Eurotiumins A–E, Five New Alkaloids from the Marine-Derived Fungus Eurotium sp. SCSIO F452
Abstract
:1. Introduction
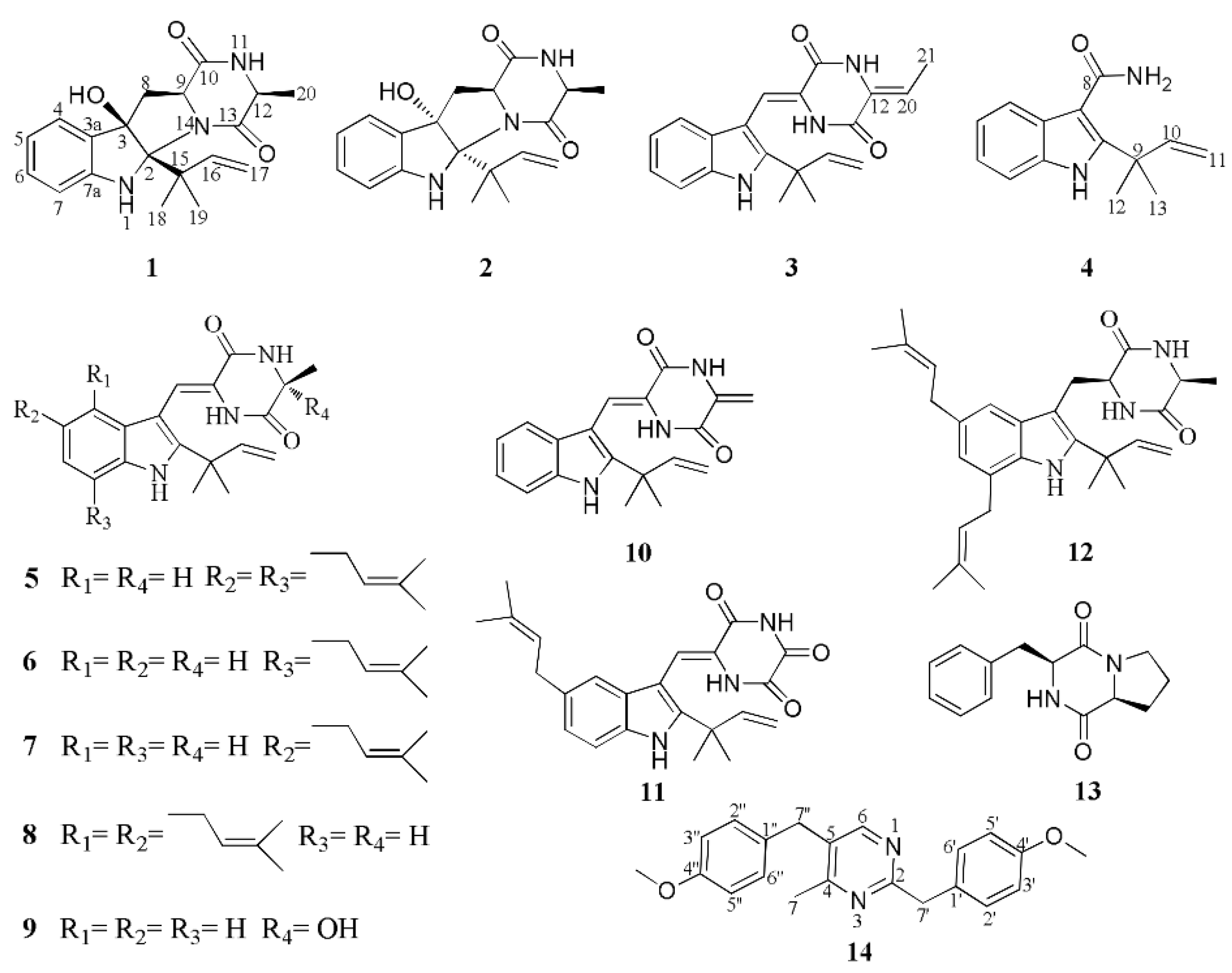
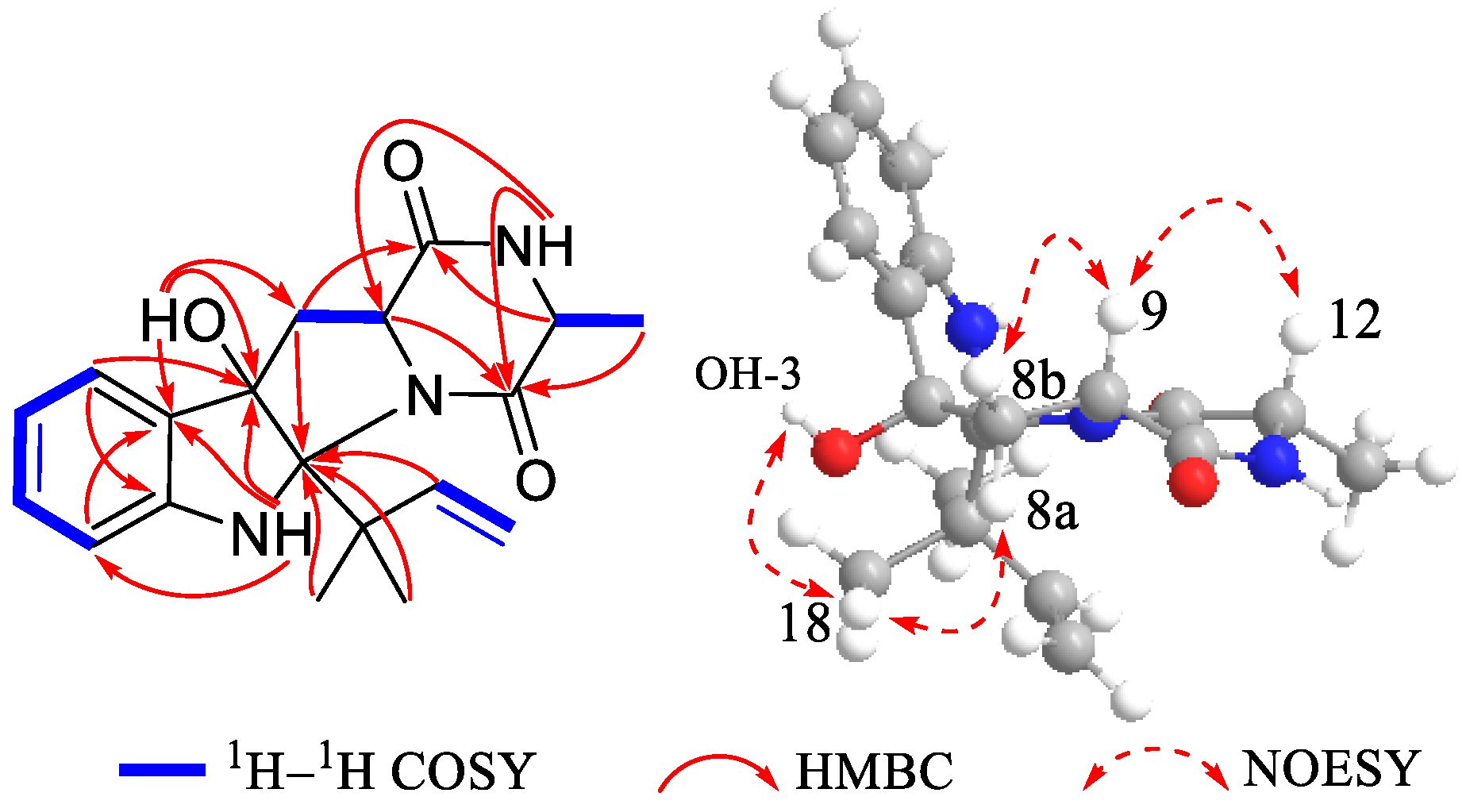
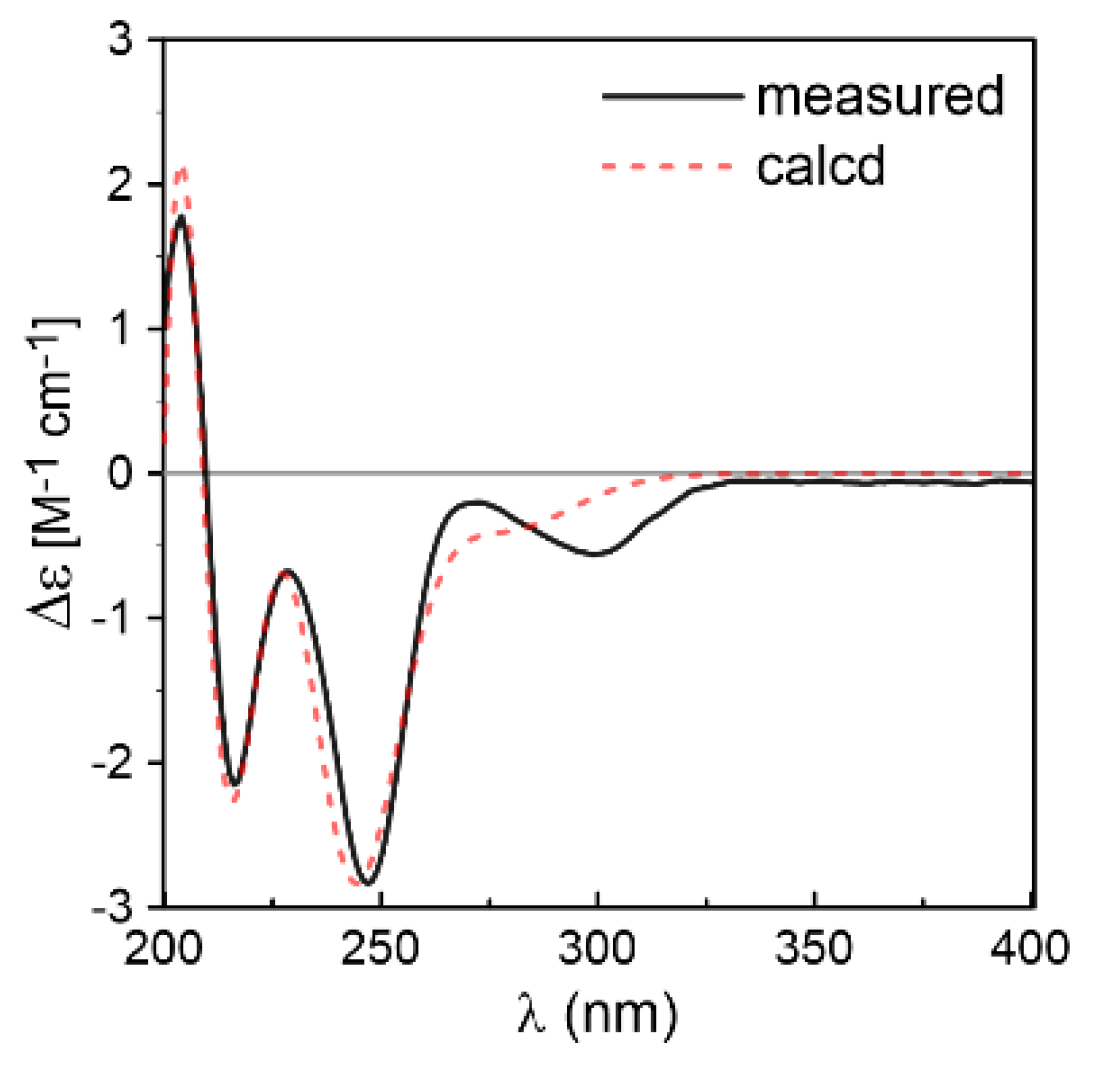
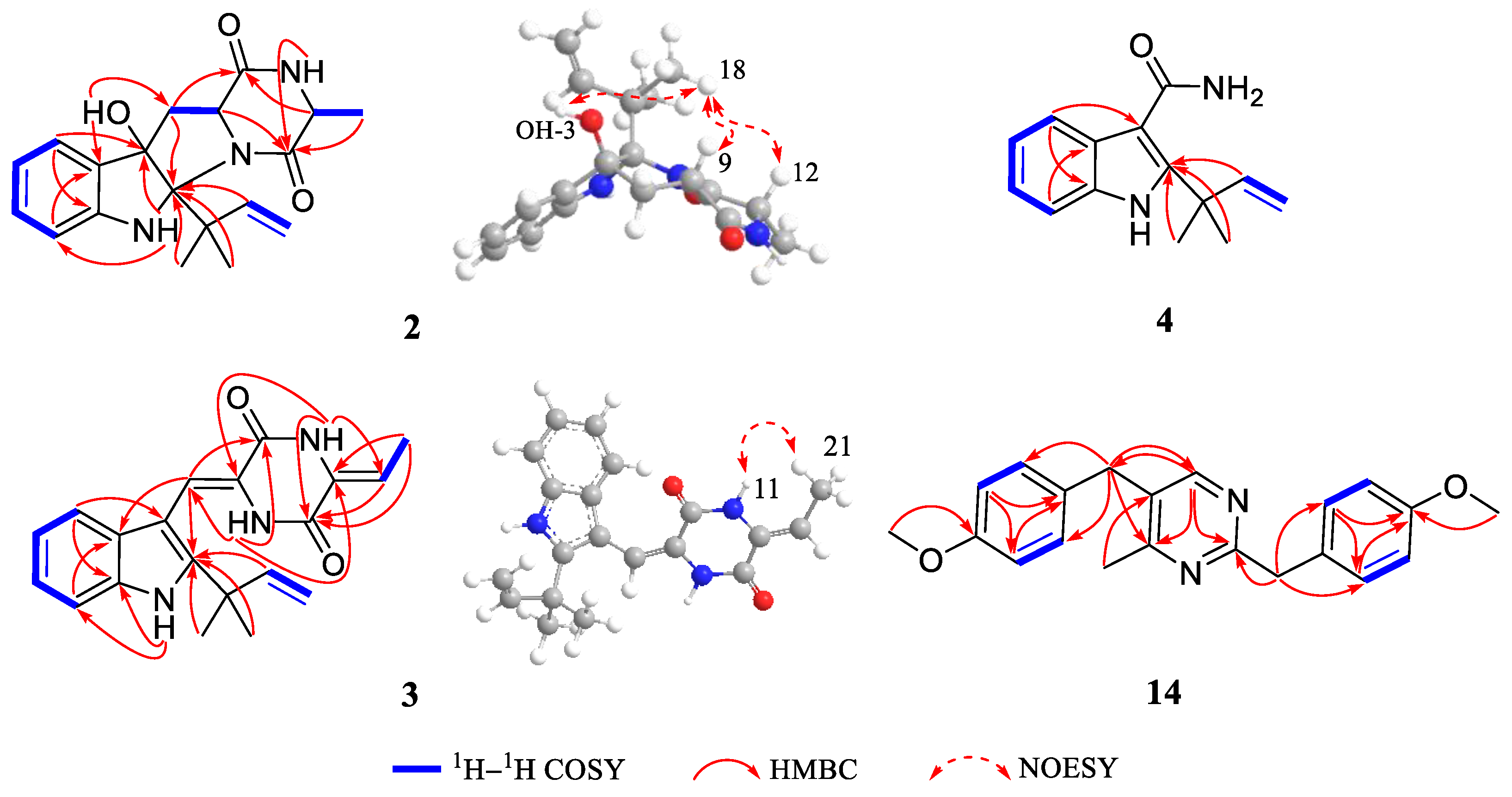
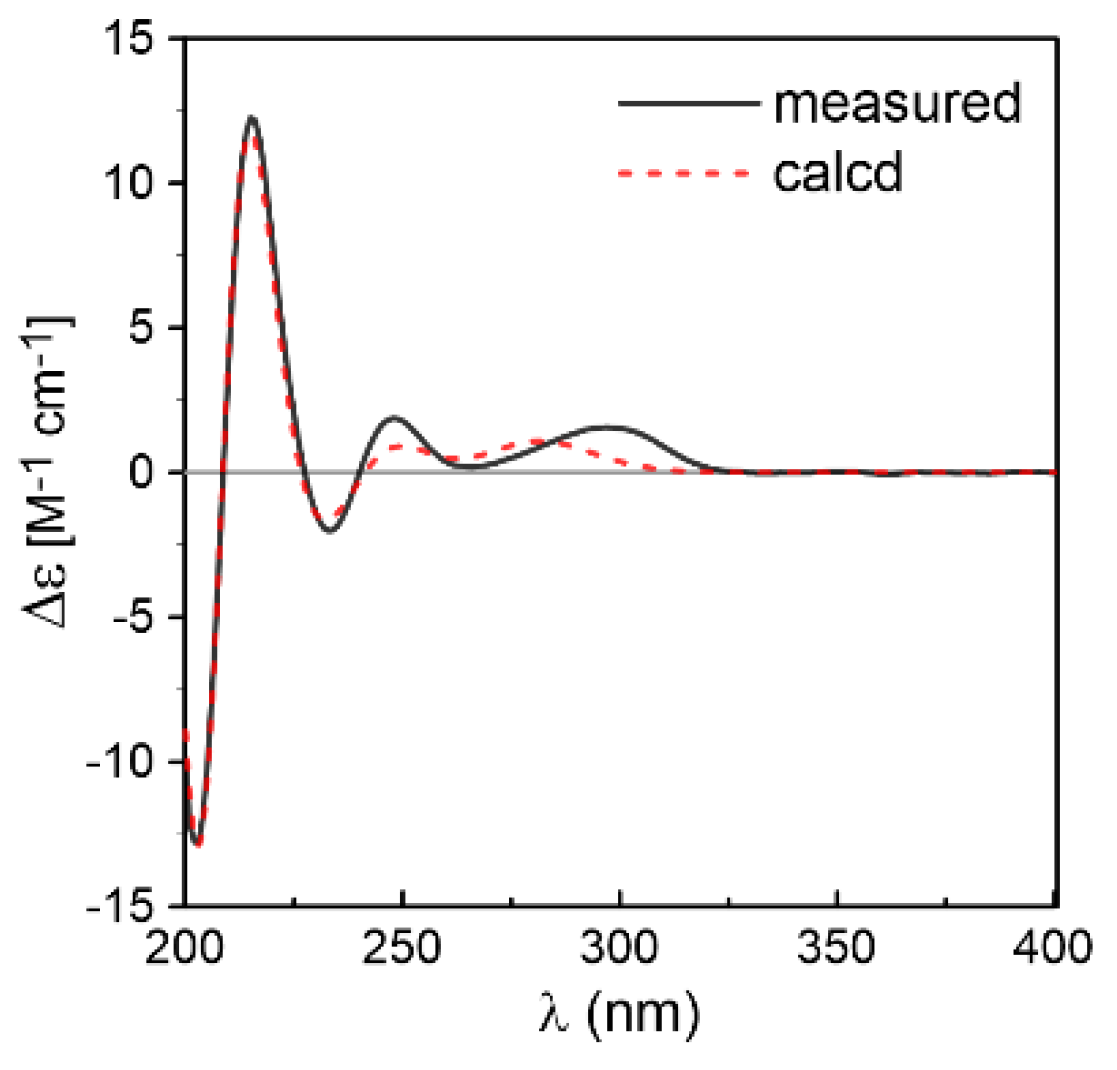
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. Purification
3.5. Spectral Data
3.6. Computational Methods
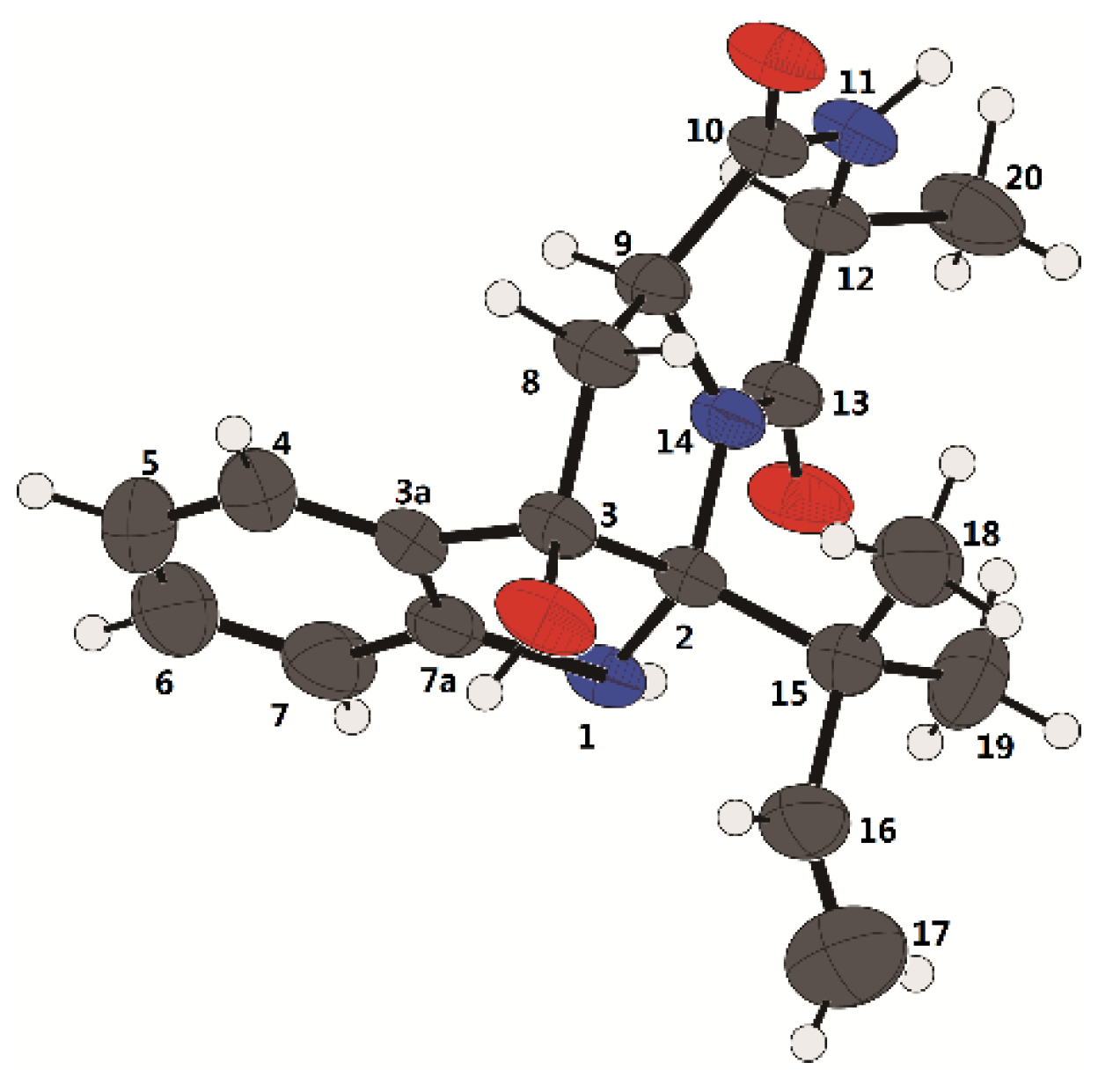
3.7. X-ray Crystal Structure Analysis
3.8. Antioxidative Assay
3.9. Cytotoxic Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010, 27, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.M.; Liang, X.A.; Kong, Y.; Jia, B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Li, X.; Li, X.M.; Zhu, L.W.; Wang, B.G. Indolediketopiperazine alkaloids from Eurotium cristatum EN-220, an endophytic fungus isolated from the marine alga Sargassum thunbergii. Mar. Drugs 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Du, F.Y.; Li, X.M.; Pedpradab, P.; Xu, G.M.; Wang, B.G. Rubrumazines A–C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 2015, 78, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Lu, Z.Y.; Tao, H.W.