Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway
Abstract
1. Introduction
2. Results and Discussion
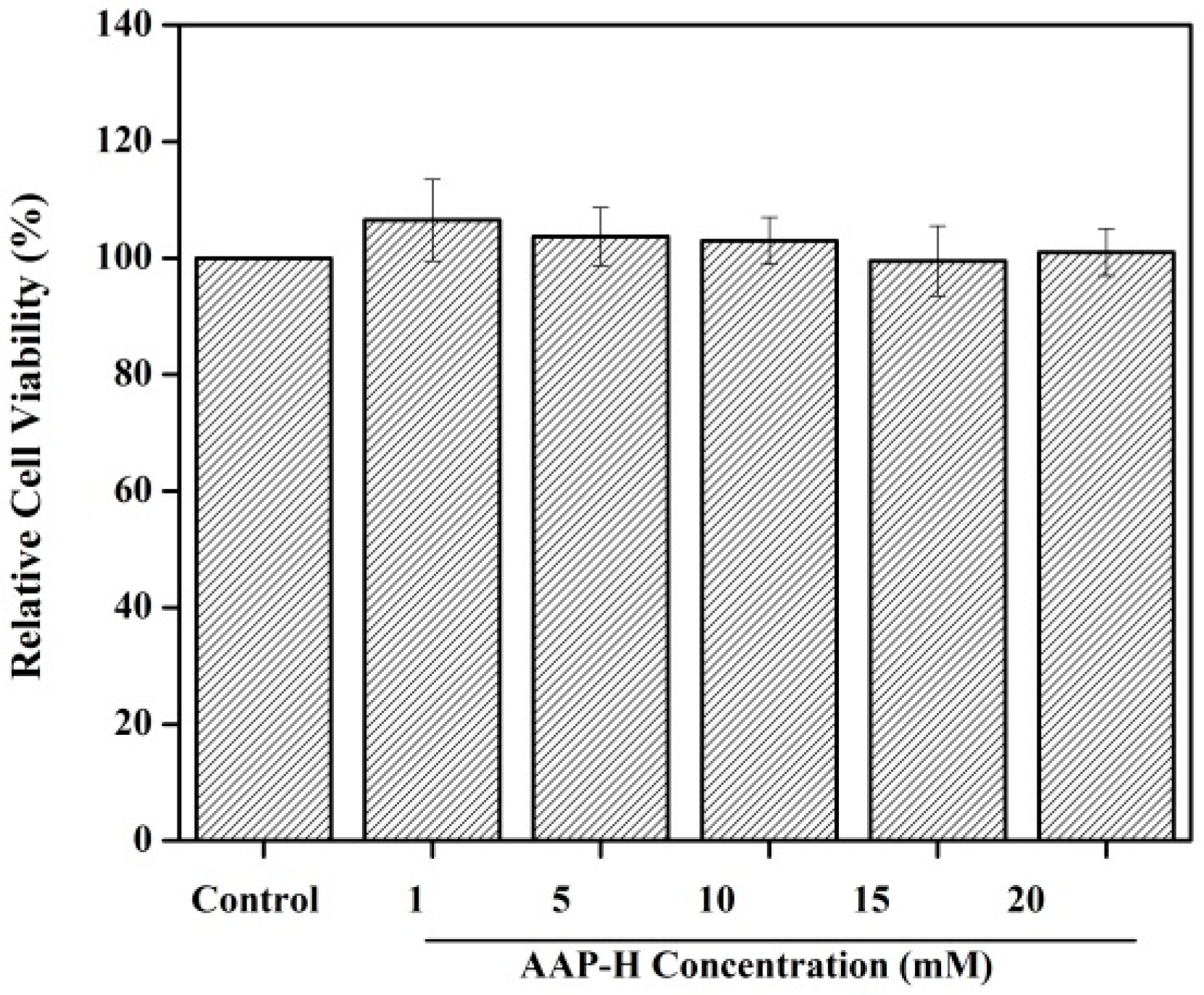
2.1. Toxicity to Normal Cells
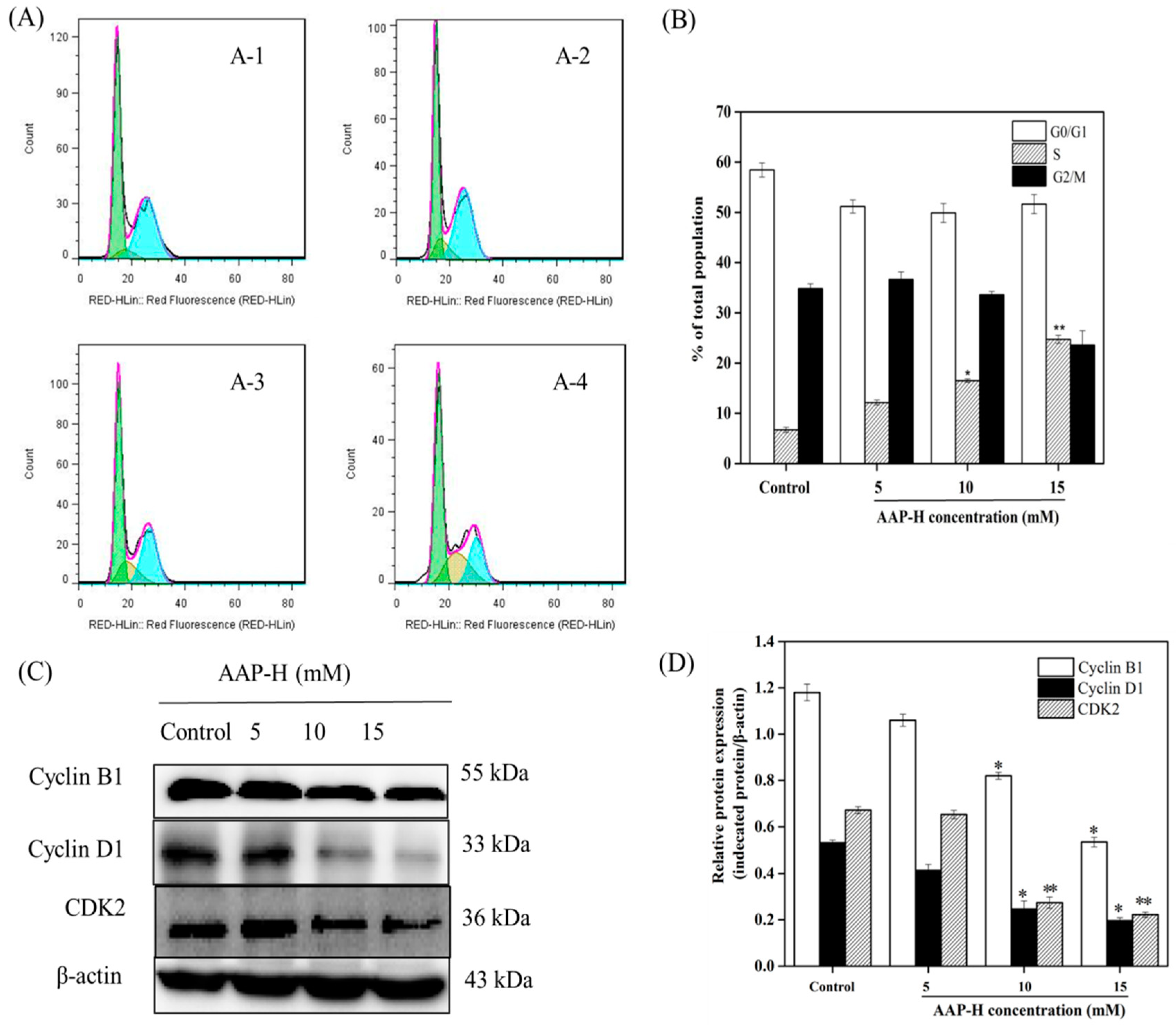
2.2. AAP-H Induced Cycle Arrest of DU-145 Cells
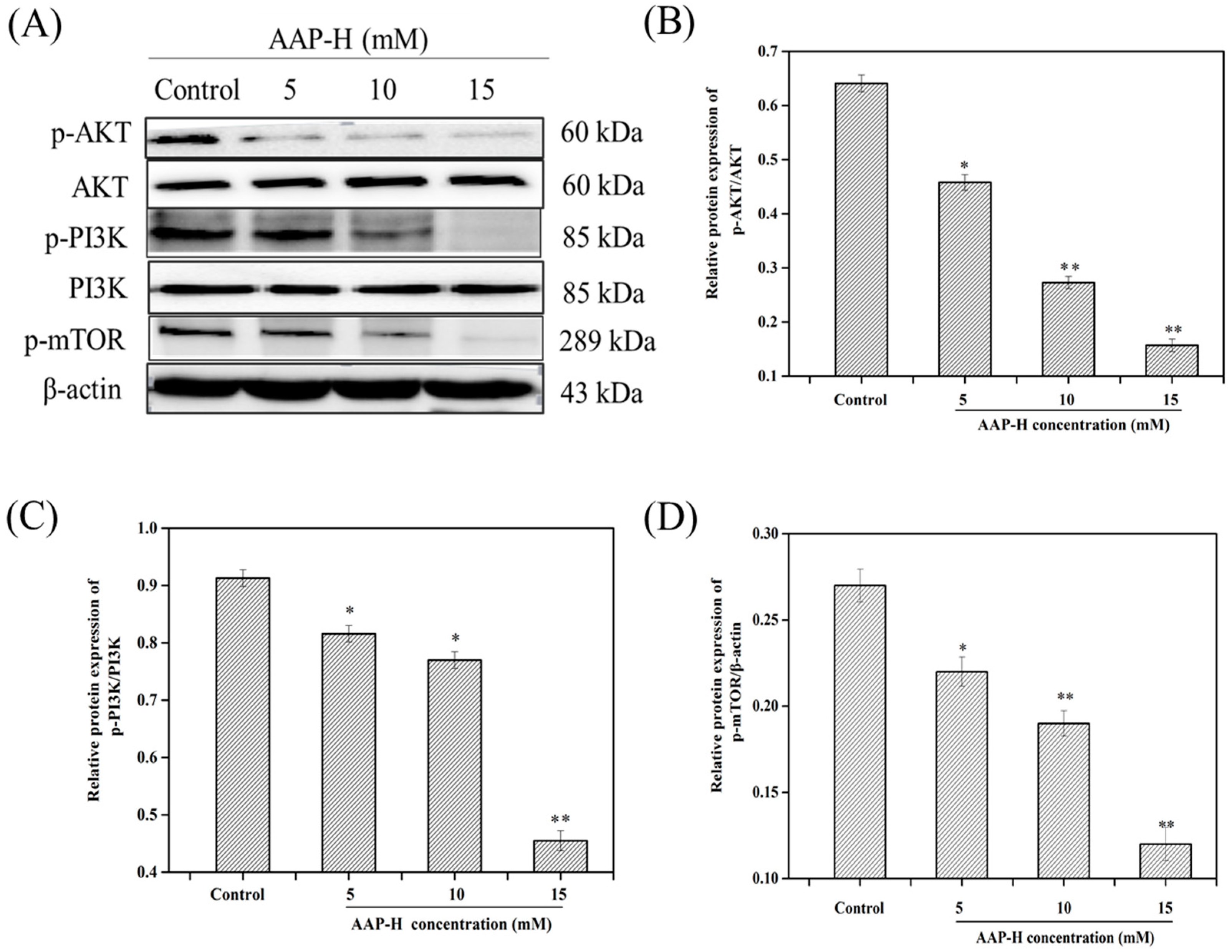
2.3. AAP-H Suppressed the PI3K/AKT/mTOR Signaling Pathway in DU-145 Cells
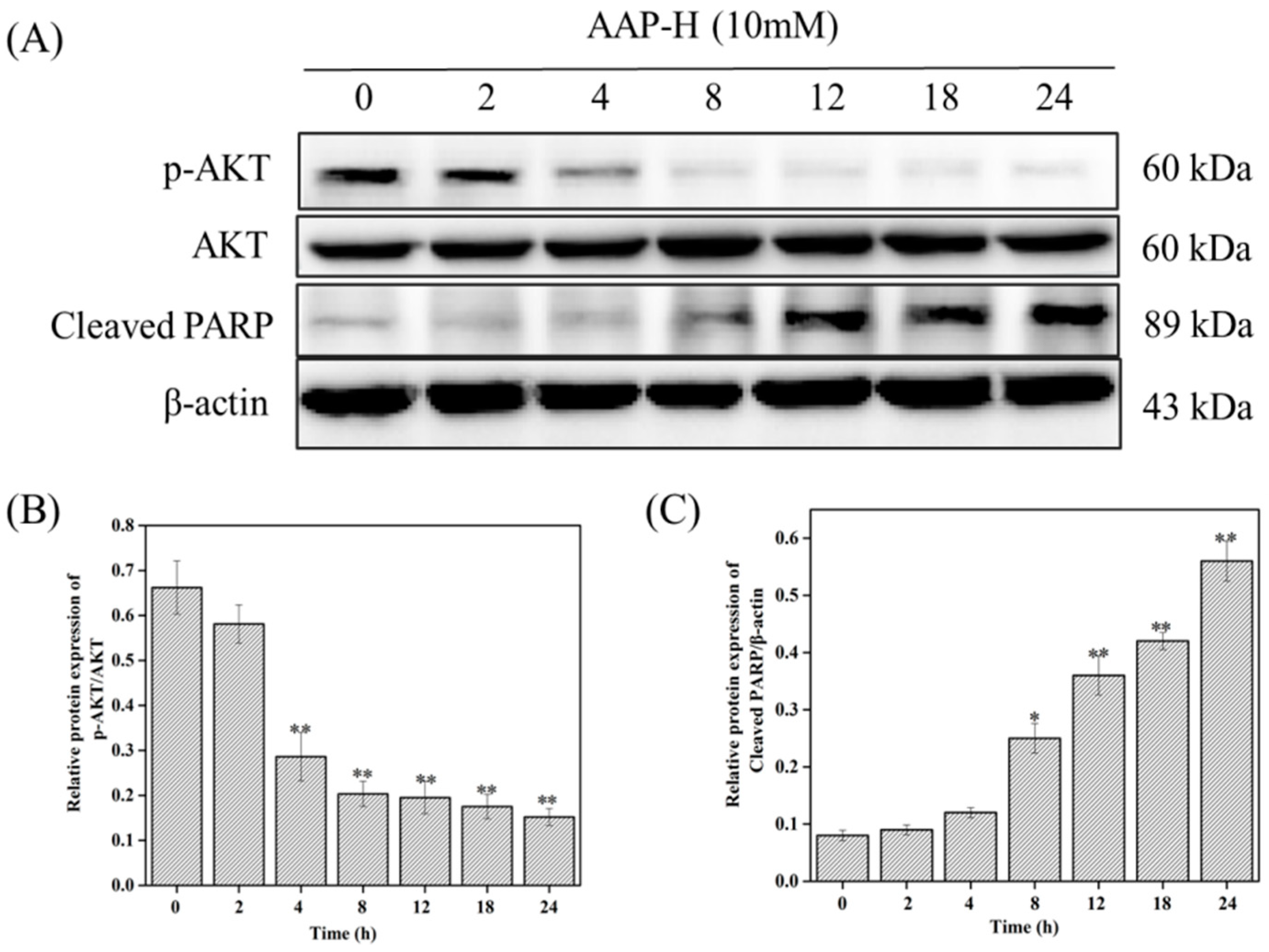
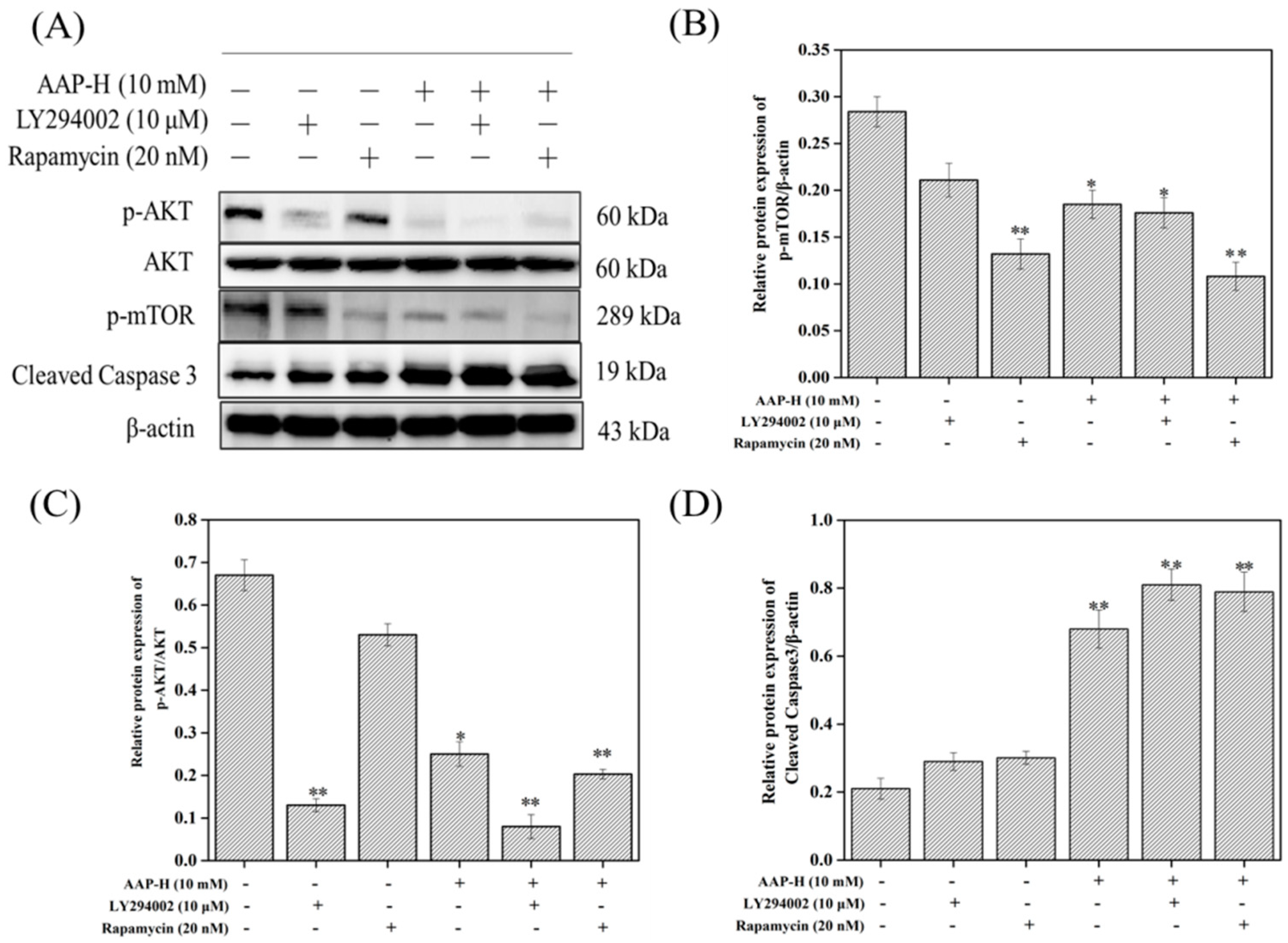
2.4. PI3K/AKT/mTOR Signaling Involved in AAP-H-Induced Apoptosis
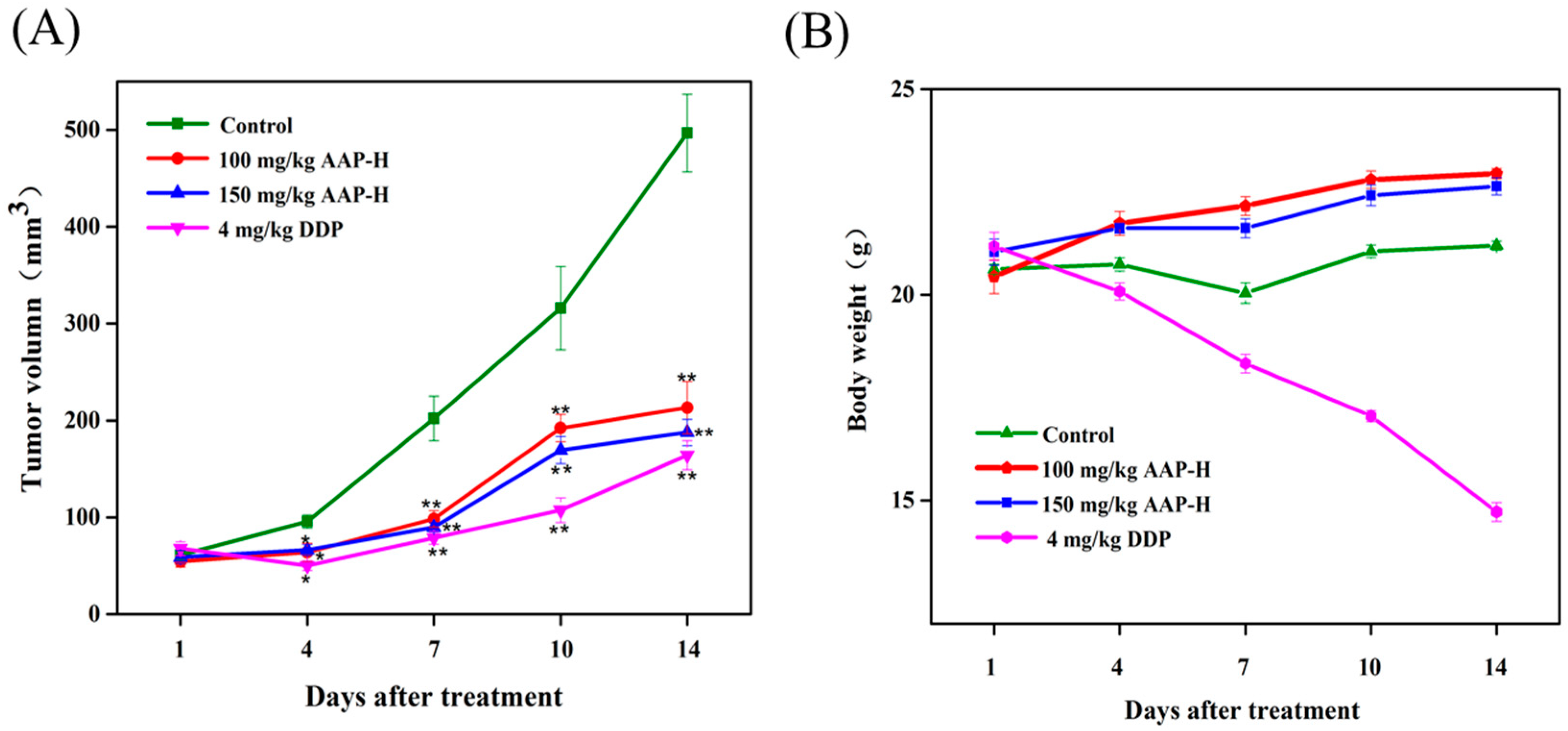
2.5. Effect of AAP-H on DU-145 Xenografts
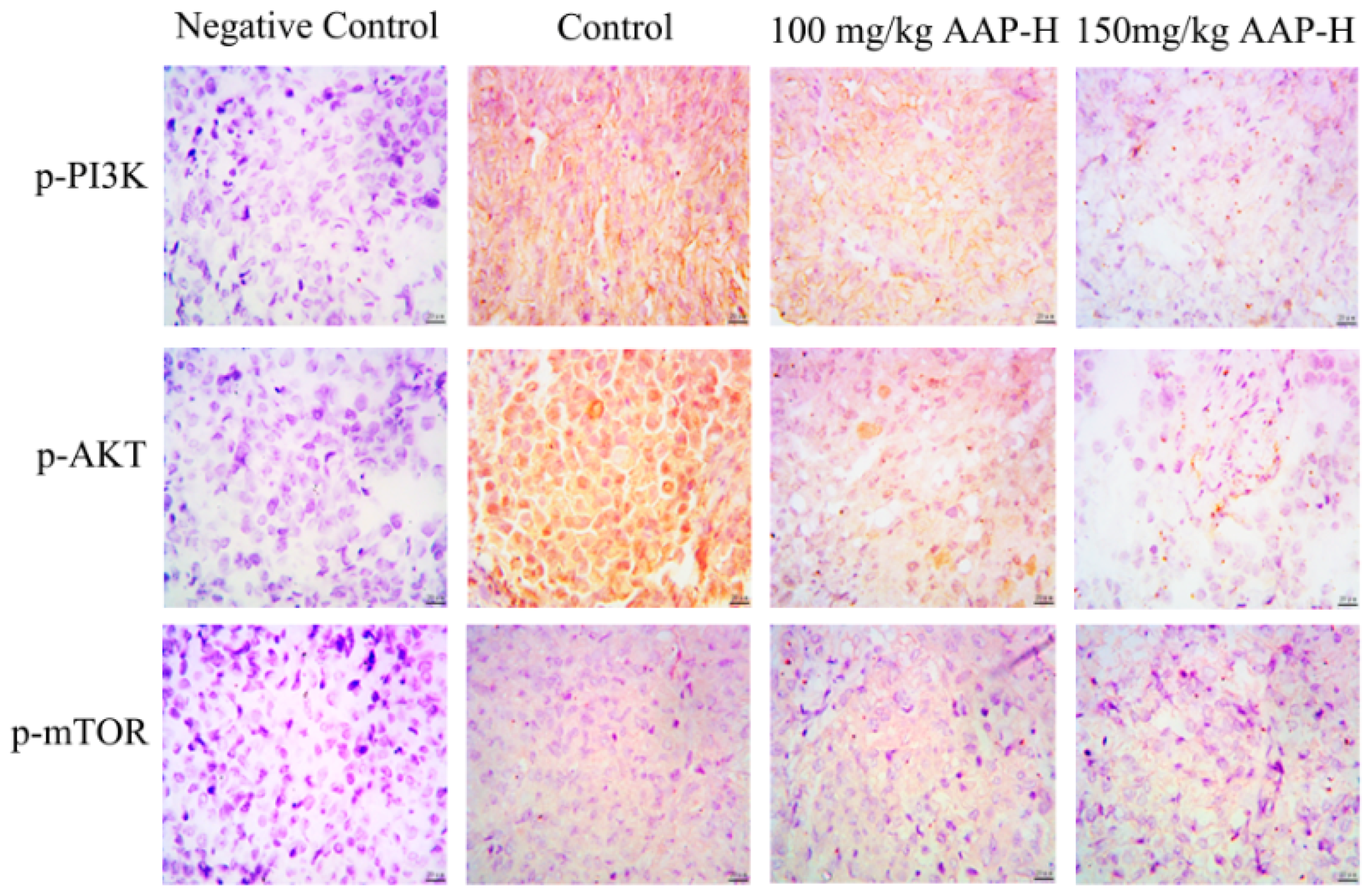
2.6. Immunocytochemistry
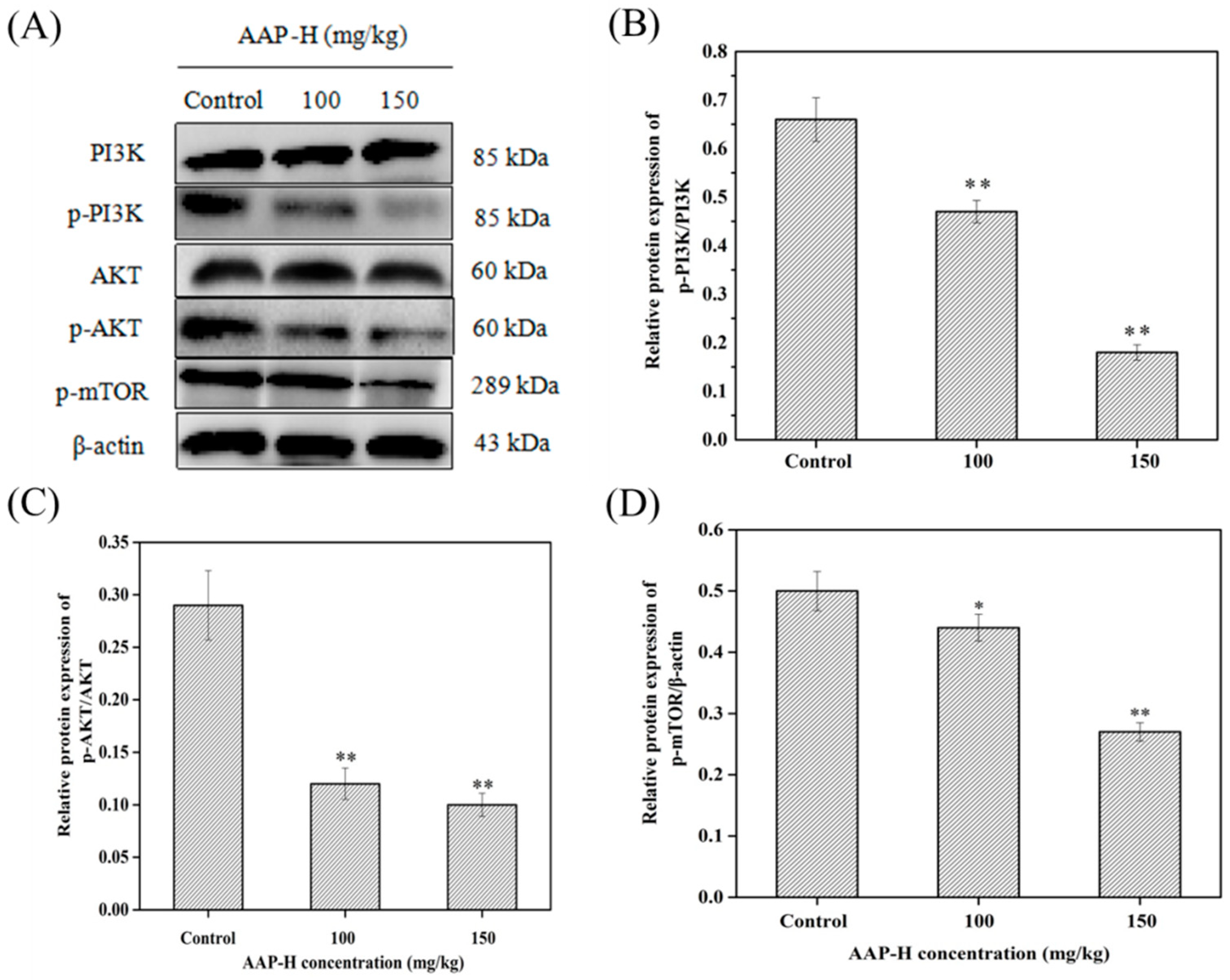
2.7. AAP-H Suppressed the PI3K/AKT/mTOR Signaling Pathway in DU-145 Xenografts
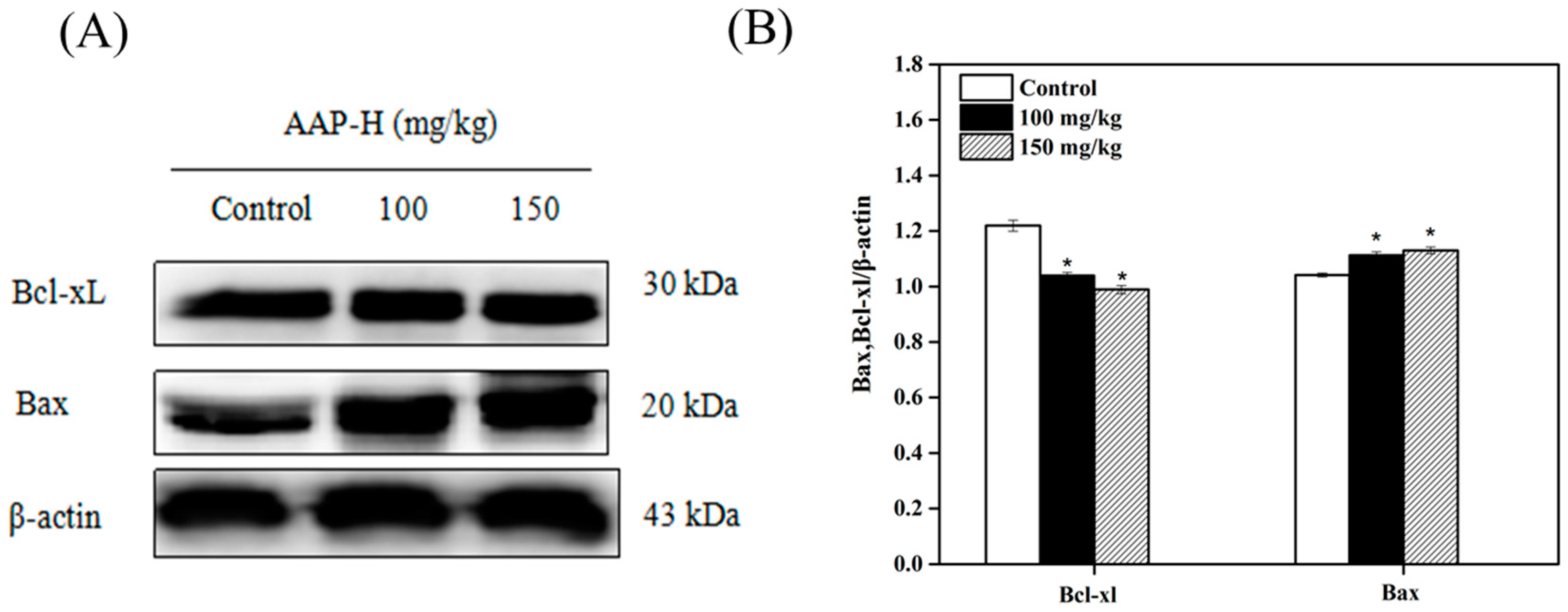
2.8. Western Blotting Analysis of Bcl-2 Family Members
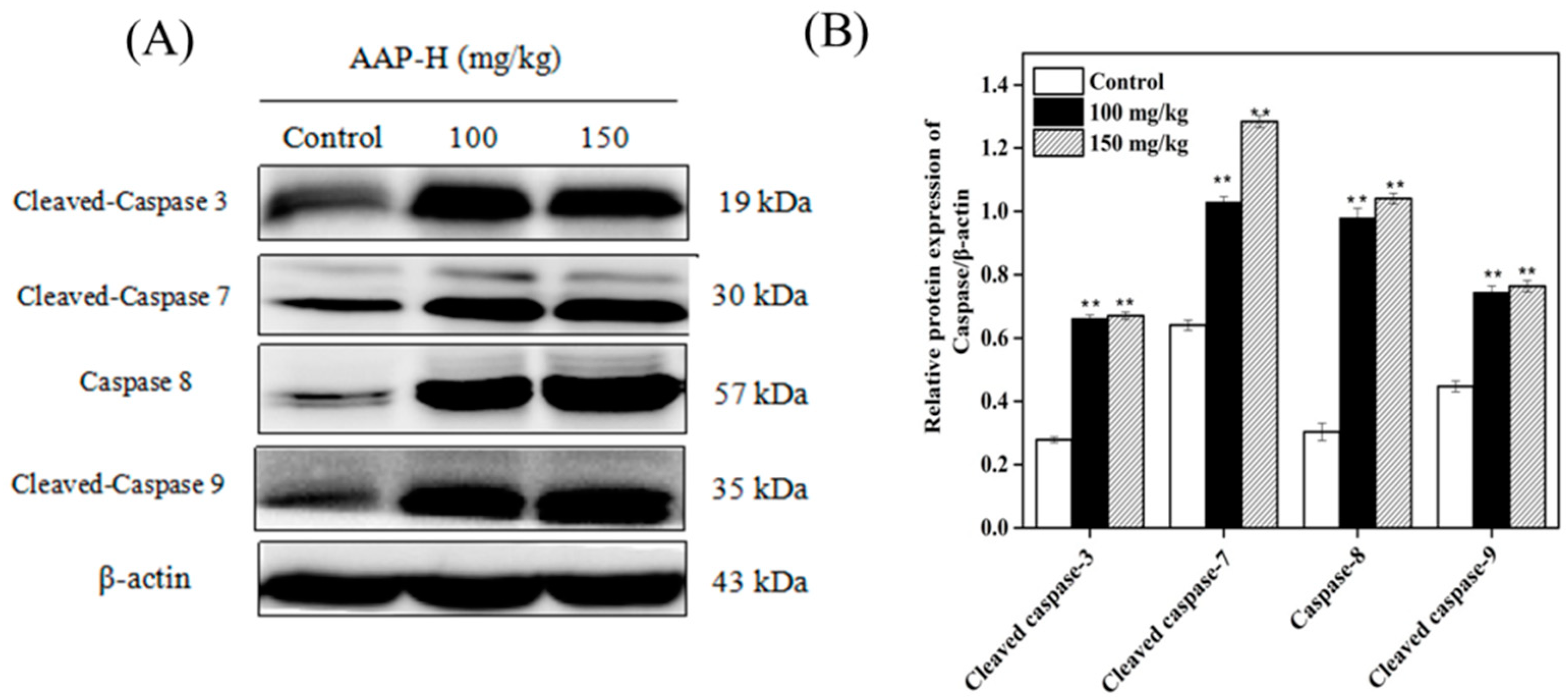
2.9. Western Blotting of Caspases
3. Materials and Methods
3.1. Materials and Reagents
3.2. Cell Toxicity Assay
3.3. Determination of Cell Cycle Arrest
3.4. Western Blot Analysis
3.5. Effect of AAP-H on DU-145 Xenografts
3.6. Immunocytochemistry (IHC)
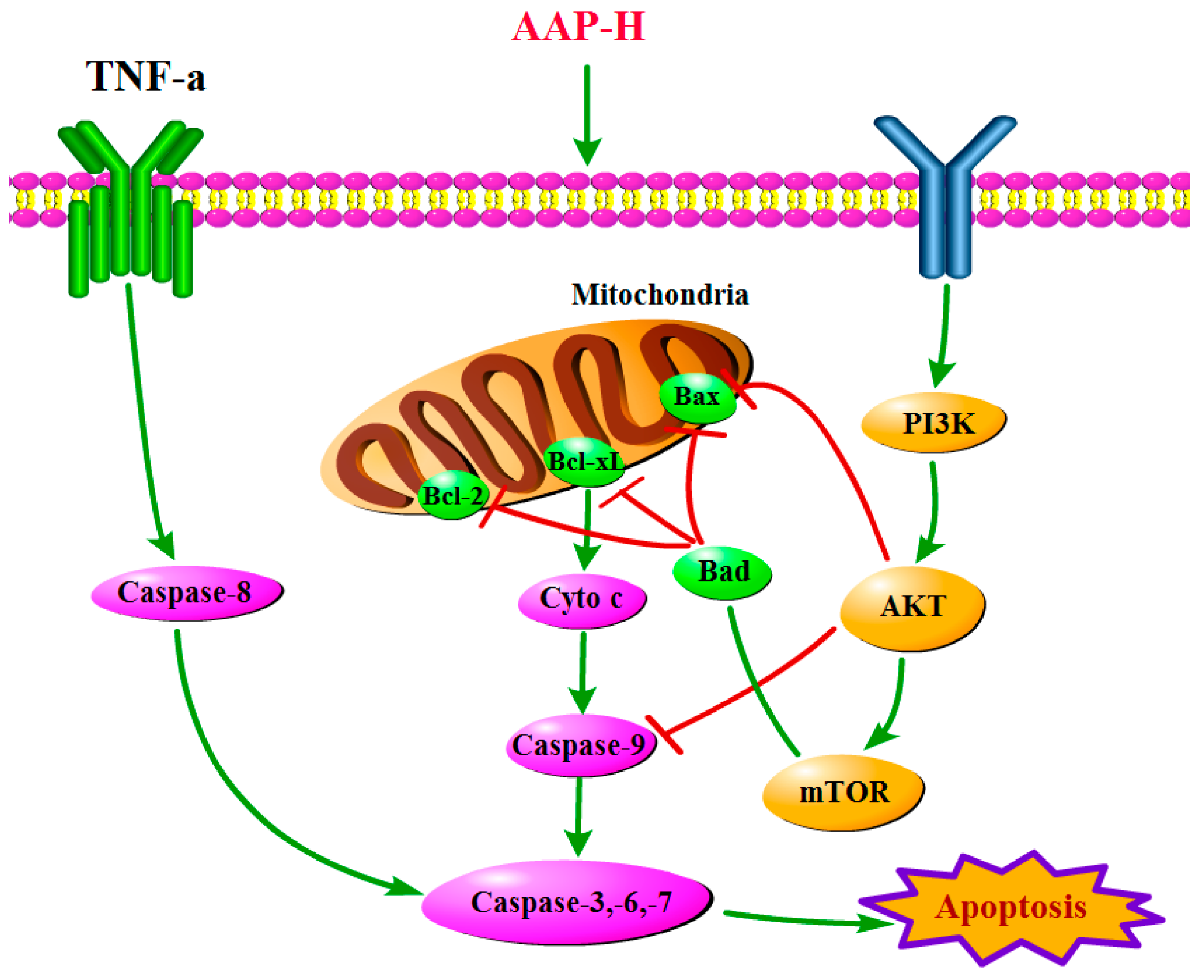
4. Conclusions
Author Contributions
Funding
Conflicts of interest
References
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Andrianasolo, E.; Mcphail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar] [PubMed]
- Arumugam, V.; Venkatesan, M.; Ramachandran, S.; Sundaresan, U. Bioactive Peptides from Marine Ascidians and Future Drug Development—A Review. Int. J. Pept. Res. Ther. 2017, 24, 13–18. [Google Scholar] [CrossRef]
