |
| 21 (n.d.) | Merida (15), Kanasin (6), Yuc. | S. barracuda | MD | 1996 | [36] |
| 11 (1:0) * | Progreso, Yuc. | S. barracuda | MD | 2000 | [37] |
| 2 (1:1) | Cancun, Q. Roo | S. barracuda | MD | 2001 | [38] |
| 1 (0:1) | Yucatan | n.d. | MD | 2004 | [39] |
| 9 (n.d.) | Isla Mujeres, Q. Roo | S. barracuda | MD | 2006 | HMR |
| 13 (n.d.) | Cozumel, Q. Roo | S. barracuda | MD | 2007 | HMR |
| 5 (n.d.) | Cozumel, Q. Roo | S. barracuda | MD | 2007 | HMR |
| 1 (0:1) | Mexico | n.d. | MD | 2008 | [40] |
| 2 (n.d.) | Cozumel, Q. Roo | S. barracuda | MD | 2009 | HMR |
| 12 (12:0) | Isla Mujeres, Q. Roo | S. barracuda | MD | 2009 | HMR |
| 11 (n.d.) | Merida, Yuc. | S. barracuda | MD and MBA | 2010 | HMR |
| 12 (4:8) | Cancun, Q. Roo | S. barracuda | MD | 2010 | HMR |
| 5 (2:3) | Puerto Aventuras, Q. Roo | S. barracuda | MD | 2010 | HMR |
| 29 (n.d.) | Playa del Carmen, Q. Roo | S. barracuda | MD | 2011 | HMR |
| 27 (14:13) | Isla Mujeres, Q. Roo | Lutjanus sp. & S. barracuda | MD | 2011 | HMR |
| 3 (2:1) | Playa del Carmen, Q. Roo | S. barracuda | MD | 2012 | HMR |
| 4 (3:0) | Tulum, Q. Roo | S. barracuda | MD | 2013 | HMR |
| 16 (3:9)* | Cuba | Lutjanus sp. | MD | 1986 | [41] |
| Total: 464 (90:66) |
| Locality/Year | Signs & Symptoms | Duration | Treatment | Ref. |
|---|---|---|---|---|
| La Paz, B.C.S., 1984 | Ciguatera symptoms (not specified). | n.d. | n.d. | [29] |
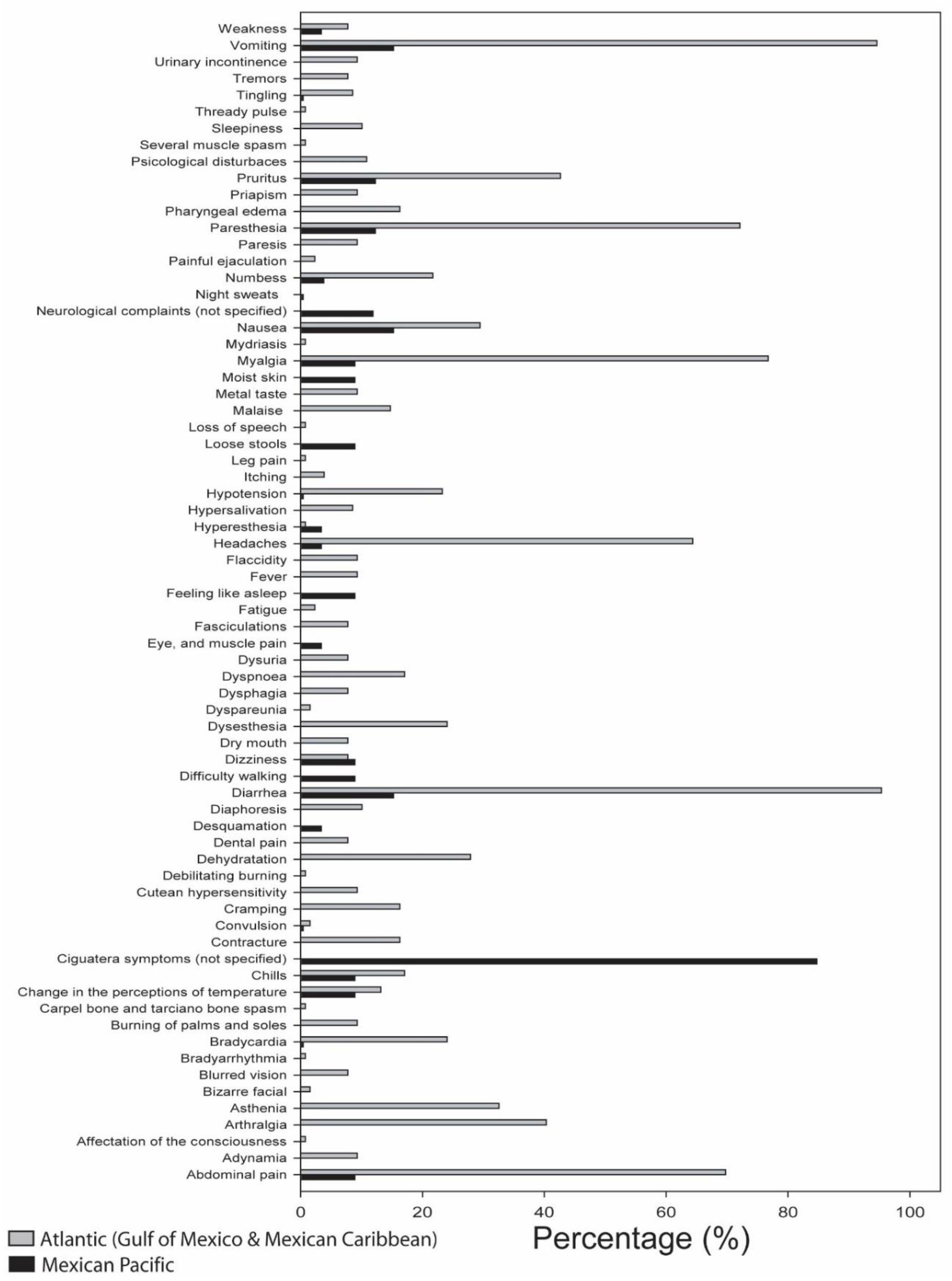
| Rocas Alijos, B.C.S., 1992 | Nausea; vomiting; diarrhea; loose stools; neurological complaints; paresthesia of the face, arms, and legs; bradycardia and hypotension; dizziness; stomachache; tingling and numbness in legs and fingers, feeling like they were asleep; moist skin; difficulty walking; myalgia; chills; sensitive cold; temperature reverse; pruritus; and night sweats. | Several days | Histamine blockers and palliative supports | [30] |
| Rocas Alijos, B.C.S., 1993 | Diarrhea, nausea, vomiting, neurophysiological disorders. | 15 days | n.d. | [31] |
| El Pardito Island and Punta San Evaristo B.C.S., 1993–1997 | Diarrhea, nausea, vomiting, eye; muscle and abdominal pain; headache, numbness, weakness, pruritus, desquamation, hyperesthesia, lip and tongue paralysis, and convulsion in one case. | Several days | Palliative support, histamine blockers, and antibiotics | [28,32] |
| Isla Mujeres, Q. Roo, 1994 | Gastrointestinal disturbances, watery diarrhea (dehydration and shock in 2 cases), cold-to-hot temperature reversal, dysesthesia in all cases with differences in the occurrence of nausea, vomiting, cramps, abdominal pain, weakness, paresthesia, arthralgia, myalgia, dizziness, dysuria, dyspnea, headache, pruritus, lip numbness, dry mouth, dental pain, chills, tremors, fasciculations, blurred vision, hypersalivation and dysphagia, emotional lability (2 cases). Painful ejaculation and dyspareunia (2 cases). Nipple hyperesthesia (1 female). | Chronic (4 cases, several months) | Palliative supports. Indomethacin (1 chronic case) | [33] |
