Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice
Abstract
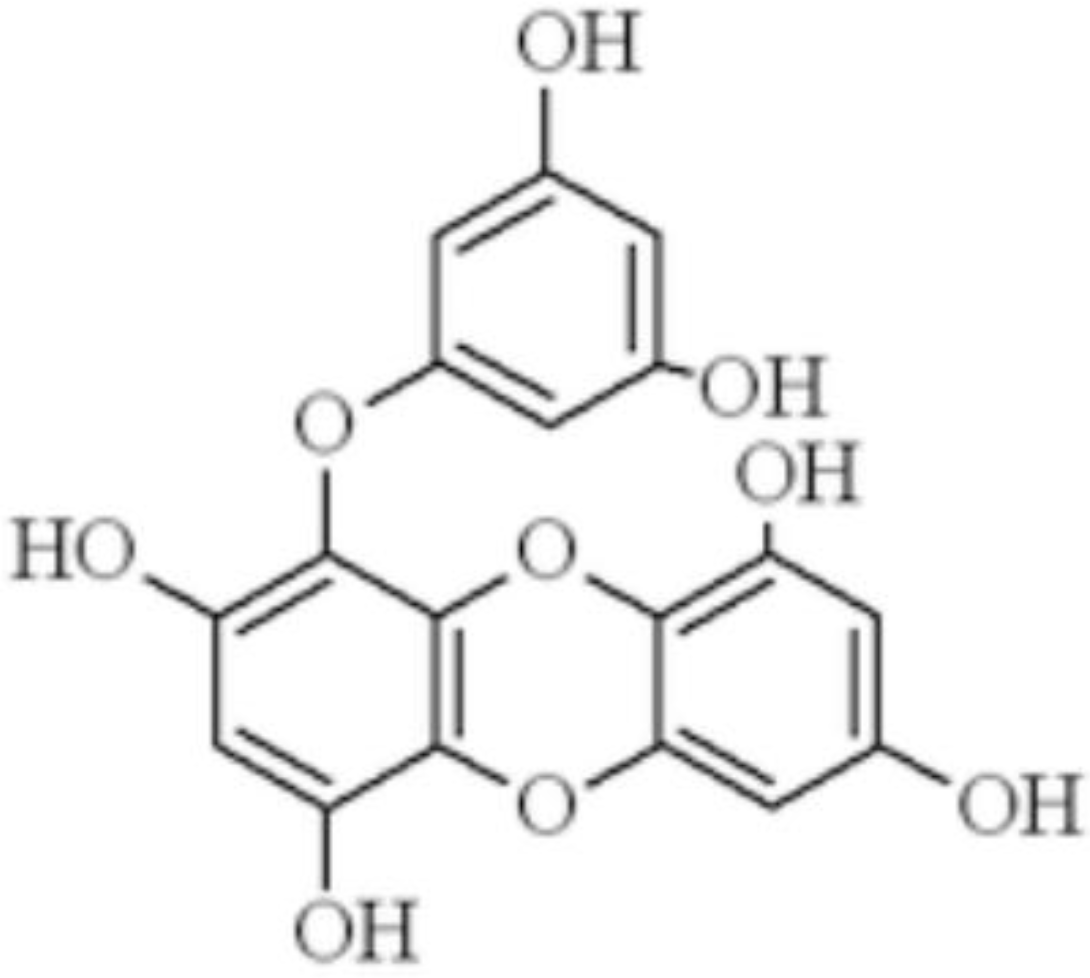
1. Introduction
2. Results and Discussion
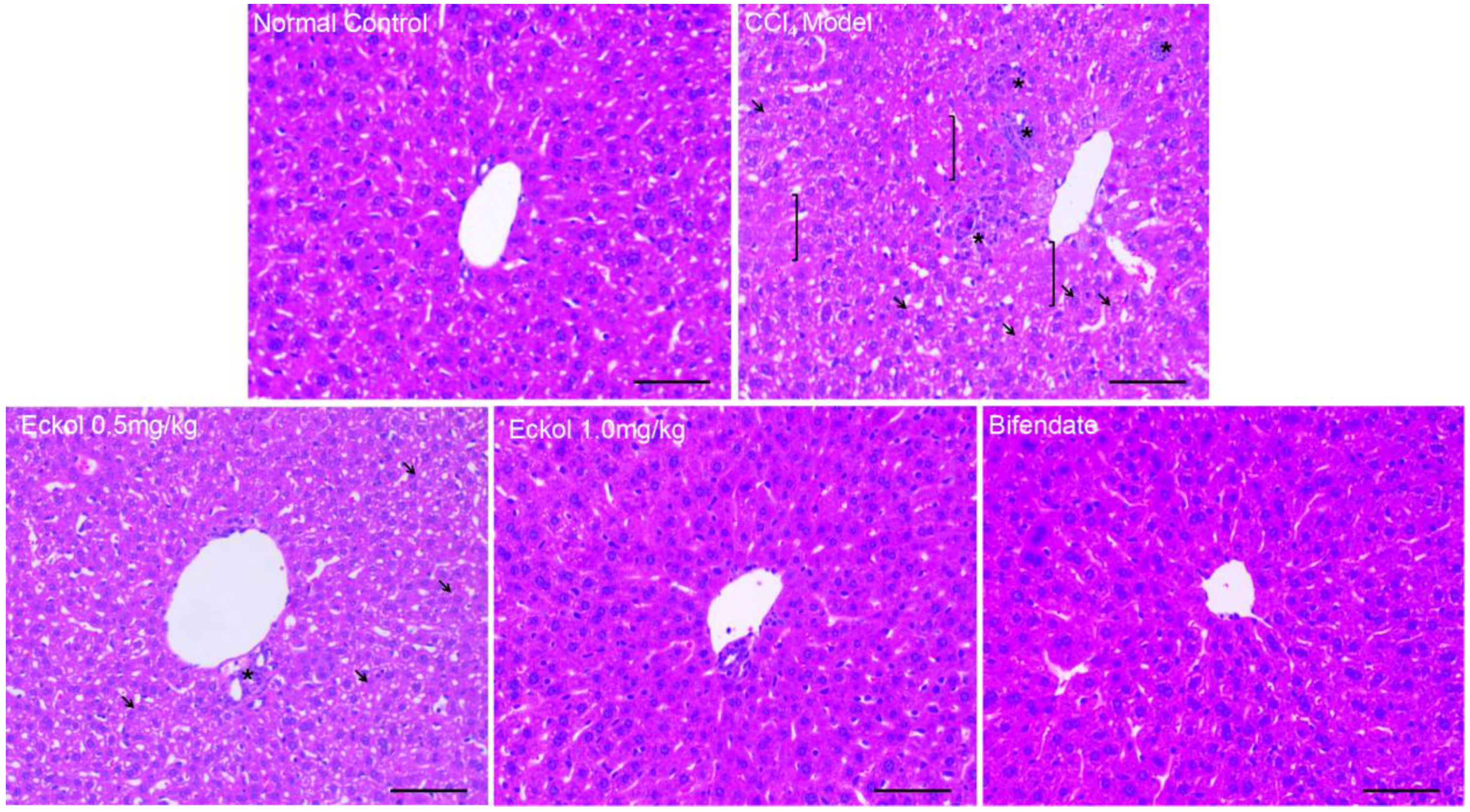
2.1. Eckol Ameliorates Acute Liver Injury Induced by CCl4 Exposure
2.2. Eckol Protects Hepatocytes from CCl4-Mediated Apoptosis
2.3. Eckol Relieves CCl4-Induced Hepatic Oxidative Stress
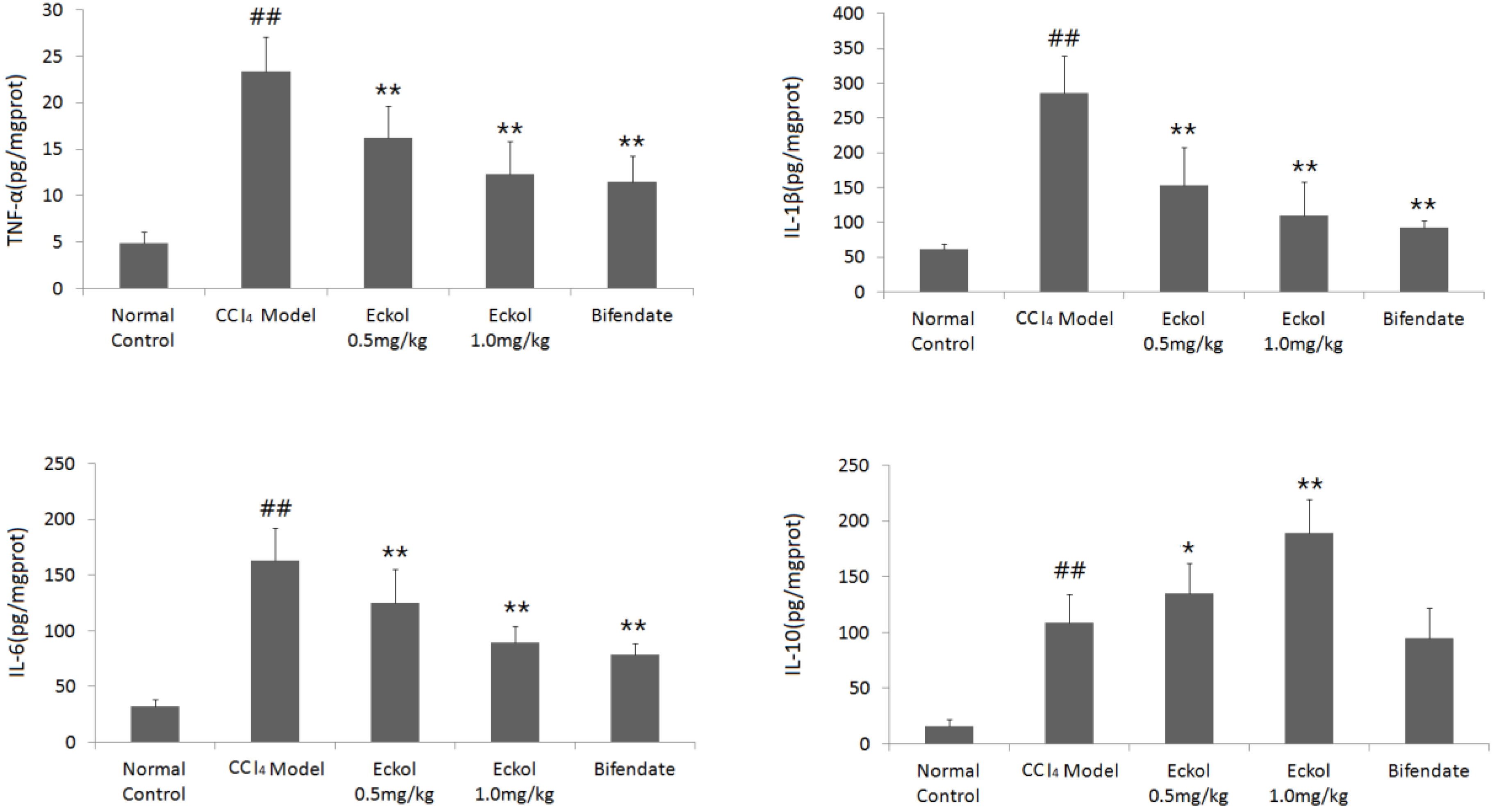
2.4. Eckol Regulates the Levels of Cytokines in Livers of CCl4-Treated Mice
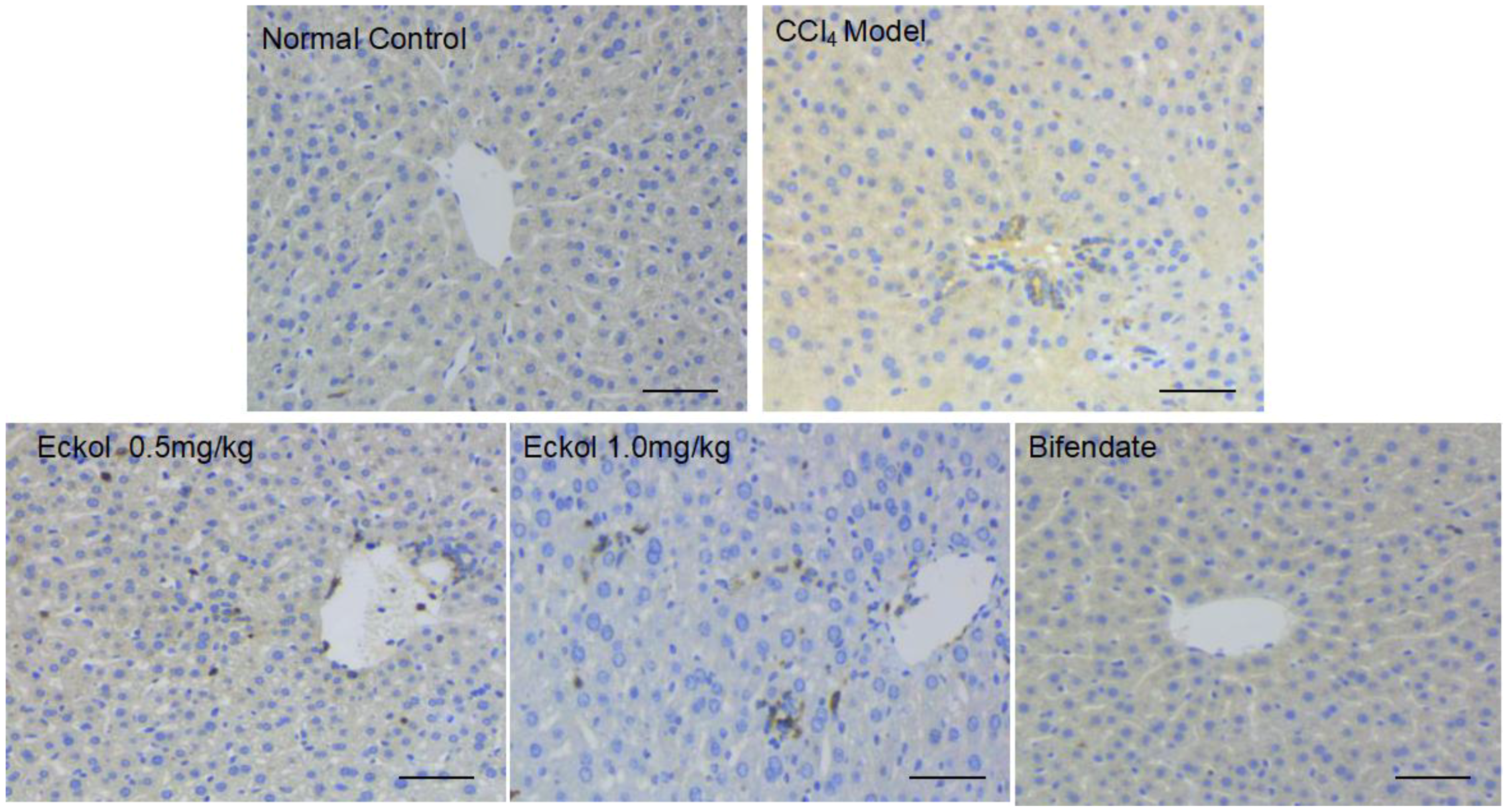
2.5. Eckol Recruites Dendritic Cells into the Liver Tissues
3. Materials and Methods
3.1. Chemicals
3.2. Animal and Treatment
3.3. Measurement of Serum AST and ALT
3.4. Histological Examination
3.5. Detection of Apoptotic Hepatocytes
3.6. Western Blotting
3.7. Measurement of Parameters Related to Oxidative Stress in the Liver
3.8. Measurement of TNF-a, IL-1β, IL-6 and IL-10 Levels
3.9. Immunohistochemistry
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, H.S.; Chung, H.Y.; Jung, J.H.; Son, B.W.; Choi, J.S. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem. Pharm. Bull. (Tokyo) 2003, 51, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Lee, Y.; Kim, S.Y.; Kim, H.S.; Joo, H.G.; Park, J.W.; et al. Eckol isolated from Ecklonia cava attenuates oxidative stress induced cell damage in lung fibroblast cells. FEBS Lett. 2005, 579, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Eom, S.H.; Kim, Y.M. Protective effect of phlorotannins from Eisenia bicyclis against lipopolysaccharide-stimulated inflammation in HepG2 cells. Environ. Toxicol. Pharmacol. 