- Beltran, H.; Beer, T.M.; Carducci, M.A.; de Bono, J.; Gleave, M.; Hussain, M.; Kelly, W.K.; Saad, F.; Sternberg, C.; Tagawa, S.T. New therapies for castration-resistant prostate cancer: Efficacy and safety. Eur. Urol. 2011, 60, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Ito, K. Prostate cancer in Asian men. Nat. Rev. Urol. 2014, 11, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.B.; Luo, H.Y.; Yang, Z.S. Isolation and identification of an antiproliferative peptide derived from heated products of peptic hydrolysates of half-fin anchovy (Setipinna taty). J. Funct. Foods 2014, 10, 104–111. [Google Scholar] [CrossRef]
- Karavelioglu, E.; Gonul, Y.; Aksit, H.; Boyaci, M.G.; Karademir, M.; Simsek, N.; Guven, M.; Atalay, T.; Rakip, U. Cabazitaxel causes a dose-dependent central nervous system toxicity in rats. J. Neurol. Sci. 2016, 360, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Frederiks, C.N.; Lam, S.W.; Guchelaar, H.J.; Boven, E. Genetic polymorphisms and paclitaxel- or docetaxel-induced toxicities: A systematic review. Cancer Treat. Rev. 2015, 41, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Cho, C.H. Development of peptides as potential drugs for cancer therapy. Curr. Pharm. Des. 2010, 16, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Engelman, J.A.; Cantley, L.C. Targeting the PI3K signaling pathway in cancer. Curr. Opin. Genet. Dev. 2010, 20, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Knight, Z.A.; Shokat, K.M. Chemically targeting the PI3K family. Biochem. Soc. Trans. 