| Isla Mujeres, Q. Roo, 1995 | Hypotension, bradycardia, headache, arthralgia, pruritus, myalgia, asthenia, paresthesia in tongue, lips and extremity, abdominal pain, vomiting, diarrhea. | 1–7 months | Antidiarrheal drugs, vitamins C, B1, B6 and B12, anti-histaminics, anxiolytic drugs, atropine, mannitol | [34,35] |
| Merida and Kanasin, Yuc., 1996 | Paresthesia and muscle spasm, pharyngeal edema dysesthesia, contracture. | n.d. | Palliative supports | [36] |
| Merida, Yuc., 2000 | Abdominal and leg pain; vomiting; muscle contracture; diaphoresis; hypersalivation; severe muscle, carpel bone and tarciano bone spasm; mydriasis, bradyarrhythmia; bradycardia; thready pulse; awareness alteration (1 child). | Days | methylprednisolone, diazepam, adrenaline, atropine, calcium gluconate, mannitol | [37] |
| Cancun, Q. Roo, 2001 | Vomiting, abdominal pain, profuse watery diarrhea, fatigue, bizarre facial and extremity paresthesia, as well as the peculiar sensation that cold foods seemed hot, and hot drinks tasted ice cold, headaches, malaise, and debilitating burning and numbness in hand and feet, pruritus. During ejaculation, severe pubic pain. | Several weeks | n.d. | [38] |
| Yucatan, 2004 | Paresthesia extended from face to hands and legs, muscle pain, pruritus, myalgia, fatigue, sleepiness. | Several weeks | Clarithromycin, ibuprofen | [39] |
| Isla Mujeres, Q. Roo, 2006 | Diarrhea, vomiting, severe electrolyte imbalance. | Days | Palliative supports, serum | HMR |
| Cozumel, Q. Roo, 2007 | Diarrhea, vomiting, dehydration. | Days | Palliative supports, serum | HMR |
| Cozumel, Q. Roo, 2007 | Diarrhea, muscle pain, nausea, vomiting, headache, itching; change in temperature perception, convulsion (two cases), speech loss (1 case), mouth, hand and feet numbness. | Days | Palliative supports | HMR |
| Mexico n.d. | Headache, pain in back and joints, abdominal discomfort and sweating, nausea and itching. | Days | Palliative supports | [40] |
| Cozumel, Q. Roo, 2009 | Diarrhea and dehydration. | Days | Palliative supports | HMR |
| Isla Mujeres, Q. Roo, 2009 | Abdominal pain, headache, diarrhea, vomiting, chills, fever, muscle pain, numbness of limbs and tongue, dehydration. | Days | Palliative supports, serum | HMR |
| Merida, Yuc., 2010 | Abdominal pain, diarrhea, nausea, vomiting, cramps, tingling, muscle pain. | Days | Palliative supports | HMR |
| Cancun, Q. Roo, 2010 | Abdominal and muscle pain, diarrhea, vomiting, paresthesia, headache, general discomfort. | Days | Palliative supports | HMR |