2013, 35, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia cava extract and dieckol attenuate cellular lipid peroxidation in keratinocytes exposed to PM10. Evid. Based Complement. Alternat. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Ku, S.K.; Bae, J.S. Antithrombotic and profibrinolytic activities of eckol and dieckol. J. Cell Biochem. 2012, 113, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Ahn, G.N.; Lee, N.H.; Kim, J.M.; Yun, J.S.; Hyun, J.W.; Jeon, Y.J.; Wie, M.B.; Lee, Y.J.; Park, J.W.; et al. Radioprotective properties of eckol against ionizing radiation in mice. FEBS Lett. 2008, 582, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Shin, T.; Utsuki, T.; Choi, J.S.; Byun, D.S.; Kim, H.R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and hepatoprotective properties in tacrine-treated HepG2 cells. J. Agric. Food Chem. 2012, 60, 5340–5349. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Kim, J.I.; Choung, S.Y.; Choi, J.S. Protective effect of the edible brown alga Ecklonia stolonifera on doxorubicin-induced hepatotoxicity in primary rat hepatocytes. J. Pharm. Pharmacol. 2014, 66, 1180–1188. [Google Scholar] [PubMed]
- Jun, Y.J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.H.; Kim, H.R. Eckol enhances heme oxygenase-1 expression through activation of Nrf2/JNK pathway in HepG2 cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, K.; Jeon, J.S.; Jho, E.H.; Kim, C.Y.; Nho, C.W.; Um, B.H. Isolation of phlorotannins from Eisenia bicyclis and their hepatoprotective effect against oxidative stress induced by tert-butyl hyperoxide. Appl. Biochem. Biotechnol. 2011, 165, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; An, R.B.; Yoon, N.Y.; Nam, T.J.; Choi, J.S. Hepatoprotective constituents of the edible brown alga Ecklonia stolonifera on tacrine-induced cytotoxicity in Hep G2 cells. Arch. Pharm. Res. 2005, 28, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.D.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Hyun, C.L.; Kang, H.K.; Lee, N.H.; et al. Cytoprotective effect of eckol against oxidative stress-induced mitochondrial dysfunction: Involvement of the FoxO3a/AMPK pathway. J. Cell Biochem. 2014, 115, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.G.; Zhang, C.; Wang, J.X.; Wang, B.W.; Wang, H.; Zhang, Z.H.; Chen, Y.H.; Lu, Y.; Tao, L.; Wang, J.Q.; Chen, X.; Xu, D.X. Obeticholic acid protects against carbon tetrachloride-induced acute liver injury and inflammation. Toxicol. Appl. Pharmacol. 2017, 314, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Sun, Z.; Jin, Z.; Lei, L.; Liu, Y.; Hu, B.; Wang, B.; Shen, Y.; Wang, Y. Matrix remodeling associated 7 deficiency alleviates carbon tetrachloride-induced acute liver Injury in mice. Front Immunol. 