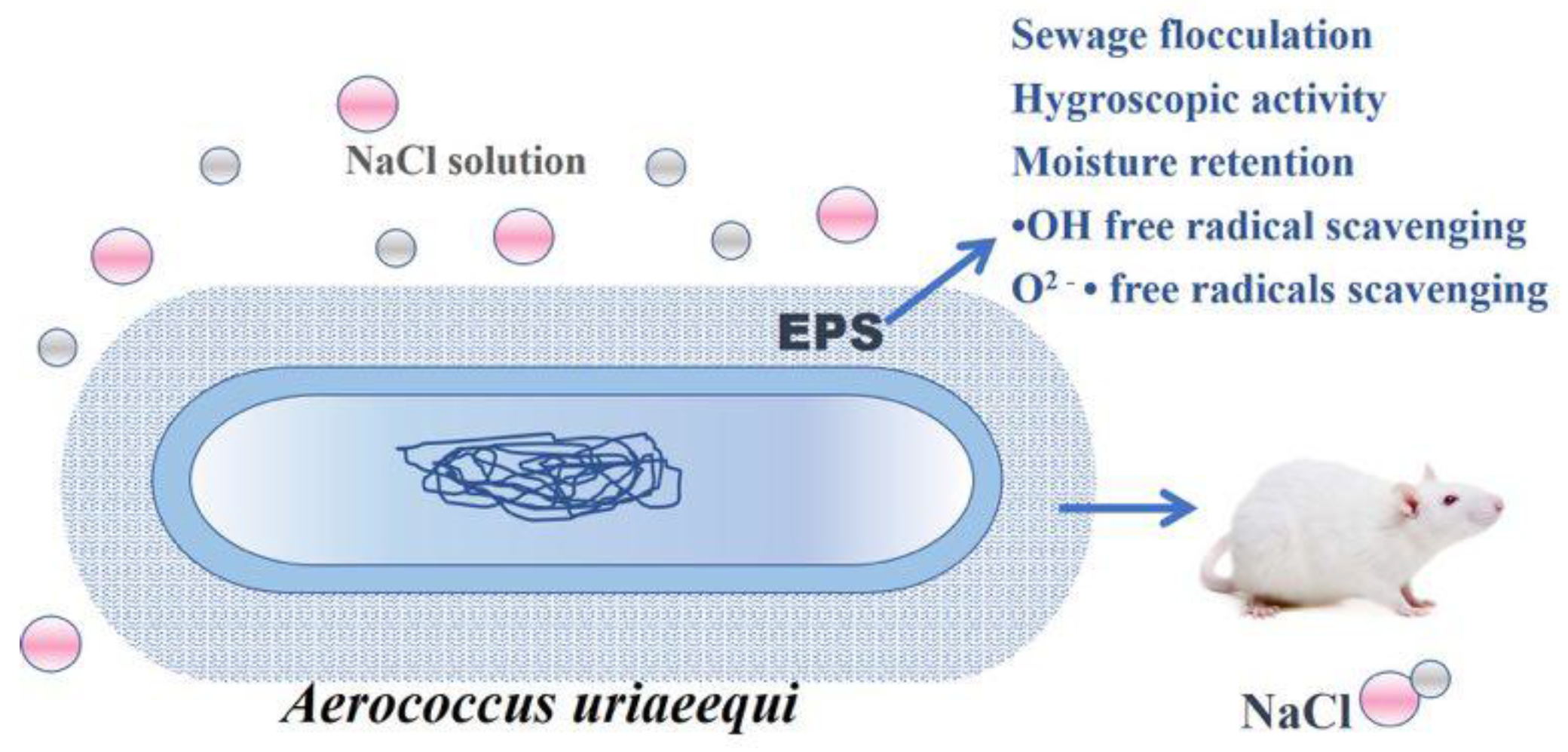
Isolation, Characterization, and Pharmaceutical Applications of an Exopolysaccharide from Aerococcus Uriaeequi
Abstract
1. Introduction
2. Results
2.1. Extraction and Purification of Microbial Exopolysaccharide (EPS-A)
2.2. Monosaccharide Composition Analysis of EPS-A
2.3. Fourier-Transform–Infrared (FT–IR) Analysis
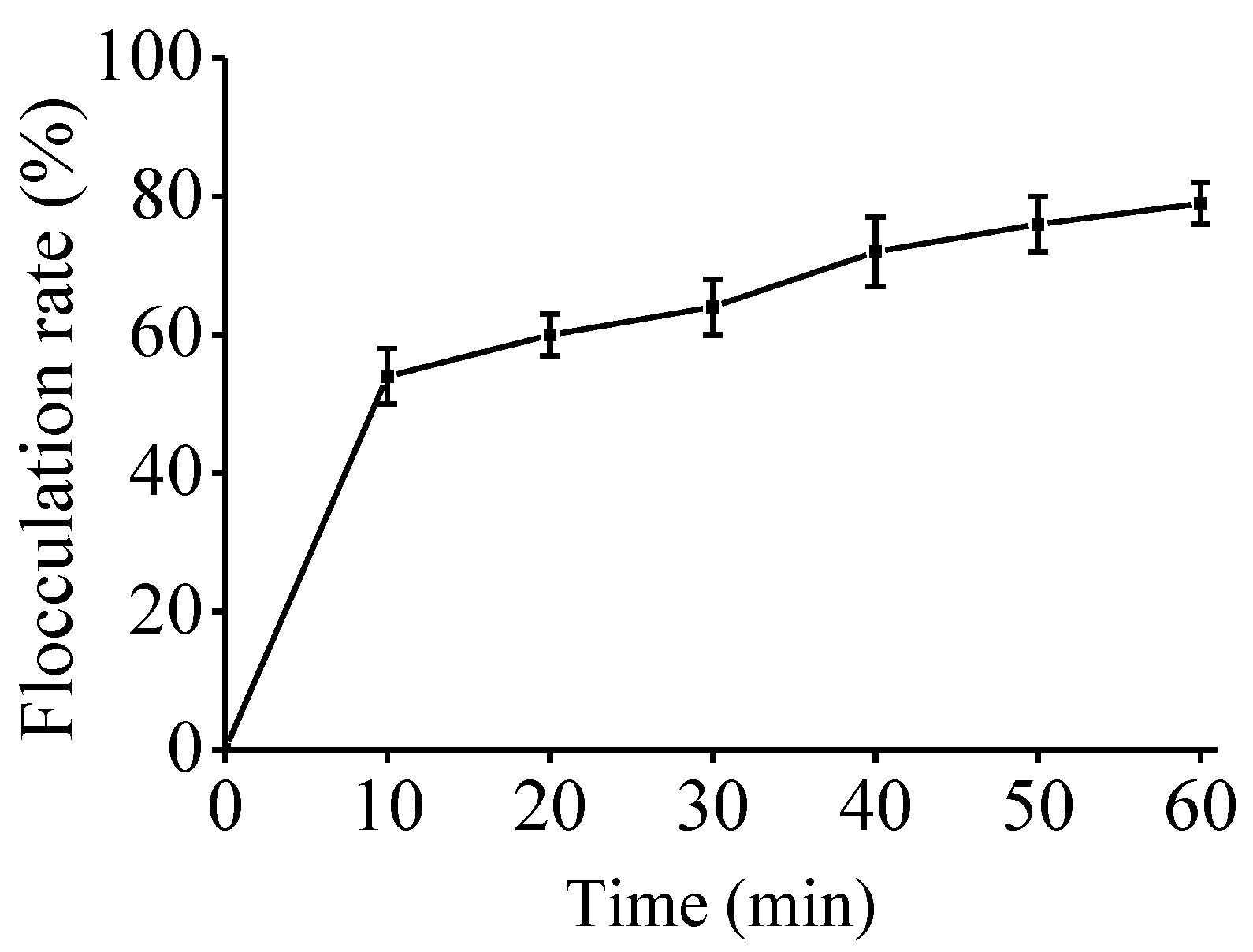
2.4. Bio-Flocculating Activity
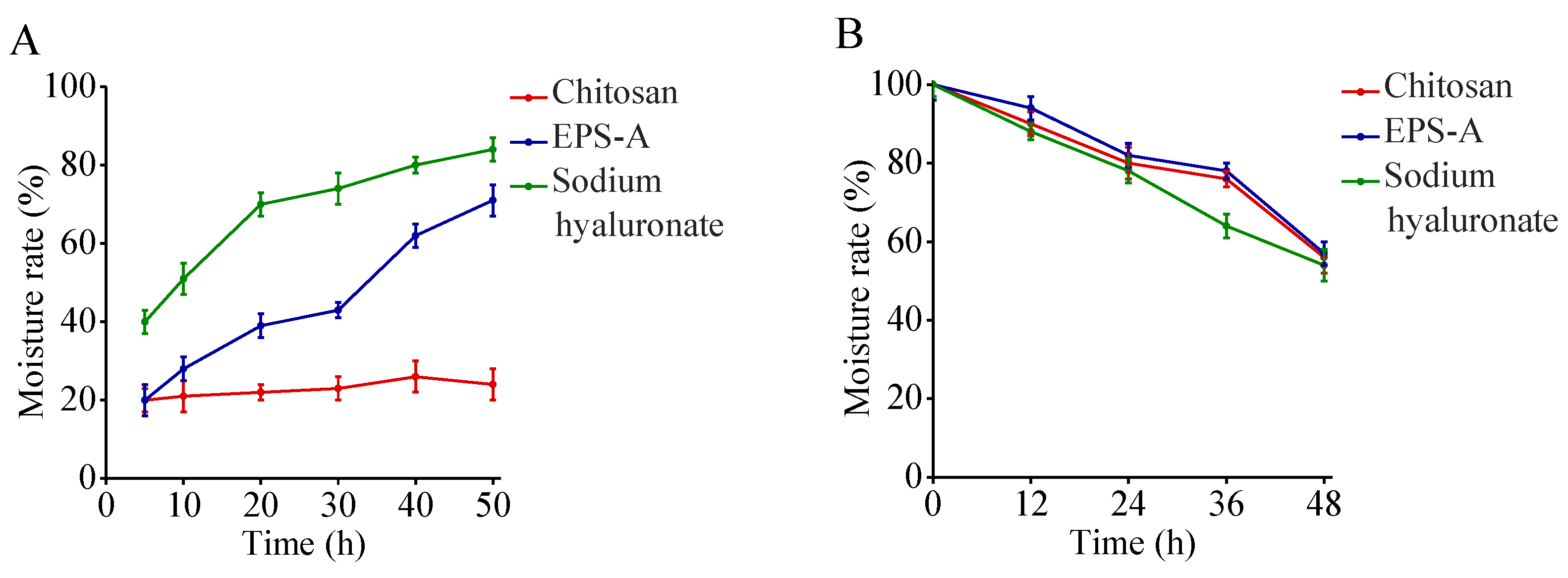
2.5. Moisture Absorption and Retention Properties EPS-A
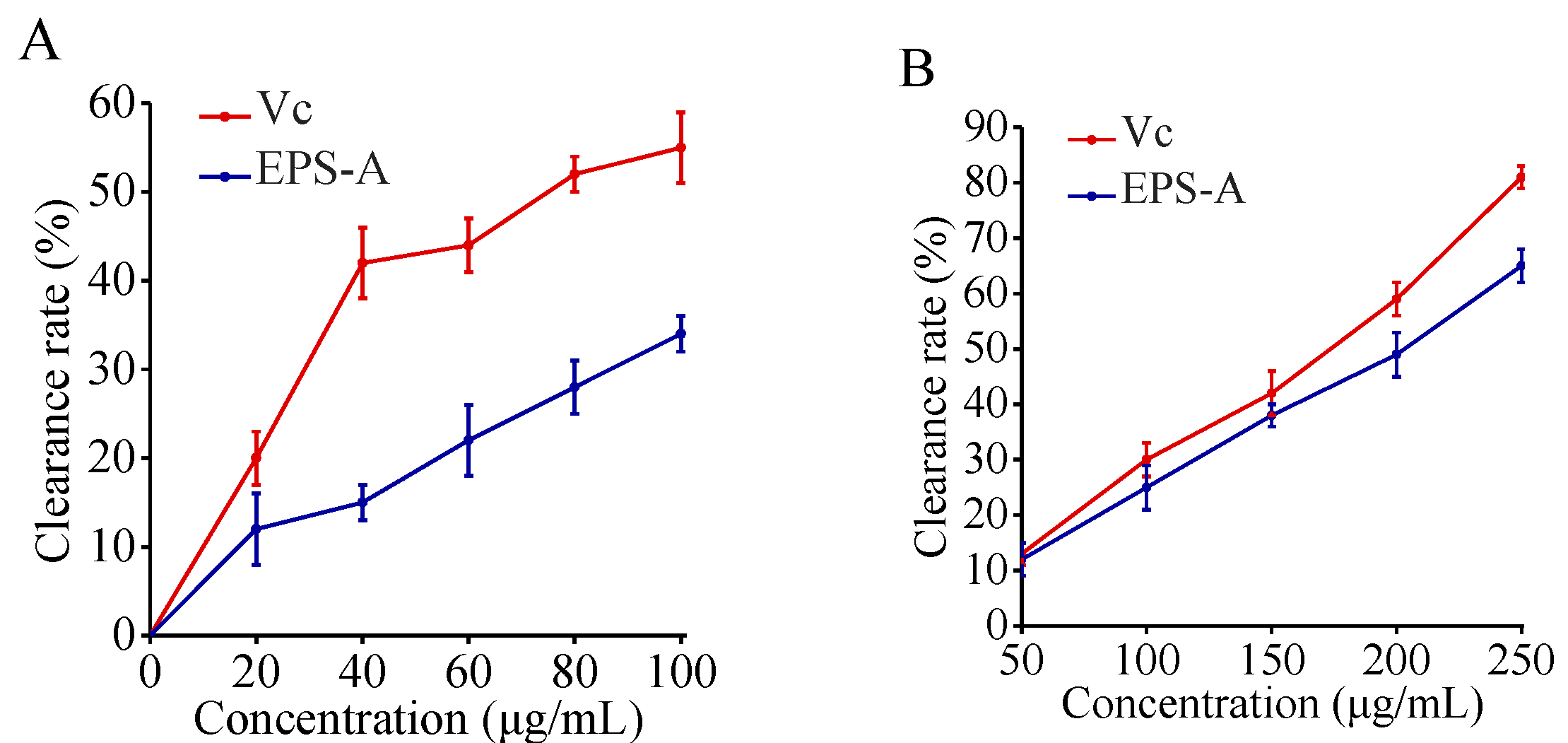
2.6. Removal Results on Free Radicals
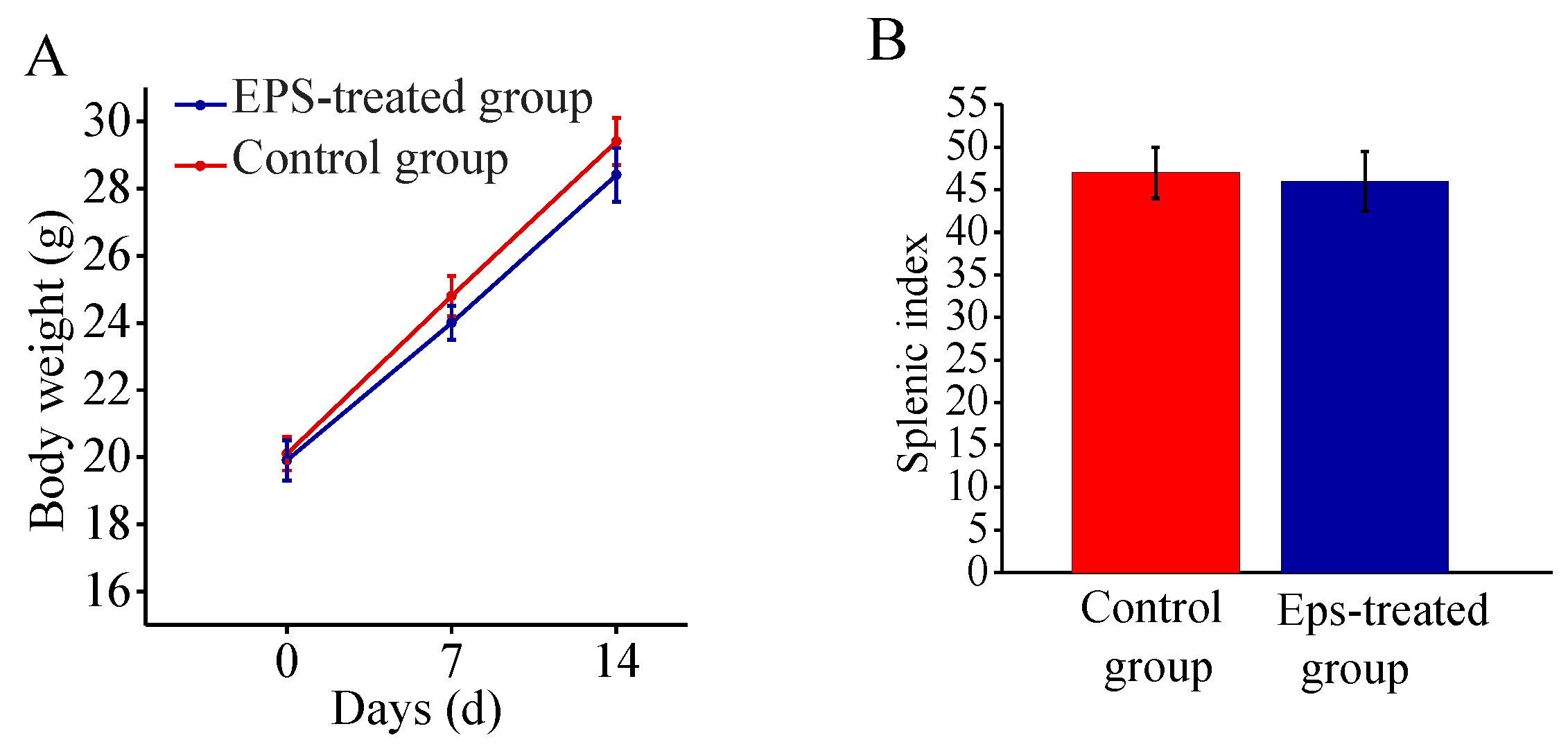
2.7. Safety Assessment of EPS-A
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Production and Purification of EPS-A
4.3. Molecular Weight Determination of EPS-A
4.4. Hydrolysis and Derivatization of EPS-A
4.5. High-Performance Liquid Chromatography (HPLC) Analysis of the Monosaccharide Composition
4.6. Ultraviolet (UV)-Visible and FT–IR Spectroscopy
4.7. Sewage Flocculation Analysis of EPS-A
4.8. Hygroscopic Activity of EPS-A
4.9. Moisture Retention Determination of EPS-A
4.10. OH• Free Radical Scavenging Activity
4.11. O2•− Free Radicals Scavenging Activity
4.12. Safety Assessment of EPS-A
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EPS | exopolysaccharide |
| EPS-A | exopolysaccharide from Aerococcus uriaeequi |
| FT–IR | Fourier-transform–infrared spectrometry |