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Isoechinulin-type alkaloids, variecolorins A–L, from halotolerant Aspergillus variecolor. J. Nat. Prod. 2007, 70, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Samizo, M.; Nojiri, Y.; Onuki, H.; Hirota, H.; Ohta, T. Notoamides F−K, prenylated indole alkaloids isolated from a marine-derived Aspergillus sp. J. Nat. Prod. 2008, 71, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
- Ravikanth, V.; Niranjan Reddy, V.L.; Ramesh, P.; Prabhakar Rao, T.; Diwan, P.V.; Khar, A.; Venkateswarlu, Y. An immunosuppressive tryptophan-derived alkaloid from Lepidagathis cristata. Phytochemistry 2001, 58, 1263–1266. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, Z.H.; Liu, Z.; Chen, Y.C.; Liu, H.X.; Li, H.H.; Zhang, W.M. Dichotocejpins A–C: New diketopiperazines from a deep-sea-derived fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; He, W.J.; Qin, X.C.; Wei, X.Y.; Tian, X.P.; Liao, L.; Liao, S.R.; Yang, B.; Tu, Z.C.; Chen, B.; et al. Three new indolyl diketopiperazine metabolites from the antarctic soil-derived fungus Penicillium sp. SCSIO 05705. RSC Adv. 2015, 5, 68736–68742. [Google Scholar] [CrossRef]
- Tian, Z.H.; Chu, Y.Y.; Wang, H.; Zhong, L.L.; Deng, M.Y.; Li, W.B. Biological activity and interaction mechanism of the diketopiperazine derivatives as tubulin polymerization inhibitors. RSC Adv. 2018, 8, 1055–1064. [Google Scholar] [CrossRef]