2018, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Dai, C.; Liu, Q.; Li, J.; Qiu, J. Curcumin attenuates on carbon tetrachloride-induced acute liver injury in mice via modulation of the Nrf2/HO-1 and TGF-β1/Smad3 pathway. Molecules 2018, 23, 215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhan, Q.; Li, Y.; Sun, S.; Zhao, L.; Zhang, H.; Zhang, G. Schisandra lignan extract protects against carbon tetrachloride-induced liver injury in mice by inhibiting oxidative stress and regulating the NF-κB and JNK signaling pathways. Evid. Based Complement. Alternat. Med. 2017, 2017, 5140297. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.F.; Safhi, M.M.; Anwer, T.; Siddiqui, R.; Khan, G.; Moni, S.S. Therapeutic potential of Vanillylacetone against CCl4 induced hepatotoxicity by suppressing the serum marker, oxidative stress, inflammatory cytokines and apoptosis in Swiss albino mice. Exp. Mol. Pathol. 2018, 105, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [PubMed]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qiu, Y.; Shu, Z.; Zhang, X.; Li, R.; Liu, S.; Chen, L.; Liu, H.; Chen, N. Protective effect of Trillium tschonoskii saponin on CCl4-induced acute liver injury of rats through apoptosis inhibition. Can. J. Physiol. Pharmacol. 2016, 94, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Nowicki, M.J. Caspase-12 mediates carbon tetrachloride- induced hepatocyte apoptosis in mice. World J. Gastroenterol. 2014, 20, 18189–18198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Lee, I.K.; Kim, B.J.; Jeong, I.Y.; Shin, T.; Park, J.W.; et al. Eckol protects V79-4 lung fibroblast cells against gamma-ray radiation-induced apoptosis via the scavenging of reactive oxygen species and inhibiting of the c-Jun NH(2)-terminal kinase pathway. Eur. J. Pharmacol. 