2007, 35, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Hidalgo, M.; Stadler, W.M.; Logan, T.F.; Dutcher, J.P.; Hudes, G.R.; Park, Y.; Liou, S.H.; Marshall, B.; Boni, J.P.; et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J. Clin. Oncol. 2004, 22, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Inhibition of PI3K/Akt/mTOR Signaling by Natural Products. Anti-Cancer Agent Med. Chem. 2012, 13, 967–970. [Google Scholar] [CrossRef]
- Stein, R.C. Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment. Endocr.-Relat. Cancer 2001, 8, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, S.I.; Zvelebil, M.J.; Shuttleworth, S.J.; Hancox, T.; Saghir, N.; Timms, J.F.; Waterfield, M.D. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem. J. 2007, 404, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ben, M.; Rodrigo, D.; Josep, T. Targeting the PI3K/Akt/mTOR Pathway-Beyond Rapalogs. Oncotarget 2010, 1, 530–543. [Google Scholar]
- Maček, P. Polypeptide cytolytic toxins from sea anemones (Actiniaria). Toxicon 1992, 40, 111–124. [Google Scholar]
- Ramezanpour, M.; Silva, K.B.D.; Sanderson, B.J. Differential susceptibilities of human lung, breast and skin cancer cell lines to killing by five sea anemone venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 157–163. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Ding, G.F.; Huang, F.F.; Yang, Z.S.; Yu, F.M.; Tang, Y.P.; Jia, Y.L.; Zheng, Y.Y.; Chen, R. Anticancer Activity of Anthopleura anjunae Oligopeptides in Prostate Cancer DU-145 Cells. Mar. Drugs 2018, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jin, S.; Li, X.; Li, X.; Hu, X.; Chen, Y.; Huang, F.; Yang, Z.; Yu, F.; Ding, G. Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Yang, Z.; Di, Y.; Wang, J.; Rong, L.; Ding, G. Sepia Ink Oligopeptide Induces Apoptosis in Prostate Cancer Cell Lines via Caspase-3 Activation and Elevation of Bax/Bcl-2 Ratio. Mar. Drugs 2012, 10, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Y.; Liu, B.; Liu, C.; Liu, D.; Zhu, J.; Yang, C.; Yan, J.; Liao, X.; Meng, X.; et al. Bmi-1-shRNA Inhibits the Proliferation of Lung Adenocarcinoma Cells by Blocking the G1/S Phase Through Decreasing Cyclin D1 and Increasing p21/p27 Levels. Nucleic Acid Ther. 2014, 24, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Black, A.R.; Black, J.D. Protein kinase C signaling and cell cycle regulation. Front. Immunol. 2013, 3, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; Mccubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Cammer, M.; Gevrey, J.C.; Lorenz, M.; Dovas, A.; Condeelis, J.; Cox, D. The mechanism of CSF-1 induced wasp activation in vivo: A role for PI 3-kinase and CDC42. J. Biol. Chem. 2009. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, F.; Bai, C.; Yao, C.; Zhong, H.; Zou, C.; Chen, X. Sophoridine induces apoptosis and S phase arrest via ROS-dependent JNK and ERK activation in human pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhao-Min, L.; Nan, G.; Deng-Lu, Z.; Jie, H.; Feng, K. Ursolic Acid Induces Apoptosis of Prostate Cancer Cells via the PI3K/Akt/mTOR Pathway. Am. J. Chin. Med. 2015, 43, 1471–1486. [Google Scholar]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014, 343, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Yasumizu, Y.; Kosaka, T.; Miyazaki, Y.; Kikuchi, E.; Miyajima, A.; Oya, M. 1318 hypoxic microenvironment promotes PI3K/AKT/mTOR signaling pathways in human castration resistant prostate cancer. J. Urol. 2013, 189, e539. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Zhang, Y.; Guan, H.; Wu, J.; Zhu, X.; Yuan, J.; Li, M. MiR-503 targets PI3K p85 and IKK-β and suppresses progression of non-small cell lung cancer. Int. J. Gynecol. Cancer 2014, 135, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.L.; Wang, Y.C.; Lu, Z.Y.; Yu, X.F.; Sui, D.Y. 20(S)-Protopanaxadiol-Induced Apoptosis in MCF-7 Breast Cancer Cell Line through the Inhibition of PI3K/AKT/mTOR Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 1053. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.M.; Wang, X.F.; Huang, Q.X. Thymoquinone induces cytotoxicity and reprogramming of EMT in gastric cancer cells by targeting PI3K/Akt/mTOR pathway. J. Biosci. 2017, 42, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, S.; Zhang, Y.; Zhang, G.; Liu, S. Lycorine induces apoptosis in human pancreatic cancer cell line PANC-1 via ROS-mediated inactivation of the PI3K/Akt/mTOR signaling pathway. Int. J. Clin. Lab. Res. 2016, 9, 21048–21056. [Google Scholar]
- Song, L.; Chang, J.; Li, Z. A serine protease extracted from Trichosanthes kirilowii induces apoptosis via the PI3K/AKT-mediated mitochondrial pathway in human colorectal adenocarcinoma cells. Food Funct. 2015, 22, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Yeh, P.Y.; Yeh, K.H.; Lu, Y.S.; Huang, S.Y.; Cheng, A.L. Down-regulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2008, 68, 6698–6707. [Google Scholar] [CrossRef] [PubMed]