- Wang, F.Z.; Huang, Z.; Shi, X.F.; Chen, Y.C.; Zhang, W.M.; Tian, X.P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.B.; Wang, F.Z.; Luo, M.H.; Chen, Y.C.; Song, Y.X.; Zhang, W.M.; Zhang, S.; Ju, J.H. Halogenated anthraquinones from the marine-derived fungus Aspergillus sp. SCSIO F063. J. Nat. Prod. 2012, 75, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Huang, Z.; Shi, X.F.; Chen, Y.C.; Zhang, W.M.; Tian, X.P.; Li, J.; Zhang, S. Analysis of secondary metabolites produced by Eurotium sp. SCSIO F452 isolated from the South China Sea sediment. Zhongguo Haiyang Yaowu 2013, 32, 7–12. [Google Scholar]
- Wollinsky, B.; Ludwig, L.; Xie, X.; Li, S.M. Breaking the regioselectivity of indole prenyltransferases: Identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org. Biomol. Chem. 2012, 10, 9262–9270. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Cui, X.; Lee, D.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-inflammatory effect of neoechinulin A from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-small ka, CyrillicB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules 2013, 18, 13245–13259. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Q.; Liu, W.Z.; Zhu, T.J.; Mo, X.; Mandi, A.; Kurtan, T.; Li, J.; Ai, J.; Gu, Q.Q.; Li, D.H. Diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Org. Biomol. Chem. 2012, 10, 9501–9506. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.; Artico, M.; Sbardella, G.; Massa, S.; Novellino, E.; Greco, G.; Loi, A.G.; Tramontano, E.; Marongiu, M.E.; La Colla, P. 5-Alkyl-2-(alkylthio)-6-(2,6-dihalophenylmethyl)-3,4-dihydropyrimidin-4(3H)-ones: novel potent and selective dihydro-alkoxy-benzyl-oxopyrimidine derivatives. J. Med. Chem. 1999, 42, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Zaton, A.M.; Villamor, J.P. Study of heterocycle rings binding to human serum albumin. Chem. Biol. Interact. 2000, 124, 1–11. [Google Scholar] [CrossRef]
- Selassie, C.D.; Gan, W.X.; Kallander, L.S.; Klein, T.E. Quantitative structure−activity relationships of 2,4-Diamino-5-(2-X-benzyl)pyrimidines versus bacterial and avian dihydrofolate reductase. J. Med. Chem. 1998, 41, 4261–4272. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Martı́nez-Álvarez, R.; Ramiro, P.; Chioua, M.; Torres, R. On the mechanism of reaction between ketones and nitriles. Unexpected results from benzyl nitriles. Tetrahedron 2002, 58, 3755–3764. [Google Scholar] [CrossRef]
- Tanaka, H.; Takashima, H.; Ubasawa, M.; Sekiya, K.; Inouye, N.; Baba, M.; Shigeta, S.; Walker, R.T.; De Clercq, E.; Miyasaka, T. Synthesis and antiviral activity of 6-benzyl analogs of 1-[(2-hydroxyethoxy)methyl]-5-(phenylthio)thymine (HEPT) as potent and selective anti-HIV-1 agents. J. Med. Chem. 1995, 38, 2860–2865. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Wang, B.G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2008, 91, 1888–1893. [Google Scholar] [CrossRef]
- Zhou, L.N.; Zhu, T.J.; Cai, S.X.; Gu, Q.Q.; Li, D.H. Three new indole-containing diketopiperazine alkaloids from a deep-ocean sediment derived fungus Penicillium griseofulvum. Helv. Chim. Acta 2010, 93, 1758–1763. [Google Scholar] [CrossRef]
- Ye, W.C.; Wang, G.C.; Li, T.; Huang, X.J. Whitmanoside A, a new α-pyrone glycoside from the Leech Whitmania pigra. Heterocycles 2013, 87, 1537–1543. [Google Scholar]
- Wang, W.L.; Zhu, T.J.; Tao, H.W.; Lu, Z.Y.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Three novel, structurally unique spirocyclic alkaloids from the halotolerant B-17 fungal strain of Aspergillus variecolor. Chem. Biodivers. 2007, 4, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Elshahawi, S.I.; Cao, H.; Shaaban, K.A.; Ponomareva, L.V.; Subramanian, T.; Farman, M.L.; Spielmann, H.P.; Phillips, G.N., Jr.; Thorson, J.S.; Singh, S. Structure and specificity of a permissive bacterial C-prenyltransferase. Nat. Chem. Biol. 2017, 13, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Awakawa, T.; Nakashima, Y.; Mori, T.; Zhu, Q.; Liu, X.; Abe, I. Two distinct substrate binding modes for the normal and reverse prenylation of hapalindoles by the prenyltransferase AmbP3. Angew. Chem. Int. Ed. 2018, 57, 560–563. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J, Hz) | δC | δH (J, Hz) | δC | δH (J, Hz) | |
| 1 | 6.53 s | 6.58 br s | 11.12 s | |||
| 2 | 92.4 | 95.4 | 144.3 | |||
| 3 | 89.4 | 88.3 | 103.3 | |||
| 3a | 132.2 | 132.7 | 125.9 | |||
| 4 | 124.5 | 7.28 d (7.6) | 125.5 | 7.17 d (7.4) | 118.8 | 7.18 d (7.9) |
| 5 | 120.1 | 6.76 overlap | 119.9 | 6.66 overlap | 119.6 | 7.02 td (7.5, 1.0) |
| 6 | 130.8 | 7.12 td (7.6, 1.2) | 130.3 | 7.02 td (7.5, 1.3) | 120.9 | 7.10 td (7.6, 1.1) |
| 7 | 111.8 | 6.75 overlap | 111.3 | 6.64 overlap | 111.7 | 7.43 d (8.1) |
| 7a | 150.6 | 149.9 | 135.1 | |||
| 8 | 36.4 | a 2.76 dd (12.7, 11.2) | 36.8 | a 3.35 dd (13.3, 2.3) | 110.8 | 6.94 s |
| b 2.56 dd (12.7, 7.6) | b 2.64 dd (13.4, 10.9) | |||||
| 9 | 59.2 | 3.73 dd (11.2, 7.6) | 59.4 | 4.41 dd (10.9, 2.5) | 124.5 | |
| 10 | 170.6 | 169.3 | 157.6 | |||
| 11 | 7.16 br s | 6.85 br s | 10.21 s | |||
| 12 | 53.0 | 3.91 q (6.9) | 51.0 | 4.11 q (6.7) | 128.4 | |
| 13 | 174.1 | 170.3 | 156.4 | |||
| 14 | 8.68 s | |||||
| 15 | 45.7 | 45.8 | 40.0 | |||
| 16 | 146.5 | 6.36 dd (17.6, 10.9) | 146.3 | 6.52 dd (17.6, 10.8) | 145.1 | 6.08 dd (17.4, 10.5) |
| 17 | 111.8 | 4.98 dd (17.7, 1.2) | 112.4 | 5.14 dd (17.7, 1.6) | 111.7 | 5.06 dd (10.4, 1.2) |