| HPGFC | high performance gel permeation chromatography |
References
- Helm, R.F.; Huang, Z.; Edwards, D.; Leeson, H.; Peery, W.; Potts, M. Structural characterization of the released polysaccharide of desiccation-tolerant Nostoc commune DRH-1. J. Bacteriol. 2000, 182, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Chen, X.L.; He, H.L.; Zhang, X.Y.; Xie, B.B.; Yu, Y.; Chen, B.; Zhou, B.C.; Zhang, Y.Z. Structure and ecological roles of a novel exopolysaccharide from the arctic sea ice bacterium Pseudoalteromonas sp. Strain SM20310. Appl. Environ. Microbiol. 2013, 79, 224–230. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, A.; Ru, Q.; Dong, D.; Sun, P. Structural characterization of a water-soluble polysaccharide from the fruiting bodies of Agaricus bisporus. Int. J. Mol. Sci. 2014, 15, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Su, C.A.; Xu, X.Y.; Liu, D.Y.; Wu, M.; Zeng, F.Q.; Zeng, M.Y.; Wei, W.; Jiang, N.; Luo, X. Isolation and characterization of exopolysaccharide with immunomodu-latory activity from fermentation broth of Morchella conica. DARU J. Pharm. Sci. 2013, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- lamas, I.L.; Mata, J.A.; Tallon, R.; Bressollier, P.; Urdaci, M.C.; Quesada, E.; Béjar, V. Characterization of the exopolysaccharide produced by Salipiger mucosus A3(T), a halophilic species belonging to the alphaproteobacteria, isolated on the spanish mediterranean seaboard. Mar. Drugs 2010, 8, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.S.; Li, D.S.; Li, H.M.; Tang, Y.J. Response surface modeling the significance of nitrogen source on the cell growth and Tuber polysaccharides production by submerged cultivation of Chinese truffle Tuber sinense. Process Biochem. 2008, 43, 868–876. [Google Scholar] [CrossRef]
- Mancuso Nichols, C.A.; Garon, S.; Bowman, J.P.; Raguenes, G.; Guezennec, J. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol. 2004, 96, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yi, Y.; Guo, Y.; Wang, R.; Hu, Q.; Xiong, X. Chemical Characterization and Antitumor Activities of Polysaccharide Extracted from Ganoderma lucidum. Int. J. Mol. Sci. 2014, 15, 9103–9116. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Xu, R.; Li, P. Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr. Polym. 2013, 91, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, R.; Schols, H.A.; Blanco, A.; Siika-Aho, M.; Rättö, M.; Buchert, J.; Lenon, G.; Voragen, A.G.J. Sugar composition and FT-IR analysis of exopolysaccharides produced by microbial isolates from paper mill slime deposits. Biotechnol. Bioeng. 2005, 91, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Identification of phycocolloids by vibrational spectroscopy. In World Seaweed Resources–An Authoritative Reference System; Alan, T., Critchley, M.O., Danilo, B.L., Eds.; ETI Information Services Ltd. Japan international cooperation agency: Yokosuka, Japan, 2006. [Google Scholar]