- Imrali, A.; Mao, X.; Yeste-Velasco, M.; Shamash, J.; Lu, Y. Rapamycin inhibits prostate cancer cell growth through cyclin D1 and enhances the cytotoxic efficacy of cisplatin. Am. J. Cancer Res. 2016, 6, 1772. [Google Scholar] [PubMed]
- Esmaeili, M.A.; Farimani, M.M.; Kiaei, M. Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling pathways, ROS-mediated pathway and mitochondrial dysfunction in hepatoblastoma cancer (HepG2) cells. Mol. Cell Biochem. 2014, 397, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Win, K.T.; Lee, S.W.; Huang, H.Y.; Lin, L.C.; Lin, C.Y.; Hsing, C.H.; Chen, L.T.; Li, C.F. Nicotinamide N-methyltransferase overexpression is associated with Akt phosphorylation and indicates worse prognosis in patients with nasopharyngeal carcinoma. Tumor Biol. 2013, 34, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Pontes, H.A.R.; Pontes, F.S.C.; de Jesus, A.S.; Soares, M.C.P.; Gonçalves, F.L.N.; de Lucena Botelho, T.; do Carmo Ribeiro, J.; dos Santos Pinto, D., Jr. p-Akt and its relationship with clinicopathological features and survival in oral squamous cell carcinoma: An immunohistochemical study. J. Oral Pathol. Med. 2015, 44, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cao, F.; Fang, W.; Hu, Y.; Chen, Y.; Ding, H.; Yu, G. Activation of SNAT1/SLC38A1 in human breast cancer: Correlation with p-Akt overexpression. BMC Cancer 2013, 13, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.T.; Zhang, W.H.; Miller, C.R.; Watters, J.W.; Gao, F.; Viswanathan, A.; Govindan, R.; Mcleod, H.L. PTEN and phosphorylated AKT expression and prognosis in early- and late-stage non-small cell lung cancer. Oncol. Rep. 2007, 17, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Mcleod, H.L. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anti-Cancer Drug 2005, 16, 797–803. [Google Scholar] [CrossRef]