| 4.90 dd (10.9, 1.5) | 5.02 dd (10.7, 1.6) | 5.03 dd (17.4, 1.2) | ||||
| 18 | 24.7 | 1.27 s | 24.7 | 1.36 s | 27.5 | 1.48 s |
| 19 | 25.7 | 1.29 s | 25.5 | 1.39 s | 27.5 | 1.48 s |
| 20 | 15.8 | 1.33 d (6.9) | 14.9 | 1.15 d (6.7) | 112.9 | 5.87 q (7.6) |
| 21 | 11.1 | 1.81 d (7.6) | ||||
| 3-OH | 4.49 s | 4.32 s | ||||
| Position | 4 | Position | 14 | ||
|---|---|---|---|---|---|
| δC | δH (J, Hz) | δC | δH (J, Hz) | ||
| 1 | 1 | ||||
| 2 | 146.1 | 2 | 152.9 | ||
| 3 | 109.8 | 3 | |||
| 3a | 128.5 | 4 | 154.4 | ||
| 4 | 120.2 | 7.70 d (7.7) | 5 | 152.2 | |
| 5 | 120.8 | 7.04 td (7.1, 1.2) | 6 | 141.5 | 8.26 s |
| 6 | 122.1 | 7.07 td (7.0, 1.3) | 7 | 22.1 | 2.44 s |
| 7 | 112.0 | 7.37 d (8.1) | 1′ | 132.2 | |
| 7a | 135.1 | 2′/6′ | 130.8 | 7.23 d (8.7) | |
| 8 | 169.1 | 3′/5′ | 114.8 | 6.85 d (8.6) | |
| 9 | 40.0 | 4′ | 159.3 | ||
| 10 | 147.0 | 6.41 dd (17.5, 10.6) | 7′ | 41.0 | 4.08 s |
| 11 | 112.3 | 5.12 dd (17.5, 1.2) | 4′-OMe | 55.4 | 3.75 s |
| 5.06 dd (10.6, 1.2) | 1″ | 131.3 | |||
| 12 | 27.3 | 1.64 s | 2″/6″ | 130.5 | 7.12 d (8.7) |
| 13 | 27.3 | 1.64 s | 3″/5″ | 114.7 | 6.82 d (8.7) |
| 4″ | 159.3 | ||||
| 7″ | 40.7 | 4.01 s | |||
| 4″-OMe | 55.4 | 3.73 s | |||