2008, 591, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, N.H.; Joo, H.G.; Jee, Y. Modulation of apoptosis of eckol against ionizing radiation in mice. Biochem. Biophys. Res. Commun. 2008, 372, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Kim, S.H.; Kim, J.C.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phytother Res. 2008, 22, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid. Med. Cell Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, L.; Jin, P.; Li, N.; Meng, Y.; Wang, C.; Xu, M.; Zhang, Y.; Bian, J.; Deng, X. Methane alleviates carbon tetrachloride induced liver injury in mice: Anti-inflammatory action demonstrated by increased PI3K/Akt/GSK-3β- mediated IL-10 expression. J. Mol. Histol. 2017, 48, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kunitani, H.; Shimizu, Y.; Murata, H.; Higuchi, K.; Watanabe, A. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronicliver diseases. J. Hepatol. 2002, 36, 734–741. [Google Scholar] [CrossRef]
- Lukacs-Kornek, V.; Schuppan, D. Dendritic cells in liver injury and fibrosis: Shortcomings and promises. J. Hepatol. 2013, 59, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Knolle, P.A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 2010, 10, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Joo, H.G. Immunomodulatory effects of eckol, a pure compound of ecklonia cava, on dendritic cells. Immune Netw. 2006, 6, 199–203. [Google Scholar] [CrossRef]