- Hager, M.; Haufe, H.; Kemmerling, R.; Mikuz, G.; Kolbitsch, C.; Moser, P.L. PTEN expression in renal cell carcinoma and oncocytoma and prognosis. Pathology 2007, 39, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Brech, A.; Ahlquist, T.; Lothe, R.A.; Stenmark, H. Autophagy in tumour suppression and promotion. Mol. Oncol. 2009, 3, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Matuzaki, H. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem. Biophys. Res. Commun. 1999, 264, 550–555. [Google Scholar]
- Goyal, A.; Wang, Y.; Graham, M.M.; Doseff, A.I.; Bhatt, N.Y.; Marsh, C.B. Monocyte survival factors induce Akt activation and suppress caspase-3. Am. J. Resp. Cell Mol. 2002, 26, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Tomomi, K.; Newmeyer, D.D. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 2003, 15, 691–699. [Google Scholar]
- Bosq, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 2012, 11, 109. [Google Scholar]
- Fulda, S. Caspase-8 in cancer biology and therapy. Cancer Lett. 2009, 281, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Simone, F. Therapeutic opportunities based on caspase modulation. Cell Dev. Biol. 2017. [Google Scholar] [CrossRef]
- Fulda, S.; Küfer, M.U.; Meyer, E.; Van, V.F.; Dockhorn-Dworniczak, B.; Debatin, K.M. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene 2001, 20, 5865–5877. [Google Scholar] [CrossRef] [PubMed]