| Puerto Aventuras, Q. Roo, 2010 | Diarrhea, vomiting, muscle pain, paresthesia, general discomfort. | Days | Palliative supports | HMR |
| Playa del Carmen Q. Roo, 2011 | Diarrhea, nausea, vomiting, dehydration, muscle pain, paresthesia. | Days | Palliative supports | HMR |
| Isla Mujeres, Q. Roo, 2011 | Diarrhea, nausea, vomiting, abdominal pain, paresthesia. | Days | Hartmann solution, hydrocortisone, mannitol and adrenaline | HMR |
| Playa del Carmen, Q. Roo, 2012 | Profuse watery diarrhea, vomiting, colic, paresthesia extended from face to hands and legs, hypothermia sensation. | Days to weeks | Palliative supports | HMR |
| Tulum, Q. Roo, 2013 | Diarrhea, abdominal pain, paresthesia, respiration difficulty, weakness. | Days | Mannitol | HMR |
| Cuba, 1986 | Profuse watery diarrhea, abdominal pain, nausea, vomiting, metal taste, diaphoresis, headache, myalgia, arthralgia, paresthesia, paresis, flaccidity, asthenia, adynamia, dyspnea, skin hypersensitivity, burning of palms and soles, intense pruritus, priapism, urinary incontinence, sleepiness, affliction, depression. | Months | Antihistaminic, antiemetic, analgesic, tranquillizer, palliative supports | [41] |
| Dinoflagellate | Distribution | Reference |
|---|---|---|
| Gambierdiscus toxicus | Quintana Roo, Yucatán, Tabasco, Veracruz, Nayarit (Isla Isabel), B.C.S., Revillagigedo Islands | [46,47,48,49,50,51,52,53,63,64,65] |
| G. carolinianus | Quintana Roo | [57,58,59,61] |
| G. belizeanum | Quintana Roo | [49,50,58] |
| G. caribaeus | Quintana Roo, Yucatán | [56,57,58,59,80,85] |
| G. carpenteri | Quintana Roo | [61] |
| Gambierdiscus sp. | Campeche, Baja California Sur | [54,66] |
| F. yasumotoi (=G. yasumotoi) | Quintana Roo | [53,54] |
| Ostreopsis ovata | Baja California, Baja California Sur | [25,63,86] |
| O. lenticularis | Baja California Sur, Revillagigedo Islands | [63,82] |
| O. marina | Quintana Roo, Baja California Sur | [81,85,86] |
| O. belizeanum | Quintana Roo | [81] |
| O. siamensis | Quintana Roo, Nayarit (Isla Isabel), B.C.S. (Isla San José), Revillagigedo Islands | [57,59,63,66,77] |
| Ostreopsis sp. | Revillagigedo Islands, B.C.S. | [78] |
| O. heptagona | Veracruz, Quintana Roo | [51,57,79,80,81,85] |
| P. lima | Quintana Roo, Yucatán, Veracruz, Baja California, Baja California Sur, Sonora, Oaxaca | [25,49,51,56,57,63,64,66,71,73,75,79,80,85,86] |
| P. hoffmannianum | Quintana Roo, Campeche, Yucatán, Veracruz | [49,51,54,56,57,79,80,85] |
| P. concavum | Quintana Roo, Yucatán, Veracruz, Baja California Sur | [25,51,56,57,63,79,86] |
| P. belizeanum | Quintana Roo, Yucatán, Baja California Sur | [49,57,63,64,79,85] |
| P. rhathymum | Quintana Roo, Yucatán, Baja California Sur | [49,56,57,63,66,79,80,85] |