- Barcala Tabarrozzi, A.E.; Andreone, L.; Deckers, J.; Castro, C.N.; Gimeno, M.L.; Ariolfo, L.; Berguer, P.M.; Antunica-Noguerol, M.; Liberman, A.C.; Vettorazzi, S.; et al. GR-independent down-modulation on GM-CSF bone marrow-derived dendritic cells by the selective glucocorticoid receptor modulator Compound A. Sci. Rep. 2016, 6, 36646. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Ferreira, M.L.; Verdan, M.H.; Dos Reis Lívero, F.A.; Galuppo, L.F.; Telles, J.E.; Alves Stefanello, M.É.; Acco, A.; Petkowicz, C.L. Inulin-type fructan and infusion of Artemisia vulgaris protect the liver against carbontetrachloride-induced liver injury. Phytomedicine 2017, 24, 68–76. [Google Scholar] [CrossRef] [PubMed]

| Groups | Doses | ALT(U/L) | AST(U/L) |
|---|---|---|---|
| Normal control | - | 9.86 ± 2.74 | 18.65 ± 7.21 |
| CCl4 model | - | 129.37 ± 38.91 ## | 117.22 ± 15.78 ## |
| Eckol | 0.5 mg/kg | 75.58 ± 23.45 ** | 86.79 ± 26.38 * |
| 1.0 mg/kg | 49.43 ± 16.03 ** | 63.21 ± 18.89 ** | |
| Bifendate | 200 mg/kg | 50.34 ± 18.35 ** | 61.05 ± 28.61 ** |
| Groups | Doses | Necrosis | Microvesicular Steatosis | Tumefaction | Inflammatory Infiltration |
|---|---|---|---|---|---|
| Normal control | - | − | - | 0 | 0 |
| CCl4 model | - | + | 1.7 ± 0.5 | 1.8 ± 0.4 | 1.9 ± 0.6 |
| Eckol | 0.5 mg/kg | − | 1.0 ± 0.7 ** | 1.2 ± 0.6 ** | 1.0 ± 0.7 ** |