- Pereira, L.; Gheda, F.S.; Ribeiro-Claro, P.J.A. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical and cosmetic industries. Int. J. Carbohydr. Chem. 2013, 2013, 537202. [Google Scholar] [CrossRef]
- Muthukumar, A.; Baskaralingam, V.; Mani, D.; Sekar, V.; Marimuthu, G.; Naiyf, S.A.; Jamal, M.K.; Mohammed, N.A.; Giovanni, B. Structural characterization of Bacillus licheniformis Dahb1exopolysaccharide—Antimicrobial potential and larvicidal activity on malaria and Zika virus mosquito vectors. Environ. Sci. Pollut. Res. 2018, 25, 18604–18619. [Google Scholar]
- Koening, J.L. Vibrational Spectroscopy of Carbohydrates. In Infrared and Raman Spectroscopy of Biological Molecules; Springer International Publishing: Cham, Switzerland, 1979; pp. 125–137. [Google Scholar]
- Mathlouthi, M.; Koening, J.L. Vibrational spectra of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987, 44, 7–89. [Google Scholar]
- Wang, Z.; Sheng, J.; Tian, X.; Wu, T.; Liu, W.; Shen, L. Optimization of the production of exopolysaccharides by Bacillus thuringiensis in sand biological soil crusts and its bioflocculant activity. Afr. J. Microbiol. Res. 2011, 5, 2359–2366. [Google Scholar]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007, 98, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Roberson, E.B.; Firestone, M.K. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [PubMed]