| Dinoflagellate | Strain | Origin | Culture Collection | Reference |
|---|---|---|---|---|
| G. toxicus | CM2K, CM3K & CM4KI M510K, IM512K IM513K & IM514K CM515K, reef CM516K, reef CM517K, reef CM518K, reef CM519K, reef CM520K, reef | Cozumel, Q. Roo Parque El Garrafón, Isla MujeresClub Med, Cancun, Q. Roo | Tropical Dinoflagellate (Southern Illinois University, Carbondale) | [47] |
| PO528K, Lagoon CZ2, CZ3 & CZ4 | Pat O’Brien’s, Cancun, Q. Roo Cozumel, Q. Roo | National Marine Fisheries Service (Charleston Lab.) | [46] | |
| G. caribaeus | Mex Algae 1 Gam 1 | Cancun, Q. Roo | [59] | |
| G. carpenteri | Mex Algae 2 Gam 1 | Cancun, Q. Roo | [61] | |
| G. carolinianus | Mex Algae Gam 1 | Cancun, Q. Roo | [59] | |
| P. lima | CM563K, reef PRL-1 PLV-1,a PLV-3 | Club Med, Cancun, Q. Roo Isla El Pardito, B.C.S. B. de La Paz, B.C.S. | Tropical Dinoflagellate CODIMAR-CIBNOR CODIMAR-CIBNOR | [47] [86] [86] |
| P. belizeanum | CM559K, reef CM560K, reef CM561K, reef CM568K, reef IM553K CZ565 | Club Med, Cancun, Q. Roo Parque El Garrafón, Isla Mujeres, Q. Roo Chankanaab reef, Cozumel | Tropical Dinoflagellate | [47] |
| P. concavum P. cf. concavum | CM559, reef PCJV-1 a PCJV-3 | Club Med, Cancun, Q. Roo Isla San José, B.C.S. | Tropical Dinoflagellate CODIMAR-CIBNOR | [47] [86] |
| P. mexicanum | PO567AK, lagoon PXCV-1, PXPV-1 & PXPV-2 | Pat O’Brien’s, Cancun, Q. Roo B. Concepción & B. de La Paz, B.C.S. | Tropical Dinoflagellate CODIMAR-CIBNOR | [47] [86] |
| O. marina | OMPV-1 | B. de La Paz, B.C.S. | CODIMAR-CIBNOR | [86] |
| O. cf. ovata | OOJV-1 a OOJV-9 OOPV-1 | Isla San José, B.C.S. B. de La Paz, B.C.S. | CODIMAR-CIBNOR | [86] |
| Ostreopsis sp. | San José del Cabo, B. C. S | Strain Collection of the CIBNOR | Virgen-Félix, 2008 * |
| Responsible Fish Genera | Frequency | Percentage | Intoxications (%) |
|---|---|---|---|
| Lutjanus | 4 | 14.28 | 48.81 |
| Epinephelus | 3 | 10.71 | 6.88 |
| Mycteroperca | 2 | 7.14 | 2. 36 |
| Sphyraena | 17 | 60.71 | 41.53 |
| Unknown | 2 | 7.14 | 0.39 |
| Total: | 28 | 100 | 100 |
| Data | Quintana Roo | Yucatán | BCS |
|---|---|---|---|
| Inhabitants | 1,215,237 | 1,818,948 | 535,808 |
| Hotels | 763 | 330 | 290 |
| Hotel rooms | 73,108 | 8880 | 15 384 |
| Tourists | 7,546,720 | 1,589,940 | 1,834,515 |
| Touristic docks | 14 | 12 | 7 |
| Biosphere reserves | 3 | 2 | 2 |
| National parks | 6 | 2 | 2 |
| Water treatments plants | 29 | 260 | 94 |
| Volume of water discharged under federal control (million m3) | 215,193,50 | 51.0 | 1279.0 |
| Fisheries production (ton) | 3861 | 27,179.2 | 134,803 |
| Aquaculture production (ton) | 56.79 | 73.4 | 4421 |
| Swine production (heads) | 156,375 | 792,202 | 15,210 |
| Avian production (heads) | 4,003,730 | 17,512,206 | 62,087 |