| 1.0 mg/kg | − | 0.8 ± 0.7 ** | 0.8 ± 0.7 ** | 0.6 ± 0.5 ** | |
| Bifendate | 200 mg/kg | − | 0.9 ± 0.8 ** | 0.8 ± 0.7 ** | 0.8 ± 0.7 ** |
| Groups | Doses | MDA (nmol/mg prot) | GSH (nmol/mg prot) | SOD (U/mg prot) | GSH-Px (U/mg prot) |
|---|---|---|---|---|---|
| Normal control | - | 2.46 ± 0.62 | 119.32 ± 34.35 | 143.69 ± 30.81 | 101.49 ± 16.38 |
| CCl4 model | - | 4.55 ± 0.73 ## | 61.38 ± 29.91 ## | 91.52 ± 26.37 ## | 50.26 ± 13.87 ## |
| Eckol | 0.5 mg/kg | 3.62 ± 1.09 * | 88.59 ± 28.32 * | 112.36 ± 37.72 | 76.56 ± 20.97 ** |
| 1.0 mg/kg | 2.86 ± 0.74 ** | 93.78 ± 25.50 * | 139.79 ± 45.52 ** | 92.67 ± 29.38 ** | |
| Bifendate | 200 mg/kg | 3.73 ± 0.96 * | 85.39 ± 26.69 | 106.89 ± 38.21 | 74.55 ± 32.73 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, J.; Zhang, M.; Chen, Y.; Zhu, T.; Wang, J. Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Mar. Drugs 2018, 16, 300. https://doi.org/10.3390/md16090300
Li S, Liu J, Zhang M, Chen Y, Zhu T, Wang J. Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Marine Drugs. 2018; 16(9):300. https://doi.org/10.3390/md16090300
Chicago/Turabian StyleLi, Shulan, Juan Liu, Mengya Zhang, Yuan Chen, Tianxing Zhu, and Jun Wang. 2018. "Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice" Marine Drugs 16, no. 9: 300. https://doi.org/10.3390/md16090300
APA StyleLi, S., Liu, J., Zhang, M., Chen, Y., Zhu, T., & Wang, J. (2018). Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Marine Drugs, 16(9), 300. https://doi.org/10.3390/md16090300