- Wu, Q.X.; Lin, D.Q.; Yao, S.J. Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Mar. Drugs 2014, 12, 6236–6253. [Google Scholar] [CrossRef] [PubMed]
- Bakos, D.; Soldan, M.; Hernandez-Fuentes, I. Hydroxyapatite-collagen-hyaluronic acid composite. Biomaterials 1999, 20, 191–195. [Google Scholar] [CrossRef]
- Kodali, V.P.; Sen, R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol. J. 2008, 3, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Asker, M.M.S.; Shawky, B.T. Structural characterization and antioxidant activity of an extracellular polysaccharide isolated from Brevibacterium otitidis BTS 44. Food Chem. 2010, 123, 315–320. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-carboxylate int eractions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Prajapat, J. Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv. Dairy Res. 2013, 1–8. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef] [PubMed]
- More, T.T.; Yan, S.; John, R.P.; Tyagi, R.D.; Surampalli, R.Y. Biochemical diversity of the bacterial strains and their biopolymer producing capabilities in wastewater sludge. Bioresour. Technol. 2012, 121, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Zhao, F.; Shi, M.; Zhang, X.Y.; Zhou, B.C.; Zhang, Y.Z.; Chen, X.L. Characterization and biotechnological potential analysis of a new exopolysaccharide from the arctic marine bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Du, Y.M.; Wu, H.Q.; Xiao, L. Relationship Between Molecular Structure and Moisture-Retention Ability of Carboxymethyl Chitin and Chitosan. J. Appl. Polym. Sci. 2002, 83, 1233–1241. [Google Scholar] [CrossRef]