| Nos. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 9 | 10 | 12 | 13 | 14 | VC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | 37 | 69 | 13 | >100 | 19 | 4 | 3 | 24 | 13 | 18 | 35 | >100 | 23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.-M.; Wang, J.-F.; Shi, X.-F.; Wei, X.-Y.; Chen, Y.-C.; Zeng, Q.; Xiang, Y.; Chen, X.-Y.; Tian, X.-P.; Xiao, Z.-H.; et al. Eurotiumins A–E, Five New Alkaloids from the Marine-Derived Fungus Eurotium sp. SCSIO F452. Mar. Drugs 2018, 16, 136. https://doi.org/10.3390/md16040136
Zhong W-M, Wang J-F, Shi X-F, Wei X-Y, Chen Y-C, Zeng Q, Xiang Y, Chen X-Y, Tian X-P, Xiao Z-H, et al. Eurotiumins A–E, Five New Alkaloids from the Marine-Derived Fungus Eurotium sp. SCSIO F452. Marine Drugs. 2018; 16(4):136. https://doi.org/10.3390/md16040136
Chicago/Turabian StyleZhong, Wei-Mao, Jun-Feng Wang, Xue-Feng Shi, Xiao-Yi Wei, Yu-Chan Chen, Qi Zeng, Yao Xiang, Xia-Yu Chen, Xin-Peng Tian, Zhi-Hui Xiao, and et al. 2018. "Eurotiumins A–E, Five New Alkaloids from the Marine-Derived Fungus Eurotium sp. SCSIO F452" Marine Drugs 16, no. 4: 136. https://doi.org/10.3390/md16040136
APA StyleZhong, W.-M., Wang, J.-F., Shi, X.-F., Wei, X.-Y., Chen, Y.-C., Zeng, Q., Xiang, Y., Chen, X.-Y., Tian, X.-P., Xiao, Z.-H., Zhang, W.-M., Wang, F.-Z., & Zhang, S. (2018). Eurotiumins A–E, Five New Alkaloids from the Marine-Derived Fungus Eurotium sp. SCSIO F452. Marine Drugs, 16(4), 136. https://doi.org/10.3390/md16040136