- Zong-Ze, W.U.; Ding, G.F.; Yang, Z.S.; Fang-Miao, Y.U.; Tang, Y.P.; Jia, Y.L.; Zheng, Y.Y.; Chen, R. Enzymatic preparation of oligopeptide from anthopleura anjunae and its anti-cancer activity of prostate cancer cells. Oceanol. Limnol. Sin. 2017, 48, 1114–1123. [Google Scholar]
- Tang, Y.; Yu, F.; Zhang, G.; Yang, Z.; Huang, F.; Ding, G. A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells. Molecules 2017, 22, 1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. CSH Perspect. Med. 2016, 6, a026104–a026119. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.J.; Xing, X.M.; Wang, C.F.; Hu, L.T.; Zhao, G.Q.; Liu, X.P.; Wu, K.; Li, H. Effect of recombinant human platelet derived growth factor B on cat corneal endothelial cell viability mediated by adeno-associated virus. Int. J. Ophthalmol. 2012, 5, 419–423. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tang, Y.; Yu, F.; Sun, Y.; Huang, F.; Chen, Y.; Yang, Z.; Ding, G. Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway. Mar. Drugs 2018, 16, 325. https://doi.org/10.3390/md16090325
Li X, Tang Y, Yu F, Sun Y, Huang F, Chen Y, Yang Z, Ding G. Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway. Marine Drugs. 2018; 16(9):325. https://doi.org/10.3390/md16090325
Chicago/Turabian StyleLi, Xiaojuan, Yunping Tang, Fangmiao Yu, Yu Sun, Fangfang Huang, Yan Chen, Zuisu Yang, and Guofang Ding. 2018. "Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway" Marine Drugs 16, no. 9: 325. https://doi.org/10.3390/md16090325
APA StyleLi, X., Tang, Y., Yu, F., Sun, Y., Huang, F., Chen, Y., Yang, Z., & Ding, G. (2018). Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway. Marine Drugs, 16(9), 325. https://doi.org/10.3390/md16090325