- Sutherland, I.W. Novel and established applications of microbial polysac-charides. Trends Biotechnol. 1998, 16, 41–46. [Google Scholar] [CrossRef]
- El-Zaher, E.H.A.; Mostafa, A.A.; El-Souod, S.M.A.; Enas, M. Optimization and characterization of exopolysaccharides from Pleurotus salmoneo-stramineus and its possible application. Egypt. J. Exp. Biol. (Bot.) 2015, 11, 181–188. [Google Scholar]
- Zhang, X.F. The Screen of Polysaccharide-Producing Marine Bacteria and the Study of Its Polysaccharide Fermentation; Shandong Polytechnic University: Jinan, China, 2012. [Google Scholar]
- Boyle, C.D.; Reade, A.E. Characterization of two extracellular polysaccharides from marine bacteria. Appl. Environ. Microbiol. 1983, 46, 392–399. [Google Scholar] [PubMed]
- Park, S.; Kelley, K.A.; Vinogradov, E.; Solinga, R.; Weidenmaier, C.; Misawa, Y.; Lee, J.C. Characterization of the Structure and Biological Functions of a Capsular Polysaccharide Produced by Staphylococcus saprophyticus. J. Bacteriol. 2010, 192, 4618–4626. [Google Scholar] [CrossRef] [PubMed]
- Vincent, P.; Pignet, P.; Talmont, F.; Bozzi, L.; Fournet, B.; Guezennec, J.; Jeanthon, C.; Prieur, D. Production and characterization of an exopolysaccharide excreted by a deep-sea hydrothermal vent bacterium isolated from the polychaete annelid Alvinella pompejana. Appl. Environ. Microbiol. 1994, 60, 4134–4141. [Google Scholar] [PubMed]