| Year/Hurricane | Site of Impact | Wind Intensity (km/h) | Category Saffir-Simpson |
|---|---|---|---|
| Gulf of Mexico and Caribbean Sea | |||
| 1988 Gilbert | Quintana Roo | 287 | H5 |
| Yucatan | |||
| 1995 Roxana | Tulum, Q. Roo | 185 | H3 |
| 1996 Doly | Felipe Carrillo Puerto, Q. Roo | 130 | H1 |
| 2000 Keith | Chetumal, Q. Roo | 148 | H1 |
| 2002 Isidore | Telchac Puerto, Yucatan | 205 | H3 |
| 2005 Wilma | Isla Cozumel, Q. Roo | 230 | H4 |
| Puerto Morelos, Q. Roo | 220 | H4 | |
| 2005 Emily | Tulum, Q. Roo | 215 | H4 |
| 2005 Stan | Felipe Carrillo Puerto, Q. Roo | 75 | Tropical Storm |
| 2007 Dean | Quintana Roo | 280 | H4 |
| Yucatan | |||
| 2008 Dolly | Cancun, Q. Roo | 85 | Tropical Storm |
| Pacific Ocean | |||
| 1995 Henriette | Cabo San Lucas, B.C.S. | 158 | H2 |
| 1996 Fausto | Todos Santos B.C.S. | 130 | H1 |
| 1997 Nora | Bahía Tortugas, B.C.S. | 120 | H1 |
| 1998 Isis | Los Cabos, B.C.S. | 110 | Tropical Storm |
| 1999 Greg | San José del Cabo, B.C.S. | 120 | H1 |
| 2001 Juliette | La Paz, Ciudad Constitución, B.C.S. | 120 | H1 |
| 2003 Ignacio | Ciudad Constitución, B.C.S. | 165 | H2 |
| 2003 Marty | San José del Cabo, B.C.S. | 160 | H2 |
| 2006 John | El Saucito, B.C.S. | 175 | H2 |
| 2007 Henriette | Los Cabos, B.C.S. | 150 | H1 |
| 2008 Norbert | Bahía Magdalena, B.C.S. | 215 | H2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Vázquez, E.J.; Almazán-Becerril, A.; López-Cortés, D.J.; Heredia-Tapia, A.; Hernández-Sandoval, F.E.; Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Gárate-Lizárraga, I.; García-Mendoza, E.; Salinas-Zavala, C.A.; et al. Ciguatera in Mexico (1984–2013). Mar. Drugs 2019, 17, 13. https://doi.org/10.3390/md17010013
Núñez-Vázquez EJ, Almazán-Becerril A, López-Cortés DJ, Heredia-Tapia A, Hernández-Sandoval FE, Band-Schmidt CJ, Bustillos-Guzmán JJ, Gárate-Lizárraga I, García-Mendoza E, Salinas-Zavala CA, et al. Ciguatera in Mexico (1984–2013). Marine Drugs. 2019; 17(1):13. https://doi.org/10.3390/md17010013
Chicago/Turabian StyleNúñez-Vázquez, Erick J., Antonio Almazán-Becerril, David J. López-Cortés, Alejandra Heredia-Tapia, Francisco E. Hernández-Sandoval, Christine J. Band-Schmidt, José J. Bustillos-Guzmán, Ismael Gárate-Lizárraga, Ernesto García-Mendoza, Cesar A. Salinas-Zavala, and et al. 2019. "Ciguatera in Mexico (1984–2013)" Marine Drugs 17, no. 1: 13. https://doi.org/10.3390/md17010013
APA StyleNúñez-Vázquez, E. J., Almazán-Becerril, A., López-Cortés, D. J., Heredia-Tapia, A., Hernández-Sandoval, F. E., Band-Schmidt, C. J., Bustillos-Guzmán, J. J., Gárate-Lizárraga, I., García-Mendoza, E., Salinas-Zavala, C. A., & Cordero-Tapia, A. (2019). Ciguatera in Mexico (1984–2013). Marine Drugs, 17(1), 13. https://doi.org/10.3390/md17010013