- Steinmetz, I.; Rohde, M.; Brenneke, B. Purification and characterization of an exopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. Infect. Immun. 1995, 63, 3959–3965. [Google Scholar] [PubMed]
- Lv, Y.; Yang, X.B.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z.Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Xie, J.Z.; Zou, L.H.; Luo, X.; Qiu, L.; Wei, Q.; Luo, D.; Wu, Y.Q.; Jiao, Y. Structural characterization and immunomodulating activities of a novel polysaccharide from Nervilia fordii. Int. J. Biol. Macromol. 2018, 114, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Tang, C.; Li, Y.; Yang, Y.D.; Zhang, Y. Analysis of Monosaccharide Compositions of Polysaccharides in Coptidis Rhizoma by Pre-column Derivatization HPLC Method. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 74–78. [Google Scholar]
- Chen, Q.; Chen, J.; Du, H.; Li, Q.; Chen, J.; Zhang, G.; Liu, H.; Wang, J. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. Int. J. Mol. Sci. 2014, 15, 11446–11455. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
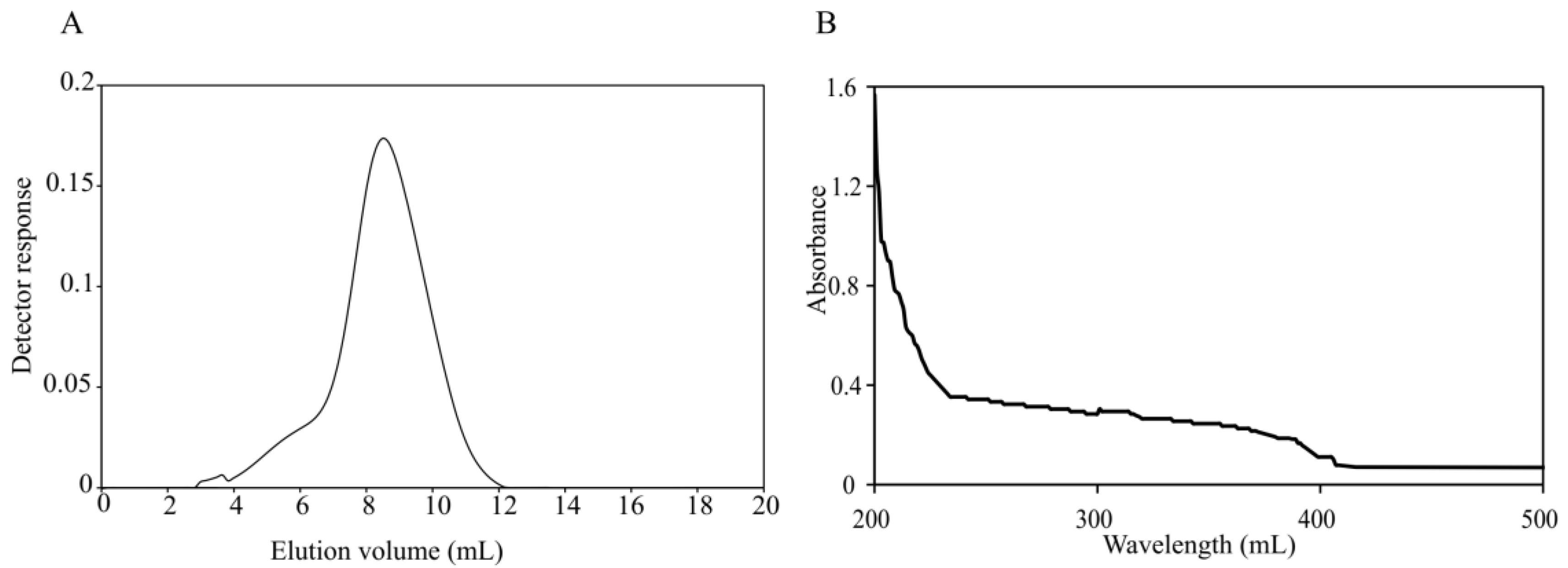
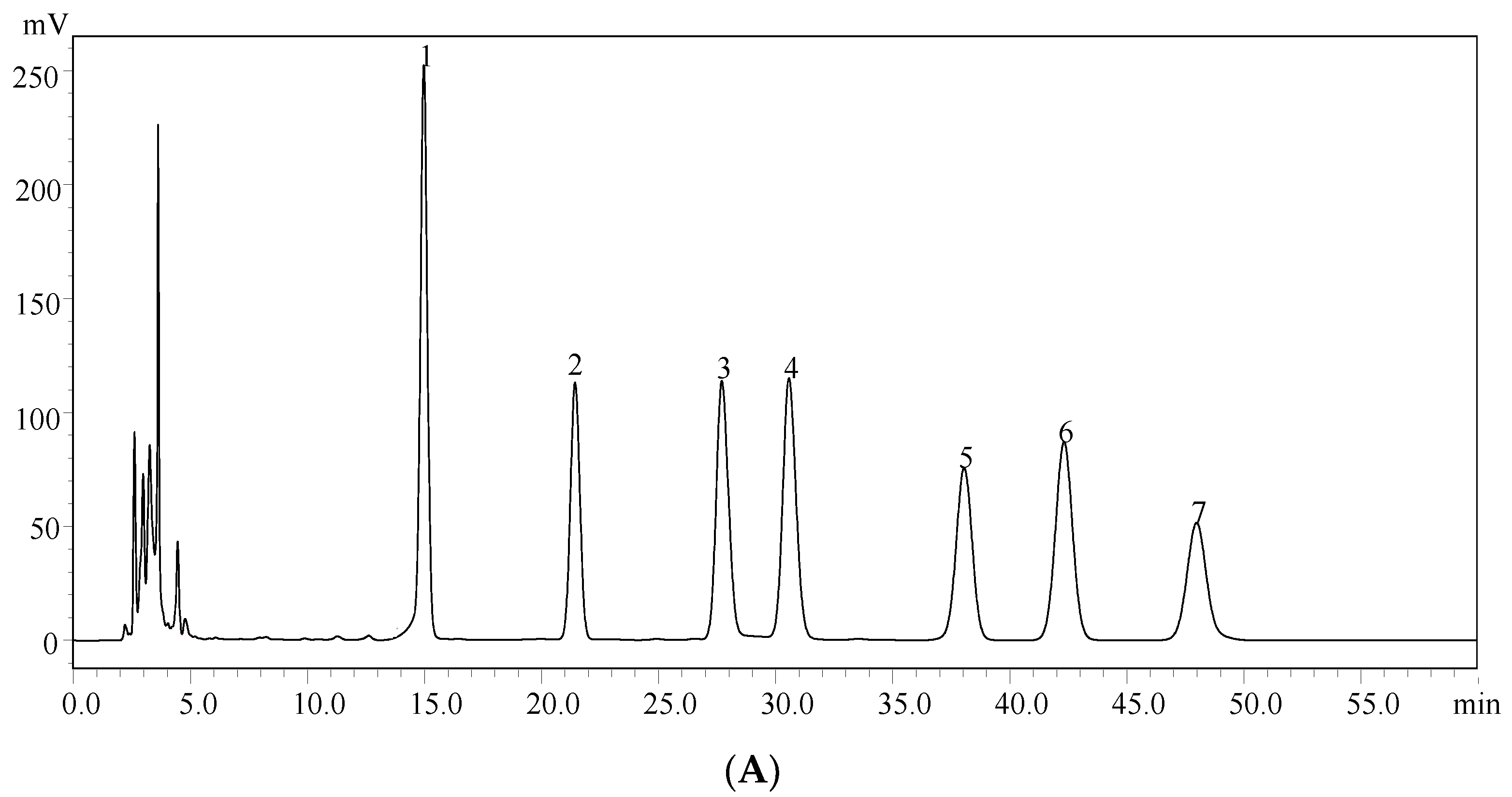
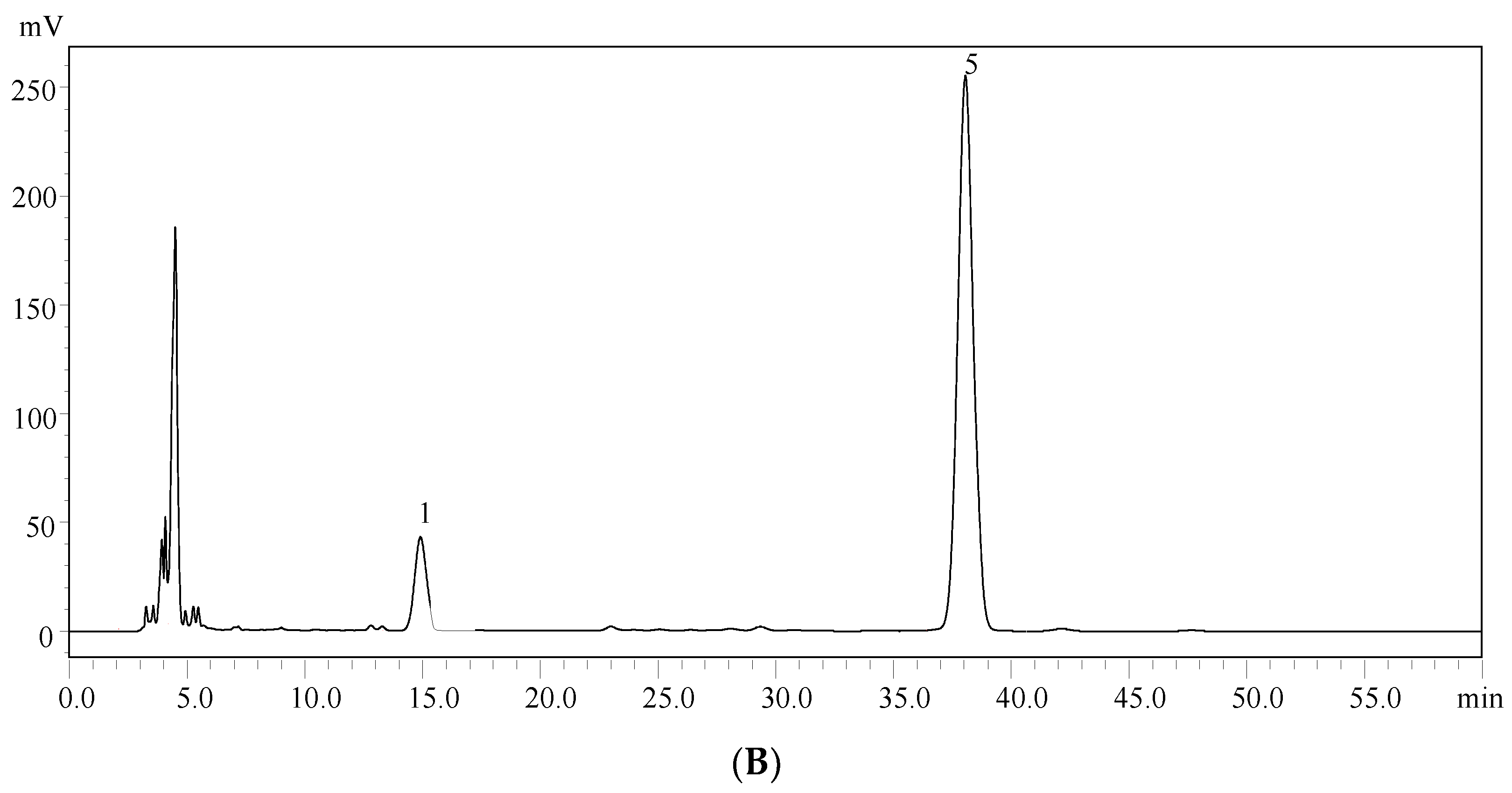
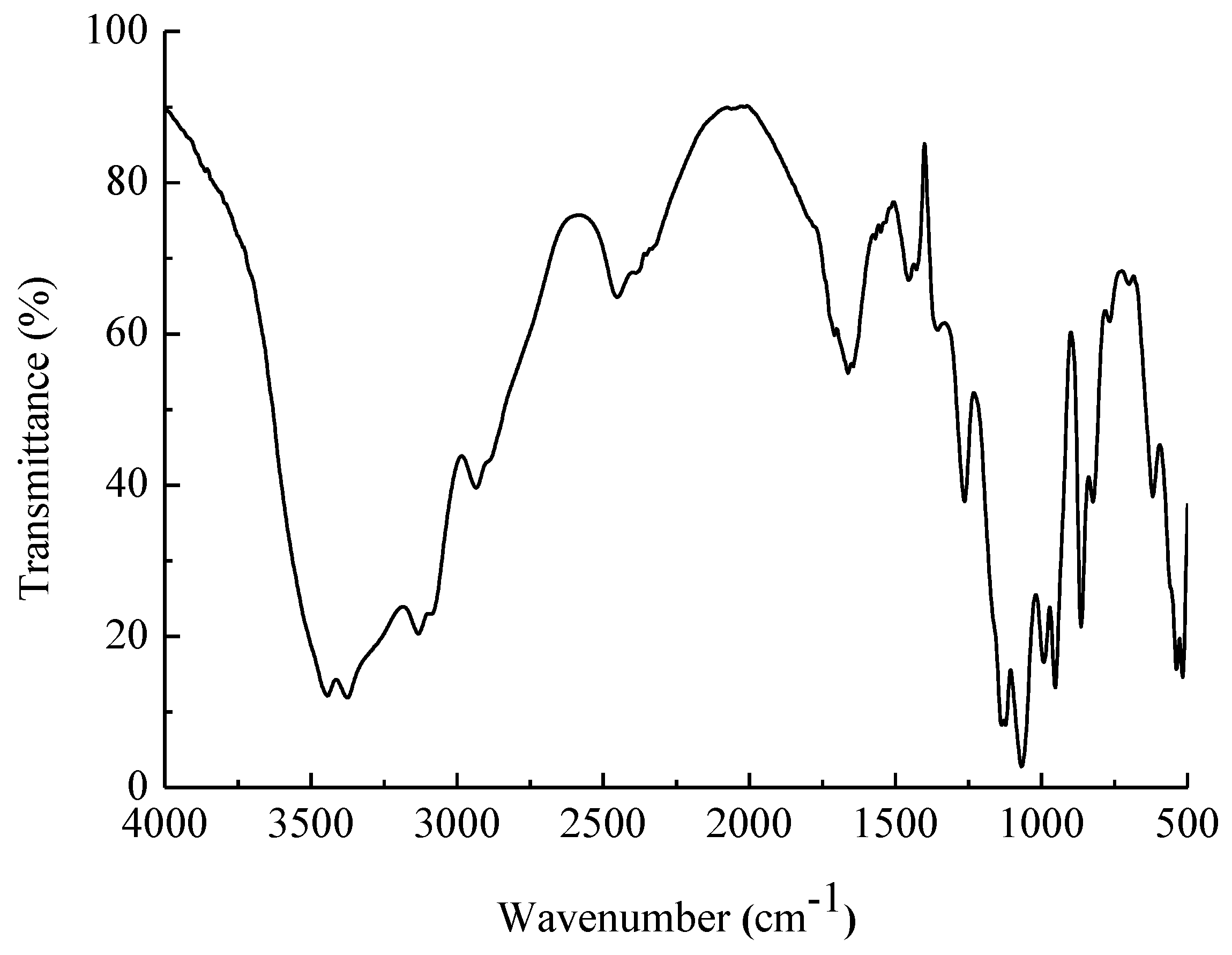
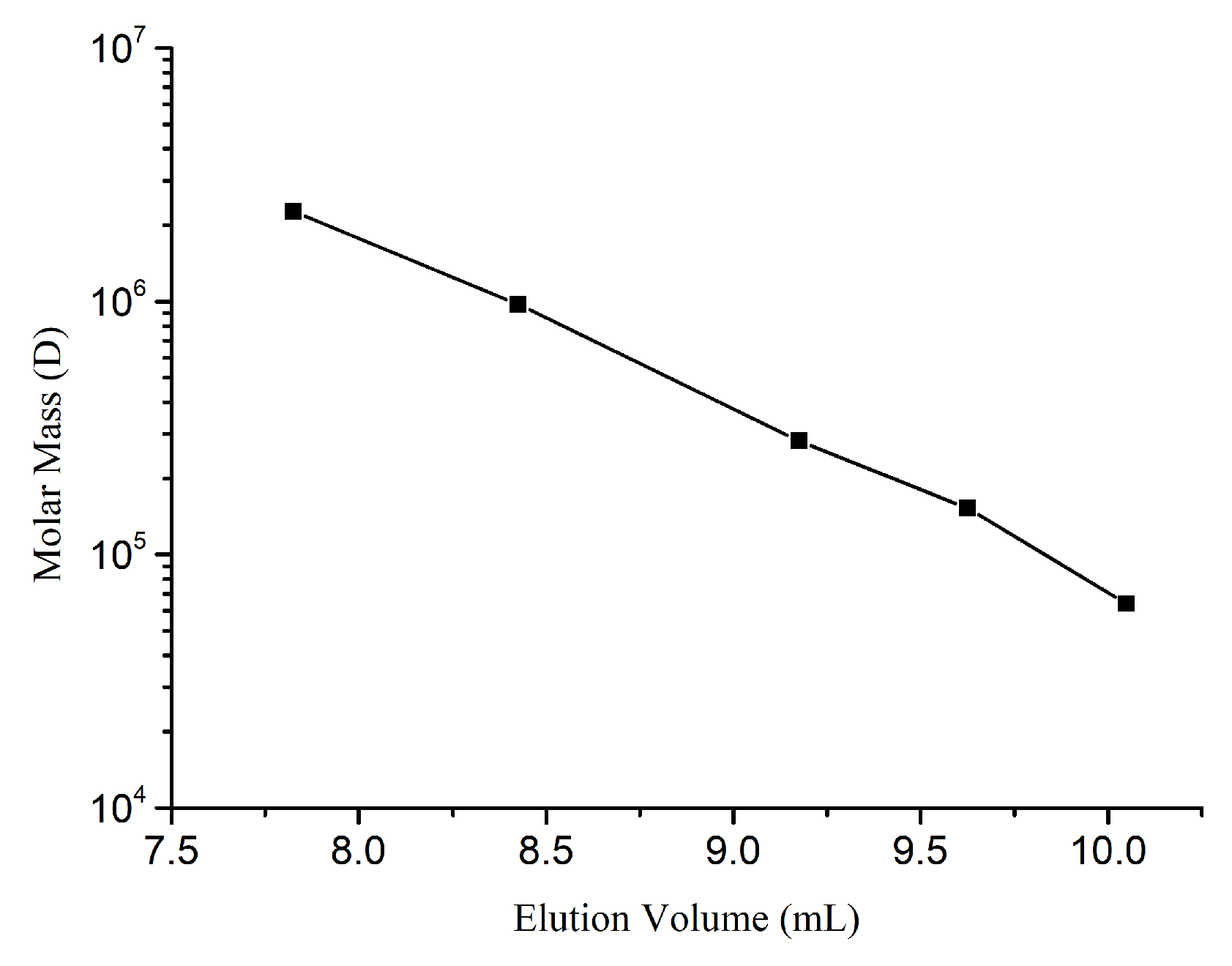
| Total Sugar Content (g/L) | Number Average Molecular Mass (g/mol) | Weight Average Molecular Mass (g/mol) | Distribution Coefficient |
|---|---|---|---|
| 2.34 | 2.22 × 105 | 2.84 × 105 | 1.28 |
| Standard Samples LOT | MW (g/mol) | Weight (mg) |
|---|---|---|
| PSS929n | 63,900 | 10.1 |
| PSS8065n | 152,000 | 10.2 |
| PSS13092 | 282,000 | 10.1 |
| PSS14052 | 976,000 | 10.0 |
| PSS9304-4 | 2,260,000 | 10.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Fan, Q.; Zhang, X.; Lu, X.; Xu, Y.; Zhu, W.; Zhang, J.; Hao, W.; Hao, L. Isolation, Characterization, and Pharmaceutical Applications of an Exopolysaccharide from Aerococcus Uriaeequi. Mar. Drugs 2018, 16, 337. https://doi.org/10.3390/md16090337
Wang C, Fan Q, Zhang X, Lu X, Xu Y, Zhu W, Zhang J, Hao W, Hao L. Isolation, Characterization, and Pharmaceutical Applications of an Exopolysaccharide from Aerococcus Uriaeequi. Marine Drugs. 2018; 16(9):337. https://doi.org/10.3390/md16090337
Chicago/Turabian StyleWang, Chunlei, Qiuping Fan, Xiaofei Zhang, Xiaoping Lu, Yanrui Xu, Wenxing Zhu, Jie Zhang, Wen Hao, and Lujiang Hao. 2018. "Isolation, Characterization, and Pharmaceutical Applications of an Exopolysaccharide from Aerococcus Uriaeequi" Marine Drugs 16, no. 9: 337. https://doi.org/10.3390/md16090337
APA StyleWang, C., Fan, Q., Zhang, X., Lu, X., Xu, Y., Zhu, W., Zhang, J., Hao, W., & Hao, L. (2018). Isolation, Characterization, and Pharmaceutical Applications of an Exopolysaccharide from Aerococcus Uriaeequi. Marine Drugs, 16(9), 337. https://doi.org/10.3390/md16090337





